Abstract
Background and Aims
Variation in fitness depends on corresponding variation in multiple traits which have both genetically controlled and plastic components. These traits are subjected to varying degrees of local adaptation in specific populations and, consequently, are genetically controlled to different extents. In this study it is hypothesized that modulation of different traits would have contrasting relevance for the fitness of populations of diverse origins. Specifically, assuming that environmental pressures vary across a latitudinal gradient, it is suggested that inherited variation in traits differentially determines fitness in annual Lupinus angustifolius populations from contrasting latitudinal origins in western Spain.
Methods
Seeds of L. angustifolius from three contrasting origins were grown in a common garden. Traits related to more plastic vegetative growth and more genetically conserved phenology were measured, together with estimates of reproductive success. Fitness was estimated by the number of viable seeds per plant. Structural Equation Models were used to infer causal relationships among multiple traits and fitness, separating the direct and indirect effects of morphological, phenological and reproductive traits.
Key Results
Phenological, vegetative and reproductive traits accounted for most of the fitness variation. Fitness was highest in plants of southernmost origin, mainly due to earlier flowering. Fitness within each seed origin was controlled by variation in different traits. Southern origin plants that grew to a larger size achieved higher fitness. However, plant size in plants of northernmost origin was irrelevant, but early flowering promoted higher fitness. Variation in fruit and seed set had a greater effect on the fitness of plants of central origin than phenological and size variation.
Conclusions
It is concluded that modulation of a functional trait can be relevant to fitness in a given population (i.e. affecting intensity and direction), but irrelevant in other populations. This points to the need to consider integrated phenotypes when trying to unravel local adaptation effects over single traits.
Key words: Lupinus, Structural Equation Models, fitness, phenology, functional traits, reproductive success, SLA, seed size
INTRODUCTION
Variation in plant fitness is determined by the interaction between heritable trait variation and the external environment (Lechowicz and Blais, 1988; Blais and Lechowicz, 1989; Farris and Lechowicz, 1990). Lechowicz and co-workers, after conducting extensive pioneering experiments, showed that: (a) when the environment shifts or different populations of origin are considered, different traits of annual species can respond in opposite, parallel or independent directions; and (b) the relative contribution of each trait to plant fitness varies when environmental or genotypic change takes place (Lechowicz, 1984; Lechowicz and Blais, 1988; Blais and Lechowicz, 1989). Thus, the relevance of modulating traits such as photosynthetic rates, plant size or flowering phenology in achieving a given level of performance varies among genotypes and habitats. Thereafter, this pattern has been found in multiple studies on the interaction between variation in plant traits and fitness (e.g. Jordan, 1996; Arntz et al., 1998; Arntz and Delph, 2001; Cheplick, 2002).
The different response of multiple traits to a given environmental stimulus is relevant for understanding how a plant species responds to the local environment and how trait responses are combined to maximize fitness. When traits do not have a simple plastic phenotypic response, widespread local genetic differentiation occurs. Traits that directly affect fitness in annual plants differ in their plastic and genetic components of variation. For instance, phenology is known to have a strong genetic component, whereas growth rate and related vegetative traits are more plastic. Moreover, reproductive processes are strongly dependent on local events and environmental conditions such as pollinator or resource abundance (Lechowick and Blais, 1988; Nagy and Rice, 1997; Santamaria et al., 2003). Although it is well established that fitness depends on a wide array of factors, we are only aware of a few recent studies that have directly addressed the relative contribution of all of the above factors through a statistically explicit approach (Volis et al., 2004; Picotte et al., 2007; Tonsor and Scheiner, 2007). Most previous studies have focused separately on the importance of phenological changes, the disruption of reproductive processes or the modification of growth rates and related vegetative traits on individual fitness (Chapin and Chapin, 1981; Stenøien et al., 2002; Becker et al., 2006; Yoshie, 2007).
Our understanding of fitness variation of annual plant species is thus extensive, but mostly fractional. The aim of this work is to understand how fitness and the main morphological, physiological and phenological traits underlying fitness vary as a function of latitudinal seed origin in an annual legume. More specifically, we wanted to know whether fitness always depends on the same traits or if local conditions restrict the way traits can contribute to fitness. The relationship between these multiple traits was observed in a common garden experiment where plants from climatically contrasting seed origins were grown and data further analysed using structural equation modelling (SEMs; Shipley, 2002). Confirmatory SEM involves testing a structure of relationships between multiple variables. Based on the premise that fitness is the result of a complex multistep developmental process where provenance can affect plant traits in various ways, we put forward an overall causal structure as our initial working hypothesis. This structure included the following expectations: (a) seed origin indirectly affects fitness through the modulation of different traits related to growth, phenology and reproduction; (b) seed origin has a greater effect on traits such as flowering phenology, whereas reproductive traits and those related to growth rate are more plastic and thus less affected by seed origin; (c) fitness of plants of contrasting origin will be affected differently by variation in traits, e.g. variation in flowering phenology may have a greater effect on fitness in plants of northern origin due to the shorter growth season, comparted with southernmost plants; and (d) during plant development, phenological, growth and reproductive traits will be inter-related, each of them producing both direct and indirect effects on fitness via multiple trait interactions. Details on the causal structure of this model are explained in full in the Materials and Methods section.
MATERIALS AND METHODS
Study species and seed origin
Lupinus angustifolius L. is an annual legume, widespread as a weed across the Mediterranean Basin. It inhabits environments subjected to frequent disturbance, such as road or forest edges. It grows preferentially on acid sandy soils. This species is well suited for this experiment due to its short life cycle, with seeds germinating in late autumn and winter, and plants senescing by early summer, which allows prompt assessment of fitness components. In addition, the germination rate of this species is almost 100 % after mechanical scarification. Functionally, it is predominantly a passive selfer and, when outcrossing takes place, flowers are pollinated by an assembly of widely distributed generalist pollinators (Forbes et al., 1971). Consequently, reproduction is scarcely dependent on the local community of pollinators at the common garden site.
