Abstract
Mechanical stretch and oxidative stress have been shown to prolong action potential duration (APD) and produce early afterdepolarizations (EADs). Here, we developed a simulation model to study the role of stretch-activated channel (SAC) currents in triggering EADs in ventricular myocytes under oxidative stress. We adapted our coupling clamp circuit so that a model ionic current representing the actual SAC current was injected into ventricular myocytes and added as a real-time current. This current was calculated as ISAC = GSAC * (Vm − ESAC), where GSAC is the stretch-activated conductance, Vm is the membrane potential, and ESAC is the reversal potential. In rat ventricular myocytes, application of GSAC did not produce sustained automaticity or EADs, although turn-on of GSAC did produce some transient automaticity at high levels of GSAC. Exposure of myocytes to 100 μM H2O2 induced significant APD prolongation and increase in intracellular Ca2+ load and transient, but no EAD or sustained automaticity was generated in the absence of GSAC. However, the combination of GSAC and H2O2 consistently produced EADs at lower levels of GSAC (2.6 ± 0.4 nS, n = 14, P < 0.05). Pacing myocytes at a faster rate further prolonged APD and promoted the development of EADs. SAC activation plays an important role in facilitating the development of EADs in ventricular myocytes under acute oxidative stress. This mechanism may contribute to the increased propensity to lethal ventricular arrhythmias seen in cardiomyopathies, where the myocardium stretch and oxidative stress generally coexist.
Keywords: arrhythmia mechanisms, action potential, patch clamp
previous studies have shown that myocardial stretch, a process termed “mechanoelectric feedback,” can induce myocyte depolarization and abnormal impulses by the stretch-activated channel (SAC) currents (7, 21). A subset of SACs has been reported as nonselective cation channels (48), which allow Na+, K+, Cs+, and Ca2+ to permeate (36). A linear current-voltage relationship has been described for SACs with reversal potentials measured in myocytes from −6 to −15 mV (17, 33, 48). In a review by Kohl et al. (24), they note that models incorporating SACs as pure charge carriers and models that include movement of ions through the channels both reproduced experimental phenomena, such as diastolic depolarization, positive chronotropic responses of pacemakers, and action potential (AP) generation due to stretch (25).
It has been suggested that oxidative stress is a mechanism for Ca2+ overload and the consequent electrical changes in cardiomyocytes (5). On the other hand, Ca2+ overload increases oxidative stress by altering mitochondrial function, which leads to increased production of oxygen radicals (9). Oxidative stress alters the function of several elements in the excitation-contraction coupling pathways (including L-type calcium channel, the ryanodine receptor, and the Ca2+-ATPase in the sarcoplasmic reticulum) and the electrophysiology of the ventricle (5). Exposure of guinea pig ventricular free wall to H2O2 induced extra ventricular beats due to early (EADs) and delayed afterdepolarizations (8).
Because both mechanical stretch and oxidative stress cause Ca2+ overload, we hypothesize that abnormal stretch of myocardium may proceed in concert with acute oxidative stress in facilitating the induction of EADs. We have incorporated a model system similar to that used by Riemer et al. (31) on cell models with a major difference that we incorporate a real-time modulation of isolated rat ventricular myocytes with a time- and voltage-varying current produced by a computation of current that would have been produced by a SAC conductance (GSAC). The advantage of our system is that we are using real myocytes and thus can expose the cells to an agent that produces acute oxidative stress (H2O2) and determine the cooperative effects of these two modulations.
METHODS
Cell isolation.
Single ventricular myocytes were isolated from Sprague-Dawley rats of either sex weighing 165–250 g, as our laboratory described previously (44). The myocytes were kept in storage solution and were used within 6 h after isolation.
Perforated patch-clamp recording for APs.
Myocytes were placed in a chamber on an inverted microscope and continuously perfused with Tyrode solution at 2 ml/min and 36 ± 0.5°C. Recording pipettes were pulled from borosilicate glass with resistances of 1.5–3 MΩ when filled with internal solution. High-resistance seals were formed by applying light suction, and we used perforated-patch technique with amphotericin B in the pipette to allow formation of a low series resistance without intracellular dialysis for AP recordings. No corrections were made for liquid junction potential, other than zeroing the potential before touching cell surface. APs were recorded using the whole cell patch clamp with Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) in current-clamp mode. Series resistance was carefully compensated after recording of the membrane potential was established. APs were initiated by current pulses of 2-ms duration and amplitude of 10–15% suprathreshold.
