Abstract
Background and Aims
Most priming studies have been conducted on commercial seed lots of unspecified uniformity and maturity, and subsequent seed longevity has been reported to both increase and decrease. Here a seed lot of Digitalis purpurea L. with relatively uniform maturity and known history was used to analyse the effects of priming on seed longevity in air-dry storage.
Methods
Seeds collected close to natural dispersal and dried at 15 % relative humidity (RH), 15 °C, were placed into experimental storage (60 % RH, 45 °C) for 14 or 28 d, primed for 48 h at 0, −1, −2, −5, −10 or −15 MPa, re-equilibrated (47 % RH, 20 °C) and then returned to storage. Further seed samples were primed for 2 or 48 h at −1 MPa and either dried at 15 % RH, 15 °C or immediately re-equilibrated for experimental storage. Finally, some seeds were given up to three cycles of experimental storage and priming (48 h at −1 MPa).
Key Results
Priming at −1 MPa had a variable effect on subsequent survival during experimental storage. The shortest lived seeds in the control population showed slightly increased life spans; the longer lived seeds showed reduced life spans. In contrast, seeds first stored for 14 or 28 d before priming had substantially increased life spans. The increase tended to be greatest in the shortest lived fraction of the seed population. Both the period of rehydration and the subsequent drying conditions had significant effects on longevity. Interrupting air-dry storage with additional cycles of priming also increased longevity.
Conclusions
The extent of prior deterioration and the post-priming desiccation environment affect the benefits of priming to the subsequent survival of mature seeds. Rehydration–dehydration treatments may have potential as an adjunct or alternative to the regeneration of seed accessions maintained in gene banks for plant biodiversity conservation or plant breeding.
Key words: Digitalis purpurea, priming, re-drying, seed longevity, seed storage, ageing
INTRODUCTION
All seeds stored under air dry conditions will have suffered a degree of deterioration. This may have been incurred, for example, during delays between collection and processing if seeds are held under inappropriate conditions, during processing or whilst in storage. Indeed even in the natural environment, macromolecules within the seed tissues will incur some damage through normal metabolism. If the damage accumulated is not too severe, repair will be possible. Villiers and Edgcumbe (1975) showed that if oxygen is present, fully hydrated seeds of lettuce (Lactuca sativa) are able to repair chromosomal aberrations, provided germination is prevented. Similarly, repair of DNA was detected prior to germination in imbibed seeds of maize (Zea mays; Zlatanova et al., 1987) and pea (Pisum sativum; Onelli et al., 2000) and in embryos of wild oat (Avena fatua) and rye (Secale cereale) (Elder and Osborne, 1993; Boubriak et al., 1997). Seeds that are not quite fully hydrated are also capable of repairing damage (Ibrahim and Roberts, 1983). At equilibrium relative humidities (eRHs) greater than approx. 85 % (approx. −20 MPa water potential), respiration and metabolism occur at a rate that increases as more water is taken up by the seeds (Vertucci and Leopold 1984, 1986). Seed longevity also increases over this range, provided oxygen is available (Roberts and Ellis, 1989). However, repair processes are unlikely to be fully functional until water potential reaches between −3 and −1·5 MPa (approx. 98 % eRH) (Vertucci and Farrant, 1995; Pammenter and Berjak, 1999).
The term ‘priming’ was originally introduced in the context of polyethylene glycol (PEG) osmotica treatments at water potentials just below full imbibition; these treatments resulted in more rapid germination on subsequent sowing (Heydecker et al., 1973; Heydecker and Gibbins, 1978). Priming has since been used to describe a wide variety of seed invigoration treatments. These have included soaking seeds in or on water (e.g. Tarquis and Bradford, 1992), or an osmoticum (e.g. Georghiou et al., 1987; Sarocco et al., 1995), in some cases with aeration (e.g. Powell et al., 2000), mixing seeds with moist vermiculite or sand (e.g. Chiu et al., 2002) or placing seeds in a saturated atmosphere (e.g. Rao et al., 1987). There are many examples of priming increasing the rate of germination (Argerich and Bradford, 1989; Argerich et al., 1989; Lanteri et al., 1993; Sarocco et al., 1995; Powell et al., 2000), and some of increases in ability to germinate (Georghiou et al., 1987; Demir, 2003). However, when combined with subsequent desiccation, the effect of priming is not consistently positive. In some cases, the benefit to germination is lost on desiccation, or partly retained on desiccation but lost rapidly in storage (Heydecker and Gibbins, 1978). Priming has been shown to be both beneficial (Georghiou et al., 1987; Probert et al., 1991; Wechsberg, 1994) and detrimental (Argerich et al., 1989; Tarquis and Bradford, 1992) to subsequent longevity.
Increases in the rate of germination through priming have been attributed to the advancement of germination metabolism (Lanteri et al., 1993; Sarocco et al., 1995; Soeda et al., 2005), enhanced antioxidant activity (Bailly et al., 2000) and, particularly where longevity is improved, repair processes (Burgass and Powell, 1984; Ashraf and Bray, 1993; Bray et al., 1993; Sivritepe and Dourado, 1995). Negative effects of priming have been explained as the consequence of germination sensu strictu having advanced to a state where seeds have lost desiccation tolerance (Śliwińska and Jendrzejczak, 2002) or are less able to resist damage during air-dry storage (Powell et al., 2000).
Differences in the effect of priming on the germination and longevity of low and high quality seed lots and on seeds with different maturity have been described (Wechsberg, 1994; Powell et al., 2000; Śliwińska and Jendrzejczak, 2002; Demir, 2003; Butler et al., 2009). It has been suggested that improvements in seed longevity after priming will be most apparent in deteriorated seed lots, as in these seeds repair will be most effective (Burgass and Powell, 1984). Powell et al. (2000) suggest that low vigour seeds will benefit from priming because of a requirement for repair before germination advancement occurs, whereas in high vigour seeds advancement of germination occurs rapidly when primed and progresses to a stage where seeds become susceptible to more rapid deterioration in subsequent storage.
The effect of priming, particularly with respect to subsequent longevity, can be influenced by the conditions immediately after priming. For example, a mild water stress or heat shock following priming restored the ability of seeds from several species to withstand deterioration during storage (Bruggink et al., 1999). Similarly, Gurusinghe and Bradford (2001) noted an improvement in longevity for seeds given a slow-drying, high temperature treatment after priming. Drying seeds slowly after priming may induce the synthesis of LEA (late embryogenesis abundant) proteins, while incubating seeds at high temperatures may induce heat shock proteins, both of which may provide protective mechanisms that are beneficial to seed longevity (Gurusinghe et al., 2002). Soeda et al. (2005) noted that seeds dried at different rates after priming showed differential gene expression: genes expressed solely after slow drying were similar to those with functions in DNA protection and stress tolerance. However, Schwember and Bradford (2005) found that the longevity of lettuce seeds given either a slow- or fast-drying treatment after priming was reduced compared with non-primed seeds.