Seeds from L. angustifolius were obtained from the Spanish National Germplasm Repository of the ‘Instituto Nacional de Investigaciones Agrarias’ in Alcalá de Henares, Spain. Seed collection protocols at the repository ensure that a representative sample of the target population is included in each accession. Seed collection was performed by randomly harvesting mature legumes across the population (H. Pascual, IMIDRA, Madrid, pers. obs.). This precludes over-representation of plants producing few seed and the harvesting of seeds coming solely from a few large individuals. In essence, this imitates a natural dispersion event, with more successful plants contributing more seed to the accession than less productive plants. Seeds were selected from three sites located along the latitudinal gradient spanning the distribution of L. angustifolius in western Spain (see Table 1). The three locations are Asturianos, Zamora province, Berruecopardo, Salamanca province, and Jaraiz de la Vera, Cáceres province (hereafter northern, central and southern seed origins, respectively). This latitudinal gradient encompasses a pronounced climate gradient (see climate parameters in Table 1).
Table 1.
Climate at the locations of seed origin, and at the common garden site
| Site | Co-ordinates | Altitude (m asl) | MAT (°C) | Tmin (°C) | Tmax (°C) | Rainfall (mm year−1) |
|---|---|---|---|---|---|---|
| Cold origin, Asturianos, (Zamora province) | 42°02′51″N 6°28′39″W | 942 | 10·0 | 4·0 | 16·0 | 897 |
| Cool origin, Berruecopardo, (Salamanca province) | 41°04′33″N 6°39′27″W | 700 | 13·0 | 7·0 | 19·0 | 671 |
| Warm origin, Jaraiz de la Vera, (Cáceres province) | 40°05′50″N 5° 44′06″W | 516 | 15·0 | 9·0 | 21·0 | 1232 |
| Common garden site, Móstoles, (Madrid province) | 40°18′48″N 3° 52′57″W | 632 | 14·0 | 8·0 | 21·0 | 481 |
Data are long-term averages extracted from the Atlas Climatico Digital de la Peninsula Iberica (http://opengis.uab.es/wms/iberia/mms/index.htm).
MAT, mean annual temperature; Tmin, yearly mean of the monthly minima; Tmax,yearly mean of the monthly maxima.
Experimental set-up
Seeds (125 per seed origin) were scarified by gently cracking the seed coat on the side opposite to the embryo with a pair of cutting pliers, and sown on 9 January 2007 under the following common growing conditions. One seed was sown per 1500-cm3 pot containing a substrate of 28 % sand, 15 % perlite and 56 % commercial peat. Pots were then placed on greenhouse shelves. Shelves were subjected to regular automatic water sprinkling as needed to maintain seedlings under optimal growing conditions.
At the onset of flowering, from 1 to 7 May 2007, plants were transplanted to 8100 cm3 containers filled with the same substrate described above and taken out of the greenhouse. Pots of each origin were placed together in the vicinity of the greenhouse and separated from pots of the other two origins by about 150 m (keeping site differences among placements negligible) to suppress the effect of uncontrolled between-population outcrossing on fitness. From the date of transplant to the end of the experiment, containers were watered manually to field capacity twice a week and were also exposed to rainfall. This watering regime progressively reduced the water content in the pots as temperatures increased and precipitation ceased by the end of spring. This procedure simulated the arrival of summer conditions, typical of the habitats where L. angustifolius thrives, and helped imitate the timing of plant senescence of this species in nature. This is particularly relevant for our study species, since variation in yield of L. angustifolius has been shown to depend strongly on the progression of summer drought (Dracup et al., 1998a). Regular watering was required, however, to avoid early, drastic desiccation of the pots, as plants growing in pots are much more sensitive to decreases in rainfall than soil-rooted plants.
Plant traits and fitness
Several measurements were taken on seeds and growing plants in order to (a) characterize phenological, morphological and reproductive traits that affect individual performance; and (b) evaluate individual fitness.
Variables related to plant productivity were measured as follows. Seed size (mass, mg) was a surrogate of early provisioning for seedling growth. Seeds were weighed to the nearest 0·1 mg prior to sowing. Specific leaf area (SLA, m2 kg−1) was an indicator of relative plant growth rate. The tightness of the SLA–growth rate relationship depends on growth form and light environment, being tighter for herbs growing in low light (Shipley, 2006). Here, SLA was sampled when the plants were juvenile and still in the greenhouse, and thus subjected to moderate neutral shading as needed by the automatic cooling mechanism of the greenhouse. Under these conditions, SLA should be a good surrogate of relative growth rate (RGR; Shipley, 2006). When plants had four mature leaves, one fully mature green leaflet was harvested per plant and scanned. The leaflets were subsequently oven-dried at 70 °C to constant weight, and weighed to the nearest microgram using a microbalance (Micro UMX-MX, Metler Toledo, Barcelona, Spain). Scanned leaves were processed with ImageJ software (http://rsb.info.nih.gov/ij/) to measure leaflet area. SLA was then calculated as the ratio of leaf area to leaf dry mass. SLA was measured only once in each plant, which does not account for putative ontogenetic variability in this trait. At the time of flowering, maximum and minimum plant canopy diameters and plant height were measured to the nearest 0·5 cm on each plant. After these measurements were taken, ten plants of contrasting sizes from each of the three seed origins were selected and the above-ground fraction was clipped, oven-dried and weighed. Linear models relating these plant dimensions and above-ground dry weight were obtained from the 30 individuals (R2 = 0·78; P < 0·01), and used for inferring the dry weight of all remaining plants of the experiment. Plant size at flowering onset (g of above-ground biomass) was used as a proxy for the pool of internal plant resources available for reproduction.
The phenological stage of each plant was assessed every 4–7 d. On each visit, each plant was allocated to one of the following phenological phases: seedling stage, juvenile vegetative stage, flower bud initiation, flowering, fruit ripening and seed dispersal.
Flowering synchrony was calculated considering all pairs of plants and the following function (Albert et al., 2001, as modified from Augspurger, 1981):
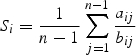 |
where n is the number of plants, aij is the number of days on which j and i individuals are simultaneously flowering and bij is the number of days on which at least one of them (j and/or i) is flowering. This index ranges from 0, when there is no synchrony, to 1 when flowering overlap is complete.
Reproductive success was measured at the end of the reproductive period. The number of pods containing fully developed seeds were counted, as well as the number of pedicel scars per plant. Fruit set was then estimated as the ratio of the number of fruits to the number of pedicels plus fruits. One pod per plant (the number of pods produced per mature individual varied from one to 12) was then harvested at random, and the number of fully developed seeds inside the pod and the number of undeveloped ovules were counted. Seed set was then computed as the ratio of seed number to total number of developed and undeveloped ovules in the pod. All other fully mature seeds of each individual were collected and counted.
