Abstract
In rats, hindlimb muscle ischemia induced by femoral artery occlusion augments the sympathetic nervous response to stimulation of transient receptor potential vanilloid type 1 (TRPV1) by injection of capsaicin into the arterial blood supply of the hindlimb muscles. The enhanced sympathetic response is due to alterations in TRPV1 receptor expression and its responsiveness in sensory neurons. The underlying mechanism by which TRPV1 receptor responses are increased after muscle vascular insufficiency/ischemia is unclear. In this report we tested the hypothesis that muscle ischemia elevates nerve growth factor (NGF) levels in primary afferent neurons, thereby increasing TRPV1 responsiveness. Muscle vascular insufficiency induced by the femoral artery ligation significantly increased NGF in the dorsal root ganglion (DRG) compared with sham controls. Furthermore, when NGF was infused in the hindlimb muscles of healthy rats (72 h using an osmotic minipump), the magnitude of the DRG neuron response to capsaicin was augmented (5.4 ± 0.54 nA with NGF infusion vs. 3.0 ± 0.17 nA in control; P < 0.05). With the addition of NGF in the culture dish containing the DRG neurons, the magnitude of the DRG neuron response to capsaicin was greater (6.4 ± 0.27 nA; P < 0.05 vs. control) than that seen in control (2.9 ± 0.16 nA). Note that this NGF effect was seen in isolectin B4-negative DRG neurons, a group of thin fiber nerves that contain neuropeptides and depend on NGF for survival. These data suggest that NGF affects a selective subpopulation of the afferent neurons in mediating augmented TRPV1 responses after femoral artery occlusion.
Keywords: peripheral arterial disease, transient receptor potential vanilloid type 1
two major clinical presentations seen in peripheral arterial occlusive disease due to atherosclerosis include intermittent claudication and critical limb ischemia (15, 32). Patients with intermittent claudication have normal or slightly diminished lower extremity blood flow at rest but an inability to adequately increase blood flow with exercise. Patients with critical limb ischemia have inadequate blood flow to meet the resting demands of the limb. The rat femoral artery ligation model exhibits impaired limb blood flow reserve capacity with exercise but normal flow at rest. Thus this model is widely used to study hindlimb muscle ischemia and vascular insufficiency that would be seen with limb ischemia due to arterial occlusive disease (44).
With the use of this rat model, the results of our previous study (47) demonstrate that the femoral artery ligation increases transient receptor potential vanilloid type 1 (TRPV1) receptor responsiveness in the primary sensory neurons and thereby enhances the reflex sympathetic responses evoked by injection of capsaicin in the arterial blood supply of the hindlimb muscle. Thus alterations in metabolite-sensitive TRPV1 are likely to contribute to augmented sympathetically mediated vasoconstriction that is a crucial factor in contribution to decreased blood flow (43). The reduced blood flow in skeletal muscle has been suggested to cause limited exercise capacity (45) seen under circumstances of muscle ischemia or muscle vascular insufficiency associated with peripheral arterial occlusive disease. Exercise training also can improve sympathetic nervous activity and reduce the resting levels of norepinephrine leading to vasoconstriction (20, 30) and has further benefits for patients with peripheral arterial disease (29). Whether the TRPV1 receptor is activated during exercise in the hindlimb muscle ischemia induced by the femoral arterial occlusion is yet to be determined. However, given its altered function after femoral arterial occlusion, it is possible that TRPV1 receptor overactivity contributes significantly to reductions in exercise capacity after the development of peripheral arterial disease.
Consistent with this idea, our previous work using this model has shown that TRPV1 responsiveness is enhanced in response to capsaicin at rest (47). The precise mechanism by which TRPV1 receptor-mediated responses are augmented after insult of muscle vascular insufficiency is unclear. A previous study has shown that nerve growth factor (NGF) levels are elevated in ischemic hindlimb muscles of rats 24 h after the femoral artery ligation (12). In addition, NGF can increase TRPV1 expression and sensitize its response in the dorsal root ganglion (DRG) neurons (2, 54, 55). Thus we postulated that the femoral artery occlusion would elevate NGF levels in primary afferent neurons-DRG and thereby increase TRPV1 responsiveness.
To test this hypothesis we employed the ELISA methods to examine whether the level of NGF would be elevated in DRG neurons of rats after femoral artery occlusion. The time course of NGF alterations induced by the femoral artery occlusion was also determined in this experiment. In addition, NGF was chronically infused into the muscle of healthy rats using an osmotic minipump previously inserted in the muscle and the magnitude of the DRG neuron response induced by capsaicin was then examined using the whole cell patch-clamp methods. This experiment allowed us to mimic the conditions induced by femoral arterial occlusion in freely perfused rats. Finally, the magnitude of the DRG neuron response to capsaicin was examined with and without the addition of NGF in the culture dish containing the DRG neurons.
