Abstract
Hypoxia-induced endothelial dysfunction plays a crucial role in the pathogenesis of hypoxic pulmonary hypertension. p38 MAPK expression is increased in the pulmonary artery following hypoxic exposure. Recent evidence suggests that increased p38 MAPK activity is associated with endothelial dysfunction. However, the role of p38 MAPK activation in pulmonary artery endothelial dysfunction is not known. Sprague-Dawley rats were exposed to 2 wk hypobaric hypoxia, which resulted in the development of pulmonary hypertension and vascular remodeling. Endothelium-dependent relaxation of intrapulmonary vessels from hypoxic animals was impaired due to a reduced nitric oxide (NO) generation. This was despite increased endothelial NO synthase immunostaining and protein expression. Hypoxia exposure increased superoxide generation and p38 MAPK expression. The inhibition of p38 MAPK restored endothelium-dependent relaxation, increased bioavailable NO, and reduced superoxide production. In conclusion, the pharmacological inhibition of p38 MAPK was effective in increasing NO generation, reducing superoxide burden, and restoring hypoxia-induced endothelial dysfunction in rats with hypoxia-induced pulmonary hypertension. p38 MAPK may be a novel target for the treatment of pulmonary hypertension.
Keywords: p38 mitogen-activated protein kinase, endothelium, nitric oxide
pulmonary hypertension of any cause, including hypoxia, is characterized by sustained vascular contraction and pulmonary vascular remodeling. Hypoxia accompanies a variety of chronic cardiopulmonary diseases and can affect normal individuals at altitude. Chronic exposure to hypoxia leads to a sustained elevation in pulmonary artery pressure, characterized by active vasoconstriction and vascular remodeling of the pulmonary arteries (31). Normal functioning of the endothelium is crucial to maintaining pulmonary vascular function and structural integrity. Endothelial dysfunction is manifested by endothelial cell proliferation and an imbalanced production of humoral factors (2, 23, 34). These processes act in concert to maintain an elevated pulmonary artery pressure. It would be most useful if new treatments for pulmonary hypertension were directed at the restoration of endothelial function.
Nitric oxide (NO) is the most important of the endothelium-derived vasoactive factors responsible for maintaining pulmonary vascular relaxation and low resting pulmonary artery pressure (40). The pivotal role of NO in hypoxic pulmonary hypertension is suggested by several elegant studies. Endothelial NO synthase (eNOS) knockout mice develop pulmonary hypertension following exposure to mild hypoxia (13, 44). Conversely, eNOS overexpression in transgenic mice or pulmonary eNOS gene transfer is protective against the development of hypoxic pulmonary hypertension (5, 36). In contrast, rodent models of hypoxic pulmonary hypertension exhibit increased eNOS expression (10, 12), but despite this, bioavailable NO is decreased (35). Impaired NO production is also a feature of human primary pulmonary hypertension, where exhaled or urinary excretion of NO metabolites are reduced (22, 38). The cellular mechanisms underlying the hypoxia-induced derangement in NO production, degradation, and function are not fully understood.
Hypoxia is known to cause pulmonary vessels to constrict and systemic vessels to dilate. As our previous work (49) in this field showed quite clearly that hypoxia caused pulmonary artery fibroblasts to proliferate while having no effect on systemic artery fibroblasts, we hypothesized that the mechanisms that we have shown to be involved in proliferation may also be involved in vasoconstriction. The MAPK pathway is activated in response to growth factors, cellular stress, and inflammatory cytokines. Three major subgroups of MAPKs have been identified: extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 MAPK. Recent interest has focused on the activation of MAPKs in response to hypoxia and its role in the pathogenesis of hypoxic pulmonary hypertension (8, 20, 27). In particular, it has been shown that p38 MAPK is activated in the pulmonary artery and not in the systemic arteries in response to both acute and chronic hypoxia (48, 50). The MAPK pathway has also been implicated in vascular smooth muscle contraction in response to agonists such as endothelin, serotonin, and angiotensin II. It is also suggested that the same pathway may be activated in pathological long-lasting contractions or vasospasm (1, 30, 47). Work from this laboratory and others have shown that hypoxia potently stimulates the activation of p38 MAPK, driving fibroblast proliferation and vascular remodeling (43, 49).
Emerging evidence points to an important role of p38 MAPK in endothelial function, including endothelial barrier function, vascular inflammation, and endothelial cell proliferation/apoptosis (3, 4, 46). The activation of p38 MAPK has been shown in blood vessels of hypertensive animals by both immunohistochemical and Western blot analysis techniques. Furthermore, the activation of p38 MAPK may also mediate endothelial dysfunction and target organ damage observed in animal models of hypertension and chronic heart failure (21, 51).
We therefore hypothesized that the activation of p38 MAPK may also mediate hypoxia-induced pulmonary artery endothelial dysfunction. To test this hypothesis, we examined endothelial-dependent and -independent relaxation in isolated pulmonary arteries exposed to acute and chronic hypoxia. We also examined the phosphorylation of p38 MAPK, superoxide generation, eNOS expression, and NO production in response to hypoxia. We then tested whether endothelial function could be restored by the inhibition of p38 MAPK activity.
METHODS
Chronically Hypoxic Rat Model
Chronic hypoxic pulmonary hypertension was induced in male Sprague-Dawley rats (starting weight, 100–125 g) placed in a hypobaric chamber for 2 wk. The chamber was gradually depressurized over 2 days to 550 mbar (equivalent to 10% O2). The chamber was maintained at room temperature and ventilated with air at ∼45 l/min. Aged-match control animals were maintained in a similar Perspex chamber at normal atmospheric pressure and subjected to a similar animal maintenance protocol. The experimental procedure had been approved by the University Ethical Committee and was carried out under authorization granted under the Animals (Scientific Procedures) Act 1986.