Fitness was measured as the total number of fully mature seeds produced per plant by the end of its life cycle. This was considered the net contribution of an individual to the next generation in terms of viable offspring.
Data analysis
Analyses of survivorship throughout the experiment were carried out through χ2 tests of contingency tables. Furthermore, to investigate the effect of seed and seedling traits on survivorship, generalized linear models (GLMs; McCullagh and Nelder, 1989) were used, including seed size and SLA as explanatory variables, and survivorship until reproductive stage as a binomial, response variable. Binomial error and logit link function were specified. Models were run independently for each seed origin.
Comparisons of phenological, morphological and reproductive variables among seed origins were also conducted with GLMs. Seed origin was included as a fixed-effect factor. Normal error and identity link function were specified for SLA and seed size, as these variables were normally distributed. Poisson error and log link function distribution were implemented for all other variables. Type 3 option of PROC GENMOD (SAS v.9) was used to analyse the main effects in all GLMs. Multiple comparisons among populations were carried out whenever a seed origin effect was detected. Dunnet's T3 correction (recommended when the sample size is small, and variance among groups is unequal) was used for multiple comparison tests.
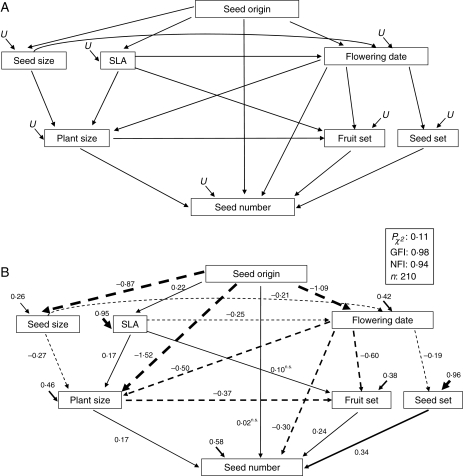
SEM was used to investigate interactions between multiple traits and fitness based on previous knowledge (Iriondo et al., 2003). SEM can be used to unravel the structure linking traits that are correlated in a multivariate way (Shipley, 2004). In addition, this approach allows direct effects to be disentangled from the indirect effects of seed origin on fitness in our experiment. Multiple interactions among traits traditionally rely either on correlational, thus purely statistical, procedures, or on a reductionistic previous understanding of bivariate relationships (Lechowicz and Blais, 1988). SEM provides an intermediate aprioristic–statistical approach. Thus, an overall causal structure relating the groups of variables in a model was first designed. The model finally selected by goodness of fit estimates should (a) address the extent to which each of three separate types of traits (traits related to reproductive success, phenology and plant productivity) influence final production of viable offspring; (b) account for the putative effect that seed origin may exert on these phenotypic traits; and (c) account for likely between-trait relationships that occur during plant development (e.g. if flowering is triggered by photoperiodic cues, then a productivity-related trait such as plant size might be constrained by an abrupt shift in resource allocation to supply reproductive development). Considering this set of aprioristic constraints, several tentative specific models were generated, and the model which received the highest statistical support is shown in Fig. 1.
Fig. 1.
(A) Hypothetical structural equation model of the causal relationships among seed origin, plant traits and fitness (seed number). U represents the unexplained variance of dependent variables. (B) Fitting of the graph (A) model to the data of this experiment. Solid and dashed arrows indicate positive and negative effects, respectively. Arrow widths are proportional to the magnitude of standardized path coefficients. Paths non-significant at P = 0·05 are denoted with the superscript ‘n.s.’. Fit statistics of the model (Pχ2, GFI and NFI) are given.
SEM was first used to assess the influence of seed origin on fitness via modulation of phenological, morphological and reproductive traits. The aim of this was to determine whether and how genetic background fixed by the selective pressures at each seed origin is expressed in the common garden environment. Secondly, SEM was used to explore whether phenotypic trait variation had similar effects on fitness in each of the three seed origins. This was performed by running separate SEMs for the data set of each seed origin.
The structure of the hypothesized causal relationships between seed origin, plant traits and fitness that received the highest statistical support is shown in Fig. 1A. In this model, seed origin was transformed to an ordinal variable accounting for the underlying latitude gradient (northern, 0; central, 1; and southern, 2). This structure hypothesized that seed origin would directly affect flowering phenology, seed size and SLA through a local adaptation component. As flowering date generally depends on both photoperiod and internal resource thresholds, large seed size and SLA were used as proxies of early resource capture ability, and were expected to advance flowering dates. Reproductive success was considered to depend indirectly on seed origin via resource availability (plant size and SLA) and flowering phenology (Julian date at onset of flowering), which are well known drivers of reproductive success in annual plant species. A direct path connecting seed origin to fitness accounts for plant traits that may mediate seed origin effects on fitness, but were not explicitly considered in our experiment. Plant size at the start of flowering was also assumed to be constrained by flowering date, because the triggering of the flowering process in annuals involves a switch of resource allocation from vegetative to reproductive growth. Preliminary analyses including flowering synchrony showed that its biologically meaningful paths were not significant and, thus, this variable was not included in the model.
Standardized path coefficients were estimated using generalized least-squares (GLS). A total of 210 plants (ranging from 50 to 103 per seed origin) were used for SEM, after discarding those that did not survive to flowering and those where data were missing for at least one variable. The degree of fit between the observed and expected covariance structures was assessed by a χ2 goodness-of-fit test. A significant goodness-of-fit test indicates that the model does not fit the data. However, a significant χ2 can also result from violation of certain assumptions, whereas failure to reject a model (a non-significant χ2) may result from inadequate statistical power (Bentler, 1989; Mitchell, 1993). Therefore, model fit to the data was also evaluated by means of the goodness-of-fit index (GFI) and the Bentler and Bonett's normed-fit index (NFI), which are often used in SEM (Schermelleh-Engel et al., 2003). Values of GFI and NFI range between 0 and 1, and values >0·9 indicate an acceptable fit of the model to the data.