METHODS
Femoral artery ligation.
The rat femoral artery occlusion model has been well characterized (37, 51). We used this method to induce hindlimb muscle vascular insufficiency/muscle ischemia. Male Sprague-Dawley rats (5–7 wk old) were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen). Right and left femoral arteries were surgically exposed and isolated immediately distal to the inguinal ligament. A ligature (3-0 silk) was placed tightly around the femoral artery ∼3 mm distal to the inguinal ligament. It has been reported that this procedure reduces blood flow reserve capacity to ∼10–20% of normal but remains sufficient to meet resting blood flow requirements (37, 49–51). Sham control animals underwent the same procedure as described except that a suture was placed below the artery but was not tied. The rats were allowed to recover 12, 24, and 48 h before the experiments were started. In some experiments, the ligature was performed on one leg and the sham control procedure was performed on another leg. All procedures outlined in this study were approved by the Animal Care Committee of this institution.
Measurements of NGF.
Seven sham control rats and eighteen rats with arterial occlusion were anesthetized by inhalation of an isoflurane-oxygen mixture (2–5% isoflurane in 100% oxygen) and killed by decapitation. The L4-L6 DRGs were removed quickly, weighed, and frozen at −80°C for NGF measurements. We chose to harvest the L4-L6 DRGs because they largely innervate the hindlimb muscles studied in this experiment. NGF levels were determined using a two-site immunoenzymatic assay (ELISA) as previously described (53). It is noted that the ELISA measurements were made on L4-L6 DRGs from individual animals. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified polyclonal goat anti-NGF antibody (Promega). Parallel wells were coated with purified goat IgG for evaluation of nonspecific signal. After overnight incubation at room temperature and 2 h of incubation with the coating buffer containing 50 mM carbonate buffer (pH 9.5) in 2% BSA, plates were washed with 50 mM Tris·HCl (pH 7.4; 200 mM NaCl, 0.5% gelatin, and 0.1% Triton X-100). After extensive washing, the diluted samples and the NGF standard solutions (Promega), ranging from 0 to 1 ng/ml, were distributed in each plate and left at room temperature overnight. The plates were then washed and incubated with 4 mU of anti-β-NGF-galactosidase per well (Boehringer Mannheim). After a 2-h incubation at 37°C, the plates were washed and then incubated with 100 μl of substrate solution (4 mg of chlorophenol red per ml of substrate buffer containing 100 mM HEPES, 150 mM NaCl, 2 mM MgCl2, and 0.1% sodium azidey1% BSA) that was added to each well. After an incubation of 2 h at 37°C, the optical density was measured at 575 nm using an ELISA reader (Dynatech).
Infusion of NGF in hindlimb muscle.
The Alzet osmotic minipump model 1003D (3-day delivery) containing either NGF (R&D systems) or saline was implanted subcutaneously in the hindlimbs of eight healthy rats under anesthesia and aseptic technique. This method allowed NGF to be chronically infused into skeletal muscles. On one leg, NGF was delivered at a rate of 0.25 μg/h (16, 34). A total of 18 μg NGF was delivered using the minipump over 72 h. In another leg, physiological saline was delivered at the same infusion rate via a minipump. This served as the control. The period of delivery was 72 h. Note that the physical size of the Alzet osmotic minipump to be better implanted subcutaneously in the hindlimbs of rats was available with 72 h, 1, 2, and 4 wk of delivery period. Based on our results showing that NGF was significantly increased in the DRG at 24 and 48 h after ischemia, the 72-h delivery period was chosen in this experiment. This was closer to the time course of increased NGF.
Fluorescent tracer injection into hindlimb muscle.
The nineteen healthy control rats and seven rats with the femoral artery occlusion were anesthetized by inhalation of an isoflurane-oxygen mixture, and the following injections were performed 4 to 5 days before electrophysiological recordings. The skin was incised and pulled away from underlying muscle tissue; a fluorescent retrograde tracer, 1,1-dioctadecyl-3,3,3,3 tetramethylindocarbocyanine percholate (DiI; 60 mg/ml; Molecular Probes, Eugene, OR), was injected into the white portion of the gastrocnemius muscle (47, 48). The results of our previous study suggest that DRG neurons with nerve endings in the white muscle develop greater inward current responses to capsaicin (48). The injection volume was 1 μl, and the injection was repeated three times at different locations. The injection needle was placed in the muscle for 5–10 min to prevent leakage of tracer. The skin overlying the muscle was then sutured to cover the incised area, and the rats were allowed to recover from the surgery. At the end of each experiment, the gastrocnemius muscle was dissected to confirm locations of DiI. The data were included in this experiment if DiI was in the white portion of the gastrocnemius muscle.
Isolation of DRG neurons.
