Abstract
Experiments were designed to test the hypothesis that the systemic delivery of planktonic forms of nanoparticles (NPs) derived from calcified, diseased human tissue or bovine blood are transmissible particles that exacerbate arterial response to injury. New Zealand White rabbits in which the endothelium was mechanically removed from one carotid artery were injected intravenously with either saline (control), lipopolysaccharide (LPS; surrogate for subclinical infection), hydroxyapatite crystals (HA; surrogate for NP shell), HA crystals exposed to culture media, or planktonic forms of bovine- or human-derived NPs. Carotid arteries were monitored by ultrasonography for 5 wk and then removed for histological examination. Uninjured arteries from all animals in each group remained patent with a normal anatomy. Injured arteries from 6 of 11 animals injected with human-derived NPs occluded and/or calcified; none of the injured arteries from animals in the other groups occluded (n = 28; P ≤ 0.05). Injured arteries of rabbits injected with LPS or HA crystals developed eccentric hyperplasia. Discontinuous internal elastic laminae and thinning media characterized arteries from animals injected with bovine-derived NPs or cultured HA crystals. In conclusion, the systemic administration of planktonic forms of human-derived NPs exacerbated arterial response to injury distinct from that of bovine-derived NPs and other inflammatory agents.
Keywords: inflammation, intimal hyperplasia, carotid artery
calcification of human arterial tissue is a common occurrence, increases with age, and is a strong predictor of cardiovascular and all-cause mortality (12, 17). Atherosclerosis is considered an inflammatory disease (36). However, the mechanisms or stimuli provoking the inflammation are incompletely understood. Circulating levels of low-density lipoproteins (LDL) and subsequent increases in lipid peroxidation leading to endothelial dysfunction appear to be precipitating events in some patient populations (26). Conversely, in other groups, significant coronary calcification is present even when circulating levels of cholesterol and LDL are below that which would warrant pharmacological interventions (19, 25, 27). A chronic burden of subclinical infection may stimulate inflammation, leading to cardiovascular disease in some individuals (3, 14, 30, 32).
Nano-sized particles (0.08–0.5 μm) with a carbonate or hydroxyapatite (HA) shell have been isolated from a variety of mammalian tissues including blood (20, 28, 34), calcified arteries (29), calcified aortic valves (6), kidney stones (8, 24), gall stones (46), ovarian cancer (41), and prostate stones (43). Associations of nanoparticles (NPs) with calcified soft tissue suggest that they participate in these disease processes. NPs isolated from human kidney stones and calcified arteries increase in density when placed in standard cell culture conditions; their rate of increase is reduced by inhibitors of oxidative metabolism and some antibiotics (5, 9, 24). In culture, NPs exist both as adherent biofilm that develops on the surface of the culture flasks (10) and as nonattached, floating particles, i.e., a “planktonic” form (2, 11). NPs isolated from human calcified tissues take up radiolabeled uridine and phosphate and stain for nucleic acids when placed in culture media (29), whereas NPs of bovine origin are proposed to be calcium-fetuin complexes (34).
Suspensions prepared from an established, calcified biofilm of human-derived NPs increased intimal hyperplasia and arterial calcification at sites of arterial endothelial denudation when injected intravenously into adult rabbits (40). The question arises whether other forms of NPs and NPs from other sources can also act as possible transmissible agents that provoke pathophysiological responses. Therefore, the present study was designed to evaluate the pathogenicity of standardized suspensions of planktonic forms of NPs derived from human calcified arteries and bovine blood. It was hypothesized that planktonic forms of human- and bovine-derived NPs are transmissible, disease-causing agents and, because of proposed differences in their composition, would differ in their capacity to exacerbate arterial responses to injury.
METHODS
NP Propagation and Collection
Self-propagating, self-calcifying NPs derived from human calcified aortic aneurysms (29) and from fetal bovine serum (bovine-derived NPs; Nanobac) (20) were used in this study. Human-derived NPs were isolated from the surgical waste of patients who gave informed consent at the Mayo Clinic in compliance with an approved Institutional Review Board protocol and consistent with the Helsinki Declaration. Tissue was processed as described previously (29).