Here an investigation of the potential for priming to increase the longevity of valuable ex situ seed gene bank collections, particularly those accessions in which ageing has occurred, is reported. Digitalis purpurea was selected for study since, as has already been shown, the subsequent longevity of immature seeds can be improved after collection by priming (Butler et al., 2009). The treatments comprised a range of water potentials, different durations of priming, different drying treatments and one or more cycles of priming, applied to mature seeds that had or had not been aged; subsequent longevity in air-dry storage was then determined.
MATERIALS AND METHODS
Seed collection
A collection of brown seed capsules that had started to split open was made from a population of wild foxglove (Digitalis purpurea L.) plants in the Loder Valley Nature Reserve (OS Grid Reference: TQ 336 301) on 10 August 2005. Following collection, capsules were taken back to the laboratory and seeds removed as quickly as possible. Seeds that shook out easily from capsules were retained; seeds that did not were discarded. Fresh seeds were tested for germination and moisture content. Seeds were then held in glass Petri dishes in the seed bank dry room (maintained at 15 °C and 15 % RH) until use.
Priming during storage at a range of water potentials
After approx. 8 weeks in the dry room, a sample of seeds was drawn at random and equilibrated at 20 °C over a non-saturated solution of LiCl giving 47 % RH (Hay et al., 2008), in a 300 × 300 × 102 mm sealed plastic electrical enclosure box (Ensto, Finland). After 14 d equilibration, seeds were transferred to experimental storage conditions of 45 °C and 60 % RH (in a second sealed plastic box over a different concentration of non-saturated LiCl solution; seed moisture content should be similar for the two environments, thus on transfer to 45 °C and 60 % RH, the seeds would only have to equilibrate to the higher temperature). Sub-samples of seeds were removed at 3 or 4 d intervals and tested for their ability to germinate. For one ‘control’ treatment, seeds were kept in experimental storage and sampling continued up to 60 d. Otherwise, after either 14 or 28 d in experimental storage, large sub-samples were removed and primed in the dark at 20 °C at a range of water potentials. For this, seeds were either scattered on two layers of filter paper held in 90 mm diameter Petri dishes and saturated with distilled water or with −1, −2 or −5 MPa solutions of PEG 6000 (Fisher Scientific, UK), or were placed over non-saturated solutions of LiCl made up to provide environments of −10 or −15 MPa. LiCl was used to create the lower water potentials since PEG solutions become extremely viscous at the high concentrations required for low water potentials. After priming for 48 h, seeds were washed in distilled water and excess water removed by transferring the seeds to a sintered glass Büchner funnel placed in a Büchner flask to which a vacuum was applied. Seeds were then re-equilibrated at 20 °C and 47 % RH for 24 h before returning to experimental storage at 45 °C and 60 % RH and sampled every 3 or 4 d thereafter. As further controls, as well as priming seeds removed after 14 or 28 d in experimental storage, additional seeds were sealed inside 2 ml glass vials and kept at 5 °C in the dark for the 72 h that the priming–equilibration treatments took. These seeds were not washed or re-equilibrated and were returned directly to experimental storage. As a final control, some seeds were given a priming treatment after the initial 14 d equilibration period but before any experimental storage; this was carried out using −1 MPa PEG solution only, for 48 h in the dark at 20 °C as before. These seeds were then re-equilibrated, placed into experimental storage and sampled as above. The moisture content and germination of seeds were also determined before and after each priming treatment, and after equilibration.
Duration of priming and effect of drying after priming
After approx. 1 year at 15 % RH and 15 °C, a further sample of seeds from the original collection was equilibrated at 47 % RH and 20 °C for 7 d and then transferred to experimental storage (60 % RH and 45 °C). Samples of seeds were removed at 3 or 4 d intervals and tested for germination. After 14 d, sub-samples of seeds were removed and primed at −1 MPa as described above but for either 2 or 48 h. After priming, seeds from both treatments were washed as described above, sub-divided, and either immediately re-equilibrated at 47 % RH and 20 °C or first dried at 15 % RH and 15 °C for 7 d. After 7 d re-equilibration, seeds were returned to experimental storage and samples removed and tested for germination every 3 or 4 d.
Multiple cycles of priming
This investigation also began about a year after the original collection was made. Seeds were removed from the dry room, equilibrated for 7 d at 47 % RH and 20 °C for 7 d, and then placed into experimental storage, all as above. After 14 d storage at 60 % RH and 45 °C, about half of the seeds were removed and primed at −1 MPa for 48 h, washed, surface-dried, re-equilibrated and returned to experimental storage and sampling, all as described above. Of the remaining seeds, a third were left in experimental storage with sampling up to 70 d; the rest were similarly primed after an initial 28 d of experimental storage. Priming was repeated with sub-samples of the seeds primed for 14 and 28 d after intervals of 14 or 18 d further storage to provide up to three cycles of storage → priming → storage.
Seed moisture content determination
Seed moisture contents were determined gravimetrically. Seeds were dried for 17 ± 1 h in an oven at 103 °C (Butler et al., 2009), using five replicates of approx. 30 seeds each, and the moisture contents were calculated on a percent fresh weight basis.
Seed germination
Germination tests were carried out on two or four replicates of 25 seeds. Seeds were sown on 1 % dH2O agar (Fisher Scientific, UK) containing 1 mm KNO3 (to break any dormancy) in 50 mm Petri dishes and incubated at 25/10 °C (8/16 h, respectively, with light provided in the warm phase). Lateral illumination with a flux density of 50–85 w m−2 was provided by 30 W cool white fluorescent tubes. Seeds were scored as germinated when the radicle had emerged by at least 2 mm. Germination tests were terminated after the seeds had been incubated for 56 d.
Data analysis
Probit analysis was carried out using GenStat for Windows 8th Edition (VSN International Ltd, UK) to estimate p50 (the time taken for viability to fall to 50 %) and fit seed survival curves to the data using the equation
| 1 |
where v is the viability [normal equivalent deviates (NED)] of a seed lot stored for period p (d), Ki is initial seed lot viability (NED) and σ is the standard deviation of the distribution of seed deaths in time (d) (Ellis and Roberts, 1980). Thus, p50 is the product of Ki and σ. The results of seeds aged, primed and then returned to experimental storage, where survival curves for each sequence of ageing data had a reduced initial viability and appeared asymmetrical, were fitted as above but including the ‘control mortality’ parameter in probit analysis (‘Immunity’ in GenStat) to estimate the initial proportion of the seeds that were sown for each sub-sample that were non-responders and not part of the newly ageing population (Mead and Gray, 1999). For these stored–primed–dried–stored treatments, p50 is the period to 50 % viability from the return to experimental storage. The total period in the experimental storage environment (60 % RH, 45 °C) for such treatments is designated tp50.