Solutions.
Normal Tyrode is as follows (mM): 148.8 NaCl, 4 KCl, 1.8 CaCl2, 0.53 MgCl2, 0.33 NaH2PO4, 5 HEPES, and 5 glucose, pH 7.4. The CaCl2 is omitted for Ca2+-free Tyrode. Storage solution is as follows (mM): 120 potassium-glutamate, 20 taurine, 5 MgCl2, 1 EGTA, 10 dextrose, and 10 HEPES, with pH adjusted to 7.4 using KOH. The pipette solution is as follows (mM): 135 KCl, 5 Na2CrPh, 5 MgATP, and 10 HEPES, pH 7.2. Amphotericin B 150 μg/ml was added to the internal solution just before use, and the solution was renewed every 2 h.
Incorporating a SAC model into a real isolated myocyte.
We adapted our coupling clamp circuit (40), so that a model ionic current that represents SACs is injected into a isolated myocyte and added as a real-time current. This SAC model was described in detail in our recent work (39). Several studies have shown that physical stretch of isolated ventricular myocytes produced stretch-activated currents with a linear current/voltage relationship and reversal potentials between −6 and −11 mV (6, 48, 49). Thus, for the majority of experiments in this study, we set the reversal potential of SAC (ESAC) = −10 mV and GSAC to a constant value for each recording, but the value of GSAC was varied to investigate the critical GSAC required to induce EADs. GSAC was applied as an “on or off” function. To test the effects of ESAC on SAC-induced EADs, ESAC of −20 and 0 mV were also employed in some experiments, as indicated.
Intracellular Ca2+ concentration transient measurements.
Myocytes were first loaded with 2 μM fura 2-AM for 10 min at room temperature, and fluorescence measurements are recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). After loading, the cells were washed and resuspended in normal Tyrode solution. These myocytes were then placed in the cell chamber, stimulated at 1 and 3 Hz with 4-ms duration, and imaged through a Fluor ×40 oil objective. Cells were exposed to light emitted by a 75-W Xenon lamp and passed though either a 340- or 380-nm wavelength filter. The emitted fluorescence is detected at 510 nm. To take into account any inference effects of background fluorescence, the background fluorescence for each myocyte was determined by moving the myocyte out of the view and recording the fluorescence from the bath solution alone.
Statistical analysis.
Data are reported as means ± SE. Comparison of AP properties before and after application of H2O2 and at different pacing rates was done with a one-way analysis of variance for repeated measures, followed by Student-Newman-Keuls post hoc test to determine significant differences (P < 0.05). If the criteria for parametric analysis were not met, a nonparametric analysis using the Friedman repeated-measures analysis of variance on ranks was used, followed by Dunn's post hoc test to determine significant differences (P < 0.05). Comparison of the critical value of GSAC required to induce abnormal activity before and after application of H2O2 was performed with a paired t-test.
Use of vertebrate animals.
All experiments on live vertebrates were performed in accordance with protocols approved by the institution's Animal Care and Use Committee.
Statement of responsibility.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the paper as written.
RESULTS
Response of rat ventricular cells to SAC.