The rats were anesthetized with isoflurane and decapitated 24 h following the ligation of the femoral artery or at the end of NGF infusion. DRGs at lumbar levels 4–6 were then removed and immediately placed into Dulbecco's modified Eagle's medium (DMEM; GIBCO, Carlsbad, CA). The DRGs were then incubated with 1 mg/ml collagenase IV (Sigma-Aldrich) and 0.5 mg/ml trypsin (Sigma-Aldrich) for 30 min at 34°C in a shaking water bath. Soybean (1.25 mg/ml; Sigma-Aldrich), a trypsin inhibitor, was then added to stop trypsin action. The cell suspension was centrifuged (500 rpm, 5 min) to remove the supernatant, replenished with DMEM, and plated onto a 35-mm culture dish containing poly-l-lysine (50 μg/ml; Sigma-Aldrich)-precoated coverslips and maintained for at least 60 min before electrophysiological recordings.
In one group of experiments, based on the previous studies (54, 55), 100 ng/ml of NGF was added in the culture dish 60 min before each recording to further determine the effects of NGF on the response of the DRG neurons to capsaicin. Note that the experiments were conducted on the DRG neurons obtained from healthy animals.
Electrophysiological recordings.
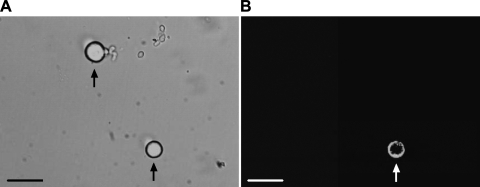
Whole cell recordings were made using fire-polished glass electrodes (2–5 MΩ resistance) pulled from glass capillaries (1.17 mm ID, 1.5 mm OD; Harvard Apparatus) on a model P-97 micropipette puller (Sutter Instruments). The recording chamber was continuously perfused (1 to 2 ml/min) with artificial cerebral spinal fluid (aCSF) containing (in mM) 140 NaCl, 5.4 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH adjusted 7.4; osmolarity 320 mOsm). Electrodes were filled with solution containing (in mM) 124 KCl, 13.2 NaCl, 2 MgCl2, 0.3 NaGTP, 1 EGTA, 10 HEPES, and 4 Mg-ATP (pH brought to 7.2; osmolarity to 300 mOsm). All solutions were made fresh daily and filtered. Immediately before recording, neurons were incubated with IB4-Alexa Fluor 488 (3 μg/ml; Invitrogen) in aCSF solution for 10 min and then rinsed for at least 3 min. Thus we can examine two distinct subpopulations of thin fiber afferent neurons, namely IB4 negative and IB4 positive. The IB4-negative neurons contain neuropeptides such as calcitonin gene-related peptide and substance P, whereas the IB4-positive neurons are relatively peptide poor (3, 5, 6, 31). Figure 1 shows that IB4-positive and IB4-negative neurons were identified. DRG neurons were first visualized using differential interference contrast (DIC; ×20–40) optics and an IB4-positive neuron was then visualized (as green color) using a combination of fluorescence illumination and DIC optics on a Nikon TE2000 inverted microscope (Olympus Optical, Tokyo, Japan). Capsaicin induced-currents were recorded from both IB4-positive and IB4-negative neurons. DiI-labeled DRG neurons were also identified (as red color) using the similar method. Under DIC, images of cells were displayed on a video monitor. A tight gigaohm seal was subsequently obtained in the selected neuron. Size of cell soma was estimated by calculating the mean of the longest and shortest cross-sectional diameters with the aid of a calibrated eyepiece reticle. As previously reported (47, 48), the DRG neurons with diameter <35 μm were recorded in this study.
Fig. 1.
Identification of IB4-positive and IB4-negative dorsal root ganglion (DRG) neurons. A: DRG neurons viewed using differential interference contrast optics. B: same microscopic field viewed using fluorescence illumination. An IB4-positive neuron is indicated by the arrows (A and B, bottom); an IB4-negative DRG neuron is indicated by the arrow (A, top; the same neuron does not appear in B). Scale bar = 50 μm.
For all chemical tests with capsaicin (Sigma-Aldrich), solutions were applied locally and rapidly (2-s duration) to the neuron of interest using silica 28-gauge syringes of 0.25 mm ID (World Precision Instruments). The tip of each syringe was placed 100 μm from the cell soma using a manipulator. The gravity-fed solutions were controlled using manual switching of one-way stopcock valves. Capsaicin was dissolved in 1% Tween 80-1% ethanol-98% saline to make a stock solution of 1 mM. For capsaicin responsiveness experiments, capsaicin was diluted in the aCSF solution to make the concentrations of 1 μM on the day of each experiment. The effect of capsaicin on DRG neurons of sham control rats and rats with vascular insufficiency and on DRG neurons with the prior exposure of NGF was examined by applying 1 μM of capsaicin. In some experiments, capsaicin-induced currents in the DRG neurons obtained from healthy rats were also examined 2 min after 50 μM of capsazepine was applied. One neuron per coverslip was studied and, after each recording, the chamber was washed with ethanol and water to eliminate any residual chemicals.