RESULTS
Seeds were collected at 21·4 ± 0·85 % moisture content, lower than that recorded for seeds harvested from a nearby population 24 d earlier, at 40 d after flowering (43·9 %; Butler et al., 2009), supporting our assertion that these seeds were more mature. All seeds germinated when tested.
Priming during storage at a range of water potentials
Priming at 0, −1, −2, −5, −10 and −15 MPa for 48 h after 14 or 28 d storage at 45 °C and 60 % RH (5·9 % moisture content) increased moisture content to between 44·1 and 11·9 % (Table 1). There was a tendency for slightly lower moisture contents when primed after 28 d compared with 14 d storage; however, this was only significant for treatment at −2 and −10 MPa (P < 0·05) and does not reflect a true difference in moisture sorption properties.
Table 1.
Moisture content (MC) and survival curve parameters* for D. purpurea seeds placed into experimental storage (45 °C, 60 % RH; 5·9 ± 0·2 % moisture content) for 0, 14 or 28 d, primed at the water potentials indicated, and then returned to experimental storage
| Treatment | MC after priming (mean % ± s.e.) | p50 (d ± s.e.) | Slope‡ (d−1 ± s.e.) | Ki (NED ± s.e.) | σ (d) | Proportion of non-responders† (±s.e.) | Responders ( %) |
|---|---|---|---|---|---|---|---|
| 0 d initial storage | |||||||
| Control | – | 30·7 ± 0·37 | −0·14 ± 0·007a | 4·2 ± 0·20 | 7·3 | Not estimated | 100 |
| −1 MPa | 29·1 ± 0·98 | 28·2 ± 0·29 | −0·25 ± 0·017c | 7·0 ± 0·49 | 4·0 | 0·008 ± 0·0055A | 99·2 |
| 14 d initial storage | |||||||
| 5 °C | – | 16·6 ± 0·40 | −0·13 ± 0·006a | 2·1 ± 0·11 | 8·0 | Not estimated | 100 |
| 0 MPa | 41·2 ± 0·74 | 25·4 ± 0·37 | −0·18 ± 0·012b | 4·7 ± 0·33 | 5·5 | 0·022 ± 0·0082A | 97·8 |
| −1 MPa | 29·4 ± 0·24 | 25·3 ± 0·33 | −0·20 ± 0·013c | 5·0 ± 0·33 | 5·1 | 0·005 ± 0·0040A | 99·5 |
| −2 MPa | 26·3 ± 0·35 | 24·2 ± 0·34 | −0·20 ± 0·014d | 4·9 ± 0·36 | 5·0 | 0·014 ± 0·0065A | 98·6 |
| −5 MPa | 18·0 ± 0·30 | 25·9 ± 0·39 | −0·15 ± 0·009e | 3·9 ± 0·24 | 6·7 | 0·012 ± 0·0064A | 98·8 |
| −10 MPa | 16·7 ± 0·44 | 34·2 ± 0·40 | −0·14 ± 0·008f | 5·0 ± 0·29 | 6·9 | 0·012 ± 0·0064A | 98·8 |
| −15 MPa | 12·1 ± 0·17 | 26·7 ± 0·45 | −0·14 ± 0·008g | 3·6 ± 0·24 | 7·5 | 0·023 ± 0·0091A | 97·6 |
| 28 d initial storage | |||||||
| 5 °C | – | 1·5 ± 0·70 | −0·12 ± 0·006a | 0·2 ± 0·09 | 8·3 | Not estimated | 100 |
| 0 MPa | 44·1 ± 1·02 | 18·5 ± 0·60 | −0·22 ± 0·028b | 4·0 ± 0·60 | 4·6 | 0·324 ± 0·0263B | 67·6 |
| −1 MPa | 28·6 ± 0·17 | 18·7 ± 0·50 | −0·22 ± 0·022c | 4·1 ± 0·48 | 4·6 | 0·210 ± 0·0227B | 79·0 |
| −2 MPa | 23·7 ± 0·26 | 16·7 ± 0·51 | −0·21 ± 0·021d | 3·5 ± 0·41 | 4·7 | 0·175 ± 0·0240B | 82·5 |
| −5 MPa | 18·3 ± 0·21 | 17·9 ± 0·73 | −0·15 ± 0·014e | 2·6 ± 0·32 | 6·8 | 0·204 ± 0·0301B | 79·6 |
| −10 MPa | 14·9 ± 0·29 | 21·1 ± 0·81 | −0·11 ± 0·011f | 2·4 ± 0·30 | 8·8 | 0·154 ± 0·0003B | 84·6 |
| −15 MPa | 11·9 ± 0·14 | 9·1 ± 0·57 | −0·09 ± 0·005g | 0·8 ± 0·08 | 11·2 | Not estimatedB | 100 |
* Estimates for p50, slope (and hence σ, where σ = 1/slope), Ki and the proportion of non-responders were determined using probit analysis as if the data were for a distinct seed lot, with the time when seeds were returned to experimental storage re-set to zero days in storage. Where the proportion of responders was <100 %, the estimate of p50 is the period to 50 % viability for that responding proportion from the time that the seeds were returned to experimental storage (the product of Ki and σ). For example, where the proportion of seeds that were responders was 79 % (28 d, −1 MPa), the estimates of p50 and s.e. given are for the period from 79 to 39·5 % viability. Complete survival curves (i.e. all of the whole populations across the total experimental storage period) are shown in Fig. 1, thereby enabling comparison of p50 amongst whole populations.
‡ Within each data set, survival curves of seeds primed at the same water potential could be constrained to a common slope without a significant increase in residual deviance (P > 0·05). These curves are shown with the same superscript.
† Within each data set corresponding to seeds removed after the same initial period of experimental storage, survival curves could be constrained to a common estimate of the proportion of non-responders without a significant increase in residual deviance (P >0·05). AFor seeds removed after 14 d, the constrained estimate of the proportion of non-responders was 0·014 ± 0·0025; Bfor seeds removed after 28 d, the estimate was 0·198 ± 0·0120.