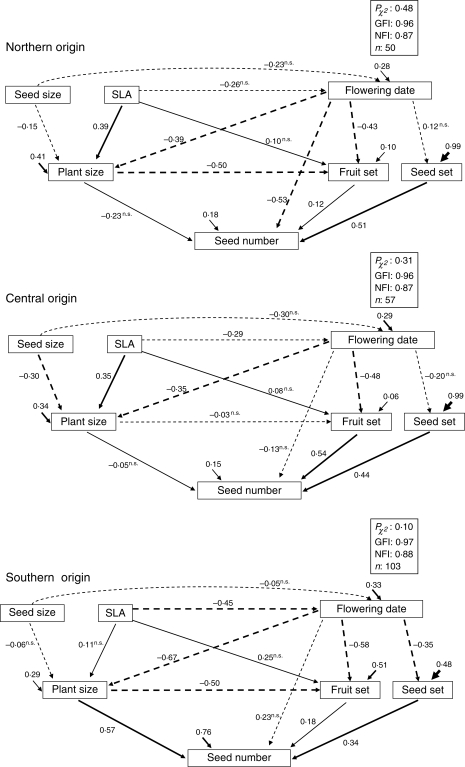
In order to test our second hypothesis, a slightly simpler model without the ‘seed origin’ variable was run separately for each seed origin. This was done to test for differences in the influence of multiple plant traits on fitness among seed origins (Fig. 2). Explicit comparisons among seed origins were performed through multigroup analysis (Shipley, 2002; Byrne, 2004). A constrained model in which all free parameters were forced to be equal across the three populations was built and contrasted with our field data. Since a lack of fit was detected in the fully constrained multigroup model, a series of nested models, where equality constraints were removed one at a time, were developed to detect which one would significantly improve the model (Shipley, 2002). The difference in the two maximum likelihood χ2 statistics was used to test for a difference in the value of a parameter between the three populations. Bonferroni correction was applied to adjust the overall significance level of these comparisons. Since several variables were not normally distributed, statistical significance of each single path coefficient was evaluated through bootstrapping (Arbuckle, 2003). Prior to analysis, all variables were z-standardized. Significance testing of path coefficients and multigroup analysis were carried out using AMOS 5·0 software (AMOS Development Corp., Mount Pleasant, SC, USA) whereas the rest of SEM analyses were performed using the CALIS procedure of SAS 9·0 software (SAS Institute Inc., Cary, NC, USA).
Fig. 2.
Structural equation model run for each seed origin separately. The meanings of path coefficients, arrow widths and headings, goodness of fit statistics and lettering are as in Fig. 1.
RESULTS
Plant survival and traits as a function of seed origin
Plant mortality differed among the three seed origins throughout the experiment (χ2 = 15·02; P < 0·01; all three comparisons among pairs of seed origins were also significantly different, not shown): 38, 24 and 14 % of the seeds from the northern, central and southern origins, respectively, died before flowering. GLM results revealed that traits at the seed and seedling stages affected survivorship differently for the three seed origins. Southern origin individuals with small seeds survived better than individuals with larger seeds, but seed size was irrelevant for survivorship of northern and central origin plants (Table 2). However, high scores of SLA in plants of northern and central origins enhanced survivorship, whereas SLA was irrelevant for the southern origin plants (Table 2).
Table 2.
Results of GLMs including seed size and SLA as explanatory variables, and probability of survivorship before flowering initiation as response variable
| SLA |
Seed size |
|||
|---|---|---|---|---|
| β (parameter estimate) | P-value | β (parameter estimate) | P-value | |
| Northern origin | 0·03 | <0·01 | −2·99 | 0·86 |
| Central origin | 0·03 | <0·05 | 31·5 | 0·18 |
| Southern origin | −0·01 | 0·4 | −79·07 | <0·01 |
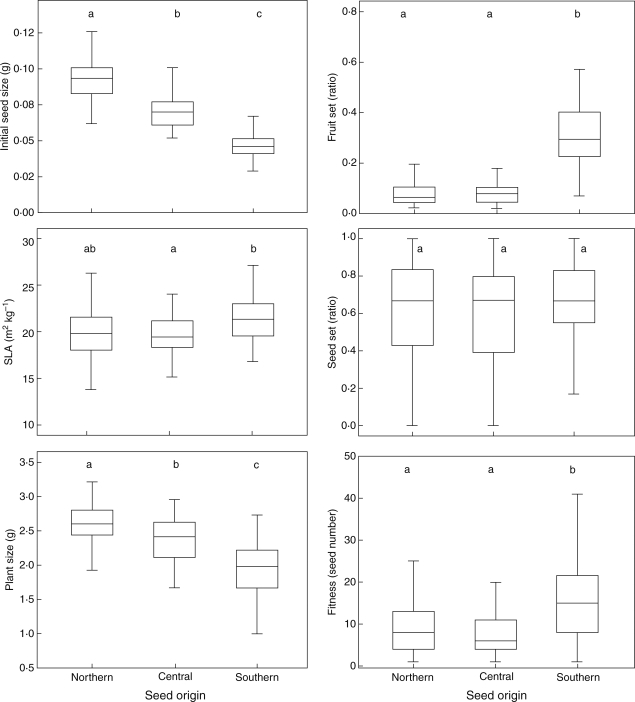
Initial seed size was larger for the seed of northern origin (Fig. 3). This trend was also observed in individual seed weight of seeds produced by the plants of the experiment (data not shown). However, heritability of seed size was low. Only plants of northern origin produced seeds of a size moderately related to initial seed size (R2 = 0·11; P = 0·02). Southern and central origin seeds showed no significant relationship between initial and final individual seed size. Above-ground dry mass at the onset of flowering was also greater for plants of northern origin than for plants of central and southern origin (Fig. 3). Plants of southern origin showed a slightly significant tendency to bear leaves with a greater SLA (Fig. 3).
Fig. 3.
Box-and-whiskers plots showing seed origin median, 75th and 25th percentiles, and ranges of several plant traits and fitness. Different letters within a plot indicate significant differences among seed origins at P = 0·05 after Dunnet's T3-corrected multiple comparisons. Plant size is above-ground dry biomass.