Isolated Vessel Study
Male Sprague-Dawley rats (250–300 g) were euthanized by the inhalation of CO2 gas in an increasing concentration. Following thorocotomy, heart and lungs were removed en bloc into Krebs solution containing (in mM) 118 NaCl, 25 NaHCO3, 11 glucose, 4.7 KCl, 1.2 MgCl2, 1.2 KH2PO4, and 2.5 CaCl2. Heart and lungs were separated, and intrapulmonary pulmonary artery (internal diameter, 400–700 μm) was carefully dissected under a dissecting microscope from left and right lung lobes. After the adherent connective tissue was carefully removed, the artery was cut into rings ∼3 mm in length.
Four artery rings were prepared and mounted on a multichannel wire myograph (Multi Myograph 610, Danish Myo Technology, Aarhus, Denmark). Each artery ring was mounted on a pair of intraluminal wires in a 5-ml organ bath filled with Krebs solution maintained at 37°C and gassed with a 20% O2-5% CO2-75% N2 mixture. One of the wires was anchored to a moveable micrometer and the other to a force transducer for continuous recording of isometric tension via Myodaq software (MyoDaq V.2.01, Danish Myo Technology).
Before the experimental protocols were started, the resting force on each pulmonary artery ring was set to be the same as that present at a specified pulmonary artery pressure using the Laplace equation (33). The resting force was equivalent to 12 mmHg in rings from normotensive rats or 22 mmHg in rings from pulmonary hypertensive rats, which are the actual arterial pressures that we find in the rats in our colony. After the normalized tension was set, the artery rings were allowed to equilibrate for 60 min and then subjected to the experimental protocols as described below.
Effects of acute hypoxia on endothelium-dependent and -independent relaxation.
Pulmonary artery rings from normoxic animals were mounted on wire myograph as described. Acute hypoxia was induced by bubbling the 5% O2-5% CO2-90% N2 gas mixture for 1 h. Oxygen tension in the organ bath dropped to 35 mmHg within 10 min and was maintained throughout the hypoxic challenge. The pulmonary artery rings were precontracted with synthetic thromboxane mimetic 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U-46619, 3 × 10−7 M, was used to obtain a reproducible, stable contraction of the arteries before cumulative generation of relaxant responses, Sigma-Aldrich). When contraction reached a stable plateau, a cumulative concentration response curve to either carbachol (10−8-10−5 M, Sigma-Aldrich) or NO donor N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino)-1,2-ethylenediamine (NOC-22, 10−8-10−5 M, Merck-Bioscience) was constructed in the continued presence of hypoxia. After being washed with oxygenated Krebs solution, the artery rings were contracted with U-46619 and relaxed with either carbachol or NOC-22 under normoxic conditions. In a parallel control, a similar experimental protocol was followed under normoxic conditions.
Effects of chronic hypoxia on endothelium-dependent and -independent relaxation.
Pulmonary artery rings from chronically hypoxic animals were mounted on wire myograph in oxygenated Krebs solution as described above. The artery rings were contracted with U-46619 to match the level of pretone recorded in the rings from normoxic rats by lowering the concentration of U-46619 if necessary. When contraction reached a stable plateau, the cumulative concentration response curve to either carbachol or NOC-22 was constructed.
Role of p38 MAPK.
In parallel experiments, before the precontracting with U-46619, the specific p38 MAPK inhibitor SB-203580 (Merck-Bioscience) was added and allowed to equilibrate for 20 min. Thereafter, the above experimental protocol was followed. In additional experiments, p38 MAPK stimulator anisomycin (Merck-Bioscience) was added and allowed to equilibrate for 20 min. The concentration of U-46619 was adjusted, if necessary, to match the level of pretone recorded in the rings from normoxic rats that had received no drug treatment.
Immunohistochemistry
Pulmonary artery and lung lobes from normoxic and hypoxic animals were immediately fixed in neutral-buffered saline (formalin) and dehydrated through graded alcohol to xylene. Artery and lung tissue were oriented and embedded in wax, and 4-μm sections were cut using a rotary microtome (Leica RM2125 RTF, Leica Instruments). The sections were mounted onto silanated slides and dewaxed and rehydrated through graded alcohols to water. Endogenous peroxidase activity was blocked in 0.3% H2O2 for 10 min and washed in Tris-buffered saline (TBS) for 5 min. Antigen retrieval was performed by microwave pressure cooking in Tris-EDTA buffer (0.37 g EDTA and 0.55 g Tris were added to 1 liter of distilled water) for 5 min. Nonspecific binding was blocked in TBS supplemented with 20% goat serum and 3% BSA for 30 min. The sections were then incubated with primary antibody diluted in DAKO antibody diluent for 60 min. The primary antibodies used were mouse anti-eNOS antibody (1:3,000 lung sections, and 1:5,000 artery sections, BD Biosciences) and mouse anti-smooth muscle actin (1:500, DAKO). After the primary antibody was drained off, the sections were washed in TBS for 5 min. The biotinylated goat anti-rabbit/anti-mouse secondary antibody (LSAB2 kit, DAKO) was then added for 30 min; the sections were then washed in TBS for 5 min. Strepavidin-horseradish peroxidase complex tertiary antibody (LSAB2 kit, DAKO) was then applied to the sections for 1 h and followed by a further wash in TBS for 5 min. The slides were then immersed in 0.5% 3,3-diaminobenzidinetetrahydrochloride, activated with 700 μl of 30% H2O2 for 10 min, rinsed and counterstained with hematoxylin for 1 min, followed by 0.5% copper sulfate for 5 min. The slides were dehydrated through immersion graded alcohol to xylene, mounted with xylene-based mountant (DPX), and examined by light microscopy.
Pulmonary artery muscularization was determined in actin-stained sections by counting 50 arteries in consecutive high-power fields. The arteries were regarded as thickened if the artery wall thickness was at least one-third the radius of the artery wall and at least 50% of the total wall area was thickened. Data are reported a percentage of arteries that are thickened. eNOS staining was quantified using a four-point grading scale, where 0 is no antibody staining (or similar to negative control), 1 is faint antibody staining, 2 is moderate antibody staining, and 3 is maximum antibody staining.