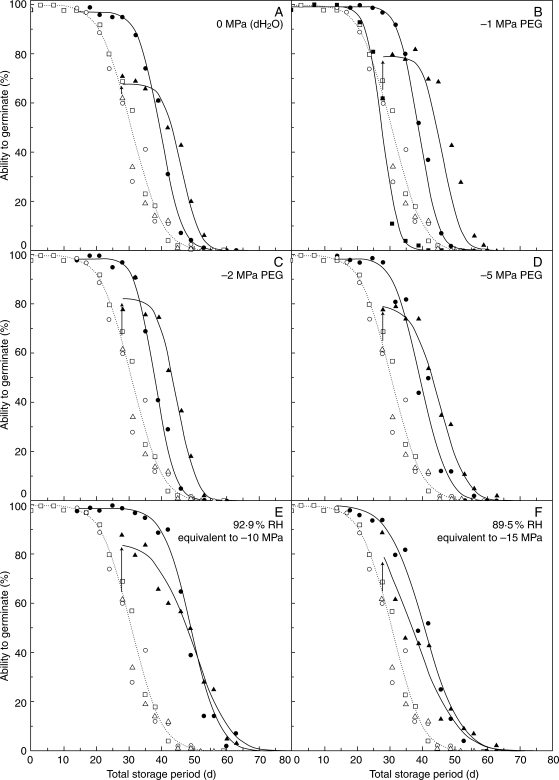
In the control experimental storage treatment (i.e. seeds not removed from storage), the pattern of loss in viability (ability to germinate) was sigmoidal, and quantified well by a negative cumulative normal distribution, with no viable seeds remaining after 52 d (Fig. 1). From the fitted survival curve for this data set, viability had fallen to 98·9 or 64 % after 14 or 28 d, respectively (Fig. 1). These are close to equivalence with the subsequent Ki values of 2·1 or 0·2 NED (98·2 or 58·0 %) for the seeds removed at these times and held at 5 °C for 72 h before returning to experimental storage (Table 1). The slope (Table 1) of each subsequent survival curve for seeds held at 5 °C did not differ significantly from the control. Hence, when the data were analysed using the total period in experimental storage as the independent variable (subtracting the period spent at 5 °C), the survival curves for these three different control treatments could be constrained to a single line without a significant increase in residual deviance (Fig. 1). Priming seeds not subjected to experimental storage at −1 MPa for 48 h resulted in a small but significant reduction in p50, from 30·7 to 28·2 d (Fig. 1B; Table 1). The survival curves for these primed and control treatments could not be constrained to a common slope [F(1,37) = 33·04, P < 0·05]; priming increased Ki but reduced σ, prolonging the shoulder of the survival period, but steepening the subsequent slope such that the survival curves crossed just below 80 % (Fig. 1B).
Fig. 1.
Survival curves, fitted by probit analysis, for D. purpurea seeds harvested in 2005 and stored at 45 °C and 60 % RH (5·9 ± 0·20 % moisture content on a percent fresh weight basis). After 14 (filled circles) or 28 d (filled triangles) experimental storage, sub-samples were withdrawn and primed for 48 h at 20 °C on filter papers saturated with (A) dH20; (B) −1 MPa PEG; (C) −2 MPa PEG; (D) −5 MPa PEG; or held over LiCl solutions in atmospheres equivalent to (E) −10 MPa; and (F) −15 MPa. All were then dried for 24 h at 45 % RH and 20 °C before being returned to experimental storage. The arrows indicate the increase in germination after priming, as estimated by probit analysis. In (B), solid squares represent seeds primed at −1 MPa before any experimental storage. The storage periods shown are total time at 60 % RH, 45 °C (i.e. disregarding the time whilst priming and re-drying). The control curve (broken line), repeated for clarity on each graph, combines the response of seeds stored without treatment (open squares) and those stored for 14 (open circles) or 28 d (open triangles), sealed in a glass vial and put at 5 °C for 72 h, and then returned to experimental storage. These three ‘control’ treatments could be constrained to the single curve shown (P > 0·05).
Priming after 14 or 28 d in experimental storage improved the subsequent survival of the seeds when they were returned to air-dry storage (Fig. 1). Priming after 14 d storage had no detectable effect on the subsequent germination percentage of seeds returned to experimental storage; the estimate of responders for all the seed lots primed after 14 d could be constrained to a common value (98·6 %; Table 1), and Ki was ≥5·0 NED (equivalent to 100 %, thus all 98·6 % of responders were viable when they were returned to experimental storage). After 28 d storage, however, priming increased the viability of seeds returned to experimental storage from 62 to 67–84 % (Fig. 1). Those seeds that did not germinate were largely accounted for by the increase in the proportion of non-responders, since Ki remained high (≥2·4 NED; equivalent to 99·2 %, i.e. very nearly all of the 67–84 % responders were viable), except for seeds primed at −15 MPa (Table 1). The high estimates for Ki (in part a consequence of a significant proportion of non-responders for seeds primed after 28 d) are manifest as periods of lag (compared with the control) before significant loss in viability (Fig. 1). Survival curve slopes were thereafter steeper than for control seeds. For example, σ was reduced from 8 d for control seeds down to 5·0–5·5 d for seeds primed after 14 d at 0 to −2 MPa (Table 1). For seeds primed at these water potentials after 28 d, σ was reduced yet further to 4·6 d. However, for seeds primed at the lower water potentials, the reduction in σ was not as great. In fact, σ increased for seeds primed at −10 or −15 MPa after 28 d in experimental storage.
Treating the survival data for seeds returned to experimental storage as if they were distinct seed lots, there were only small differences in the survival curves for seeds primed after 14 d at 0 to −5 MPa, with the resulting p50 values being very similar (approx. 25 d; Table 1). For seeds primed after 28 d at these same water potentials, estimates of p50 were lower but again similar amongst treatments at approx. 17–18 d. Estimates of p50 were generally lower for seeds primed at the lower water potentials. The effects of these changed estimates for the survival curve parameters, as shown in Table 1, when plotted with the total experimental storage period taken into account (as in Fig. 1) are that tp50 increased compared with control seeds. For example, for seeds removed after 14 d, tp50 was approx. 39 d for seeds primed at 0, −1, −2, −5 or −15 MPa, and 49 d for seeds primed at −10 MPa, compared with 31 d for seeds not primed. For seeds removed after 28 d, the survival curves for the viable seeds were shifted further to the right and the tp50 increased to 43–45 d for seeds primed at 0, −1, −2 or −5 MPa. However, for those primed at −10 or −15 MPa, there were slight decreases in tp50 compared with seeds primed at 14 d, and there was little difference amongst survival curves once viability fell below 60 %.