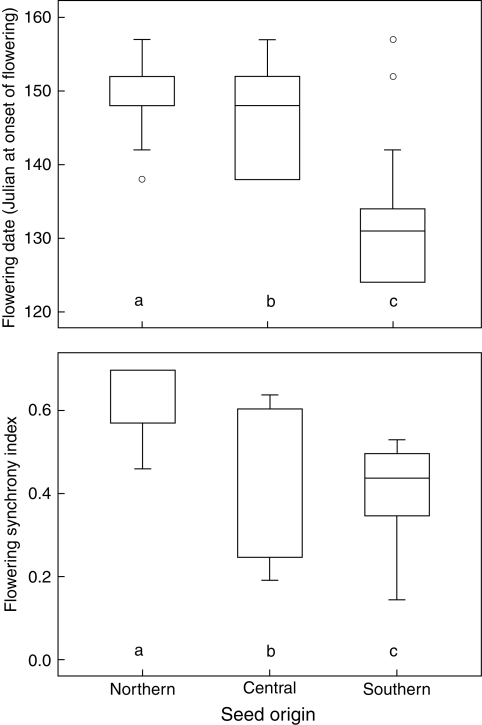
Plants of southern origin flowered the earliest, while within-population flowering synchrony was higher in plants of central and northern origins (Fig. 4). However, reproductive success was higher in plants of southern origin. Approximately one-third of flowers of southern origin set fruit compared with approximately one-tenth of flowers of central and northern origins. Seed set within pods was similar for all seed origins (Fig. 3).
Fig. 4.
Julian date at first flowering for the plants of this experiment, and flowering synchrony, as a function of seed origin. The flowering synchrony index varies from 0 (complete asynchrony) to 1 (complete overlap). Lettering as in Fig. 3. Dots are potential outliers (>1·5 interquartile ranges from the end of the box).
Relative importance of seed origin, phenological traits, growth rate traits and reproductive traits on fitness
Individual fitness, measured as the number of viable offspring produced per individual plant, was the highest in southern origin plants, which produced an average of 16 seeds per plant as compared with eight and nine seeds per plant in central and northern origin plants, respectively.
The factors underlying fitness variation are shown in the SEMs in Figs 1 and 2. The global model in Fig. 1 shows that the overall goodness of fit of the data to the aprioristic model was high, as denoted by a non-significant χ2 P-value, high GFI (0·98) and NFI (0·94) values. Three interesting patterns can be drawn from this model. First, seed origin did not have a significant direct effect on fitness, but affected fitness indirectly through modulation of plant traits. Indirect effects accounted for 42 % of the variation in fitness. Secondly, the path passing through phenological and reproductive traits explained a greater proportion of the variation in fitness than the path connecting seed origin to fitness through growth rate traits (seed size, SLA and plant size). Plants from the earlier flowering seed origin achieved higher fruit and seed set, which positively affected fitness. The time of flowering onset also had a significant direct effect on fitness. Thirdly, different types of traits interacted intensely during development, as suggested by the fact that the supported model included several significant interconnections among growth, phenological and reproductive success traits.
The three models run separately for each seed origin also showed high goodness of fit to the data, but contrasted with the above global model, and among each other, in the relevance of the different types of traits to defining final fitness (Fig. 2). Plants of southern origin, as a whole, flowered earlier and more asynchronously. Within this population, larger plants bearing leaves with a higher SLA attained higher fitness. Within-population variation in flowering date did not significantly affect the fitness of southern origin plants. In contrast, plants of northern origin flowered later and more synchronously. Within this population, individuals that initiated flowering earlier produced more seed, regardless of their size. In the plants of central origin, intermediate results were obtained. Within-population variation in growth rate traits and flowering phenology did not have a significant direct effect on fitness. Fitness was strongly determined by reproductive success components and indirectly through the effect of flowering date on fruit set.
The factors included in the global model accounted for 42 % of the variance in fitness, whereas the separate models for each seed origin explained 82, 85 and 24 % of fitness variance, for the northern, central and southern origins, respectively (value of U in Figs 1 and 2).
Comparisons between the three partial models were conducted jointly through multigroup analyses. Goodness of fit of the model improved significantly if the link between plant size and seed number was released (multigroup comparison tests, Table 3). Other paths directly related to fitness, such as the one connecting flowering date to seed number, clearly differed in direction, statistical significance and magnitude among seed origins, although goodness of fit did not improve significantly when freed in multigroup comparison tests (Table 3). This may have occurred because the effect of flowering date was partitioned between a direct effect and indirect effects, mediated by reproductive success and plant size.
Table 3.
Multigroup comparison of path coefficients among seed origins
| Free parameters for which between-group equality constraint was released | MLχ2 | ΔMLχ2 | Probability of ΔMLχ2 |
|---|---|---|---|
| None | 253·661 | ||
| Path from seed size to flowering date | 250·692 | 2·968 | 0·227 |
| Path from seed size to plant size | 250·185 | 3·475 | 0·176 |
| Path from SLA to plant size | 252·723 | 0·937 | 0·626 |
| Path from SLA to fruit set | 248·403 | 5·257 | 0·072 |
| Path from SLA to flowering date | 249·558 | 4·102 | 0·129 |
| Path from plant size to seed number | 239·951 | 13·710 | 0·001 |
| Path from plant size to fruit set | 252·071 | 1·590 | 0·452 |
| Path from flowering date to plant size | 247·004 | 6·657 | 0·036 |
| Path from flowering date to fruit set | 248·997 | 4·664 | 0·097 |
| Path from flowering date to seed number | 252·970 | 0·691 | 0·708 |
| Path from flowering date to seed set | 248·329 | 5·332 | 0·070 |
| Path from fruit set to seed number | 249·431 | 4·229 | 0·121 |
| Path from seed set to seed number | 250·174 | 3·486 | 0·175 |
| Variance of seed size | 245·682 | 7·979 | 0·019 |
| Variance of SLA | 244·145 | 9·516 | 0·009 |
| Error variance of flowering date | 253·188 | 0·472 | 0·790 |
| Error variance of fruit set | 247·583 | 6·077 | 0·048 |
| Error variance of plant size | 192·381 | 61·280 | 0·000 |
| Error variance of seed set | 242·030 | 11·631 | 0·003 |
| Error variance of seed number | 213·014 | 40·647 | 0·000 |
The first row shows the χ2 estimate resulting from constraining all free parameters to the same value among the three seed origins. Below, the effect on χ2 of releasing each single free parameter one at a time is shown. The right-most column indicates the probability that the release of that parameter improves the model significantly. Bonferroni-corrected P-value threshold 0·05/20 = 0·0025.