Dihydroethidium Fluorescence
Fresh pulmonary artery rings were snap frozen in liquid nitrogen and embedded in optimum cutting temperature (VWR International) compound. Cryosections (30 μm) were cut and placed on glass slides. The sections were incubated with 10 μM dihydroethidium (Molecular Probes, Invitrogen) in a light-protected chamber at 37°C for 30 min. Images were obtained with the use of a Bio-Rad MRC-1024 laser scanning confocal microscope equipped with a krypton/argon laser (excitation, 488 nm; and emission, 585 nm) and analyzed using MetaMorph software (Molecular Devices). Data are reported as fluorescent intensity.
NO microsensor and NO measurements
NO microsensor.
A 30-μm NO microsensor (ISONOP 30; WPI-Europe) was used to measure changes in NO release within the lumen of pulmonary artery rings as previously described (29, 39). The NO microsensor was calibrated on a daily basis and in conditions closely matching the experimental conditions.
Measurement of NO production simultaneously with force development.
An isolated pulmonary artery ring was mounted on one 30-μm wire and one 100-μm wire in a modified two-channel myograph (dual wire myography system 410A, Danish Myo Technology) containing Krebs solution heated to 37°C and gassed with 75% N2-20% O2-5% CO2. The modified myograph consisted of a hole drilled through one side of the myograph to allow the introduction of the NO sensor into the lumen of the vessel. The NO sensor was visualized with a stereo-zoom microscope, and with the aid of a micromanipulator, the electrode tip was inserted into the artery lumen and placed close to the endothelial surface. The output from the force transducer of the myograph was amplified through a Myo-interface 410A (Danish Myo Technology), and the current from the NO sensor was connected to an amplifier (NO meter; WPI-Europe) and recorded on a computer using Intercept Chart V3.0 (developed by J. Dempster, University of Strathclyde, UK).
Experimental protocol.
Pulmonary artery rings (2 mm in length) from control or hypoxic animals were mounted on a wire myograph as described in Isolated Vessel Study. The artery ring was preconstricted with 0.3 μM U-46619, and the contractile force was allowed to stabilize. Carbachol (10 μM; a concentration giving maximal relaxation) was added, and simultaneous measurements were made of vascular tone and NO concentration at the endothelium surface. The maximal increase in NO and in relaxation are reported. After the rings were washed, the experiment was repeated after incubating the artery rings in the presence 10 μM SB-203580 for 20 min. When necessary, the concentration of U-46619 used to preconstrict the artery ring was altered to ensure that the level of tone was the same as that of the control.
Western Blot Analysis
Isolated pulmonary artery tissues were cut into small pieces and transferred into 250 μl of ice-cold radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail, 1 mM PMSF, and 1 mM sodium orthovandate. Tissues were then homogenized using a Retsch MM301 Ball Mill with 5-mm-diameter stainless steel cone balls. Samples were centrifuged at 10,000 rpm for 1 min, and the supernatant was stored at −70°C. The samples were thawed, their protein content measured (modified Bradford method) and transferred to NuPAGE LDS sample buffer containing NuPAGE reducing agent. The samples were denatured by heating at 70°C for 10 min. An equal amount of samples was loaded onto 10% NuPAGE Bis-Tris gel. The gel was placed in XCell Surelock Mini-Cell (Invitrogen), and the inner chamber was filled with 200 ml of running buffer supplemented with 500 μl of NuPAGE antioxidant. The outer chamber was filled with running buffer. The proteins were electrophoretically separated for 1 h (200 V constant at 4°C). The gel was placed in a XCell II blot module (Invitrogen) containing transfer buffer, and the proteins were transferred onto a nitrocellulose membrane for 1 h (30 V constant at 4°C). The membrane was washed in PBS and blocked with 10% nonfat milk in PBS for 1 h at room temperature. After 3 × 5-min washes in PBS, the membrane was probed with primary antibody diluted in 5% nonfat milk in PBS-Tween overnight at 4°C. The antibodies used were monoclonal mouse phospho-p38 MAPK (1:1,000, Santa Cruz Biotechnology), polyclonal rabbit p38 MAPK (1:1,000, New England Bio Labs), or monoclonal mouse eNOS (1:1,000, BD Bioscience) antibodies. The membrane was washed in 3 × 5-min washes in PBS-Tween and probed with horseradish peroxidase-linked secondary antibody in PBS-Tween for 1 h at room temperature. After the 3 × 5-min washes in PBS, the membrane was incubated in chemiluminescent substrate (Amersham ECL, Amersham Bioscience) for 30 s. The membrane was then placed between two layers of acetate film and then exposed onto X-ray film (Kodak BioMax Light Film, Sigma-Aldrich). The film was then developed in a Kodak X-OMAT developer. The bands of interest were then scanned and the intensity of bands quantified using ImageJ software.
Statistical Analysis
Values are expressed as means ± SE. The concentration-response curves were compared by two-way ANOVA for multiple comparisons followed by a post hoc Tukey test. Comparison of multiple groups was by one-way ANOVA followed by Tukey test. Pairwise comparisons were performed using the Student's t-test. P values < 0.05 were considered statistically significant.