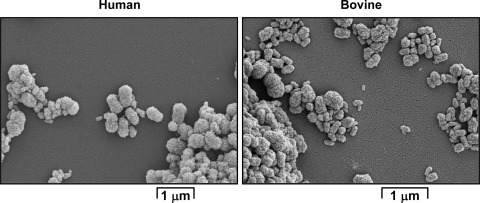
Human-derived NPs were seeded into standard tissue culture flasks containing Dulbecco's modified Eagle's medium (DMEM 10-013, Mediatech, Manassas, VA) supplemented with 10% γ-irradiated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 50 μM β-mercaptoethanol (Sigma Chemical, St. Louis, MO), and 3.6 mM CaCl2 (Sigma). In this medium, NPs exist in a floating or planktonic form before adhering to the surface of the culture flasks and forming visible domes encrusted with HA. These cultures are screened randomly and periodically for common environmental bacterial contamination, and any contaminated cultures are promptly discarded. To standardize the inoculums, culture media containing the planktonic forms of human-derived NPs were collected 3 wk after flask seeding and centrifuged (20,000 g, 20 min, 4°C). The supernatant was discarded and the pellets were washed with phosphate-buffered saline. The washed pellets from several flasks were combined. After two additional centrifugations (20,000 g, 20 min, 4°C), the resulting pellet was suspended in sterile saline (0.9% NaCl) with the volume adjusted to obtain a standard turbidity of 1,000 nephelometric turbidity units (NTU; Hach 2100N Turbidimeter, Loveland, CO). Stock solutions of bovine-derived NPs were diluted in sterile saline to a turbidity of 1,000 NTU. This method of preparation resulted in a solution containing individual and clumped NPs (Fig. 1). Inorganic HA crystals [1 μg; 3Ca3(PO4)2.Ca(OH)2; Sigma] were seeded into flasks with the same media used for human-derived NPs, incubated for 3 wk, and harvested in the same manner as NPs. A separate group of animals was inoculated with inorganic HA crystals never exposed to media or sera.
Fig. 1.
Scanning electron micrographs of isolated planktonic forms of human- (left) and bovine (right)-derived nanoparticles (NPs). Human-derived NPs were propagated from a human calcified aneurysm (Ref. 29). Bovine-derived NPs were pooled from several sources of fetal bovine serum and prepared by Nanobac Pharmaceuticals. Bar indicates 1 μm.
Animals and Surgery
All protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic, conforming to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). Adult male New Zealand White rabbits (3.7 kg, outbred, multiple suppliers, n = 39) were housed in individual cages (12-h light:12-h dark cycle, LabDiet hi-fiber rabbit chow and water ad libitum). NP-exposed animals were housed in a biohazard level 2 safety facility. The endothelium of the left carotid artery of each animal was denuded by balloon injury as previously described (40); the right carotid artery was unoperated. Just before surgery, animals were given penicillin (75,000 units, Pen-Aqueous, Durvet, Blue Springs, MO) intramuscularly as prophylaxis against surgical-associated infection, as this antibiotic does not interfere with the propagation of bovine-derived NPs (9).
Inoculation
Within 3 h of surgery, while still under anesthesia (35 mg/kg ketamine, 5 mg/kg xylazine, and 1 mg/kg acepromazine; im), animals were injected, via a catheter placed in the marginal ear vein, with 5 ml of one of the following solutions (where n = number of animals/group): 1) sterile saline only (control; 5 ml, n = 9); 2) LPS (1 μg/kg in 5 ml saline; Escherichia coli; ultrapure; Invivogen, San Diego, CA; n = 5)—this group controlled for effects that might be caused by acute subclinical bacterial-associated inflammation; 3) HA crystals (1 μg/5 ml saline; n = 5)—as NPs form a HA shell, this group controlled for the response to the inorganic material in the shell of NPs; 4) HA crystals exposed to culture medium (1 μg/5 ml saline; n = 4)—this group controlled for responses that might be due to HA forming complexes with proteins in the culture media such as might occur with the HA shell of NPs; 5) bovine-derived NPs (derived from pooled serum; 1 ml of 1,000 NTU followed by 4 ml saline; n = 5); and 6) human-derived NPs (isolated from human aneurysms A2; separate cultures derived from a single stock; 1 ml of 1,000 NTU followed by 4 ml saline; n = 11).