DISCUSSION
Variation in plant traits explains fitness differences among seed origins
Our results show that the phenological, vegetative and reproductive traits considered here accounted for most of the fitness variation, including indirect effects due to different seed origins. It is noteworthy that the direct effect of seed origin over fitness was not significant, which suggests that other factors related to origin and not explicitly included in the model were negligible. Thus, a small set of key phenotypic traits can, to a large extent, account for the genetic effect of seed origin on plant fitness. Each of the traits considered here encompasses and captures variation in the broad facets of annual plant development. For instance, seed size and SLA integrate aspects of potential growth rate and early resource provisioning (Westoby et al., 2002). Growth rate at the early stage of plant life has previously been reported to be an important determinant of fitness in annuals (Cheplick, 2002; Luzuriaga and Escudero, 2008). Studies in herbs have found onset of the flowering period to synthesize ample aspects of phenological development relevant for fitness such as length of the vegetative period or time at which seed filling occurs (Dracup et al., 1998b; Sola and Ehrlén, 2007). Flowering date is particularly relevant to reproductive fitness in L. angustifolius due to the deterioration of growing conditions in summer during reproductive growth in Mediterranean-type environments (Dracup et al., 1998a). In fact, plants with high early resource provisioning (high SLA and seed size) tended to advance flowering, as revealed by within- and among-population SEM analyses. Flowering time was the most relevant path between seed origin and fitness. This occurred because flowering date directly affected fitness, but was also closely related to plant size and fruit set. Finally, reproductive success summarizes the multiple events that occur from flower bud initiation to the completion of seed filling (Wiens et al., 1987). Flowering time may also be influenced by specific vernalization requirements at each seed origin, as the experiment was initiated in late winter. However, this is unlikely, given that a parallel experiment with similar seed material sown before winter showed similar flowering phenologies (R. Milla, pers. obs.). Fruit set depends on both pollination-related processes and resource availability, including competition among growing fruits (Sutherland, 1986). It was found that fruit set was more dependent on flowering time and seed origin than seed set. Seed set occurs as a progressive adjustment of available resources to the number of seed that the plant can afford to mature (Burd, 1994). In our global model, seed set affected fitness more intensely than fruit set and was less dependent than fruit set on seed origin.
Other traits, such as photosynthetic rates, water-use efficiency, leaf area, etc., have also been regarded as relevant predictors of fitness in annuals (Maddox and Antonovic, 1983; Lechowicz, 1984; Farris and Lechowicz, 1990; Arntz et al., 1998). However, here it is shown that the selection of a few easy-to-measure ‘umbrella’ traits (i.e. those that capture variation of a wider set of harder-to-measure traits, see Cornelissen et al., 2003) can explain most of the fitness variation in annuals, both within and among populations.
Diverse traits contribute differently to fitness for each seed origin
It was hypothesized that multiple plant traits would show different degrees of dependence on seed origin. Variation in flowering time was probably associated with climate at seed origin, as plants from the southernmost site flowered earlier. Flowering time is known to be under strong genetic control, to the point that selection in flowering times can occur even in the time span of a single generation (Franks et al., 2007). Traits related to growth rate varied in their degree of dependence on seed origin. Seeds from the northern and central sites were larger, while SLA showed no clinal variation in relation to seed origin. SLA is a highly plastic trait (Shipley, 2006; Milla et al., 2008). Thus, in accordance with our first hypothesis and with the literature, SLA showed little dependence on seed origin. As a consequence, growth rate variation is not likely to depend on a local adaptation component. Seed size, however, is plastic enough to react to variation in resource provisioning during seed filling or to variation in other plant traits (Winn, 1991; Borrás et al., 2004; Luzuriaga et al., 2006), but also has a strong genetic component (Dolan, 1984; Winn, 1991; Oleksyn et al., 1998). Nevertheless, the effect of seed size on growth rate is modest compared with SLA, and only becomes relevant in competitive settings, where larger seeded individuals tend to outcompete neighbours (Dolan, 1984). As there was virtually no competition in our one plant per pot regularly weeded experiment, variation in seed size explained little of the variance in final plant size within each seed origin. Although trends in seed and plant sizes among the three seed origins were similar, this was mainly due to the effect of seed origin on each separate variable, not to a direct mechanistic link between seed and plant size (see SEMs). Seed mass can be negatively correlated to RGR (Shipley and Peters, 1990). If this was the case in our experiment, it is likely that the relationship between seed size and plant size could disappear, provided that SLA (surrogate for RGR) influenced plant size.
Regarding reproductive traits, it was anticipated that seed origin should scarcely affect reproductive success, as it is highly dependent on local events and environmental conditions. The results support this hypothesis for the most part. Pollination, resource provisioning and phenology are the main factors controlling reproductive success (Wesselingh, 2007; Giménez-Benavides et al., 2007). Regarding pollination, our study species is predominantly autogamous, and, when outcrossed, it does not depend on specialist pollinators (Forbes et al., 1971). This probably made the reproductive success of our plants highly independent of the local assembly of pollinators (Forbes et al., 1971). Resource availability was common to all individuals in the experiment, and thus we did not expect direct seed origin effects on fitness, as found in SEMs. However, southern origin plants showed remarkably higher fruit set than central and northern origin plants. This may have been an indirect effect of earlier flowering in the southern origin plants. In general, earlier flowering was associated with higher fruit set within each of the seed origins (see paths in SEMs). It is remarkable that survival at flowering also varied between seed origins. This reflects the different capability of different origin plants to perform under conditions existing in the common garden. However, it is noteworthy that traits that affected survivorship were different depending on seed origin and that such traits were also related to the reproductive response of survivors.