RESULTS
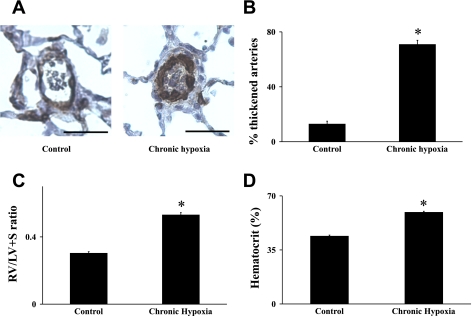
Exposure to 2 wk of chronic hypobaric hypoxia resulted in a significant increase of pulmonary arterial remodeling. In control lungs, α-actin immunoreactivity was present in medial smooth muscle cells of conduit pulmonary arteries with a weaker or a lack of staining in smaller resistance arteries. The quantification of the degree of α-actin immunostaining confirmed the significant vascular remodeling in lung sections obtained from chronically hypoxic animals compared with controls (from 12.8 ± 2.17%, n = 11 control rats, to 70.77 ± 3.12%, n = 13 chronically hypoxic rats, P < 0.001, Fig. 1, A and B). Consistent with the development of hypoxic pulmonary hypertension, there was a significant increase in both right ventricle weight (RV)/left ventricular weight (LV) + septum weight (S) ratio (from 0.299 ± 0.008%, n = 25 control rats, to 0.527 ± 0.014%, n = 20 hypoxic rats, P < 0.001, Fig. 1C) and hematocrit (from 43.85 ± 0.76%, n = 7 control rats, to 59.29 ± 0.98%, n = 10 hypoxic rats, P < 0.001, Fig. 1D).
Fig. 1.
The measurement of hypoxia-induced pulmonary hypertension in rats. Immunostaining for α-smooth muscle actin demonstrated muscularization of pulmonary arteries (PAs) in sections from chronically hypoxic rats compared with normotensive rats (A). Quantification of thickening of the PA wall showed muscularization in chronically hypoxic lung sections immunostained for α-smooth muscle actin compared with normotensive lungs (B). Chronically hypoxic rats had increased right ventricular weight (RV)/left ventricular weight (LV) + septum weight (S) ratio (C) and hematocrit (D) compared with control animals. Results are expressed as means ± SE; n = 6 experiments. Scale bar = 50 μm. *P < 0.001 vs. control.
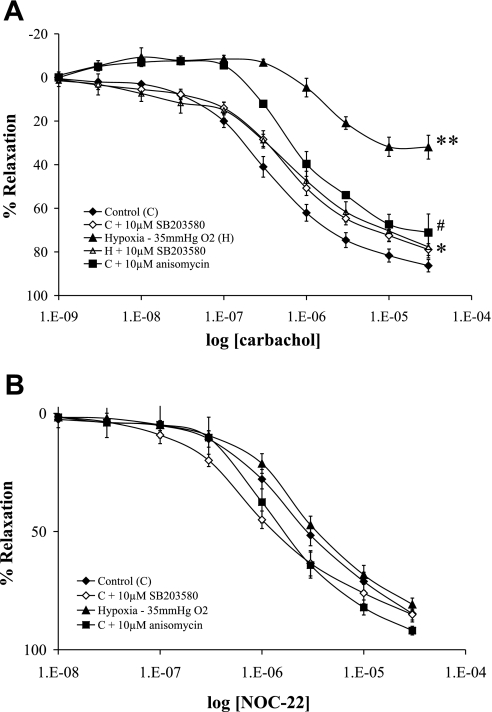
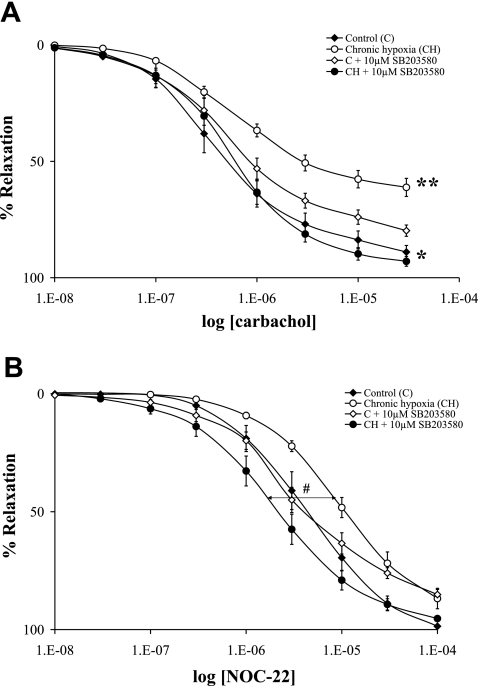
In intrapulmonary arteries from control rats preconstricted with U-46619, carbachol (endothelium-dependent relaxant) elicited a concentration-dependent relaxation (Figs. 2 and 3). A carbachol-induced relaxation of pulmonary arteries was significantly impaired following exposure to both acute and chronic hypoxia compared with that of controls (Figs. 2A and 3A). However, pulmonary artery relaxation induced by the NO donor NOC-22 (endothelium-independent relaxant) was not significantly changed following exposure to both acute and chronic hypoxia (Figs. 2B and 3B).
Fig. 2.
The effect of acute hypoxia on endothelium-dependent and -independent relaxation in rat PA. Cumulative concentration-response curves to relaxations induced by carbachol (A) and the nitric oxide (NO) donor N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino)-1,2-ethylenediamine (NOC-22; B) in isolated PAs are shown. The artery rings were precontracted with U-46619, and relaxation is expressed as percentage of the maximum U-46619-induced contraction. PA rings were examined in parallel under the following conditions: oxygenated control untreated (⧫), oxygenated and pretreated with 10 μM SB-203580 (◊), during hypoxia but with no other treatment (▴), during hypoxia and pretreated with 10 μM SB-203580 (▵), and oxygenated and pretreated with 10 μM anisomycin (▪). For carbachol (A), the significant differences were as follows: #P < 0.05, anisomycin vs. oxygenated control; *P < 0.01, hypoxia and pretreated with SB-203580 vs. hypoxia with no other treatment; and **P < 0.001, hypoxia vs. oxygenated control. Results are expressed as means ± SE; n = 6 experiments.
Fig. 3.