Ultrasound Imaging
In a subgroup of animals, the lumen diameter of the uninjured right and the injured segment of the left carotid arteries were measured on days 0, 7, 14, 21, 28, and 35 using an ultrasound microimaging system (Vevo 770, Visualsonics, Toronto, Canada) equipped with a 25-MHz linear transducer (model 710B). Under light anesthesia (50 mg/kg ketamine and 10 mg/kg xylazine, im), each animal was placed in dorsal recumbency on a heating pad maintained at 37°C. The dorsal neck of the animal was shaved, and each artery was first located using B-mode, then in M-mode, to visualize the bright vessel walls. Images were recorded for 10 s. The lumen diameter was subsequently determined by averaging three measurements each of the diameter at peak systole and at end diastole.
Tissue Collection
Blood samples (3 ml via ear artery) were collected into tubes containing EDTA immediately before inoculation (day 0) and at days 3, 7, 14, 21, 28, and 35 after surgery and inoculation. Plasma was stored at −80°C until analyzed. At postoperative day 35, the animals were anesthetized and both carotid arteries removed and fixed in formalin for subsequent analysis by light microscopy. Two rabbits each from the saline and human-derived NP groups were evaluated visually for vessel patency at the time of tissue harvest, and the tissue was used for purposes other than histology.
Histological Analysis
Both carotid arteries were fixed in formalin and paraffin embedded; 11 5-μm sections were serially cut and placed on glass slides. The slides were numbered so that the animal grouping was not known to the investigator at the time of the evaluation. All 11 sections from each artery were evaluated for the presence of an occlusion. Four sections from each artery were randomly selected for staining by hemotoxylin and eosin, elastin van Giesen, rabbit macrophage antibody (IgG1; Dako, Carpiteria, CA), and von Kossa for evaluation of general anatomy, intimal-to-media ratio, macrophage infiltration, and calcification, respectively. The extent of myointimal hyperplasia is presented as the ratio of intima to media areas calculated using KS400 software (Zeiss, Thornwood, NY). Sections were also evaluated to determine whether the internal elastic lamina and media were intact or discontinuous. Arterial occlusion with canalization was determined by ultrasound evaluation before tissue harvest, visual inspection of the artery at the time of dissection, and/or histological confirmation.
Immunological Analysis
The amount of anti-NP IgG and NP antigen in plasma was measured using Nano-Sero IgG and Nanocapture ELISA kits (Nanobac), respectively, according to the manufacturer's instructions. Samples were coded to blind investigators to the animal group and sorted by group after analysis.
Statistical Analysis
The numbers of animals assigned to each group were based on the rate of arterial occlusion in animals injected with biofilm-derived NPs (40). As intima-to-media ratios were not normally distributed, the differences among groups were determined using Wilcoxon/Kruskal Wallis tests (JMP software, SAS, Cary, NC). Contingency analyses were performed to determine the significant differences among groups related to outcomes including calcification, occlusion, and disruption of the internal elastic lamina and media layer (JMP software). Statistical significance was accepted at P ≤ 0.05.
RESULTS
Animals
Animals remained in good health; none showed signs of anorexia or liver failure (icteric scleras, poor condition of mucous membranes). Animals weighed 3.7 ± 0.1 kg before surgery and 3.9 ± 0.1 kg (mean ± SE; n = 39) at death. There were no significant differences in average weight changes among animals from each group.
Anatomy of Uninjured Arteries
Uninjured carotid arteries from all rabbits were indistinguishable among groups. Each artery was patent (Table 1, and Fig. 2), and the lumen was lined with a single layer of endothelial cells (Fig. 2). The internal elastic lamina remained intact, and the media was well defined. Leukocytic infiltrates were not observed in any of the uninjured arteries.
Table 1.
Lumen diameter, by ultrasound, of carotid arteries from animals before and 5 wk following surgery
| Treatment | Group n* | Uninjured Artery, mm† | Injured Artery, mm† |
|---|---|---|---|
| Day 0 | 15, 12 | 1.87±0.04 | 1.95±0.06 |
| Day 35 Saline‡ | 3, 3 | 1.99±0.02 | 2.08±0.08 |
| LPS‡ | 2, 2 | 1.97±0.05 | 2.09±0.12 |
| Cultured HA crystals‡ | 2, 3 | 2.01±0.20 | 2.07±0.12 |
| Bovine NPs | 5, 5 | 2.21±0.05 | 2.17±0.10 |
| Human NPs‡ | 4, 2§ | 2.22±0.06 | 2.27±0.09 |
Number of individual animals in which uninjured and injured arteries were imaged. Not all arteries could be imaged from all rabbits at both time points.
