Abstract
Prenatal cocaine exposure in rats resulted in decreased PKCɛ protein expression in the heart of adult male but not female offspring. The present study determined its functional consequence of inhibiting cardioprotection mediated by ischemic preconditioning. Pregnant Sprague-Dawley rats were administered intraperitoneally saline or cocaine (30 mg·kg−1·day−1) from day 15 to day 21 of gestational age. Hearts were isolated from 3-mo-old offspring and were subjected to ischemia and reperfusion injury in a Langendorff preparation, with or without prior ischemic preconditioning. Preischemic values of left ventricular function were the same between the saline control and cocaine-treated animals. Ischemic preconditioning of two episodes of 5-min ischemia significantly decreased infarct size and enhanced postischemic functional recovery of the left ventricle in the saline control animals. This ischemic preconditioning was associated with increased phospho-PKCɛ, but not phospho-PKCδ, levels and was blocked by a PKCɛ translocation inhibitor peptide. Prenatal cocaine treatment abolished the ischemic preconditioning-mediated increase in phospho-PKCɛ and cardioprotection in the heart of male offspring. In contrast, the cardioprotective effect was fully maintained in female offspring that were exposed to cocaine before birth. The results suggest that prenatal cocaine exposure causes a sex-specific loss of cardioprotection by ischemic preconditioning in adult offspring, which is most likely due to fetal programming of PKCɛ gene repression, resulting in a downregulation of PKCɛ function in the heart of adult male offspring.
Keywords: fetal programming, protein kinase C, ischemia
cocaine is a widely abused drug that has significant cardiovascular effects. Cocaine abuse clearly increases the risk of several cardiac disease states in the adult user, most notably myocardial infarction (30). Additionally, exposure to cocaine in utero has a detrimental impact on the developing heart. While the effect of fetal cocaine exposure on birth weight and gross cardiac malformations is still under investigation, recent animal studies in our laboratory have demonstrated that the heart undergoes significant changes at the cellular and genetic levels in response to cocaine exposure (4, 39). Fetal cocaine exposure causes myocardial cell apoptosis, resulting in a decreased number of myocardiocytes at birth and increases the heart's sensitivity to ischemia and reperfusion injury in adult male offspring in a sex-dependent manner (2, 4). These studies also showed a correlation between a decrease in both total and phosphorylated protein kinase Cɛ and increased myocardial sensitivity to ischemia in adult male offspring after fetal cocaine exposure (2).
A short period of ischemia has been shown to be both a potent and reproducible method of protecting the heart from subsequent prolonged ischemia and reperfusion injury. This phenomenon, known as ischemic preconditioning (IPC), causes the translocation and activation of the ɛ-isoform of PKC (PKCɛ) (29), which has been shown to be an important component of the IPC pathway (12, 13, 17). Studies in a PKCɛ knockout (KO) mouse model have demonstrated no significant difference in baseline susceptibility to ischemia and reperfusion injury between the wild-type and PKCɛ KO hearts (10). However, targeted disruption of the PKCɛ gene blocked cardioprotection caused by IPC (10), suggesting dichotomy of mechanisms in heart susceptibility to ischemia-reperfusion injury and inducible protection against oxidative stress observed during cardiac preconditioning.
The present study investigated the effect of fetal cocaine exposure and IPC in the heart of the adult offspring. Our previous studies suggest a possible link between an increased sensitivity to ischemia and decreased levels of activated (phosphorylated) PKCɛ (2). Because IPC is known to activate PKCɛ in the myocardium to induce protection from ischemia-reperfusion injury, we investigated the possibility that IPC may rescue the myocardium from the increased ischemic sensitivity induced by fetal cocaine exposure. As IPC is a powerful activator of PKCɛ, we hypothesized that it would temporarily restore the level of active PKCɛ, although the effect may be less dramatic than in controls due to lower total PKCɛ expression. The goal of this study was to gain additional insight into the physiological effect of fetal cocaine exposure on the myocardium and increased understanding of the mechanism of increased ischemic sensitivity previously observed in this model.
MATERIALS AND METHODS
Experimental animals and cocaine treatment.
All experiments and procedures used in this study adhered to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Loma Linda University. Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI). As described previously, the animals were randomly divided into two groups: 1) saline control, and 2) 15 mg/kg cocaine administered intraperitoneally twice daily at 10:00 AM and 4:00 PM from day 15 to 21 of gestational age, the dose that produces plasma cocaine levels in the range of those reported in human cocaine users (2). The animals were allowed to give birth naturally, and no fetal loss in the control and cocaine-treated groups was observed. Pups were weaned and separated by sex 21 days after birth. The offspring were given food and water ad libitum and were subjected to no further treatment before death.
Langendorff preparation and IPC protocol.
At age of 3 mo old, offspring from both cocaine- and saline-treated dams were anesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine injected intramuscularly. The heart was rapidly excised and transferred to ice cold Krebs-Heinseleit buffer. After which it was perfused via the aorta in a modified Langendorff apparatus under constant pressure (70 mmHg) with gassed (95% O2, 5% CO2) Krebs-Heinseleit buffer at 37°C, as previously described (22). A latex balloon attached to a pressure transducer was inserted into the left ventricle and inflated to obtain a left ventricular end-diastolic pressure (LVEDP) of ∼5 mmHg. Hearts either were treated with IPC before ischemia, or underwent continuous perfusion before ischemia. IPC was achieved by two cycles of 5 min of global ischemia with 5-min recovery between cycles, after which the heart was allowed to recover for 20 min and then was subjected to 20 min of ischemia, followed by 40-min reperfusion. Non-IPC-treated hearts were perfused continuously before undergoing the same 20 min of global ischemia and 40 min of reperfusion. Additionally, some hearts from rats were treated with 5 μM of a PKCɛ translocation inhibitor peptide (PKCɛ-TIP) (H-Glu-Ala-Val-Ser-Leu-Lys-Pro-Thr-OH) (Calbiochem, San Diego, CA) for 10 min before and continuing during IPC. After the 20-min period of global ischemia, the heart was reperfused with the buffer not containing PKCɛ-TIP. The following parameters of cardiac function were monitored: heart rate, left ventricular developed pressure (LVDP), the maximal rate of pressure increase (dP/dtmax), the rate of maximal relaxation (dP/dtmin), and the LVEDP. Perfusate solution was collected over 1 min at predetermined intervals, and the volume recorded as an indicator of coronary flow rate. The heart was allowed to stabilize before the treatment protocol was started.