The effect of pulmonary hypertension induced by chronic hypoxia on endothelium-dependent and -independent relaxation in rat PA. Cumulative concentration-response curves to relaxations induced by carbachol (A) and the NO donor NOC-22 (B) in isolated PAs from either normoxic rats or chronically hypoxic rats are shown. The artery rings were precontracted with U-46619, and relaxation is expressed as percentage of the maximum U-46619-induced contraction. PA rings were examined in parallel under the following conditions: normoxic rat control untreated (⧫), chronic hypoxic rat with no other treatment (○), normoxic rat with the PA being pretreated with 10 μM SB-203580 (◊), and chronic hypoxic rat with the PA being pretreated with 10 μM SB-203580 (•). The significant differences for carbachol (A) were as follows: *P < 0.01, chronic hypoxic rat vs. chronic hypoxic rat with the artery ring pretreated with SB-203580; and **P < 0.001 chronic hypoxic rat vs. normoxic rat. The significant difference for NOC-22 (B) was as follows: #P < 0.05, chronic hypoxic rat vs. chronic hypoxic rat with the artery ring pretreated with SB-203580. Results are expressed as means ± SE; n = 6 experiments.
To determine the role of p38 MAPK in hypoxia-induced pulmonary artery endothelial dysfunction, we pretreated the pulmonary artery rings with the specific p38 MAPK inhibitor SB-203580 (10 μM) or the p38 MAPK stimulator anisomycin (1 μM) before the vasorelaxant stimulus. The pretreatment of the pulmonary artery rings with SB-203580 caused a complete reversal of the impaired endothelium-dependent relaxation secondary to both acute and chronic hypoxia (Figs. 2A and 3A). Moreover, the pretreatment of the normoxic pulmonary arteries with anisomycin significantly impaired the relaxation to carbachol, a change in the same direction though smaller than caused by hypoxia (Fig. 2A). The relaxant effect of NOC-22 was not significantly modified by the anisomycin treatment (Fig. 2B). Although chronic hypoxia did not affect NOC-22-mediated relaxation, the pretreatment with SB-203580 resulted in a significant shift in the concentration response curve to the left (Fig. 3B).
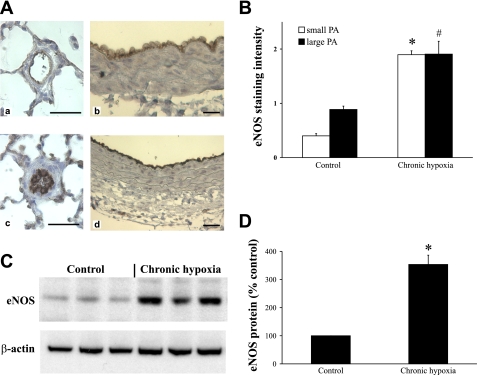
In control lungs, eNOS immunostaining was present throughout the vascular endothelium of conduit pulmonary arteries and the staining diminished down the pulmonary arterial tree with only weak endothelial positivity being found in smaller resistance arteries. Chronic hypoxia exposure significantly increased eNOS immunostaining observed in both small resistance pulmonary arteries (Fig. 4, A,a compared with A,c) and in large conduit pulmonary arteries (Fig. 4, A,b compared with A,d). An increased eNOS protein expression in the chronically hypoxic pulmonary artery was also confirmed by Western blot analysis (Fig. 4, C and D).
Fig. 4.
The effect of chronic hypoxia on endothelial NO synthase (eNOS) expression in small (<100 μm) and large (400–700 μm) PAs. A: sections of small and large PAs from normoxic (a and b) and chronically hypoxic (c and d) rats immunostained for eNOS. B: quantitative measurement of eNOS immunostaining in small and large PAs from normoxic and chronically hypoxic rats. C and D: representative Western blot (C) and corresponding densitometric analysis (D) showing of eNOS protein expression in control and chronically hypoxic PAs (400–700 μm). β-Actin was probed to confirm equal protein loading. Results are expressed as means ± SE; n = 6 experiments. Scale bar = 50 μm. *P < 0.001 vs. small PA control, #P < 0.01 vs. large PA control.
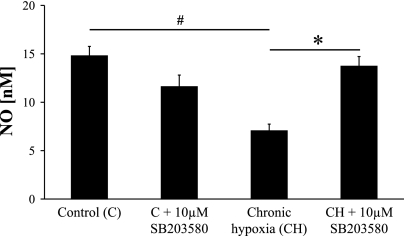
Although eNOS protein expression was increased in response to chronic hypoxia, carbachol-stimulated NO generation was significantly reduced in chronically hypoxic artery rings (Fig. 5). The NO concentration in response to a maximal concentration of carbachol was significantly smaller in pulmonary artery rings from pulmonary hypertensive rats compared with normotensive rats (Fig. 5). In keeping with the vascular ring studies, the pretreatment with SB-203580 restored carbachol-stimulated NO production (Fig. 5).
Fig. 5.
The effect of chronic hypoxia on endothelium-derived NO production in rat PA. Carbachol-stimulated NO production was measured with NO electrode in control and chronically hypoxic PA and following pretreatment with 10 μM SB-203580. Results are expressed as means ± SE; n = 6 experiments. #P < 0.001 vs. control; *P < 0.01 vs. chronic hypoxia.
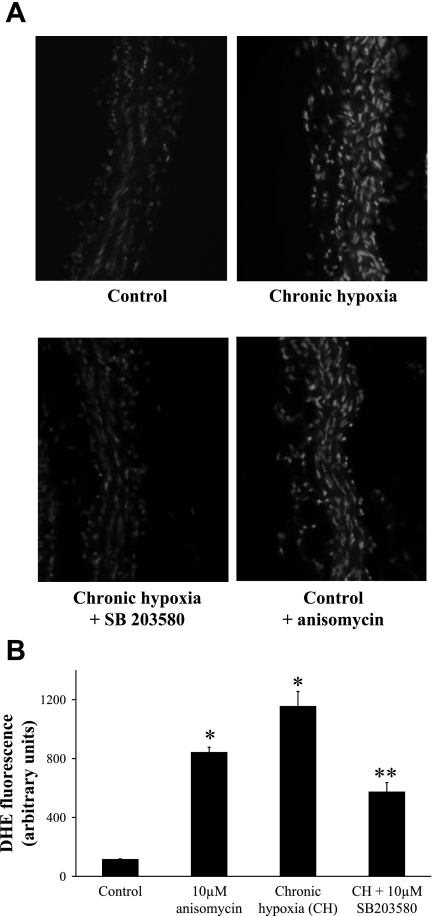
To determine the effect of chronic hypoxia on superoxide levels of pulmonary arteries, in situ staining with the fluorescent dye dihydroethidium was applied to freshly cut pulmonary artery sections. Superoxide anion production was markedly increased in artery rings isolated from chronically hypoxic animals compared with controls (Fig. 6, A and B). The pretreatment of chronically hypoxic artery rings with SB-203580 significantly reduced superoxide anion production. Consistent with this finding, the induction of p38 MAPK with anisomycin significantly increased the superoxide production in normoxic pulmonary artery rings.