Signals were recorded with a MultiClamp 700B amplifier (Axon Instruments, Foster City, CA), digitized at 10 kHz with a DigiData 1322A, and filtered at 1 to 2 kHz and saved in a PC-based computer using pClamp 10.1 software (Axon Instruments). The whole cell configuration was maintained at −60 mV. Seals ranged from 1.5 to 6.0 GΩ. An equilibration period of 5–10 min was allowed after whole cell access was established and the recording reached a steady state. The recording was then made to measure changes in inward currents evoked by chemical stimuli. Electrical access to the cell was monitored throughout each recording. The recording was abandoned if the monitored input resistance changed >10%. The magnitude of inward current was determined using Clampfit 10.1 (Axon Instruments). Neurons were considered to be capsaicin sensitive if an evoked inward current was >50 pA in peak amplitude.
Statistics.
NGF measurements were analyzed using a one-way ANOVA. Amplitude of capsaicin-evoked currents was analyzed using a two-way repeated-measure ANOVA. As appropriate, Tukey post hoc tests were utilized. Values are presented as means ± SE. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SPSS for Windows version 15.0 (SPSS, Chicago, IL).
RESULTS
NGF levels in DRG neurons after muscle ischemia.
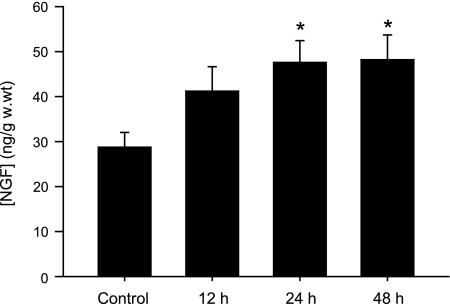
NGF levels in the DRG of seven sham control rats and rats with 12, 24, and 48 h muscle ischemia induced by the femoral artery occlusion (6 rats in each group) were examined (Fig. 2). Arterial occlusion elevated NGF in the DRG. When compared with sham control rats, 24 and 48 h of occlusion significantly increased NGF levels in the DRG of ischemic rats. There was no difference in NGF levels between 24 and 48 h of occlusion.
Fig. 2.
Nerve growth factor (NGF) levels (ng/g wet weight) in DRG tissue of 7 sham control rats and 18 rats with the femoral artery occlusion at different time course (6 rats in each group). *P < 0.05 vs. sham control. There was no difference in NGF levels between 24 and 48 h after occlusion.
Effect of ischemia on capsaicin-induced currents in DRG neurons.
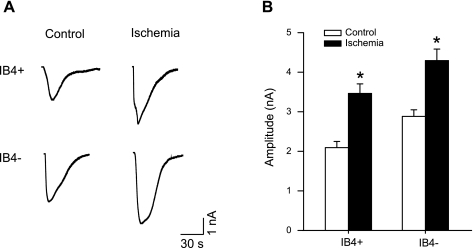
Capsaicin-induced currents in the DRG neurons innervating muscles were further examined in control and 24 h ischemia. We chose to examine the response of DRG neurons 24 h postischemia based on our previous study (47) showing that 24 h of femoral artery occlusion can significantly increase the magnitude of TRPV1 response to capsaicin in DRG neurons and that there is no difference in the response in 24 and 48 h of ischemia. In this experiment, a sham operation was performed on one leg as a control; femoral artery ligation was performed on the contralateral leg to evoke ischemia. IB4-positive and IB4-negative neurons were identified before each recording. Figure 3, A and B, shows original traces and average data of IB4-positive (n = 19 in control; and n = 18 in ischemia) and IB4-negative DRG neuron (n = 22 in control; and n = 18 in ischemia) responses to 1 μM capsaicin in control and in ischemia. When compared with control, arterial occlusion increased the peak inward current induced by capsaicin in both IB4-positive and IB4-negative DRG neurons. We also examined effects of capsaicin receptor blocker capsazepine on evoked currents in sixteen neurons. Capsaicin-induced currents in the DRG neurons were attenuated by 85% with prior exposure of 50 μM of capsazepine as previously reported (47, 48). A possible effect of the vehicle for preparations of capsaicin on DRG neurons was also examined. The membrane currents remained at ∼0 nA in seven tested DRG neurons when the vehicle was applied.
Fig. 3.