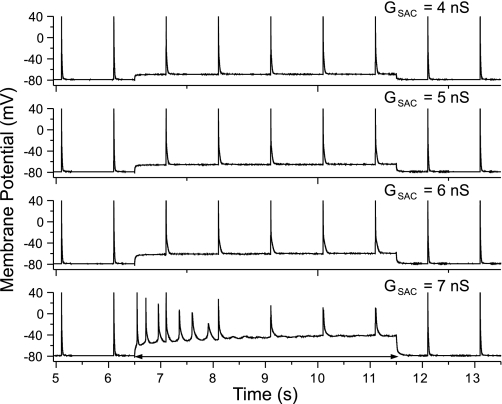
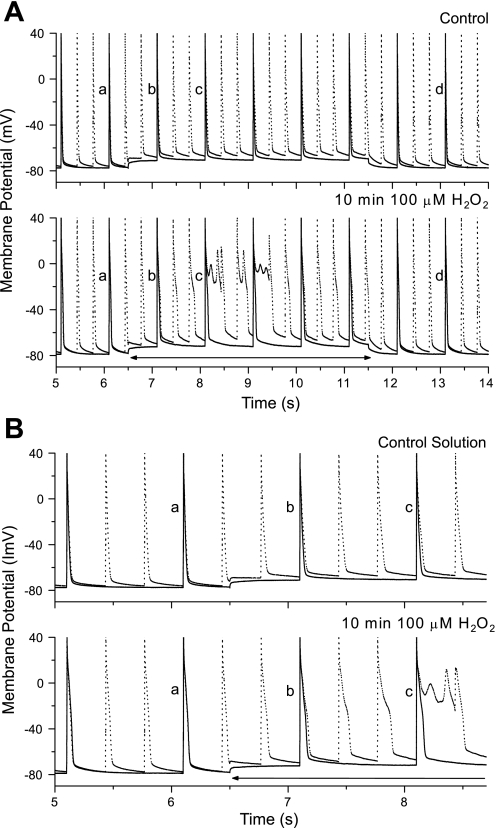
Using the perforated patch, we applied varying levels of GSAC to rat ventricular myocytes, either in control solution or in the presence of 100 μM H2O2. The representative results were shown in Fig. 1. We paced the myocyte at 1 Hz and applied the GSAC during the time period from 6.5 to 11.5 s. The responses of myocyte to GSAC levels (ESAC was set at −10 mV) of 4, 5, 6, or 7 nS (top to bottom) are shown. The depolarization of the membrane potential increases as GSAC increases. For GSAC = 7 nS, there was some induced automaticities. However, these automaticities were not sustained, owing to the inexcitable property of the myocyte at large depolarization (sodium channel is inactivated), and the cell was also not excitable to the direct stimulations (Fig. 1, bottom). None of the cells tested showed induced sustained automaticity or EADs in response to any level of GSAC in the control solution.
Fig. 1.
Recordings from an isolated rat ventricular myocyte at 1-Hz stimulation with the stretch-activated channel conductance (GSAC) applied from 6.5 through 11.5 s at various levels. Early afterdepolarization (EAD) was not induced at any levels of GSAC. However, a transient activity was observed at a high GSAC (7 nS), but failed to sustain, and the cell became refractory to direct stimulation.
Effect of oxidative stress on cellular response to SACs.
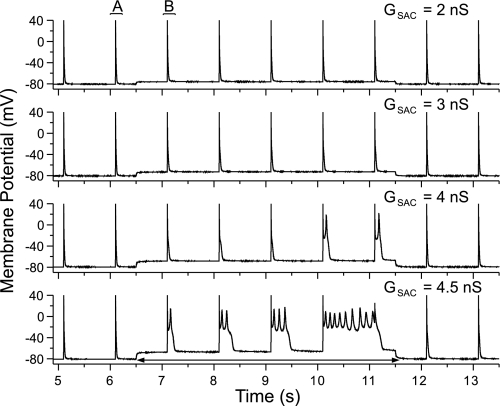
Figure 2 shows the results of the application of GSAC levels of 2, 3, 4, or 4.5 nS (top to bottom) after a 10-min application of 100 μM H2O2 for the same cell as Fig. 1. Application of GSAC under these conditions did not produce sustained automaticity, but EADs were consistently produced with more EADs and longer EAD duration at the higher GSAC level. The minimal GSAC level for inducing transient automaticity (as shown in Fig. 1) was 5.6 ± 0.3 nS in the control solution, and the GSAC level required for producing EADs in the same cells after 10-min exposure to H2O2 was 2.6 ± 0.4 nS (n = 14, P < 0.05). When normalized for cell capacitance, the critical value of GSAC decreased from 92.6 ± 12.6 pS/pF in control to 43.32 ± 10.9 pS/pF in H2O2 (n = 14, P < 0.05). None of EADs has been triggered in myocytes by GSAC in control solution, indicating that application of H2O2 sensitized the cells to EAD development in response to GSAC. However, EADs were observed in some myocytes exposed to high levels of H2O2 (>1 mM), which led to significant AP prolongation (data not shown), consistent with previous reports (42). H2O2 concentrations lower than 100 μM produced little or no effect on APD. Thus we take the concentration of 100 μM H2O2 as the desired concentration. This concentration falls into the range detected in human blood plasma (15).