Duration of priming and effect of drying after priming
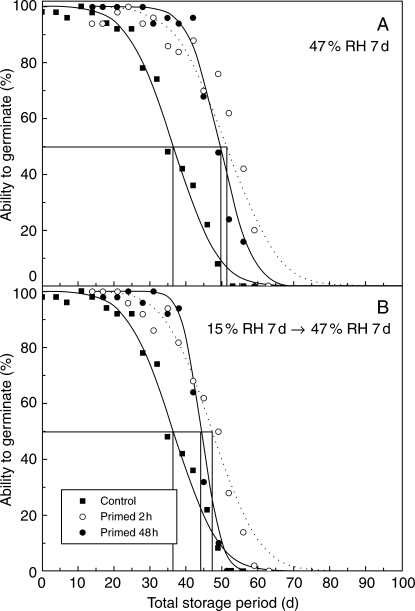
Seeds primed for 48 h using −1 MPa PEG in this experiment did not reach as high a moisture content as those given the equivalent treatment in the earlier experiment, just 23·7 ± 0·71 % (data not shown) compared with approx. 29 % (Table 1). This indicates the practical difficulties of achieving identical experimental conditions at such high water potentials. The moisture content of seeds primed for just 2 h was significantly lower at 18·7 ± .57 % (P < 0·05).
As before, the longevity of seeds removed after 14 d in experimental storage improved when primed for either 2 or 48 h (Fig. 2). The duration to 50 % viability increased from 37 d for control seeds (p50) to 44–51 d for primed seeds (tp50) and was approx. 2 d longer for seeds primed for 2 h compared with those primed for 48 h. However, the greatest difference between seeds primed for 2 or 48 h was in the shape of the survival curves; the estimates for σ following 2 h priming were similar to the control and could be constrained to a common slope [F(2,29) = 0·55, P >0·05], whereas 48 h priming reduced σ, and slopes could not be constrained to a common value [F(2,24) = 14·2, P < 0·05].
Fig. 2.
Survival of seeds of D. purpurea, harvested in 2005, stored at 45 °C and 60 % RH throughout (filled squares, repeated for clarity in both graphs) or removed after 14 d, primed (−1 MPa PEG) for 2 (open circles and dotted lines) or 48 h (filled circles and lines), then either (A) immediately re-equilibrated at 47 % RH and 20 °C for 7 d or (B) dried at 15 % RH and 15 °C for 7 d before re-equilibration at 47 % RH and 20 °C for 7 d, and then returned to experimental storage. The seed survival curves shown, fitted by probit analysis, are individual lines of best fit, although the 2 h priming treatments could be constrained to the same slope as the control without a significant increase in residual deviance (P > 0·05). Note that the storage period shown is the total time at 60 % RH, 45 °C (i.e. disregarding the time whilst priming and re-drying). The horizontal and vertical lines indicate the times when viability had fallen to 50 %.
The benefit of priming to subsequent seed survival was affected by whether the seeds were dried at 15 % RH post-priming, or immediately placed into the re-equilibration environment (47 % RH; Fig. 2). The estimate of tp50 for seeds primed for 2 h and immediately re-equilibrated was 51 d; this was reduced to 47 d if the seeds were first dried down at 15 % RH. A slightly greater reduction in tp50 was apparent for seeds primed for 48 h, from 49·5 to 44 d.
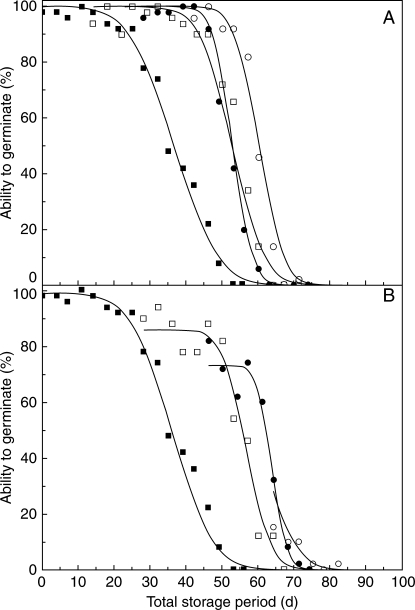
Multiple cycles of priming
Additional cycles of priming interrupting air-dry storage repeatedly improved seed longevity (Fig. 3). As in the first investigation, there had been little decline in viability by the time seeds were removed from experimental storage at 14 d (Fig. 3A). Priming seeds then shifted the resultant survival curve to the right and tp50 increased by approx. 17 d. Priming these seeds again after 14 d, before any significant decline in viability, had no effect on tp50. However, priming a third time after another 14 d improved tp50 by a further 7 d. Thus tp50 for seeds which had experienced a total of 42 d in experimental storage before the third priming treatment was 60 d; for control seeds (not primed), p50 was 36 d and all the seeds had died by 70 d. As before, survival curves were steeper for primed seeds than the control.
Fig. 3.
Survival of seeds of D. purpurea, harvested in 2005, stored at 45 °C and 60 % RH throughout (filled squares, repeated for clarity in both graphs) or removed after either 14 (A) or 28 d (B) and then primed for 2 d (−1 MPa PEG), re-equilibrated at 20 °C and 47 % RH for 24 h, and returned to storage. (A) Seeds were primed after 14 d storage (open squares), re-primed after a further 14 d storage (filled circles), or primed for a third time after another 14 d storage (open circles). (B) Seeds were primed after 28 d storage (open squares), re-primed after a further 18 d storage (filled circles), or primed for a third time after another 18 d storage (open circles). Survival curves were fitted by probit analysis. Note that the storage period shown is the total time at 60 % RH, 45 °C (i.e. disregarding the time whilst priming and re-drying).
After 28 d in storage, the ability to germinate had already fallen to 80 %. As before (Fig. 1), priming resulted in an increase in the germinability of seeds returned to experimental storage (Fig. 3B). Again, priming resulted in a lag period before loss in viability was apparent, and the subsequent survival curves were steeper. These effects were largely duplicated by the second priming treatment, except that ability to germinate on the return to experimental storage was approx. 10 % lower than that of seeds removed from storage. Thus tp50 increased to 55 or 62 d for seeds initially stored for 28 d and given one or two cycles of priming, respectively. By the third priming treatment, after a total of 64 d in experimental storage, viability had been reduced to approx. 20 % and differences between the second and third priming treatments were difficult to discern (Fig. 3B).
DISCUSSION
Whilst priming is consistently reported to improve the rate and uniformity of seed germination, the effect on subsequent seed longevity appears to be more variable, with reports of increases (e.g. Georghiou et al., 1987; Probert et al., 1991; Wechsberg, 1994) and decreases in seed longevity (e.g. Argerich et al., 1989; Tarquis and Bradford, 1992; Schwember and Bradford, 2005; Hill et al., 2007). Powell et al. (2000) reported different responses related to initial seed quality; priming increased the rate of subsequent deterioration in high vigour seeds of Brassica oleracea L, but was not deleterious to lower vigour seeds.