Effect of ischemia on capsaicin-induced currents in IB4-positive and IB4-negative DRG neurons. A: original traces of DRG neuron response to 1 μM capsaicin in sham control and 24-h arterial occlusion. B: average data show that 24 h of arterial occlusion insult induced larger peak amplitude of inward currents in both IB4-positive and IB4-negative DRG neurons compared with sham control. *P < 0.05 vs. sham control.
Effect of NGF on capsaicin-induced currents in DRG neurons.
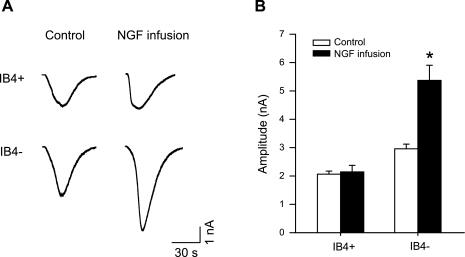
To determine the role of NGF in modulating TRPV1 responses of DRG neurons, NGF was chronically infused into the muscle of healthy rats using an osmotic minipump, and the magnitude of the DRG neuron response induced by capsaicin was then examined. Figure 4, A and B, shows original traces and average data of IB4-positive (n = 29 for control; and n = 24 with NGF) and IB4-negative (n = 31 for control; n = 25 with NGF) DRG neuron responses to 1 μM capsaicin in sham control and NGF infusion. When compared with control, 72-h infusion of NGF (0.25 μg/h) increased peak inward current amplitudes in IB4-negative DRG neurons but not in IB4-positive neurons.
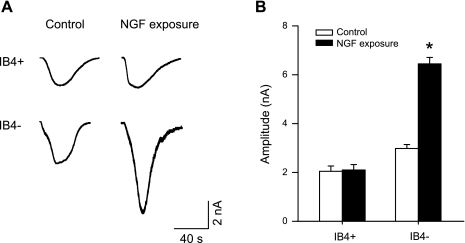
Fig. 4.
Effect of NGF infusion on capsaicin-induced currents in DRG neurons. A: original traces of DRG neuron response to 1 μM capsaicin in sham control and 72-h NGF infusion. B: average data show that NGF infusion augmented peak amplitude of inward currents in IB4-negative DRG neurons compared with sham control. *P < 0.05 compared with a sham control.
Finally, in another group of experiments 100 ng/ml of NGF were added in the culture dish containing the DRG neurons 60 min before each recording. The magnitude of the DRG neuron TRPV1 response was examined with and without the prior exposure of NGF. Both original traces and average data are shown in Fig. 5, A and B. Adding NGF to the culture dish of 1 μM of capsaicin led to larger peak inward current amplitudes in IB4-negative DRG neurons (n = 36 with NGF; and n = 40 without NGF) but not in IB4-positive neurons (n = 47 with NGF; and n = 42 without NGF).
Fig. 5.
Capsaicin-induced currents in IB4-positive and IB4-negative DRG neurons with and without addition of NGF. With the prior exposure of NGF, 1 μM capsaicin induced greater peak inward current amplitudes in IB4-negative DRG neurons but not in IB4-positive neurons. A and B: original traces and average data, respectively. *P < 0.05, NGF addition vs. without NGF.
DISCUSSION
The results of our present study show that the femoral artery ligation increased NGF levels in DRG neurons of rats. Furthermore, hindlimb ischemia augmented capsaicin-induced currents of both IB4-negative and IB4-positive DRG neurons innervating muscles. In addition, infusion of NGF in the muscles as well as the addition of NGF to the culture dish containing DRG neurons increased the magnitude of the TRPV1 response in IB4-negative but not in IB4-positive DRG neurons. These findings suggest that NGF plays a role in augmented TRPV1 responses after hindlimb muscle ischemia by affecting a selective subpopulation of the afferent neurons.
A prior report has shown that NGF levels are elevated in hindlimb muscles of rats 24 h after the femoral artery ligation (12). However, this study did not determine NGF levels in the primary sensory neurons after ischemia. In our current study, the ELISA method was used to assess NGF levels in DRG neurons as reported previously (53). The femoral artery occlusion elevated NGF in the DRG, and 24 and 48 h of postocclusion significantly increased NGF levels in the DRG of ischemic rats. It is noted that there was no difference in NGF levels between 24 and 48 h after the occlusion.
Our previous study has demonstrated that the femoral artery occlusion increased the magnitude of TRPV1 response to capsaicin in DRG neurons innervating muscles (47). A time course of altered TRPV1 response induced by ischemia was also determined in this prior experiment. The augmented TRPV1 response was seen 24 and 48 h after occlusion. The time courses are very similar in elevated NGF and increased TRPV1 responsiveness in the DRG neurons. The similarity may indicate that there is a close relationship between NGF and TRPV1 responses in the DRG neurons in the processing of the muscle ischemia. It has been reported that NGF can increase TRPV1 expression and response in DRG neurons (2, 54, 55). Thus ischemia-induced NGF is likely to increase TRPV1 expression and responsiveness after femoral artery occlusion. The results of our current experiment further show that ischemia augmented capsaicin-induced currents in both IB4-negative and IB4-positive DRG neurons.