It was also confirmed that plant traits contribute to fitness differently depending on seed origin. This is evidenced by: (a) the strong relationship between seed origin and growth and phenology traits in the global model; and (b) the different magnitude and direction of effects of internal paths in each seed origin model. This idea has been put forward previously in studies addressing the relative contribution of plant traits to fitness (e.g. Farris and Lechowicz, 1990). Fewer studies, however, have reported on the explicit differences in the causal structure of multiple trait interactions that culminate in contrasting fitness outcomes. For instance, Tonsor and Scheiner (2007) showed that the pattern of intertrait causal relationships in Arabidopsis thaliana changed across four CO2 supply treatments, with diverse causal paths contributing differently to fitness under each of the four CO2 environments. Similarly, Picotte et al. (2007) found that modulation of one or another trait related to water-use efficiency is more or less relevant to fitness depending on the overall morphology of genotypes grown in a common garden. Moreover, the ecological consequences of the effects of trait interaction on fitness are poorly acknowledged. It is common knowledge that plant species showing different typical scores for traits with a functional relevance differ in terms of habitat segregation (Grime, 2001). How a given species modulates a certain trait in response to an environmental change is also extensively addressed in phenotypic plasticity research programmes (Sultan, 2000). However, how the hierarchy, intensity and direction in which modulation of one or another trait changes across local populations within a species range is rarely highlighted. In this study, it was found that flowering time, which has been repeatedly shown to have a strong impact on fitness (e.g. Hall and Willis, 2006; Giménez-Benavides et al., 2007), becomes almost irrelevant for plants coming from a southern climate. This contrasts with most literature on the over-riding relevance of flowering phenology in the fitness of annuals (see Hall and Willis, 2006 and references therein), and again points to the relevance of trait interaction and the relative contribution of different traits under contrasting environmental pressures. In the case of the present study, the irrelevance of flowering timing for the southern plants probably occurs because most individuals of this seed origin initiate flowering early enough to preclude reproductive development during the lattermost period of summer drought. Avoiding summer drought is crucial to achieve high yield in this species (Dracup et al., 1998a). Thus, variation in flowering time at the early stage of the reproductive period becomes negligible for fitness. In this scenario, other traits such as plant growth rate arise as more direct determinants of fitness. For the northern and central seed origins, provided that the range of variation in flowering onset forcibly includes later dates due to colder climate, the direct and indirect effects of flowering date produce an over-riding effect on fitness, irrespective of the size or resource pool of the plants available for reproductive effort. Similar trait interactions are more commonly found when comparing environments instead of plant procedures. For instance, in Cleome serratula, size and growth rate are determinants of fitness at the moist end of a moisture gradient, while drought tolerance characteristics over-ride growth traits at the dry end (Farris, 1987). In the seed origin locations of this experiment, flowering is most probably finely tuned to local conditions, as demonstrated by the remarkable effect of seed origin on flowering phenology in the common garden, since the temperature regime at the common garden site was similar to that of the southern origin site. Thus, variation in phenology is probably not a main determinant of fitness in situ, as shown for southern origin plants in the common garden. Instead, within populations, traits such as growth-related parameters are likely to be more rewarded, as long as environmental conditions remain the same. However, if the temperature regime varies, then the hierarchy of trait relevance for fitness may shift, and the resulting phenotypes may be completely different from those selected under previous conditions. This is a most significant mechanism that needs to be taken into account when assessing the ecological causes of variation in individual fitness.
In synthesis, multiple trait interactions during plant development can modulate fitness to a large extent, as shown by the several significant interconnections among productivity, phenological and reproductive traits in our models. It was also shown that relevant paths are context dependent and shift when compared across different scenarios (e.g. seed origin here). Further work focusing on multiple interactions among developmental traits is needed to advance our knowledge on the mechanisms underlying local adaptation and the ability of plants to cope with changes in climate or resource availability.
ACKNOWLEDGEMENTS
We thank David de Llano and Jose Luis Margalet for their help during the set up and monitoring of the experiment, and the staff of the Instituto Nacional de Investigaciones Agrarias (INIA) for providing seeds. We also thank Lori De Hond for her linguistic assistance, and two anonymous referees for constructive comments on an earlier version of this paper. R.M. was supported by the Ministerio de Educación y Ciencia (Spain) through a Juan de la Cierva contract. This study was supported by the Spanish Government MEC project CGL2006-09431/BOS, the Madrid Regional Government project Remedinal and the INIA project RF2004-00016-00-00.
LITERATURE CITED
- Albert MJ, Escudero A, Iriondo JM. Female reproductive success of narrow endemic Erodium paularense in contrasting microhabitats. Ecology. 2001;82:1734–1747. [Google Scholar]
- Arbuckl JL. AMOS 5 (Version 5—Build 5168). Chicago, IL: Smallwaters Corporation; 2003. [Google Scholar]
- Arntz MA, Delph LF. Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologia. 2001;127:455–467. doi: 10.1007/s004420100650. [DOI] [PubMed] [Google Scholar]
- Arntz AM, DeLucia EH, Jordan N. Contribution of photosynthetic rate to growth and reproduction in Amaranthus hybridus. Oecologia. 1998;117:323–330. doi: 10.1007/s004420050665. [DOI] [PubMed] [Google Scholar]
- Augspurger CK. Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators on Hybantus prunifolius (Violaceae) Ecology. 1981;63:775–788. [Google Scholar]
- Becker U, Reinhold T, Matthies D. Effects of pollination distance on reproduction and offspring performance in Hypochoeris radicata: experiments with plants from three European regions. Biological Conservation. 2006;132:109–118. [Google Scholar]
- Bentler PM. EQS structural equations program manual. Los Angeles, CA: BMDP Statistical Software; 1989. [Google Scholar]
- Blais PA, Lechowicz MJ. Variation among populations of Xanthium strumarium L. (Compositae) from natural and ruderal habitats. American Journal of Botany. 1989;76:901–908. [Google Scholar]
- Borrás L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research. 2004;86:131–146. [Google Scholar]
- Burd M. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review. 1994;60:83–139. [Google Scholar]
- Byrne B. Testing for multigroup invariance using AMOS graphics: a road less traveled. Structural Equation Modeling. 2004;11:272–300. [Google Scholar]