Fig. 6.
A: representative confocal microscopic images of superoxide production in isolated PA sections stained with dihydroethidium (DHE, 10−5 M). B: the production of superoxide was quantified in arbitrary units of DHE fluorescence in control, chronically hypoxic, SB-203580-pretreated chronically hypoxic, and anisomycin-treated control PAs. Results are expressed as means ± SE; n = 4 to 6 experiments. *P < 0.001 vs. control; **P < 0.01 vs. chronic hypoxia.
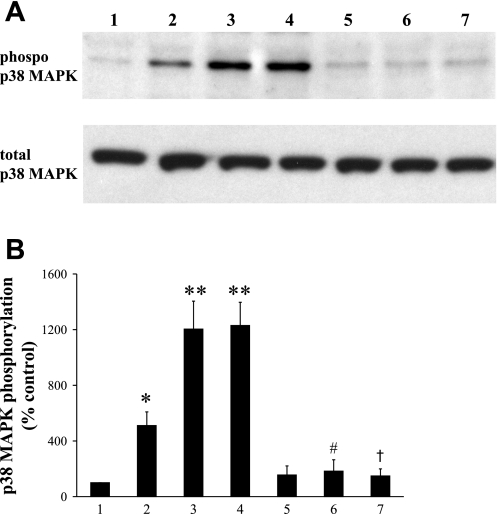
Because previous experiments showed that p38 MAPK inhibitors reversed and stimulators impaired endothelium-dependent relaxation, the effect of acute and chronic hypoxia on p38 MAPK expression was studied. Pulmonary artery rings treated with acute hypoxia and anisomycin showed a significant increase in p38 MAPK phosphorylation as assessed by Western blot analysis (Fig. 7, A and B). Pulmonary arteries isolated from chronically hypoxic animals also showed a marked increase in phospho-p38 MAPK expression, similar to levels observed following exposure to acute hypoxia (Fig. 7, A and B).
Fig. 7.
The effects of hypoxia (acute and chronic) and anisomycin stimulation on p38 MAPK phosphorylation in the PA. Representative Western blot (A) and corresponding densitometric analysis (B) of phosphorylation of p38 MAPK in PA: 1) control, 2) 10 μM anisomycin, 3) acute hypoxia (35 mmHg O2), 4) chronic hypoxia, 5) control + 10 μM SB-203580, 6) acute hypoxia + 10 μM SB-230580, and 7) chronic hypoxia + 10 μM SB-230580. Total p38 MAPK was probed to confirm equal protein loading. Results are expressed as means ± SE; n = 4 experiments. *P < 0.01 vs. control; **P < 0.001 vs. control; #P < 0.001 vs. acute hypoxia; †P < 0.001 vs. chronic hypoxia.
Normoxic and chronically hypoxic pulmonary artery rings pretreated with p38 MAPK inhibitor SB-203580 abrogated increased p38 MAPK phosphorylation. SB-203580 (10 μM) had no significant effect on basal p38 MAPK expression (Fig. 7).
DISCUSSION
In the present study, both acute and chronic hypoxia caused a marked increase in phosphorylation of p38 MAPK in isolated rat pulmonary arteries. This is consistent with previous reports showing the phosphorylation and activation of p38 MAPK by hypoxia both in cells and in vivo (48–50). Pulmonary artery fibroblasts express all four isoforms of p38 MAPK; however, in hypoxia, only p38 α- and δ-isoforms are phosphorylated (32). Previous studies have linked the activation of p38 MAPK by hypoxia to the resulting pulmonary vascular remodeling and increased cell proliferation (49, 50). Furthermore, an increase in p38 MAPK phosphorylation has been shown in cell lines transfected with bone morphogeneic protein receptor type II mutants and associated with an increased cell proliferation (52). Moreover, the activation of p38 MAPK persists after a prolonged cell culture consistent with a phenotypic shift in pulmonary vascular cell mitogenic signaling induced by hypoxia (8, 49). In the present study, we report a role for p38 MAPK in endothelium depression, thus extending the postulated role for p38 MAPK as a common link for hypoxia-induced vasomotor dysfunction and vascular remodeling.
Our data reported in the present article show that rat pulmonary arteries exposed to acute hypoxia and those isolated from chronically hypoxic rats had an impaired relaxation when the endothelium was stimulated. However, vessel relaxation induced by the NO donor NOC-22 was unaffected by both acute and chronic hypoxia, pointing to an impairment of NO generation by the endothelial layer. This was confirmed by the measurement of endothelium-derived NO, which was reduced in pulmonary arteries isolated from chronically hypoxic rats. Nevertheless, the eNOS expression was markedly increased in the pulmonary arteries of chronically hypoxic rats, in keeping with previous studies (10, 12). Increased eNOS expression can occur through many mechanisms, but probably the hypoxia-sensitive transcription factors have a major role, especially hypoxia-inducible factor, activator protein-1, and vascular endothelial growth factor (45). In turn, hypoxic induction of p38 MAPK may also increase the expression of hypoxia-inducible factor, activator protein-1, and vascular endothelial growth factor (18). Although eNOS protein expression is increased, its enzymatic activity might also be reduced since it is established that eNOS activity is impaired in various pathological conditions by alterations in phosphorylation, protein-protein interactions, or cofactor availability. In the present work, the decrease in bioavailable NO combined with the increase in eNOS protein expression reveals a decline in eNOS function.