Thin fiber afferent nerves (neurons) are distinct as IB4 negative and IB4 positive because of their distinct neurochemical characteristics and neurotrophic factor responsiveness. The IB4-negative neurons express trkA receptors for NGF, depend on NGF for survival during postnatal development, and contain neuropeptides such as calcitonin gene-related peptide and substance P (3, 5, 6, 31). The IB4-positive neurons express receptors for glial cell line-derived neurotrophic factor (GDNF), depend on GDNF for survival during postnatal development, and are relatively peptide poor but express a surface carbohydrate group that binds IB4 (3, 5, 6, 31).
Our current findings show that NGF infused in the muscles and NGF added to the culture dish increase the magnitude of TRPV1 response in IB4-negative DRG neurons but not in IB4-positive DRG neurons. This result is consistent with the idea that NGF is more responsible for the IB4-negative neurons (3, 5, 6, 31). However, it is interesting that ischemia can also augment capsaicin-induced currents of both IB4-negative and IB4-positive DRG neurons. Ischemia-augmented TRPV1 response of IB4-positive DRG neurons suggests GDNF may also be involved in the processing of TRPV1 responsiveness after the femoral artery occlusion. Prior studies have shown that GDNF can also increase activities of TRPV1 receptors in sensory nerves (1, 28). Additional experiments are needed to determine GDNF levels after the hindlimb muscle ischemia and its modulating effects on ischemia-induced TRPV1 responsiveness.
TRPV1 receptors appear preferentially on metabolite-sensitive thin fiber sensory neurons (27). These receptors are located on afferents in a variety of tissues and mediate the effect of the vanilloid compound capsaicin (8). Capsaicin injected into the pulmonary circulation activates C fibers that play a role in evoking a pulmonary chemoreflex (9, 35). The epicardial application of capsaicin stimulates cardiac TRPV1 receptors evoking a sympathoexcitatory reflex (52). The competitive capsaicin antagonist capsazepine has been shown to reduce capsaicin-induced activation of the cloned nonselective cation channel TRPV1 (8). Capsazepine also abolishes capsaicin-induced C fiber activity both in vitro and in vivo (13, 22). Although the endogenous TRPV1 ligand has not been determined, both the metabolic by-products accompanying the inflammatory process (lactic acid, H+) and inflammatory mediators themselves (histamine, seratonin, prostaglandin E2) have been identified as potential endogenous ligands for the C fiber capsaicin receptor. Hydrogen ions (H+) in general and lactic acid in particular have been shown to activate C fiber afferents similar to the effect seen with capsaicin (7, 17, 39). In vitro studies have demonstrated that H+ inhibits the binding of the capsaicin analog resiniferatoxin to vanilloid receptors, a finding attributed to competition for the same binding site (42).
Activation of thin fiber muscle afferent nerves increases blood pressure and heart rate via a reflex muscle response (18, 19). When capsaicin is injected into the arterial supply of the dog hindlimb, blood pressure rises by 20%, an effect abolished by sectioning afferent nerves (10). The muscle pressor response is likely to be due to the stimulation of both group III and IV fibers since capsaicin stimulates 71% of group IV and 26% of group III dog hindlimb muscle afferent fibers (18). In a recent study, we observed that when capsaicin is injected into the arterial supply of the hindlimb muscles of rats, blood pressure increases and the effect is mediated via the engagement of TRPV1 receptors on sensory afferents (24).
It is well established that group III and IV thin fiber afferents in skeletal muscle mediate, in part, the cardiovascular response to exercise. However, the TRPV1 receptor appears to play little role in stimulating thin fiber afferents in cats during static contraction of freely perfused muscles (21). Protons (lower pH) in the muscle interstitium may be required in mediating the reflex responses with activation of TRPV1 because TRPV1 response is sensitive to pH. As interstitial pH is lowered to 6.5, TRPV1 responsiveness is augmented (14). Notably, the value of pH required for the stimulation of TRPV1 receptors in the interstitial space is lower than that seen during either dynamic exercise or static contraction. Thus it is postulated that during exercise the likely stimuli to TRPV1 receptors are temperature and muscle metabolites (such as protons, ATP, and inorganic phosphates) accumulated in exercising muscles or some combination. A recent study suggests that P2X, TRPV1, and acid-sensing ion channel (ASIC) play a combining role in sensory neurons innervating skeletal muscles since neurons are exposed to physiological concentrations of muscle metabolites (25). Blocking ASIC can also attenuate the effects of lowering the pH on TRPV1 (14). Circulatory occlusion for 24 h is likely to increase muscle metabolites, thereby augmenting the expression and sensitivity of TRPV1 receptors (47) and then altering sensitivity of TRPV1 receptors to protons. In situations with muscle ischemia, we speculate that TRPV1 is activated when metabolites are accumulated in resting and active muscles to a greater degree. Thus augmented muscle afferent responses to stimulation of TRPV1 are seen at 24-h postischemia (47). This is likely to lead to increased afferent discharge and a resultant augmentation of the pressor response to exercise. A prior study has demonstrated that the pressor response in patients with intermittent claudication is markedly augmented during exercise (4). On the other hand, it possible that an increase in metabolites can dilate the vessels in active muscles and tend to neutralize the effect of enhanced vasoconstriction. Clearly, additional experiments are needed to examine the role of TRPV1 in the exercise pressor reflex after hindlimb muscle ischemia.