Measurement of myocardial infarct size.
Myocardial infarct size was measured as described previously (2). At the end of reperfusion, the left ventricle was sectioned into four pieces and incubated in 1% triphenyltetrazolium chloride at 37°C for 15 min. After which, the tissues were fixed in 10% formalin for 1 h at room temperature. The slices were then photographed and analyzed for area of myocardial infarction. The combined value was obtained, corrected for the tissue weight, and expressed as a percentage of the total left ventricle weight.
Protein collection and Western blot analysis.
Our previous studies demonstrated that prenatal cocaine exposure resulted in a significant decrease in PKCɛ protein abundance in left ventricles of male, but not female, offspring (2, 39). To determine the effect of cocaine on PKCɛ and PKCδ protein expression in adult offspring, protein was collected from left ventricles of both saline and cocaine-treated offspring. To determine whether IPC activates PKCɛ and/or PKCδ, the hearts were removed from the Langendorff apparatus before prolonged (20 min) ischemia, with and without IPC, and left ventricles were isolated. Left ventricles were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween 20, 0.1% β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 5 μg/ml aprotinin, pH 7.4. Homogenates were then centrifuged at 4°C for 10 min at 10,000 g, and supernatants were collected. Protein concentration was measured in the supernatant using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 10% polyacrylamide gel with 0.1% sodium dodecyl sulfate and were separated by electrophoresis at 100 V for 2 h. Proteins were then transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked with overnight incubation at 4°C in a Tris-buffered saline solution containing 5% dry milk. The membranes were incubated with primary antibodies against PKCɛ, PKCδ (Santa Cruze Biotechnology, Santa Cruz, CA), phospho-PKCɛ and phospho-PKCδ (Upstate/Millipore, Billerica, MA). α-Sacromeric actin antibody (Sigma, St. Louis, MO) was used to normalize the loading. After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. Results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software.
Real-time RT-PCR.
RNA was extracted from left ventricles using TRIzol reagents (Invitrogen, Carlsbad, CA). PKCɛ and PKCδ mRNA abundance was determined by real-time RT-PCR using Icycler Thermal cycler (Bio-Rad, Hercules, CA). PKCɛ primers were 5′-GCGAAGCCCCTAAGACAAT-3′ (forward) and 5′-CACCCCAGATGAAATCCCTAC-3′ (reverse). PKCδ primers were 5′-ACAGAAGAAGCCCACCAT-3′ (forward) and 5′-GAACTCAGCCTTGCCGTT-3′ (reverse). Real-time RT-PCR was performed in a final volume of 25 μl. Each PCR reaction mixture consisted of 600 nM of primers, 33 units of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI), and iQ SYBR Green Supermix (Bio-Rad) containing 0.625 unit Taq polymerase, 400 μM each of dATP, dCTP, dGTP, and dTTP, 100 mM KCl, 16.6 mM ammonium sulfate, 40 mM Tris-HCl, 6 mM MgSO4, SYBR Green I, 20 nM fluoresein, and stabilizers. RT-PCR was performed under the following conditions: 42°C for 30 min, 95°C for 15 min, followed by 50 cycles of 95°C for 20 s, and 52°C for 1 min. GAPDH was used as an internal reference, and serial dilutions of the positive control were performed on each plate to create a standard curve. PCR was performed in triplicate, and threshold cycle numbers were averaged.
Statistical analysis.
Data were expressed as means ± SE. Experimental number (n) represents offspring from different dams. Statistical significance (P < 0.05) was determined using one-way or two-way ANOVA followed by a Tukey post hoc analysis, or t-test, as appropriate for the data sets.
RESULTS
Body weight and baseline cardiac function.
Both male and female offspring had no significant difference in body mass between saline control animals and those exposed to cocaine before birth (data not shown). The baseline cardiac function parameters in the Langendorff preparation also showed no significant difference between saline- and cocaine-exposed rats (Table 1).
Table 1.
Preischemic left ventricular functional parameters of 3-mo-old offspring of prenatal saline control and cocaine treatments
| HR, beats/min | LVDP, mmHg | LVEDP, mmHg | dP/dtmax, mmHg/s | dP/dtmin, mmHg/s | CF, ml/min | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||||
| Control (n = 15) | 264±8 | 118±8 | 5±0 | 3,832±193 | 2,491±103 | 11±1 | ||||||
| Cocaine (n = 15) | 255±6 | 119±7 | 6±0 | 3,745±209 | 2,518±162 | 12±1 | ||||||
| TIP control (n = 10) | 265±9 | 120±5 | 6±0 | 3,789±212 | 2,459±120 | 11±1 | ||||||
| TIP cocaine (n = 10) | 272±8 | 116±9 | 5±0 | 3,822±225 | 2,386±154 | 11±1 | ||||||
| Female | ||||||||||||
| Control (n = 10) | 269±12 | 109±9 | 6±0 | 3,307±204 | 2,260±140 | 10±1 | ||||||
| Cocaine (n = 10) | 255±6 | 110±10 | 5±0 | 3,197±301 | 2,207±122 | 10±1 | ||||||
| TIP control (n = 10) | 255±10 | 107±10 | 5±0 | 3,241±474 | 2,014±280 | 10±1 | ||||||
| TIP cocaine (n = 10) | 261±14 | 104±11 | 5±0 | 3,184±258 | 2,195±185 | 10±1 | ||||||
Values are means ± SE; n, no. of animals. HR, heart rate; LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; dP/dtmax, maximal rate of contraction; dP/dtmin, maximal rate of relaxation; CF, coronary flow; TIP, PKCɛ translocation inhibitory peptide.