Acute pulmonary hypoxia causes pulmonary vasoconstriction and depresses NO formation in the pulmonary vascular endothelium (6, 14, 24, 28, 35, 38). Evidence that NO has a significant vasodilator role in the pulmonary vasculature in the absence of disease derives from the vasoconstriction seen on the administration of NO synthesis inhibitors to human subjects (7, 41) and animal models (42). Overall, it is likely that the declining endothelium NO production is a major cause of hypoxic pulmonary vasoconstriction. However, it has also been argued that hypoxic pulmonary vasoconstriction is caused by pathways independent of NO and indeed that NO release can limit pulmonary vasoconstriction induced by acute hypoxia (11, 19).
The main novel findings in the present article link endothelium depression to the activation of p38 MAPK. In the present study, we demonstrated that SB-203580 at the concentration of 10 μM prevented the phosphorylation of p38 MAPK in response to acute or chronic hypoxia, in agreement with previous data (16, 25, 49, 50). SB-203580 inhibits primarily α-isoform of p38 MAPK (32). In the presence of SB-203580, a normal endothelium relaxation was restored following an exposure to hypoxia (acute and chronic) with a confirmation of restored NO concentrations in pulmonary hypertensive arteries. Furthermore, a p38 MAPK stimulator anisomycin caused a significant impairment in endothelium-dependent relaxation. The selectivity of SB-203580 and other related p38 MAPK inhibitors has been widely studied and shown to cause a near-complete blockade of p38 MAPK activity at 10 μM without a significant effect on other cellular protein kinases (9).
Our present data report an increased superoxide formation in pulmonary arteries from chronically hypoxic rats, as measured by dihydroethidium fluorescence. This is consistent with previous data showing oxidative stress and NADPH oxidase activation in experimental pulmonary hypertension (10, 17, 26). Dihydroethidium fluorescence was partially normalized by the p38 MAPK inhibitor SB-203580. Moreover, activation of p38 MAPK, like hypoxia, increased dihydroethidium fluorescence. These results provide an explanation for the endothelium depression and reduced NO output found in acute and chronic hypoxia in the present study. Our data are in keeping with findings from systemic vascular beds, where p38 MAPK activation has been shown to mediate endothelial dysfunction (21) via the stimulation of superoxide production (37, 51). One mechanism that has been identified is the upregulation of p47phox, a key component of NADPH oxidase (51), the main enzyme responsible for superoxide formation in the artery wall. In the presence of SB-203580, the relaxation induced by NOC-22 in chronic hypoxic vessels was significantly shifted to the left. This suggests that oxidative stress in the pulmonary artery in the chronic hypoxic rat limits NO availability from the NO donor and that by reducing the superoxide burden, we allow significantly more NO to reach the smooth muscle and cause relaxation. Further data are needed to show the effect of p38 on NOX expression and/or activity in this model.
The uncoupling of eNOS leads to production of superoxide anion instead of NO and is increasingly appreciated as an important source of superoxide anion in the pathogenesis of cardiovascular disorders (15). This would be expected to initiate a vicious cycle of superoxide anion production and impairing its own capability to produce NO. It is unlikely that an absolute deficiency of substrate or cofactor was crucial in eNOS uncoupling as the synthesis of NO was restored without any changes in substrate/cofactor. However, the substrate/cofactors are sensitive to redox changes, and it is possible that a relative deficiency could arise due to oxidation (15, 17).
In conclusion, the present study extends evidence that p38 MAPK is an important intermediary in the functional consequences of acute hypoxia in the pulmonary circulation and in experimental pulmonary hypertension. Hypoxia-induced pulmonary artery endothelial depression and dysfunction were p38 MAPK dependent. The induction of p38 MAPK stimulated superoxide production and reduced the bioavailability of NO. Pharmacological inhibition can restore endothelium-dependent NO generation, reduce superoxide burden, and restore endothelial function. Therefore, p38 MAP inhibition may prove to be novel target for the treatment of pulmonary hypertension.