Fig. 2.
Recordings from the same cell in Fig. 1 after 10-min exposure to 100 μM H2O2 with GSAC levels of 2, 3, 4, and 4.5 nS. EADs occurred at GSAC levels of 4 and 4.5 nS with increasing EAD duration during SAC activation. No EAD was produced before or after the GSAC application.
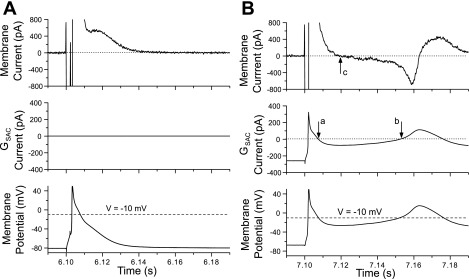
To investigate the interaction of the GSAC current with the repolarization process of the cells, we replotted portions of Fig. 2, labeled A and B, for Fig. 3. For each part of this figure, we have used the data from Fig. 2, bottom (GSAC = 4.5 nS) and replotted the APs just before (part A) and after GSAC application (part B), along with the membrane current and the GSAC current. The membrane current (top) was computed by multiplying cell capacitance by the negative of the computed time derivative of voltage of the measured membrane potential vs. time. Note that this definition of membrane current generates values that include the GSAC current (middle). For Fig. 3A, GSAC was turned off; so there was no GSAC current. The membrane current showed a decreasing outward current during the repolarization process. For Fig. 3B, we illustrated these parameters for the first AP after GSAC was turned on. Before stimulation of the cell, the membrane current (top) was zero, even though the GSAC current was a steady −260 pA. This is because the membrane current has “adjusted” to the presence of the GSAC current (which is inward) by the outward current produced by the steady depolarization of the cell. After activation of the cell, a time-dependent change in the GSAC current was generated with the time course of changes of cell membrane potential. We have included a dashed line at −10 mV in the bottom panels of Fig. 3, A and B, indicating the voltage level of the ESAC. In Fig. 3B, as the membrane potential went positive to ESAC, the GSAC current became transiently positive and then, as repolarization proceeded, it became negative during the time marked by arrows a and b of the middle panel, owing to the membrane potential becoming negative to ESAC. This inward GSAC current makes the cell repolarize more slowly, and the membrane current actually became net negative at the time marked by arrow c in the top panel. This net negative membrane current produced interruption of repolarization process in the cell, and the cell then began to depolarize again, which led to an EAD in this cell. The GSAC current became outward during the positive phase of the EAD, which tends to repolarize the cell.
Fig. 3.
Demonstration of the time course of membrane current (top), GSAC current (middle), and membrane potential (V; bottom) for the action potentials (APs) occurred just before (A) and after (B) SAC (4.5 nS) activation. B: points labeled a, b, and c indicate times points when the current changes polarity.
Measurements of AP parameters in response to SAC and H2O2.
We analyzed the effects of the following three interventions on AP parameters: 1) application of a subthreshold level of GSAC (2 nS); 2) 10 min of exposure of 100 μM H2O2; and 3) 100 μM H2O2 at 10 min with GSAC = 2 nS. These data were summarized in Table 1. The resting membrane potential (RMP) was unchanged by H2O2, but was significantly depolarized by application of GSAC = 2 nS in both control and H2O2 solution. The AP amplitude was decreased by GSAC application (due to the depolarization of RMP and the outward SAC current for the overshoot), but increased by H2O2. The AP durations at 30% (APD30) and 50% repolarization (APD50) were unchanged by GSAC in control solution. The early repolarization in rat ventricular myocytes is very fast, due to large transient outward current (Ito) (29). The GSAC current during early repolarization is outward because the membrane potential is greater than the ESAC (−10 mV) and would tend to facilitate early repolarization. But because there is already a large Ito-induced fast early repolarization, GSAC application has little effect and does not change APD30 or APD50. Whereas during the late repolarization phase, the stretch current is inward (membrane potential is negative to ESAC), which tends to prolong the APD. Thus APD at 90% repolarization (APD90) was significantly increased (by ∼15%) by GSAC.