IPC in hearts of saline control animals.
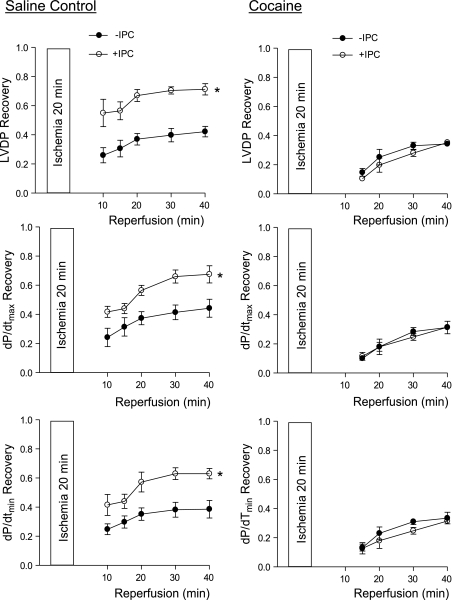
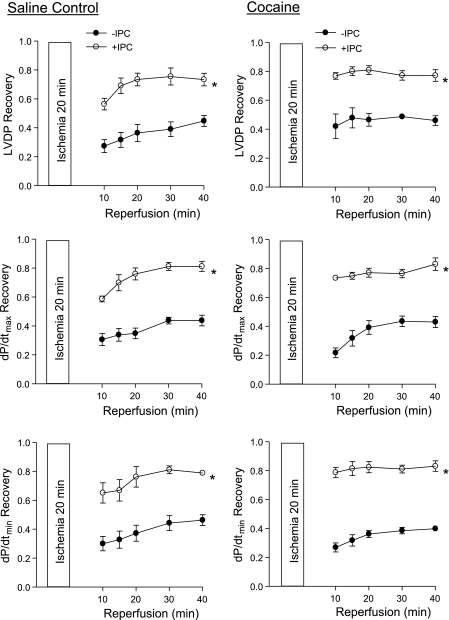
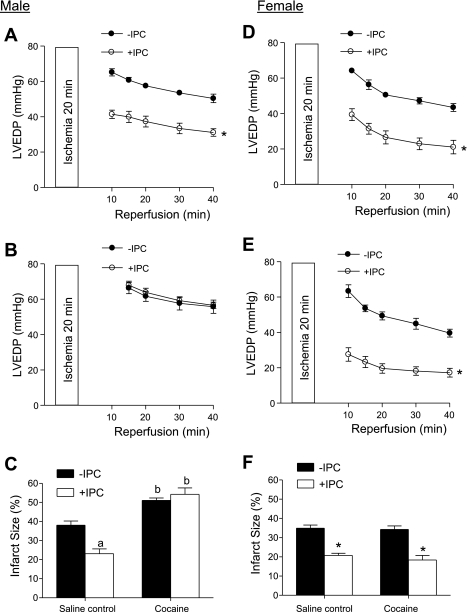
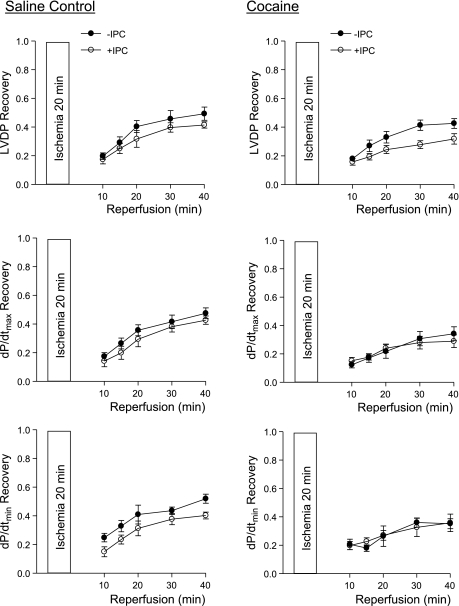
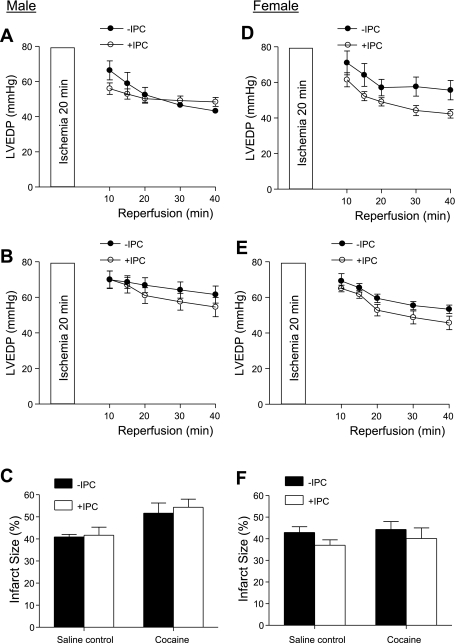
We first determined the IPC of two cycles of short ischemia with 5 min each in cardioprotection in the heart of offspring of saline control animals. In both males and females, IPC had no significant effect on the baseline function of the left ventricle before the prolonged ischemia of 20 min, but resulted in a significant increase in postischemic recovery of LVDP, dP/dtmax, and dP/dtmin during reperfusion after prolonged ischemia (Figs. 1 and 2, left). Additionally, the IPC-mediated increases in postischemic recovery of dP/dtmax and dP/dtmin were significantly greater in females than males (P < 0.05; Figs. 1 and 2, left). IPC also caused a significant reduction in the increased LVEDP seen after prolonged ischemia in both males (Fig. 3A) and females (Fig. 3D). Female hearts showed significantly greater reduction in LVEDP in the presence of IPC than did the male hearts (P < 0.05, Fig. 3, A and D). Consistent with the improved LVEDP, there was a significant decrease in infarct size of the left ventricle in both males (Fig. 3C) and females (Fig. 3F) after the IPC treatment.