Nevertheless, we have reported that the femoral artery occlusion can upregulate TRPV1 receptors and augment the responsiveness of those receptors in the primary sensory neurons (47). In turn TRPV1-mediated reflex sympathetic and pressor responses are enhanced after the insult of hindlimb muscle ischemia (47), suggesting that TRPV1 receptors are sensitized after muscle ischemia. Thus we suggest that alterations in TRPV1 contribute to enhanced sympathetically mediated vasoconstriction. This response may lead to a reduction in blood flow directed to skeletal muscle and limited exercise capacity (43, 45) seen under circumstances of muscle vascular insufficiency or muscle ischemia associated with peripheral arterial occlusive disease. In the present study, we further show that NGF plays a role in augmented TRPV1 responses in the DRG neurons, suggesting that NGF is a key factor in the involvement of ischemia-induced sympathetic responses. It is also noted that that NGF affects a selective subpopulation of the afferent neurons in augmented TRPV1 responses after femoral artery occlusion.
Approximately 30% of patients with intermittent claudication have symptoms of pain or leg fatigue with walking (15). Thus another issue to be considered is the role of NGF in accompanying pain. There seems to be widespread agreement that the primary role played by NGF is to initiate and maintain hypersensitivity of sensory neurons after tissue injury or inflammation (33, 36). This hypersensitivity by sensory neurons is attributed to either a lower threshold or an increased discharge or both in response to a given stimulus. In animals, NGF has been shown to reduce nociceptive thresholds in several models of pain (23, 46). It is reported that the hyperalgesic effects of NGF are due to increased neuropeptide release from thin fiber afferents. For example, immunoneutralization of NGF decreased substance P content in small sensory neurons (38). On the other hand, exogenous NGF increases substance P and CGRP levels in these neurons (26). TRPV1 in sensory nerves plays an important role in modulating peripheral pain (41). Our data further show that NGF increases TRPV1 response in primary sensory neurons that innervate the hindlimb muscles. Little is known about the interplay of pain and ischemia-enhanced afferent stimulation in intermittent claudication during exercise. A recent report has shown that exercise training has benefits for patients with peripheral artery disease (29). For example, exercise training can improve sympathetic nerve activity and reduce the resting levels of norepinephrine leading to vasoconstriction (20, 30). A decrease in chronic sympathetic tone produced during training may also contribute to reduced progression of peripheral artery disease, thereby reducing overall susceptibility to inflammation and coagulation predisposing to further injury.
There is a limitation of this study. We did not determine whether chronic infusion of the NGF in the muscle can increase NGF in the DRG neurons to levels similar to those seen in the hindlimb muscle ischemia. A single injection of NGF (5 μg) into the masseter muscle of humans reduced significantly pressure pain thresholds, and the effect lasted about 7 days (40). Likewise, a single intradermal injection of NGF (1 μg) into the forearm skin of humans produced pressure allodynia and heat-induced hyperalgesia, effects which started 3 h after injection and peaked at ∼3 days (11). In animals, NGF that has been shown to reduce nociceptive thresholds in several models of pain is in a range of micrograms (23, 46). The effects of NGF on pain or its threshold are presumably via sensory nerves (neurons). In the present experiment a total of 18 μg NGF was delivered using the minipump over 72 h. Consequently, TRPV1 response was increased as shown in our data. Nevertheless, we postulate that NGF infusion into the muscle increases NGF in the neurons, thereby augmenting TRPV1 response.
In conclusion, our data demonstrate that the femoral artery occlusion significantly increased NGF in the DRG neurons compared with sham control. The arterial occlusion then augmented responses with the activation of metabolite-sensitive TRPV1 receptors in IB4-positive and -negative DRG neurons. We further show that increased NGF in the muscles and in the culture dish containing DRG neurons amplified the magnitude of TRPV1 response to capsaicin in IB4-negative but not in IB4-positive DRG neurons. These findings suggest that NGF plays a role in augmented TRPV1 responses in the processing of muscle ischemia or vascular insufficiency induced by the femoral artery occlusion. Our data suggest that a selective subpopulation of the afferent neurons is engaged in NGF-augmented TRPV1 response. Thus the evidence of our study provides strong support for the proposition that NGF regulation in muscle metabolic changes associated with TRPV1 receptors contributes to augmented sympathetic activity and may lead to a reduction in exercise capacity seen in the peripheral artery disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-075533 and R01-HL-078866 and American Heart Association Established Investigator Award 0840130N.