Values are means ± SE of lumen diameter.
Ultrasound images were not obtained on all animals in this group.
Data from 2 arteries that remained patent; 3 injured arteries from animals in this group occluded within 1–3 wk of the injection. HA, hydroxyapatite; NP, nanoparticle.
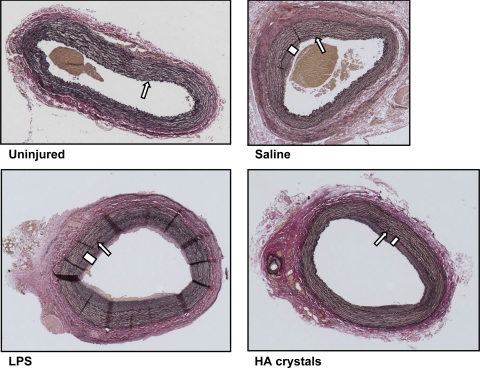
Fig. 2.
Light micrographs of representative sections of uninjured (top, left) and injured carotid arteries from rabbits injected with saline (top, right), lipopolysaccharide (LPS; bottom, left), and hydroxyapatite crystals (HA; bottom, right). Sections are stained with an elastin van Giesen stain and shown at ×5 magnification. All arteries were collected at 35 days postoperatively. The uninjured arteries from all rabbits are indistinguishable from this uninjured artery. Arrows indicate the internal elastic lamina, and bars designate intimal hyperplasia.
Anatomy of Injured Arteries
Control groups.
All injured arteries of animals inoculated with either saline, LPS, or HA crystals remained patent even though eccentric intimal hyperplasia was present (Tables 1 and 2, and Figs. 2 and 3). The mean intima-to-media ratios in these injured arteries did not differ significantly among these groups and averaged 0.63 ± 0.08 (mean ± SE; Table 1). With the exception of one animal injected with saline, the internal elastic laminae remained continuous. None of the injured arteries in animals inoculated with saline, LPS, or HA crystals showed histological evidence of calcification by von Kossa staining (data not shown).
Table 2.
Condition of injured arteries in each experimental group
| Treatment | Group n | Intima-to-Media Ratio* | Canalized Occlusion | Damage to IEL/Media | Intimal Calcification |
|---|---|---|---|---|---|
| Saline | 9† | 0.57±0.13 | 0 | 1 | 0 |
| LPS | 5 | 0.75±0.17 | 0 | 0 | 0 |
| HA crystals | 5 | 0.60±0.15 | 0 | 0 | 0 |
| Cultured HA crystals | 4 | NA | 0 | 2§ | 0 |
| Bovine NPs | 5 | NA | 0 | 4§ | 0 |
| Human NPs | 11† | 0.32±0.1‡ | 6§ | 2 | 2 (punctate within occlusion)§ |
Values are means ± SE; n, number of animals.
Patency of arteries of 2 animals from each group was validated at dissection; the tissue was used for purposes other than histology.
Ratio was determined in 5 injured arteries that were not occluded and had an intact internal elastic lamina (IEL). NA, not applicable.
P ≤ 0.05, statistical significance from control groups (saline, LPS, and HA crystals).
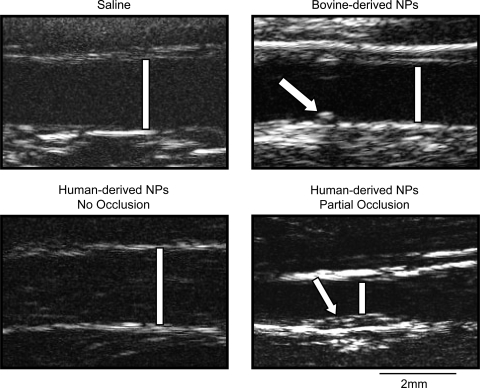
Fig. 3.