Fig. 1.
Effect of ischemic preconditioning (IPC) on postischemic recovery of left ventricular function in male offspring. Hearts were isolated from 3-mo-old male offspring of prenatal saline (left) or cocaine (right) treatments and were treated without (−) or with (+) IPC, followed by ischemia-reperfusion (20/40 min). Postischemic recovery of left ventricular function during reperfusion was measured relative to the preischemic values. LVDP, left ventricular developed pressure; dP/dtmax, maximal rate of contraction; dP/dtmin, maximal rate of relaxation. Values are means ± SE; n = 5. *P < 0.05 vs. no IPC for the entire curve.
Fig. 2.
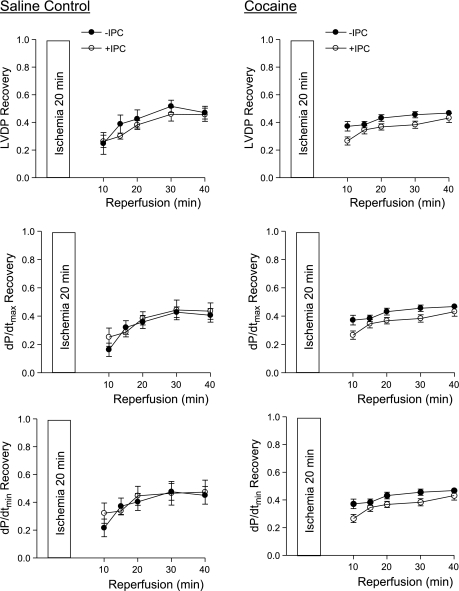
Effect of IPC on postischemic recovery of left ventricular function in female offspring. Hearts were isolated from 3-mo-old female offspring of prenatal saline (left) or cocaine (right) treatments and were treated without or with IPC followed by ischemia-reperfusion (20/40 min). Postischemic recovery of left ventricular function during reperfusion was measured relative to the preischemic values. Values are means ± SE; n = 5. *P < 0.05 vs. no IPC for the entire curve.
Fig. 3.
Effect of IPC on left ventricle end-diastolic pressure (LVEDP) and infarct size after ischemia in male and female offspring. Hearts were isolated from 3-mo-old male (left) and female (right) offspring of prenatal saline or cocaine treatments and were treated without or with IPC, followed by ischemia-reperfusion (20/40 min). LVEDP was measured during reperfusion. At the end of reperfusion, infarct size of the left ventricle was measured as described in materials and methods. A and D: LVEDP in hearts of saline control animals. *P < 0.05 vs. no IPC for the entire curve. B and E: LVEDP in hearts of cocaine-treated animals; *P < 0.05 vs. no IPC for the entire curve. C and F: infarct size in hearts of both saline control and cocaine-treated animals. aP < 0.05 vs. no IPC; bP < 0.05 vs. saline control. Values are means ± SE; n = 5.
IPC in hearts of cocaine-treated animals.
In contrast to the findings in the hearts of saline control animals, IPC failed to produce cardioprotection and did not improve postischemia recovery of LVDP, dP/dtmax, and dP/dtmin during reperfusion after prolonged ischemia in the heart of male offspring with prenatal cocaine treatment (Fig. 1, right). Additionally, the IPC-mediated reduction of LVEDP and infarct size of the left ventricle after prolonged ischemia was abolished in the hearts of cocaine-treated male offspring (Fig. 3, B and C). Unlike that in the male offspring, the IPC-mediated cardioprotection was fully preserved in the female offspring with prenatal cocaine treatment (Fig. 2, right, Fig. 3, E and F). Compared with the saline control females, the cocaine-treated females showed significantly greater IPC-induced improvement of postischemia recovery of LVDP (77 ± 2 vs. 56 ± 4%, P < 0.05) and dP/dtmax (74 ± 1 vs. 59 ± 2%, P < 0.05) in the early phase of recovery at 10 min of reperfusion. Additionally, LVEDP at 10 min of reperfusion was significantly lower in the heart of cocaine-treated females than that of saline control females in the presence of IPC (27.6 ± 3.8 vs. 39.4 ± 3.3 mmHg, P < 0.05). These differences were not seen at the end of reperfusion at 40 min.
Protein and mRNA abundance of PKCɛ and PKCδ in the heart.