Acknowledgments
We express gratitude to Dr. Lawrence Sinoway for reading the manuscript and Jennie Stoner for outstanding secretarial skills.
REFERENCES
- 1.Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci 20: 2303–2310, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett 399: 51–56, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci 7: 1484–1494, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg 33: 20–25, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci 8: 2204–2208, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 18: 3059–3072, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci 17: 509–512, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Coleridge HM, Coleridge JC, Schultz HD. Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacol Ther 42: 1–63, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Crayton SC, Mitchell JH, Payne FC III. Reflex cardiovascular response during injection of capsaicin into skeletal muscle. Am J Physiol Heart Circ Physiol 240: H315–H319, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O′Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heatpain threshold in humans. Neurology 48: 501–505, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106: 2257–2262, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience 67: 741–752, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol 102: 2288–2293, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Garcia LA Epidemiology and pathophysiology of lower extremity peripheral arterial disease. J Endovas Ther 13: II3–II9, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hobara N, Goda M, Kitamura Y, Sendou T, Gomita Y, Kawasaki H. Adenomedullin facilitates reinnervation of phenol-injured perivascular nerves in the rat mesenteric resistance artery. Neuroscience 144: 721–730, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hong JL, Kwong K, Lee LY. Stimulation of pulmonary C fibres by lactic acid in rats: contributions of H+ and lactate ions. J Physiol 500: 319–329, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res 50: 133–139, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res 18: 663–668, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Kiilavuori K, Naveri H, Leinonen H, Harkonen M. The effect of physical training on hormonal status and exertional hormonal response in patients with chronic congestive heart failure. Eur Heart J 20: 456–464, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol 288: H1867–H1873, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lee LY, Morton RF, Lundberg JM. Pulmonary chemoreflexes elicited by intravenous injection of lactic acid in anesthetized rats. J Appl Physiol 81: 2349–2357, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 13: 2136–2148, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol 97: 1709–1714, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–364, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Ma QP Vanilloid receptor homologue, VR1, is expressed by both Aδ- and C-fiber sensory neurons. Neuroreport 12: 3693–3695, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Liu K, Ferrucci L, Criqui MH, Greenland P, Guralnik JM, Tian L, Schneider JR, Pearce WH, Tan J, Martin GJ. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med 144: 10–20, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation 101: 784–789, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I, Regensteiner JG. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 8: 115–126, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv 7: 26–41, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci 23: 4117–4126, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paintal AS Vagal sensory receptors and their reflex effects. Physiol Rev 53: 159–227, 1973. [DOI] [PubMed] [Google Scholar]
- 36.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz JP, Pearson J, Johnson EM. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res 244: 378–381, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Stahl GL, Longhurst JC. Ischemically sensitive visceral afferents: importance of H+ derived from lactic acid and hypercapnia. Am J Physiol Heart Circ Physiol 262: H748–H753, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Svensson P, Cairns BE, Wang K, Rendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain 104: 241–247, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–211, 1999. [PubMed] [Google Scholar]
- 42.Szallasi A, Blumberg PM, Lundberg JM. Proton inhibition of [3H]resiniferatoxin binding to vanilloid (capsaicin) receptors in rat spinal cord. Eur J Pharmacol 289: 181–187, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol 97: 731–738, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J Appl Physiol 97: 773–780, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JR, Mancini DM. Factors contributing to the exercise limitation of heart failure. J Am Coll Cardiol 22: 93A–98A, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 62: 327–331, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol 295: H1262–H1269, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing J, Sinoway L, Li J. Differential responses of sensory neurons innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol 586: 3245–3252, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol Heart Circ Physiol 278: H85–H93, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Yang HT, Ogilvie RW, Terjung RL. Peripheral adaptations in trained aged rats with femoral artery stenosis. Circ Res 74: 235–243, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551: 515–523, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zettler C, Bridges DC, Zhou XF, Rush RA. Detection of increased tissue concentrations of nerve growth factor with an improved extraction procedure. J Neurosci Res 46: 581–594, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol 92: 3148–3152, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Zhu W, Oxford GS. Phosphoinositide-3-kinase and mitogen activated protein kinase signaling pathways mediates acute NGF sensitization of TRPV1. Mol Cell Neurosci 34: 689–700, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]