Similarly here, priming only improved the subsequent longevity of mature D. purpurea seeds that had already experienced some experimental storage. It had a generally negative effect (see later) on newly collected mature seeds which had just been processed as if for long-term storage (Fig. 1B). Although loss of viability was not detected after 14 d of experimental storage, the seeds had clearly incurred some damage which was then repaired by priming. Upon return to experimental storage, such treatments resulted in large increases in the period for viability to fall to 50 % (tp50; Fig. 1). After 28 d experimental storage, there had been a measurable decline in viability. However, after priming, a greater proportion of the seeds returned to experimental storage were germinable. This is an extreme case of the shortest lived (least vigorous) seeds benefiting most from priming. We suggest that these seeds were on the ‘brink’ of losing the capacity to complete germination under standard conditions, because germination sensu strictu commenced (but then failed) before sufficient repair had occurred during imbibition. By delaying germination, priming provided the additional repair time such that these individuals could recover the capacity to germinate under standard conditions and indeed survive a further period of storage.
Thus the priming treatments had a ‘rejuvenating’ effect on the population of responding seeds that were returned to experimental storage. The control mortality term (Mead and Gray, 1999) was a significant parameter in the probit analysis of the data for these seed lots returned to experimental storage. Thus the responding individuals amongst the returned seeds followed a distinct (normal) distribution of seed deaths over time. Unlike the seeds held at 5 °C, whereby ageing was in effect suspended, the returned primed seeds did not behave as part of the same population of seeds that were originally placed in experimental storage. The estimates for p50, slope and Ki given in Table 1 for these primed seed lots are the estimates of the distribution of seed deaths over time for the ‘responders’, not for all the seeds stored and then sown in the germination tests. Seeds that had been irreversibly damaged by the initial period of experimental storage were no longer part of the subsequent repaired and ageing seed population. The distributions of seed deaths over time when primed after 14 d storage were not so different (but see below) from that of the control; p50 was reduced by only approx. 5 d and estimates for Ki were still high (3·5–5·0 NED; Table 1). A high Ki (anything ≥2 NED) results in the ‘shoulder’ to the survival curves, which then becomes exaggerated as the proportion of non-responders increases (Fig. 1). The distributions of seed deaths over time for seeds removed from experimental storage after 28 d, primed and then returned to experimental storage showed greater differences: estimates of p50 for the responders were typically approx. 18 d compared with 30 d for control seeds. The longer initial period of experimental storage had a negative effect on the estimates of both Ki and σ of the subsequent responders, suggesting that these seeds were not able to repair fully the damage that had been incurred. That is, there is a limit to the amount of damage that can be fully repaired, at least with the treatments used here.
Our analyses re-set the duration of storage to zero when the seeds were returned to experimental storage; the estimates of p50 values from probit analysis do not reflect the total period of experimental storage. Thus the term tp50 was introduced to reflect the full ageing history of the seed lot, whereas p50 only reflects the latest phase of experimental storage. Clearly, priming the stored seeds of D. purpurea just before the accumulated damage had lethal consequences (i.e. on the cusp of the survival curves) dramatically increased tp50. Re-priming seeds at this point repeatedly allowed sufficient repair to occur such that close to 100 % viability was maintained for 50 d and tp50 was extended to 60 d (Fig. 3A). In contrast, in the control (for which p50 = tp50), almost no viable seeds remained at 55d and p50 was only 36 d. The maximum number of priming cycles applied here was only three. Each cycle improved longevity to some degree. It might be supposed that at some point the efficiency of the repair processes would be impaired. However, lettuce seed survived for many months when subjected to repeated 14 d cycles of imbibition, redrying and storage (Villiers and Edgcumbe, 1975). It is suggested, therefore, that limiting the extent of seed deterioration in air-dry storage before (re-)priming may be more important than the number of cycles.
Priming after a significant loss of viability had accumulated within a population resulted in yet greater longevity of those seeds that had remained viable: the estimate of tp50 was greater for seeds primed after 28 than 14 d storage. This is seen as a further shift to the right by the survival curves in Fig. 1. For those seeds that do survive, priming is not having an additive effect. We deduce this from the remarkable similarity, <70 %, of the survival curve for seeds aged a total of 42 d and receiving three priming treatments (open circles, Fig. 3A) and that for seeds primed at approximately the same time (total treatment period), but primed only twice (filled circles Fig. 3B). However, the benefit of priming to subsequent longevity once the majority of the population had lost viability was meagre (compare open and filled circles, Fig. 3B). The implication of this is that whereas seeds from the shortest lived fraction of the original population close to the end of their life spans benefitted most from priming in terms of their subsequent survival (e.g. Fig. 1B), those from the longest lived fraction when similarly close to the end of their life spans showed much less benefit (Fig. 3B).
In this study, seeds were collected at 21·4 % moisture content. Data from Hay and Probert (1995), together with the fact that the capsules had already split, would suggest that the seeds were close to natural dispersal and hence mature. Butler et al. (2009) proposed that priming of less than mature seeds allowed maturation events to resume and thereby improve subsequent seed longevity. In this study, priming mature seeds before experimental storage generally had a negative effect on seed longevity, with the exception of the shortest lived individuals which can be surmised to be the least mature individuals within the collection and therefore able to benefit from the priming treatment. In consequence, the population became more uniform and resulted in a sharper survival curve compared with the control (Fig. 1B). Thus priming and re-drying can be used as either a maturation or repair treatment to extend the longevity of immature or aged seeds, respectively, but should not be used for high quality seeds collected at the optimum stage of development (point of natural dispersal for D. purpurea), except as a potential ‘invigoration’ treatment prior to germination (i.e. not returned to storage). They should be dried and placed into storage without delay. Since it may not be practicable to sort a heterogeneous seed collection, post-harvest, into those at, or not yet at, this optimum stage of development, attempts to optimize slow-drying and/or priming treatments will be a compromise between what is best for the different fractions within a single seed population. This might be easier to achieve at the lower water potentials investigated here because the benefit to the longevity of individuals within the population tended to be more uniform (i.e. σ was similar to the control) and the absolute benefit greater at lower water potentials. Priming seeds at −10 MPa after withdrawal from experimental storage provided the greatest benefit to subsequent longevity (Fig. 1). Note, however, that this treatment was provided by a moist atmosphere over LiCl, whereas water potentials closer to those of fully imbibed seeds were provided on filter papers saturated with dH2O or PEG in solution. Too rapid uptake of water has been suggested to be damaging during priming, whilst a more controlled uptake of water from a moist atmosphere can be beneficial (Basu and Pal, 1980; Rao et al., 1987). Additionally, oxygen required for repair may be more available to seeds equilibrated in a moist atmosphere than those primed on PEG in a sealed Petri dish. Nonetheless, this investigation showed that all water potentials investigated were beneficial to the subsequent longevity of aged seeds, and differences among them were not large. This contrasts with the situation in seeds stored at high moisture contents where, in the presence of oxygen, survival is greater the higher the water potential, and which has been attributed to repair mechanisms being more effective at these values (Ibrahim and Roberts, 1983). Hence, it is difficult to explain all of the benefits reported here by repair mechanisms alone – particularly since the driest beneficial treatment (−15 MPa, approx. 12 % moisture content, or an eRH of 80–85 %; Butler et al. 2009) is just below the lower limit of the moisture range over which repair mechanisms are believed to function.