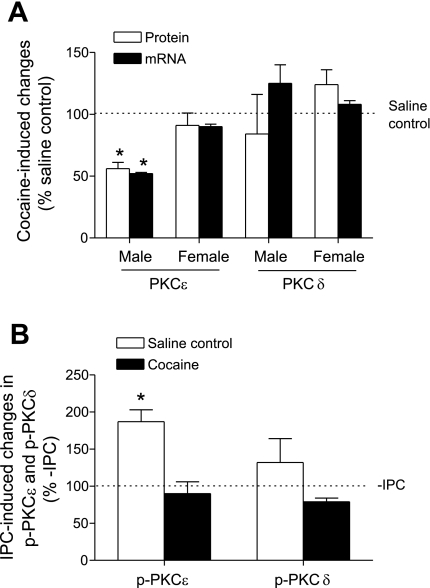
To determine whether prenatal cocaine exposure alters PKCɛ and PKCδ expression in adult hearts, protein and mRNA levels of PKCɛ and PKCɛ in the left ventricle were measured by Western blot analyses and quantitative real-time RT-PCR, respectively. As shown in Fig. 4A, prenatal cocaine exposure resulted in significant decreases in both mRNA and protein abundance of PKCɛ in the left ventricle of male, but not female, offspring. Unlike PKCɛ, there was no significant difference in PKCδ mRNA and protein abundance in the left ventricle of adult offspring between saline control and cocaine-treated animals, in either males or females (Fig. 4A).
Fig. 4.
Effect of prenatal cocaine on PKCδ expression and IPC-induced PKC activation in the heart of adult offspring. A: left ventricles were obtained from 3-mo-old male and female offspring of prenatal saline and cocaine treatments. PKCɛ and PKCδ mRNA and protein abundance were determined with qPCR and Western blot analyses, respectively. Values are means ± SE; n = 5. B: hearts were isolated from 3-mo-old male offspring of prenatal saline and cocaine treatments and were treated without or with IPC. Protein abundance of phospho-PKCɛ (p-PKCɛ) and phospho-PKCδ (p-PKCδ) in the left ventricle was determined with Western blot analysis and was normalized to α-sacromeric actin. Values are means ± SE; n = 5. *P < 0.05 vs. no IPC.
IPC activates PKCɛ.
To determine a role of PKCɛ in the cocaine-mediated loss of IPC-induced protection in the heart of male offspring, phospho-PKCɛSer729 levels were determined in the left ventricle in the absence or presence of IPC. Phosphorylation plays a key role in converting nascent PKC isozymes into the mature forms during the process of PKC activation, and the active form of phospho-PKCɛSer729 has been identified in cardiomyocytes (27, 33). IPC in the heart of saline control animals resulted in a significant increase in phospho-PKCɛSer729 levels (Fig. 4B), suggesting a role for PKCɛ activation in the IPC-mediated cardioprotection in the control hearts. In contrast, IPC had no significant effect on phospho-PKCɛSer729 levels in the heart of male offspring that had been exposed to cocaine before birth (Fig. 4B), consistent with the lack of IPC-induced protection in the heart of cocaine-treated male offspring (Fig. 1, right; Fig. 3, B and C). Unlike PKCɛ, IPC had no significant effect on phospho-PKCδ levels in the left ventricle of either saline control or cocaine-treated offspring (Fig. 4B).
Effect of PKCɛ-TIP on IPC.
To further demonstrate the cause-and-effect relation between PKCɛ activation and the IPC-mediated protection in the heart, we determined the effect of selective inhibition of PKCɛ on the IPC-induced cardioprotection using a selective PKCɛ-TIP. Hearts were treated with 5 μM PKCɛ-TIP before and during IPC. The dosage was chosen based on a previous study that showed 5 μM of PKCɛ-TIP inhibited PKCɛ translocation in the heart of adult male rat in the Langendorff preparation (28). Treatment of the heart with PKCɛ-TIP did not significantly alter the baseline left ventricular function before ischemia (Table 1). In both male and female control animals, comparison of the effects of IPC in the absence or presence of PKCɛ-TIP showed that the inhibition of PKCɛ completely reversed the IPC-mediated protection in postischemic functional recovery of the left ventricle (data in Figs. 5 and 6, left, compared with those in Figs. 1 and 2, left). This reversal in the IPC-mediated protection was also demonstrated in the cocaine-treated female offspring (data in Fig. 6, right, compared with those in the Fig. 2, right). The inhibition of PKCɛ in the cocaine-treated male offspring had no significant impact on the postischemic functional recovery, as IPC remained ineffective at providing protection from ischemic injury (data in Fig. 5, right, compared with those in Fig. 1, right). LVEDP and infarct size had the same findings as functional recovery in the PKCɛ-TIP experiments. In both the control and cocaine group of male offspring, there was no significant difference from IPC in postischemic LVEDP or infarct size in the presence of PKCɛ-TIP (Fig. 7, left). The addition of PKCɛ-TIP to female cocaine-treated offspring also abolished any IPC-induced protection in LVEDP or infarct size (Fig. 7, E and F). In the female control offspring, IPC appeared to have a minor protective effect in the presence of PKCɛ-TIP in LVEDP that did not reach statistical significance (Fig. 7D, P = 0.10).
Fig. 5.
Effect of PKCɛ-translocation inhibitor peptide (TIP) on IPC-induced protection in the cardiac function in male offspring. Hearts were isolated from 3-mo-old control (left) and cocaine-treated (right) male offspring and were treated with 5 μM PKCɛ-TIP before and during IPC, followed by ischemia-reperfusion (20/40 min). Postischemic recovery of left ventricular function during reperfusion was measured relative to the preischemic values. Values are means ± SE; n = 5.
Fig. 6.
Effect of PKCɛ-TIP on IPC-induced protection in the cardiac function in female offspring. Hearts were isolated from 3-mo-old control (left) and cocaine-treated (right) female offspring and were treated with 5 μM PKCɛ-TIP before and during IPC, followed by ischemia-reperfusion (20/40 min). Postischemic recovery of left ventricular function during reperfusion was measured relative to the preischemic values. Values are means ± SE; n = 5.
Fig. 7.