GRANTS
R. P. Weerackody was supported by British Heart Foundation Doctoral Fellowship FS/02/038.
REFERENCES
- 1.Aaron ME, Douglas T, Colleen MB. Mitogen-activated protein kinase activation: an alternate signaling pathway for sustained vascular smooth muscle contraction. J Vasc Surg 26: 327–332, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest 87: 155–162, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr TM, Berova M, Doe CP, Ju H, Angermann CE, Boehm J, Willette RN. p38 mitogen-activated protein kinase inhibitors for the treatment of chronic cardiovascular disease. Curr Opin Investig Drugs 4: 1059–1064, 2003. [PubMed] [Google Scholar]
- 4.Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 287: L911–L918, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad USA 99: 13248–13253, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clini E, Cremona G, Campana M, Scotti C, Pagani M, Bianchi L, Giordano A, Ambrosino N. Production of endogenous nitric oxide in chronic obstructive pulmonary disease and patients with cor pulmonale. Correlates with echo-Doppler assessment. Am J Respir Crit Care Med 162: 446–450, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CJ, Landzberg MJ, Anderson TJ, Charbonneau F, Creager MA, Ganz P, Selwyn AP. Role of nitric oxide in the local regulation of pulmonary vascular resistance in humans. Circulation 93: 266–271, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Das M, Bouchey DM, Moore MJ, Hopkins DC, Nemenoff RA, Stenmark KR. Hypoxia-induced proliferative response of vascular adventitial fibroblasts is dependent on G protein-mediated activation of mitogen-activated protein kinases. J Biol Chem 276: 15631–15640, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demiryurek AT, Karamsetty MR, McPhaden AR, Wadsworth RM, Kane KA, MacLean MR. Accumulation of nitrotyrosine correlates with endothelial NO synthase in pulmonary resistance arteries during chronic hypoxia in the rat. Pulm Pharmacol Ther 13: 157–165, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Emery CJ, Teng GQ, Liu X, Barer GR. Vasoreactions to acute hypoxia, whole lungs and isolated vessels compared: modulation by NO. Respir Physiol Neurobiol 134: 115–129, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Fagan K, Morrissey B, Fouty B, Sato K, Harral J, Morris K, Hoedt-Miller M, Vidmar S, McMurtry I, Rodman D. Upregulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res 2: 306–313, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol Lung Cell Mol Physiol 274: L517–L526, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Frantz B, Klatt T, Pang M, Parsons J, Rolando A, Williams H, Tocci MJ, O'Keefe SJ, O'Neill EA. The activation state of p38 mitogen-activated protein kinase determines the efficiency of ATP competition for pyridinylimidazole inhibitor binding. Biochemistry 37: 13846–13853, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res 25: 297–310, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hubloue I, Biarent D, Kafi SA, Bejjani G, Kerbaul F, Naeije R, Leeman M. Endogenous endothelins and nitric oxide in hypoxic pulmonary vasoconstriction. Eur Respir J 21: 19–24, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jin N, Hatton N, Swartz DR, Xia X, Harrington MA, Larsen SH, Rhoades RA. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol 23: 593–601, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Ju H, Behm DJ, Nerurkar S, Eybye ME, Haimbach RE, Olzinski AR, Douglas SA, Willette RN. p38 MAPK inhibitors ameliorate target organ damage in hypertension: Part 1. p38 MAPK-dependent endothelial dysfunction and hypertension. J Pharmacol Exp Ther 307: 932–938, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158: 917–923, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Karamsetty MR, Klinger JR, Hill NS. Phytoestrogens restore nitric oxide-mediated relaxation in isolated pulmonary arteries from chronically hypoxic rats. J Pharmacol Exp Ther 297: 968–974, 2001. [PubMed] [Google Scholar]
- 24.Kharitonov SA, Cailes JB, Black CM, du Bois RM, Barnes PJ. Decreased nitric oxide in the exhaled air of patients with systemic sclerosis with pulmonary hypertension. Thorax 52: 1051–1055, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knock GA, De Silva AS, Snetkov VA, Siow R, Thomas GD, Shiraishi M, Walsh MP, Ward JP, Aaronson PI. Modulation of PGF2α- and hypoxia-induced contraction of rat intrapulmonary artery by p38 MAPK inhibition: a nitric oxide-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 289: L1039–L1048, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Shimpo H, Shimamoto A, Chong AJ, Hampton CR, Spring DJ, Yada M, Takao M, Onoda K, Yada I. Specific inhibition of p38 mitogen-activated protein kinase with FR167653 attenuates vascular proliferation in monocrotaline-induced pulmonary hypertension in rats. J Thorac Cardiovasc Surg 128: 850–859, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Mathew R, Gloster ES, Sundararajan T, Thompson CI, Zeballos GA, Gewitz MH. Role of inhibition of nitric oxide production in monocrotaline-induced pulmonary hypertension. J Appl Physiol 82: 1493–1498, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Mathewson AM, Wadsworth RM. Induction of iNOS restricts functional activity of both eNOS and nNOS in pig cerebral artery. Nitric Oxide 11: 331–339, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol 279: H741–H751, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest 38: 188–200, 1978. [PubMed] [Google Scholar]
- 32.Mortimer HJ, Peacock AJ, Kirk A, Welsh DJ. p38 MAP kinase: essential role in hypoxia-mediated human pulmonary artery fibroblast proliferation. Pulm Pharmacol Ther 20: 718–725, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977. [DOI] [PubMed] [Google Scholar]
- 34.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 25: 2335–2342, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki M, Kawashima S, Yamashita T, Ohashi Y, Rikitake Y, Inoue N, Hirata KI, Hayashi Y, Itoh H, Yokoyama M. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension 37: 322–327, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 25: 995–1001, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Riley MS, Porszasz J, Miranda J, Engelen MP, Brundage B, Wasserman K. Exhaled nitric oxide during exercise in primary pulmonary hypertension and pulmonary fibrosis. Chest 111: 44–50, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol 516: 271–282, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperling RT, Creager MA. Nitric oxide and pulmonary hypertension. Coron Artery Dis 10: 287–294, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 89: 2035–2040, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Steeds RP, Thompson JS, Channer KS, Morice AH. Response of normoxic pulmonary arteries of the rat in the resting and contracted state to NO synthase blockade. Br J Pharmacol 122: 99–102, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology 21: 134–145, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest 101: 2468–2477, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol 24: 405–412, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase-dependent mechanism. Atherosclerosis 161: 387–394, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Watts SW Serotonin activates the mitogen-activated protein kinase pathway in vascular smooth muscle: use of the mitogen-activated protein kinase kinase inhibitor PD098059. J Pharmacol Exp Ther 279: 1541–1550, 1996. [PubMed] [Google Scholar]
- 48.Welsh D, Mortimer H, Kirk A, Peacock A. The role of p38 mitogen-activated protein kinase in hypoxia-induced vascular cell proliferation: an interspecies comparison. Chest 128: 573S–574S, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med 164: 282–289, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Welsh DJ, Scott PH, Peacock AJ. p38 MAP kinase isoform activity and cell cycle regulators in the proliferative response of pulmonary and systemic artery fibroblasts to acute hypoxia. Pulm Pharmacol Ther 19: 128–138, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Widder J, Behr T, Fraccarollo D, Hu K, Galuppo P, Tas P, Angermann CE, Ertl G, Bauersachs J. Vascular endothelial dysfunction and superoxide anion production in heart failure are p38 MAP kinase-dependent. Cardiovasc Res 63: 161–167, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005. [DOI] [PubMed] [Google Scholar]