The major benefit from priming to subsequent seed longevity was achieved quite rapidly, apparently occurring during early imbibition and perhaps the early stages of subsequent re-drying. Earlier experiments suggested that 2 h was sufficient for D. purpurea seeds to equilibrate at the priming water potential. The moisture content estimates suggest that seeds continued to take up water, at least up to 48 h, but nonetheless seed longevity was improved after just 2 h priming (Fig. 2). Furthermore, this 2 h treatment had a uniform effect on all the individual seeds within the population. This contrasts with the longer duration priming treatment where the survival periods of the shortest lived individuals were further improved whilst those for the longest lived individuals were impaired (and hence the steeper survival curves).
Drying primed seeds back at 15 % RH, 15 °C (dry room conditions) also impaired subsequent longevity (compared with 47 % RH, 20 °C). Indeed, the viability of seeds primed for 48 h and dried under these conditions was lost in the longest lived individuals after the same period of storage as those in the control (Fig. 2B). The milder water stress of the re-equilibration environment (47 % RH) following priming may contribute to the overall beneficial effect of the treatment since gradual loss of water may improve resistance to dehydration damage by allowing time for protective mechanisms to operate (Kermode and Finch-Savage, 2002; Soeda et al., 2005). Although both of our regimes dried seeds more rapidly than occurs in normal seed maturation, there appears to be the possibility of parallels between drying after priming, where this is beneficial to longevity, and during the post-abscission phase of seed maturation where similar benefits are seen. We speculate that those aspects of seed quality which are gained during the final stages of maturation drying may be the first functionality to be lost during seed ageing. However, provided the damage is not too great, this can be regained quickly by a cycle of rehydration and desiccation (as here), or perhaps one of rehydration and heat shock (Gurusinghe and Bradford, 2001).
The capability of fractions of a population of seeds to respond differently to the same priming treatment – the longevity of some individuals increasing whilst decreasing in others – poses questions about the methods traditionally adopted to investigate the molecular or biochemical bases for seed behaviour. In such situations, the certainty of correctly interpreting differences between samples, when measures are determined using homogeneous extracts from a number of seeds, appears low.
It would be premature on the basis of the current study on a single species to advocate that seed accessions maintained in gene banks should be routinely primed at some period during their storage, particularly as the literature on the effect of priming on both desiccation tolerance and subsequent longevity is contradictory. However, adopting an approach of improving the quality of seed collections through repeated cycles of rehydration and desiccation could retain seed viability and reduce the need for costly, and risky, regeneration procedures. Seed bank collections are typically regenerated when viability falls to 85 % (e.g. IBPGR, 1976) or 75 % (e.g. Terry et al., 2003). Moreover, such values are on the part of the seed survival curve where a high proportion of those seeds that remain viable are close to death, and these individuals are more likely to fail to produce seedlings when sown (Ellis and Roberts, 1981). Hence there is selection against the shorter lived individuals. In the case of genetically heterogeneous accessions, the regeneration of such accessions inevitably results in the selection of longer lived genotypes and, with them, any other tightly linked characters. As we have shown here, priming tends to benefit the survival of the whole (surviving) population but often benefits the shorter lived fraction more. Hence such rehydration–desiccation cycles have the potential to minimize the risk of genetic selection during regeneration.
ACKNOWLEDGEMENTS
This work was supported by a research studentship to L.H.B. from the University of Reading Research Endowment Trust Fund and from the Seed Conservation Department, RBG Kew. The SCD, RBG Kew receives financial support from the Millennium Commission, The Wellcome Trust, Orange plc and through grant-in-aid from Defra, UK.
LITERATURE CITED
- Argerich CA, Bradford KJ. The effects of priming and ageing on seed vigour in tomato. Journal of Experimental Botany. 1989;40:599–607. [Google Scholar]
- Argerich CA, Bradford KJ, Tarquis AM. The effects of priming and ageing on resistance to deterioration of tomato seeds. Journal of Experimental Botany. 1989;40:593–598. [Google Scholar]
- Ashraf M, Bray CM. DNA synthesis in osmoprimed leek (Allium porrum L.) seeds and evidence for repair and replication. Seed Science Research. 1993;3:15–23. [Google Scholar]
- Bailly C, Benamar A, Corbineau F, Côme D. Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Science Research. 2000;10:35–42. [Google Scholar]
- Basu RN, Pal P. Control of rice seed deterioration by hydration–dehydration pretreatments. Seed Science and Technology. 1980;8:151–160. [Google Scholar]
- Boubriak I, Kargiolaki H, Lyne L, Osborne DJ. The requirement for DNA repair in desiccation tolerance of germinating embryos. Seed Science Research. 1997;7:97–105. [Google Scholar]
- Bray CM, Ashraf M, Davison PA, Taylor RM. Molecular markers of seed quality. In: Côme D, Corbineau F, editors. Fourth international workshop on seeds: basic and applied aspects of seed biology. Paris: ASFIS; 1993. pp. 887–896. [Google Scholar]
- Bruggink GT, Ooms JJJ, van der Toorn P. Induction of longevity in primed seeds. Seed Science Research. 1999;9:49–53. [Google Scholar]
- Burgass RW, Powell AA. Evidence for repair processes in the invigoration of seeds by hydration. Annals of Botany. 1984;53:753–757. [Google Scholar]
- Butler LH, Hay FR, Ellis RH, Smith RD. Post-abscission, pre-dispersal seeds of Digitalis purpurea L. remain in a developmental state that is not terminated by desiccation ex planta. Annals of Botany. 2009;103:785–794. doi: 10.1093/aob/mcn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu KY, Chen CL, Sung JM. Effect of priming temperature on storability of primed sh-2 sweet corn seed. Crop Science. 2002;42:1996–2003. [Google Scholar]