Effect of PKCɛ-TIP on IPC-induced protection in myocardial infarction in male and female offspring. Hearts were isolated from 3-mo-old male (left) and female (right) offspring of prenatal saline or cocaine treatments and were treated with 5 μM PKCɛ-TIP before and during IPC, followed by ischemia-reperfusion (20/40 min). LVEDP was measured during reperfusion. At the end of reperfusion, infarct size of the left ventricle was measured as described in materials and methods. A and D: LVEDP in hearts of saline control animals. B and E: LVEDP in hearts of cocaine-treated animals. C and F: infarct size in hearts of both saline control and cocaine-treated animals. Values are means ± SE; n = 5.
IPC and coronary flow.
To determine whether changes in coronary flow play a role in the IPC-mediated protection in the heart, pulmonary artery effluent was measured as an index of coronary flow. There was no significant difference in coronary flow at the baseline levels between the hearts of saline control and cocaine-treated animals, as well as hearts treated with PKCɛ-TIP (Table 1). Coronary flow rate decreased after ischemia, but IPC had no significant effects on the rate of postischemic coronary flow in any group of hearts studied (data not shown).
DISCUSSION
Multiple studies have suggested that PKCɛ plays a key role in the IPC pathway. It has been demonstrated that IPC induces PKCɛ activation (29), and several cellular models have shown that the inhibition of PKCɛ activation blocks the protective effect of IPC (23). The role of PKCɛ in the IPC protection is thought to be activation of the mitochondrial ATP-sensitive K+ channels resulting in a protection of cardiomyocytes from apoptosis (13). Additionally, PKCɛ-dependent activation of the sphingosine kinase and production of endogenous sphingosine-1-phosphate also play a role in the IPC protection in the heart (14). The present finding of the IPC-mediated protection in the lack of a significant effect on postischemic coronary flow supports the theory that the target mechanisms reside in cardiomyocytes rather than the coronary vasculature.
The present study demonstrated that selective inhibition of PKCɛ with a PKCɛ-TIP abolished the IPC-mediated protection of left ventricular recovery in both male and female offspring and thus provided the cause-and-effect evidence of the functional importance of PKCɛ in the IPC-mediated cardioprotection in the intact heart. PKCɛ-TIP inhibits activation of PKCɛ at the intracellular concentration of 3–10 nM and has been widely used to study the role of PKCɛ in cardiac function (26, 31, 41). A previous study has shown that 5 μM of PKCɛ-TIP inhibits PKCɛ translocation in the heart of adult male rats in a Langendorff preparation (28). Consistent with the previous studies (4, 28), the present study showed that PKCɛ-TIP had no significant effects on left ventricular function at the baseline levels. This is in agreement with the findings obtained in a PKCɛ KO mouse model, demonstrating that PKCɛ expression is not required for normal cardiac function under physiological conditions, but PKCɛ activation is necessary and sufficient for acute cardioprotection caused by IPC (10). Similar findings were obtained in intact rat hearts showing that inhibition of PKCɛ blocked ouabain-triggered and isoflurane-induced preconditioning (24, 28).
The present finding that IPC-mediated cardioprotection was significantly greater in female hearts than male hearts is intriguing and suggests a sex dimorphism in the IPC pathway. In contrast to the present finding, previous studies in mice showed that two cycles of 2-min ischemic episodes followed by 5-min reperfusion periods preconditioned male, but not female, hearts (34). Given that cardiomyocytes from female hearts have been shown to be more resistant to ischemia and reperfusion injury compared with male cardiomyocytes (4, 32), it is possible that female hearts may require a higher injury threshold for preconditioning to occur. Indeed, a temporal threshold for preconditioning the myocardium has been demonstrated (1, 37). In the present study, we have demonstrated that two cycles of 5-min ischemic episodes followed by a 20-min reperfusion period produced a marked protection in female hearts. Consistent with this finding, myocardial protection from IPC has been demonstrated in canine females (20). While the mechanisms for the increased IPC-mediated protection in female hearts are not clear at present, it has been demonstrated in rats that female hearts have higher levels of the active form of phospho-PKCɛ and that reperfusion increases phospho-PKCɛ more in female than male hearts (3).
As our previous studies have shown that the hearts of male offspring born to cocaine-treated dams are more sensitive to ischemia (2), we hypothesized that our preconditioning protocol would have an effect equal to or greater than the protection in control males. To our surprise, the protective effect of IPC was entirely abolished. IPC did not rescue the heart from the increased sensitivity to ischemia or provide any increase in functional recovery or protect from infarction. Our data suggest that fetal cocaine exposure not only decreases the expression of PKCɛ, but also results in a defect in the activation of PKCɛ. This was verified by protein analysis that showed that IPC significantly increased the level of phospho-PKCɛ in control hearts, but resulted in no increase in the hearts taken from cocaine exposed animals.