- Chapin FS, III, Chapin MC. Ecotypic differentiation of growth processes in Carex aquatilis along latitudinal and local gradients. Ecology. 1981;62:1000–1009. [Google Scholar]
- Cheplick GP. Size and architectural traits as ontogenetic determinants of fitness in a phenotypically plastic annual weed (Amaranthus albus) Plant Species Biology. 2002;17:71–84. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Dolan RW. The effect of seed size and maternal source on individual size in a population of Ludwigia leptocarpa (Onagraceae) American Journal of Botany. 1984;71:1302–1307. [Google Scholar]
- Dracup M, Reader MA, Palta JA. Variation in yield of narrowleafed lupin caused by terminal drought. Australian Journal of Agricultural Research. 1998;a 49:799–810. [Google Scholar]
- Dracup M, Thomson B, Reader M, Kirby EJM, Shield I, Leach J. Daylength responses, flowering time, and seed filling in lupins. Australian Journal of Agricultural Research. 1998;b 49:1047–1056. [Google Scholar]
- Farris MA. Natural selection on the plant–water relations of Cleome serrulata growing along natural moisture gradients. Oecologia. 1987;72:434–439. doi: 10.1007/BF00377576. [DOI] [PubMed] [Google Scholar]
- Farris MA, Lechowicz MJ. Functional interactions among traits that determine reproductive success in a native annual plant. Ecology. 1990;71:548–557. [Google Scholar]
- Forbes I, Leuck DB, Edwardson JR, Burns RE. Natural cross-pollination in blue lupine (Lupinus angustifolius L.) in Georgia and Florida. Crop Science. 1971;11:851–854. [Google Scholar]
- Franks SJ, Sim S, Weis AE. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences of the USA. 2007;104:1278–1282. doi: 10.1073/pnas.0608379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Benavides L, Escudero A, Iriondo JM. Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytologist. 2007;173:367–382. doi: 10.1111/j.1469-8137.2006.01932.x. [DOI] [PubMed] [Google Scholar]
- Grime JP. Plant strategies, vegetation processes and ecosystem properties. Chichester, UK: John Wiley; 2001. [Google Scholar]
- Hall MC, Willis JH. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution. 2006;60:2466–2477. [PubMed] [Google Scholar]
- Iriondo JM, Albert MJ, Escudero A. Structural equation modelling: an alternative for assessing causal relationships in threatened plant populations. Biological Conservation. 2003;113:367–377. [Google Scholar]
- Jordan N. Effects of the triazine-resistance mutation on fitness in Amaranthus hybridus (smooth pigweed) Journal of Applied Ecology. 1996;33:141–150. [Google Scholar]
- Lechowicz MJ. The effects of individual variation in physiological and morphological traits on the reproductive capacity of the common cocklebur Xanthium strumarium L. Evolution. 1984;38:833–844. doi: 10.1111/j.1558-5646.1984.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Lechowicz MJ, Blais PA. Assessing the contributions of multiple interacting traits to plant reproductive success: environmental dependence. Journal of Evolutionary Biology. 1988;1:255–273. [Google Scholar]
- Luzuriaga AL, Escudero A. What determines emergence and net recruitment in an early succession plant community? Disentangling biotic and abiotic effects. Journal of Vegetation Science. 2008;19:445–456. [Google Scholar]
- Luzuriaga AL, Escudero A, Pérez-García F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae) Weed Research. 2006;46:163–174. [Google Scholar]
- Maddox D, Antonovics J. Experimental ecological genetics in Plantago: a structural equation approach to fitness components in P. aristata and P. patagonica. Ecology. 1983;64:1092–1099. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1989. [Google Scholar]
- Milla R, Reich PB, Niinemets Ü, Castro-Díez P. Environmental and developmental controls on specific leaf area are little modified by leaf allometry. Functional Ecology. 2008;22:565–576. [Google Scholar]
- Mitchell RJ. Path analysis: pollination. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York: Chapman and Hall; 1993. pp. 211–231. [Google Scholar]
- Nagy ES, Rice KJ. Local adaptation in two subspecies of an annual plant: implications for migration and gene flow. Evolution. 1997;51:1079–1089. doi: 10.1111/j.1558-5646.1997.tb03955.x. [DOI] [PubMed] [Google Scholar]
- Oleksyn J, Modrzynski J, Tjoelker MG, Zytkowiak R, Reich PB, Karolewski P. Growth and physiology of Picea abies populations from elevational transects: common garden evidence for altitudinal ecotypes and cold adaptation. Functional Ecology. 1998;12:573–590. [Google Scholar]
- Picotte JJ. Plastic responses to temporal variation in moisture availability: consequences for water use efficiency and plant performance. Oecologia. 2007;153:821–832. doi: 10.1007/s00442-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Santamaría L, Figuerola J, Pilon JJ, et al. Plant performance across latitude: the role of plasticity and local adaptation. Ecology. 2003;84:2454–2461. [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of Structural Equation Models: tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research Online. 2003;8:23–74. [Google Scholar]
- Shipley B. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge: Cambridge Univeristy Press; 2002. [Google Scholar]
- Shipley B. Analysing the allometry of multiple interacting traits. Perspectives in Plant Ecology, Evolution and Systematics. 2004;6:235–241. [Google Scholar]
- Shipley B. Net assimilation rate, specific leaf area and leaf mass ratio: which is most closely correlated with relative growth rate: a meta-analysis. Functional Ecology. 2006;20:565–574. [Google Scholar]
- Shipley B, Peters RH. The allometry of seed weight and seedling relative growth rate. Functional Ecology. 1990;4:523–529. [Google Scholar]
- Sola AJ, Ehrlén J. Vegetative phenology constrains the onset of flowering in the perennial herb Lathyrus vernus. Journal of Ecology. 2007;95:208–216. [Google Scholar]
- Stenøien HK, Fenster CB, Kuittinen H, Savolainen O. Quantifying latitudinal clines in hypocotyl responses to red and far-red light treatments in natural populations of Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 2002;89:1604–1608. doi: 10.3732/ajb.89.10.1604. [DOI] [PubMed] [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Sutherland S. Patterns of fruit-set: what controls fruit–flower ratios in plants? Evolution. 1986;40:117–128. doi: 10.1111/j.1558-5646.1986.tb05723.x. [DOI] [PubMed] [Google Scholar]
- Tonsor SJ, Scheiner SM. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. American Naturalist. 2007;169:E119–E140. doi: 10.1086/513493. [DOI] [PubMed] [Google Scholar]
- Volis S, Verhoeven KJF, Mendlinger S, Ward D. Phenotypic selection and regulation of reproduction in different environments in wild barley. Journal of Evolutionary Biology. 2004;17:1121–1131. doi: 10.1111/j.1420-9101.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Wesselingh RA. Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytologist. 2007;174:26–37. doi: 10.1111/j.1469-8137.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Wiens D, Calvin WD, Wilson CL, Davern CA, Frank CI, Seavey SR. Reproductive success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia. 1987;71:501–509. doi: 10.1007/BF00379288. [DOI] [PubMed] [Google Scholar]
- Winn AA. Proximate and ultimate sources of within-individual variation in seed mass in Prunella vulgaris (Lamiaceae) American Journal of Botany. 1991;78:838–844. [Google Scholar]
- Yoshie F. Length of the pre-reproductive period of Plantago asiatica L. from different latitudes. Plant Species Biology. 2007;22:135–139. [Google Scholar]