- Demir I. Effect of controlled hydration treatment on quality of aubergine seeds following storage. Phyton Annales Rei Botanicae. 2003;43:307–317. [Google Scholar]
- Elder RH, Osborne DJ. Function of DNA synthesis and DNA repair in the survival of embryos during early germination and in dormancy. Seed Science Research. 1993;3:43–53. [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- Ellis RH, Roberts EH. The quantification of ageing and survival in orthodox seeds. Seed Science and Technology. 1981;9:373–409. [Google Scholar]
- Georghiou K, Thanos CA, Passam HC. Osmoconditioning as a means of counteracting the ageing of pepper seeds during high-temperature storage. Annals of Botany. 1987;60:279–285. [Google Scholar]
- Gurusinghe S, Bradford KJ. Galactosyl-sucrose oligosaccharides and potential longevity of primed seeds. Seed Science Research. 2001;11:121–133. [Google Scholar]
- Gurusinghe S, Powell ALT, Bradford KJ. Enhanced expression of BiP is associated with treatments that extend storage longevity of primed tomato seeds. Journal of the American Society for Horticultural Science. 2002;127:528–534. [Google Scholar]
- Hay FR, Probert RJ. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.) Annals of Botany. 1995;76:639–647. [Google Scholar]
- Hay FR, Adams J, Manger K, Probert R. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology. 2008;36:737–746. [Google Scholar]
- Heydecker W, Gibbins BM. The ‘priming’ of seeds. Acta Horticulturae. 1978;83:213–113. [Google Scholar]
- Heydecker W, Higgins J, Gulliver RL. Accelerated germination by osmotic seed treatment. Nature. 1973;246:42–44. [Google Scholar]
- Hill HJ, Cunningham JD, Bradford KJ, Taylor AG. Primed lettuce seeds exhibit increased sensitivity to moisture content during controlled deterioration. HortScience. 2007;42:1436–1439. [Google Scholar]
- Ibrahim AE, Roberts EH. Viability of lettuce seeds I. Survival in hermetic storage. Journal of Experimental Botany. 1983;34:620–630. [Google Scholar]
- IBPGR. Report of IBPGR working group on engineering, design and cost aspects of long-term seed storage facilities. Rome: International Board for Plant Genetic Resources; 1976. [Google Scholar]
- Kermode AR, Finch-Savage WE. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CAB International Publishing; 2002. pp. 149–184. [Google Scholar]
- Lanteri S, Kraak HL, Ric De Vos CH, Bino RJ. Effects of osmotic preconditioning on nuclear replication activity in seeds of pepper (Capsicum annuum) Physiologia Plantarum. 1993;89:433–440. [Google Scholar]
- Mead A, Gray D. Prediction of seed longevity: a modification of the shape of the Ellis and Roberts seed survival curves. Seed Science Research. 1999;9:63–73. [Google Scholar]
- Onelli E, Citterio S, Labra M, Ghiani A, Sgorbati S. The presence of a p53-like protein during pea seed maturation and germination. Plant Biosystems. 2000;134:153–165. [Google Scholar]
- Pammenter NW, Berjak P. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Science Research. 1999;9:13–37. [Google Scholar]
- Powell AA, Yule LJ, Jing H, Groot SPC, Bino RJ, Pritchard HW. The influence of aerated hydration seed treatment on seed longevity as assessed by the viability equations. Journal of Experimental Botany. 2000;51:2031–2043. doi: 10.1093/jexbot/51.353.2031. [DOI] [PubMed] [Google Scholar]
- Probert RJ, Bogh SV, Smith AJ, Wechsberg GE. The effects of priming on seed longevity in Ranunculus sceleratus L. Seed Science Research. 1991;1:243–249. [Google Scholar]
- Rao NK, Roberts EH, Ellis RH. The influence of pre and post-storage hydration treatments on chromosomal aberrations, seedling abnormalities, and viability of lettuce seeds. Annals of Botany. 1987;60:97–108. [Google Scholar]
- Roberts EH, Ellis RH. Water and seed survival. Annals of Botany. 1989;63:39–52. [Google Scholar]
- Sarocco F, Bino RJ, Bergervoet JHW, Lanteri S. Influence of priming-induced nuclear replication activity on storability of pepper (Capsicum annuum L.) seed. Seed Science Research. 1995;5:25–29. [Google Scholar]
- Schwember AR, Bradford KJ. Drying rates following priming affect temperature sensitivity of germination and longevity of lettuce seeds. HortScience. 2005;40:778–781. [Google Scholar]
- Sivritepe HO, Dourado AM. The effect of priming treatments on the viability and accumulation of chromosomal damage in aged pea seeds. Annals of Botany. 1995;75:165–171. [Google Scholar]
- Śliwińska E, Jendrzejczak E. Sugar-beet seed quality and DNA synthesis in the embryo in relation to hydration–dehydration cycles. Seed Science and Technology. 2002;30:597–608. [Google Scholar]
- Soeda Y, Konings CJM, Vorst O, et al. Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination process and the stress tolerance level. Plant Physiology. 2005;137:354–368. doi: 10.1104/pp.104.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquis AM, Bradford KJ. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seed. Journal of Experimental Botany. 1992;43:307–317. [Google Scholar]
- Terry J, Probert RJ, Linington SH. Processing and maintenance of the Millennium Seed Bank collections. In: Smith RD, Linington SH, Dickie JB, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew, UK: Royal Botanic Gardens; 2003. pp. 307–325. [Google Scholar]
- Vertucci CW, Farrant JM. Acquisition and loss of desiccation tolerance. In: Kigel J, Galili G, editors. Seed development and germination. New York: Marcel Dekker Inc; 1995. pp. 237–271. [Google Scholar]
- Vertucci CW, Leopold AC. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiology. 1984;75:114–117. doi: 10.1104/pp.75.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW, Leopold AC. Physiological activities associated with hydration level in seeds. In: Leopold AC, editor. Membranes, metabolism and dry organisms. Ithaca, New York: Cornell University Press; 1986. pp. 35–49. [Google Scholar]
- Villiers TA, Edgcumbe DJ. On the cause of seed deterioration in dry storage. Seed Science and Technology. 1975;3:761–774. [Google Scholar]
- Wechsberg GE. Seed longevity and response to priming in Ranunculus sceleratus L. UK: University of Manchester; 1994. PhD Thesis. [Google Scholar]
- Zlatanova JS, Ivanov PV, Stoilov LM, Chimshirova KV, Stanchev BS. DNA repair precedes replicative synthesis during early germination in maize. Plant Molecular Biology. 1987;10:139–144. doi: 10.1007/BF00016151. [DOI] [PubMed] [Google Scholar]