The sex dichotomy has been demonstrated further in fetal programming of adult hearts in response to IPC. Prenatal cocaine treatment abolished the IPC-mediated protection in the heart of adult male offspring. Whereas inhibition of PKCɛ with PKCɛ-TIP mimiced the effect of cocaine and blocked the IPC-mediated protection in the control males, PKCɛ-TIP had no further effect on the postischemic functional recovery, as IPC remained ineffective in the cocaine-treated males. In contrast, the IPC-mediated protection of the heart in the cocaine-treated females was fully preserved. Additional studies showed that PKCɛ remained functional in the IPC-mediated protection in the cocaine-treated female offspring, as it was demonstrated that, in both control and cocaine-treated females, the inhibition of PKCɛ with PKCɛ-TIP abolished equally well the IPC-mediated protection of left ventricular recovery. Taken together, these findings indicate that the sex difference in IPC-induced cardioprotection after fetal cocaine exposure is due to a cocaine-mediated downregulation in PKCɛ functioning that is specific to males rather than a sex-specific difference in the IPC pathway. Human epidemiological studies have demonstrated a link between adverse intrauterine environments and an increased risk of ischemic heart disease in adulthood (5). Recent animal studies demonstrated that fetal exposure to hypoxia (22, 38), glucocorticoids (8), cocaine (2), and nicotine (19) caused an epigenetic programming in the heart, resulting in an increased heart susceptibility to ischemia and reperfusion injury in adult offspring. The sex dichotomy in fetal programming of adult disease has been demonstrated in several animal models. Although the results are conflicting, it has been shown that female offspring are generally less sensitive in manifestation of cardiovascular disease caused by adverse prenatal stimuli (7). Sex differences in adult offspring, with males often being more susceptible than females on a diversity of measures to the effects of prenatal cocaine exposure in the rat, have been demonstrated consistently (2, 11, 25, 35, 36). Whereas the stage of the estrous cycle of the female rats at the time of experiments was not determined, future studies are needed to investigate further the mechanisms of the sex dichotomy observed in the present study and to test for a role of sex steroid hormones in male and female gonadectomized rats.
Consistent with the finding of a loss of IPC in male but not in female hearts, prenatal cocaine exposure caused a sex-dependent decrease in PKCɛ mRNA and protein abundance in the heart of male offspring. Our recent study demonstrated a sex-dependent epigenetic mechanism of DNA methylation in programming of cardiac PKCɛ gene repression, linking fetal cocaine exposure and pathophysiological consequences in the heart of adult male offspring (39). Previous studies in several animal models of fetal stress demonstrated a correlation between a decrease in PKCɛ expression and increased heart vulnerability to ischemia injury in adult offspring (2, 19, 21). These findings suggest a common mechanism of PKCɛ in cardiac programming in response to intrauterine adverse stimuli. Unlike fetal cocaine, PKCɛ protein expression was decreased in the heart of both male and female adult offspring after prenatal nicotine treatment, which corresponded to the decreased postischemic recovery of left ventricular function in both male and female hearts (19). These findings suggest a stimuli specificity of sex-dependent programming of PKCɛ gene expression pattern in the heart and reinforce a key role for PKCɛ in programming of heart vulnerability to ischemia and reperfusion injury in adult offspring. In our laboratory's previous study, we demonstrated that prenatal cocaine exposure significantly decreased not only PKCɛ protein abundance by 59% but also phospho-PKCɛ levels by 75% in the left ventricle of male offspring (2). The finding of a greater decrease in phospho-PKCɛ levels than PKCɛ protein abundance suggests that, in addition to programming of PKCɛ gene repression, prenatal cocaine exposure also results in inhibition of PKCɛ activation in the heart. In the present study, the physiological modulation of PKCɛ activation by IPC was examined in the heart. Whereas the heart of saline control animals showed a significant increase in phospho-PKCɛ by IPC, in the heart of cocaine-treated animals, the IPC-induced phosphorylation of PKCɛ was abolished. This is consistent with the lack of IPC-mediated cardioprotection in the heart of cocaine-treated animals, providing clear evidence that inhibition of PKCɛ activation plays a key role in the cocaine-mediated loss of IPC-induced protection in the heart of male offspring.
Unlike PKCɛ, the role of PKCδ in ischemia and reperfusion injury is less clear and is somewhat controversial. Inhibition of PKCδ during reperfusion has been shown to decrease reperfusion-induced injury (26). Other studies demonstrated the cardioprotective effects of PKCδ (6, 15, 40). In contrast to the findings of PKCɛ, neither mRNA nor protein abundance of PKCδ in the heart of both male and female offspring were altered after prenatal cocaine exposure. Additionally, the lack of increase in phospho-PKCδ with IPC seen in the present study suggests that the activation of PKCδ may not be involved in the IPC-mediated cardioprotection. This is in agreement with the finding in human myocardium showing that PKCɛ but not PKCδ is essential for the IPC-induced myocardial protection (12).
Our investigation has demonstrated that prenatal cocaine exposure causes a loss of IPC-mediated cardioprotection in adult male offspring in a sex-dependent manner and provided evidence of a relationship between cocaine-induced inhibition of PKCɛ and increased myocardial sensitivity to ischemia. In addition to demonstrating that blocking PKCɛ activation results in a loss of IPC-induced cardioprotection, we demonstrated that IPC failed to activate PKCɛ in the hearts of cocaine-exposed males. Together, our findings strongly suggest a causal relationship between alterations to PKCɛ and increased myocardial sensitivity to ischemia in the adult animal with prenatal cocaine exposure. These findings could have significant clinical importance, a contention supported by the observations that preinfarction angina and exercise protect the heart from ischemic injury via the IPC pathway, as well as other evidence that IPC is an important physiological protective mechanism (9, 16, 18). Given that cocaine abuse in pregnant women is a significant problem, especially in urban areas, the present finding suggests that intrauterine cocaine exposure may result in significant long-term changes to the myocardiocytes and be a risk factor for morbidity and mortality secondary to myocardial ischemia in adult male offspring. As is often the case with novel findings, the present study may raise more questions than it answers. For instance, whether and to what extent does the impaired cardioprotective signaling pass to the next generation? Is there a critical window in the fetal development that can be isolated during which exposure to the drug can program the heart with pathophysiological consequences in the offspring? Furthermore, does the treatment before conception have a similar effect on the offspring? Undoubtedly, these questions warrant continuing investigations.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL82779 (L. Zhang) and HL83966 (L. Zhang) and by Loma Linda University School of Medicine.