Representative images of carotid arteries obtained by B-mode ultrasonography. Patent, injured arteries of rabbits inoculated with saline (top, left), bovine-derived NPs (top, right), and human-derived NPs (bottom, left) are shown at 5 wk postinjury. An injured artery of a rabbit inoculated with human-derived NPs is shown at 2 wk postsurgery (bottom, right); this artery was occluded by week 3. Bars designate the lumens. A stitch closing access to the artery following endothelial denudation was used as a marker of the injured area (arrow; top, right). Intimal thickening in the artery that subsequently occluded (arrow; bottom, right) is shown. Scale bar is 2 mm.
HA crystals exposed to culture medium.
All injured carotid arteries of animals in this group remained patent (Tables 1 and 2, and Fig. 4). Arteries were characterized by varying degrees of intimal hyperplasia and disrupted internal elastic laminae. In two animals, it was not possible to measure the intima-to-media ratio because of the discontinuous internal elastic lamina (Table 2, and Fig. 4). The intima-to-media ratios of the other two animals (0.14 and 0.78) were within the range of that measured in animals injected with saline; none of these arteries exhibited histological evidence of calcification by von Kossa staining (Table 2).
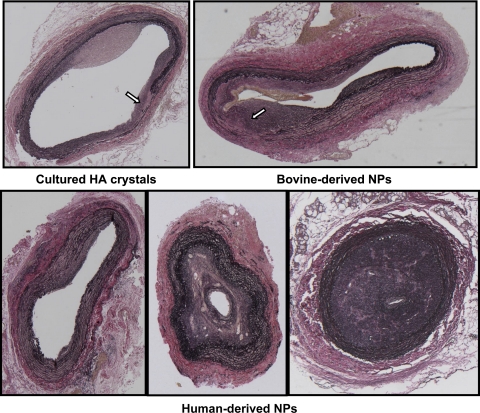
Fig. 4.
Light micrographs of representative sections of injured carotid arteries from rabbits injected with HA crystals exposed to culture medium (top, left), bovine-derived NPs (top, right), and human-derived NPs (bottom). Sections are stained with an elastin van Giesen stain and shown at ×5 magnification. All arteries were collected at 35 days postoperatively. Uninjured arteries from all rabbits are indistinguishable from the uninjured artery shown in Fig. 2. Arrows indicate areas of disrupted internal elastic lamina/media layer.
Bovine-derived NPs.
All of the injured carotid arteries of rabbits inoculated with bovine-derived NPs remained patent (Tables 1 and 2, and Figs. 3 and 4). The internal elastic lamina and media layer were discontinuous in four of five rabbits (Fig. 4). Thus it was not possible to calculate an intima-to-media ratio despite the substantial intimal hyperplasia in these arteries (Table 2). None of the injured arteries from this group stained positive for macrophage infiltration or intimal calcification (data not shown).
Human-derived NPs.
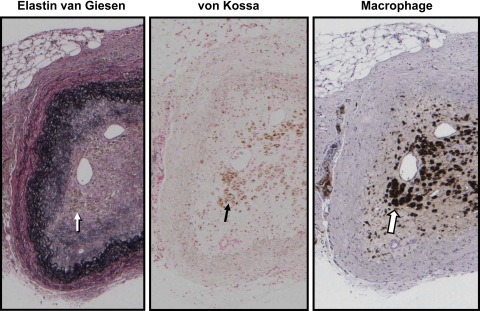
The response to injury in the arteries of animals injected with the planktonic human-derived NPs was variable (Fig. 4). In 6 of 11 animals in this group, the injured artery occluded with canalization; no occlusions were observed in any of the other groups (n = 28; P ≤ 0.05; Figs. 3 and 4, and Tables 1 and 2). In five animals where the lumen of the injured artery remained patent and the internal elastic lamina intact, the intima-to-media ratio was similar to ratios measured in rabbits of the control groups (Fig. 2, and Table 2). Unlike arteries from animals injected with bovine-derived NPs, the internal elastic lamina was intact in the injured arteries of all but two rabbits in this group (1 patent and 1 occluded vessel). Punctate clusters of calcification were detected by von Kossa staining in two of six occluded vessels analyzed by histology; this staining colocalized with positive staining for macrophages (Table 2, and Fig. 5).
Fig. 5.
Light micrographs of serial sections of an injured carotid artery from a rabbit injected with human-derived NPs. The artery was collected at 35 days postoperatively. The uninjured artery from this rabbit is that shown in Fig. 2. Consecutive sections were stained with an elastin van Giesen stain (left), von Kossa stain (middle), and a macrophage-specific antibody (right). Magnification is ×5. Arrows indicate an area that shows positive staining for both calcification and macrophages.