REFERENCES
- 1.Awan MM, Taunyane C, Aitchison KA, Yellon DM, Opie LH. Normothermic transfer times up to 3 min will not precondition the isolated rat heart. J Mol Cell Cardiol 31: 503–511, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia-reperfusion injury in adult male but not female rats. J Physiol 565: 149–158, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts. J Pharmacol Exp Ther 315: 1125–1135, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia-reperfusion injury in 1-mo-old rat. Br J Pharmacol 144: 900–907, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker D, Gluckman P, Godfrey K, Harding J, Owens J, Robinson J. Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bouwman R, Salic K, Padding F, Eringa E, van eek-Harmsen B, Matsuda T, Baba A, Musters R, de Lange J, Boer C. Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation 114: I226–I232, 2006. [DOI] [PubMed] [Google Scholar]
- 7.do Carmo Pinho Franco M, Nigro D, Fortes ZB, Tostes RCA, Carvalho MHC, Lucas SRR, Gomes GN, Coimbra TM, Gil FZ. Intrauterine undernutrition–renal and vascular origin of hypertension. Cardiovasc Res 60: 228–234, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dodic M, Samuel C, Moritz K, Wintour EM, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res 89: 623–629, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Domenech RJ Preconditioning: a new concept about the benefit of exercise. Circulation 113: e1–e3, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase Cɛ. J Biol Chem 279: 3596–3604, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Grimm V, Frieder B. Differential vulnerability of male and female rats to the timing of various perinatal insults. Int J Neurosci 27: 155–164, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Hassouna A, Matata BM, Galiñanes M. PKC-ɛ is upstream and PKC-α is downstream of mitoKATP channels in the signal transduction pathway of ischemic preconditioning of human myocardium. Am J Physiol Cell Physiol 287: C1418–C1425, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Jaburek M, Costa ADT, Burton JR, Costa CL, Garlid KD. Mitochondrial PKCɛ and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res 99: 878–883, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl E, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCe knockout mouse hearts. Am J Physiol Heart Circ Physiol 282: H1970–H1977, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura S, Yoshida KI, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-δ and -ɛ, which mediate functional protection in isolated rat heart. Am J Physiol Heart Circ Physiol 275: H2266–H2271, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson CM, Poole WK, Cannon CP, McCabe CH, Braunwald E. Previous angina alters in-hospital outcome in TIMI 4: a clinical correlate to preconditioning? Circulation 91: 37–45, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Korge P, Honda HM, Weiss JN. Protection of cardiac mitochondria by diazoxide and protein kinase C: implications for ischemic preconditioning. Proc Natl Acad Sci USA 99: 3312–3317, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambiase PD, Edwards RJ, Cusack MR, Bucknall CA, Redwood SR, Marber MS. Exercise-induced ischemia initiates the second window of protection in humans independent of collateral recruitment. J Am Coll Cardiol 41: 1174–1182, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther 324: 331–341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. Cardioprotective effects of 17 beta-estradiol produced by activation of mitochondrial ATP-sensitive K(+) channels in canine hearts. J Mol Cell Cardiol 32: 1147–1158, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol 286: H1712–H1719, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-ξ is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol 31: 1937–1948, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig L, Weihrauch D, Kersten J, Pagel P, Warltier D. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology 100: 532–539, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Molina V, Wagner J, Spear L. The behavioral response to stress is altered in adult rats exposed prenatally to cocaine. Physiol Behav 55: 941–945, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys 420: 246–254, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Parekh D, Ziegler W, Parker P. Multiple pathways control protein kinase C phosphorylation. EMBO J 19: 496–503, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, Dos-Santosd P, Xie Z. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res 73: 488–496, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms ɛ and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res 81: 404–414, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Pozner CN, Levine M, Zane R. The cardiovascular effects of cocaine. J Emerg Med 29: 173–178, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Przyklenk K, Li G, Simkhovich BZ, Kloner RA. Mechanisms of myocardial ischemic preconditioning are age related: PKC-ɛ does not play a requisite role in old rabbits. J Appl Physiol 95: 2563–2569, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Ranki HJ, Budas GR, Crawford RM. Gender-specific difference in cardiac ATP-sensitive K+ channels. J Am Coll Cardiol 38: 906–915, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Rybin VO, Sabri A, Short J, Braz JC. Cross-regulation of novel protein kinase C (PKC) isoform function in cardiomyocytes. Role of PKC epsilon in activation loop phosphorylations and PKC delta in hydrophobic motif phosphorylations. J Biol Chem 278: 14555–14564, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischaemic preconditioning and ischaemia-reperfusion injury in mice. Acta Physiol Scand 177: 459–466, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Spear L, Campbell J, Snyder K, Silveri M, Katovic N. Animal behavior models. Increased sensitivity to stressors and other environmental experiences after prenatal cocaine exposure. Ann N Y Acad Sci 846: 76–88, 1998. [PubMed] [Google Scholar]
- 36.Spear L, Silveri M, Casale M, Katovic N, Campbell J, Douglas L. Cocaine and development: a retrospective perspective. Neurotoxicol Teratol 24: 321–327, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchida A, Liu GS, Mullane K, Downey JM. Acadesine lowers temporal threshold for the myocardial infarct size limiting effect of preconditioning. Cardiovasc Res 27: 116–120, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodelling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Meyer K, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod 80: 440–448, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Renner O, Wightman L, Sugden P, Stewart L, Miller A, Latchman D, Marber MS. The expression of constitutively active isotypes of protein kinase C to investigate preconditioning. J Biol Chem 273: 23072–23079, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol 283: H165–H174, 2002. [DOI] [PubMed] [Google Scholar]