Immunological Identification of NP Antigen and Antibodies
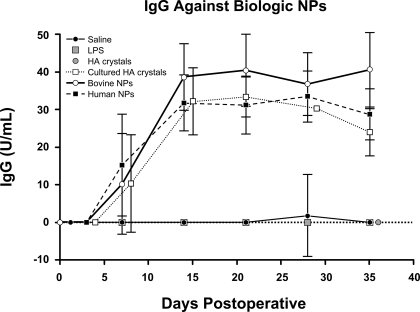
Animals inoculated with saline, LPS, and HA crystals did not produce antibodies detectable with the Nano-Sero IgG ELISA kit. Plasma antibody titers showed similar time-dependent increases in rabbits inoculated with bovine- and human-derived NPs and those injected with HA crystals exposed to culture media. Antibodies were detected as early as 7 days postinoculation, reached a maximum at 14 days, and were sustained throughout the 35 days of the experiment (Fig. 6). In contrast, the plasma levels of the NP antigen were at or below the detection limit of the assay in all the groups except the animals inoculated with human-derived NPs, where the titers ranged from 0 to 357.5 U/ml over the 35 days of the study. There was no statistically significant relationship between plasma levels of biological NP antigen and either anti-NP IgG or the degree of intimal hyperplasia.
Fig. 6.
Average plasma levels of IgG that recognizes biological NPs (in U/ml). Data are shown as means ± SE; n = 4 to 5 animals/group.
DISCUSSION
The results of this study demonstrate that a systemic inoculation of naïve animals with the planktonic form of propagated, calcified NPs derived from either human calcified tissue or bovine blood modifies the response to arterial injury. Therefore, these results support the hypothesis that NPs represent potential transmissible pathogenic agents. This study supports and extends previous observations that identified the pathological potential of NPs derived from an established biofilm (40). Furthermore, the anatomical configuration, including occlusions and calcification, of arteries recovered from animals exposed to planktonic human-derived NPs were different from those of animals exposed to planktonic bovine-derived NPs and other inflammatory agents, suggesting that the origin and/or composition of the NPs affect their biological activity (31). Since there were not discernable histological differences among the uninjured carotid arteries of all the groups, the vascular effects of NP inoculation were restricted to the areas of endothelial denudation. However, an assessment of inflammatory activation of endothelial cells in uninjured carotid arteries (15) or the effects of NPs in other arterial beds or at the level of the microcirculation remain to be determined.
Changes in vascular anatomy observed in arteries following an intravenous inoculation of NPs demonstrate that NPs circulate throughout the body. Thus it is possible that NPs could be released from one anatomical site to potentiate disease at a distant site within the same individual. Whether or not these biological NPs associate with other blood elements, such as platelets (33) or leukocytes, that would localize to areas of vascular damage remains to be determined.
The anatomical configuration of injured arteries from animals injected with saline, LPS (as a surrogate for gram-negative bacterial infection), or inorganic HA crystals (as a surrogate for nonspecific inflammation related to the HA shells of the NPs) was similar to what is observed in similar models of arterial endothelial denudation (7, 23, 42) and provides a credible comparison with animals exposed to the NPs. Unlike the anatomy of injured arteries from any of the above-mentioned control groups, considerable variation was seen among injured arteries of animals injected with human-derived NPs (6 occluded, and 5 did not). The interanimal variability within this group is not due to an inadequate vascular sampling at the site of the endothelium denudation, since an ultrasound evaluation of the arteries or a visual inspection of patency at the time of tissue harvest both correlated with a subsequent histological evaluation of patency. Indeed, despite the intrinsic biological variability, the rate of occlusion achieved statistical significance, since occlusions were observed only in animals inoculated with human-derived NPs. Some variability in response to vascular injury would be expected in these outbred rabbits from different suppliers, just as there is variable propensity to vascular diseases in humans (14, 19, 25, 32). Because animals were purchased from different suppliers, their prior exposure to environmental antigens might also have affected their immunological response to a potential pathogen, i.e., planktonic NPs.
Although inoculum dose was strictly standardized to 1,000 NTU per animal, another source of variability could have been the relative virulence of the human-derived NPs used in these studies. To minimize this possibility, all NPs were derived from the same original isolate (29). However, the development of biofilm can vary among subcultures; this may reflect the heterogenous composition of these NPs compared with the homogeneous composition of NPs derived from bovine blood (4, 10, 34). Indeed, the term “nanoparticle” defines a size, the biological activity of which will depend on its composition. Particles of this size include the general class of cell-derived microparticles, some of which may be of nano size, and calcifying matrix vesicles (19, 21, 31, 33, 35, 38, 39). Further studies are needed to define the differences in the biochemical composition of each type/strain of biologically derived NPs. However, fetuin was identified as a component of bovine-derived NPs (34). Fetuin limits soft tissue calcification (37). Consistent with this function, animals inoculated with bovine NPs and HA crystals incubated with culture medium containing 10% fetal bovine serum lacked calcification but demonstrated a disruption of the internal elastic lamina and medial smooth muscle of the arteries. In contrast, animals inoculated with human-derived NPs manifested a range of responses including vascular calcification, an effect not to be expected from fetuin alone.
Antibodies identified by the Nano-Sero IgG ELISA kit were detectable in all animals inoculated with material that had been exposed to culture media containing 10% fetal bovine serum. The specificity of this test is unclear, and antibodies developed against bovine fetuin or albumin may be detected (34). Since antibodies were detected in all groups that were inoculated with material exposed to culture media containing fetal calf serum, it is possible that the calcium shell of the human-derived NPs is capable of delivering bovine antigens to sites of injury. However, our studies also suggest that bovine proteins are not solely responsible for the vascular changes, since responses to human-derived NPs can be differentiated histologically from arteries of animals inoculated with either the bovine NPs or HA crystals incubated with culture media. There is a clear need to develop tests that more specifically detect antigens of, and antibodies to, biological NPs of human origin.
Arterial calcification is a multifactor process, and several theories suggest that calcification may proceed from matrix vesicles of apoptotic cells and the induction of osteoblast-like cells with secretions of bone matrix proteins (1, 13, 21, 22). The results of the present study do not negate these proposed mechanisms, since NPs may provoke the formation of matrix vesicles in tissue damaged from other causes (for example, biochemical oxidative stress or hyperlipidemia) (16, 45). It remains to be determined whether NPs would also target injuries resulting from environmental pollutants (18, 44).
In summary, the results of this study identify planktonic forms of nano-sized particles as potential stimuli for exacerbating arterial disease. They also raise the possibility that NPs being developed for industrial and medical purposes, depending on their distinct physical and chemical characteristics (31), could participate in some cardiovascular pathologies.
GRANTS
This work was funded in part by National Institutes of Health Grants DK-62021 and HL-88988 and grants from the Mayo Foundation, the Richard C. Wilson Sr. Foundation, and Nanobac Pharmaceuticals, Inc.
REFERENCES
- 1.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24: 1161–1170, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Akerman KK, Juronen I, Kajander EO. Scanning electron microscopy of nanobacteria—novel biofilm producing organisms in blood. Scanning Electron Microscopy 15: 90–91, 1993. [Google Scholar]
- 3.Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: a state-of-the-science review. Ann Periodontol 6: 9–15, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Benzerara K, Miller VM, Barell G, Kumar V, Miot J, Brown GE Jr, Lieske JC. Search for microbial signatures within human and microbial calcifications using soft X-ray spectromicroscopy. J Investig Med 54: 367–379, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bjorklund M, Ciftcioglu N, Kajander EO. Extraordinary survival of nanobacteria under extreme conditions. SPIE 3441: 123–129, 1998. [Google Scholar]
- 6.Bratos-Perez MA, Sanchez PL, Garcia de Cruz S, Villacorta E, Palacios IF, Fernandez-Fernandez JM, Di Stefano S, Orduna-Domingo A, Carrascal Y, Mota P, Martin-Luengo C, Bermejo J, San Roman JA, Rodriguez-Torres A, Fernandez-Aviles F. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J 29: 371–376, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation 93: 577–584, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int 56: 1893–1898, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Ciftcioglu N, Miller-Hjelle MA, Hjelle JT, Kajander EO. Inhibition of nanobacteria by antimicrobial drugs as measured by a modified microdilution method. Antimicrob Agents Chemother 46: 2077–2086, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cisar JO, Xu DQ, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci USA 97: 11511–11515, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Doherty TM, Asotra K, Fitzpatrick LA, Qiao JH, Wilkin DJ, Dentrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA 100: 11201–11206, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti GM, Hajjar D. Infection and atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol 20: 1417–1420, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Foglieni C, Maisano F, Dreas L, Giazzon A, Ruotolo G, Ferrero E, Li Volsi L, Coli S, Sinagra G, Zingone B, Alfieri O, Becker AE, Maseri A. Mild inflammatory activation of mammary arteries in patients with acute coronary syndromes. Am J Physiol Heart Circ Physiol 294: H2831–H2837, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hecht HS, Budoff MJ, Berman DS, Ehrlich J, Rumberger JA. Coronary artery calcium scanning: clinical paradigms for cardiac risk assessment and treatment. Am Heart J 151: 1139–1146, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hill AB The environment and disease: association or causation? Proc R Soc Med 58: 295–300, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, Araoz PA, Budoff MJ, Harman SM, Miller VM. Characterization of blood borne microparticles as markers of premature coronary calcification in recently menopausal women. Am J Physiol Heart Circ Physiol 295: H931–H938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajander EO, Kuronen I, Akerman KK, Pelttari A, Cifticioglu N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth. SPIE 3111: 420–428, 1997. [Google Scholar]
- 21.Kim KM Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc 35: 156–162, 1976. [PubMed] [Google Scholar]
- 22.Kockx MM, Muhring J, Bortier H, De Meyer GR, Jacob W. Biotin- or digoxigenin-conjugated nucleotides bind to matrix vesicles in atherosclerotic plaques. Am J Pathol 148: 1771–1777, 1996. [PMC free article] [PubMed] [Google Scholar]
- 23.Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95: 1768–1772, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Farell G, Yu S, Harrington S, Fitzpatrick L, Rzewuska E, Miller VM, Lieske JC. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med 54: 412–424, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med 167: 2437–2442, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Libby P Inflammation in atherosclerosis. Nature 420: 868–874, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Manson J, Allison M, Rossouw JE, Carr J, Langer R, Hsia J, Kuller L, Cochrane B, Hunt J, Ludlam S, Pettinger M, Gass M, Margolis K, Nathan L, Ockene J, Prentice R, Robbins JR, Stefanick M. Estrogen therapy and coronary-artery calcification. N Engl J Med 356: 2591–2602, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Martel J, Ding Young JD. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci USA 105: 5549–5554, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, Yagubyan M, Rodgers JC, Cockerill FR, III, Folk RL, Kumar V, Farell-Baril G, Lieske JC. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol Heart Circ Physiol 287: H1115–H1124, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Muhlestein JB, Anderson JL. Chronic infection and coronary artery disease. Cardiol Clin 21: 333–362, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science 311: 622–627, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections. Role of endothelial dysfunction. Circulation 106: 184–190, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol 146: 882–893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raoult D, Drancourt M, Azza S, Nappex C, Guieu R, Rolain JM, Fourquet P, Campagna B, La Scola B, Mege JL, Mansuelle P, Lechevalier E, Berland Y, Gorvel J, Renesto P. Nanobacteria are mineralo fetuin complexes. PLoS Pathog 4: e41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers KM, Stehbens WE. The morphology of matrix vesicles produced in experimental arterial aneurysms of rabbits. Pathology 18: 64–71, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Ross R The pathogenesis of atherosclerosis—an update. N Engl J Med 14: 488–500, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem 271: 20789–20796, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 73: 989–991, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what's circulating? Kidney Int 73: 384–390, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MA, Lieske JC, Kumar V, Farell-Baril G, Miller VM. Human-derived nanoparticles and vascular responses to injury in rabbit carotid arteries: proof of principle. Int J Nanomedicine 3: 243–248, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS 111: 951–954, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Shimokawa H, Flavahan NA, Vanhoutte PM. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Circ Res 65: 740–753, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: preliminary experience. J Urol 173: 474–477, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol 52: 719–726, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol 24: e6–e10, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Shen W, Wen J, An X, Cao L, Wang B. An animal model of black pigment gallstones caused by nanobacteria. Dig Dis Sci 51: 1126–1132, 2006. [DOI] [PubMed] [Google Scholar]