Table 1.
AP characteristics and response to GSAC and to 100 μM H2O2
| Control Solution | 100 μM H2O2 | |
|---|---|---|
| Critical | ||
| GSAC, nS | 5.6±0.3 | 2.6±0.4* |
| Normalized | ||
| GSAC, pS/pF | 92.6±12.6 | 43.32±10.9* |
| GSAC = 0 nS | GSAC = 2 nS | GSAC = 0 nS | GSAC = 2 nS | |
|---|---|---|---|---|
| RMP, mV | −78.8±0.5 | −69.9±2.2* | −79.3±0.7† | −76.3±2.7*‡ |
| APD30, ms | 4.1±0.7 | 4.0±0.9 | 5.4±0.8*† | 5.8±1.4‡ |
| APD50, ms | 8.7±1.1 | 8.5±1.3 | 11.7±2.1*† | 11.8±0.8*† |
| APD90, ms | 28.9±2.3 | 32.9±2.9* | 37.5±3.4*† | 44.8±4.2*†‡ |
| APA, mV | 107.5.4±2.5 | 96.0±2.5* | 102.7±3.5*† | 99.1±2.6‡ |
Values are means ± SE; n = 14. GSAC, stretch-activated channel conductance; RMP, resting membrane potential; APD30, APD50, and APD90: action potential duration at 30, 50, and 90% repolarization, respectively; APA, action potential amplitude.
P < 0.05 compared with control, GSAC = 0 nS.
P < 0.05 compared with control, GSAC = 2 nS.
P < 0.05 compared with H2O2, GSAC = 0 nS.
The effect of exposure to 100 μM H2O2 on APD was dramatic with significant prolongation of APD30, APD50, and APD90. This increase in APDs may reflect an increase in the L-type calcium current (ICa) and the late sodium current due to exposure to H2O2 (5, 42, 46). When GSAC = 2 nS is applied to cells in conjunction with H2O2, there was a variable effect on the APD compared with control (see Table 1). Since H2O2 increased APD30 compared with control, when the stretch current (which is outward during early membrane potential level) was applied, the outward SAC current accelerated membrane repolarization and thus shortened APD30. The addition of GSAC in the presence of H2O2 did not alter APD50 compared with H2O2 alone. This is likely because the membrane potential level at APD50 is close to the ESAC; the current from SAC is very small, thus making little contribution to the APD50. The application of GSAC in the presence of H2O2 further increases APD90 over the control value (a total 55% increase, compared with 30% increase due to H2O2 alone and a 25% increase due to GSAC alone).
Changing the ESAC model alters the SAC-induced depolarization and the EAD inducibility.
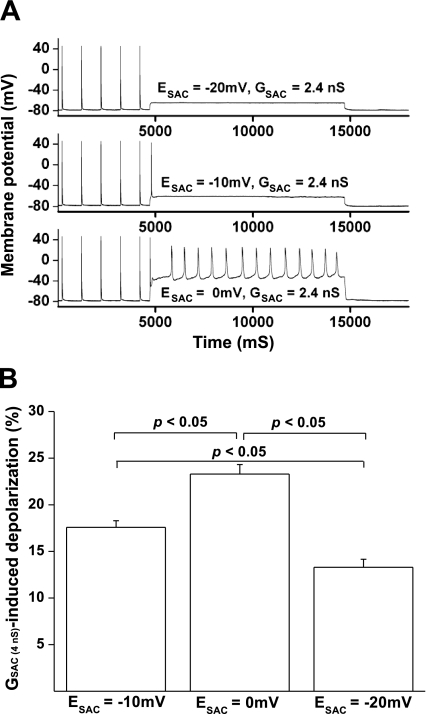
Because the ESAC varies from different species and cardiac tissue types, cell response to the same GSAC may be different. In addition, it has been reported that H2O2 alters intracellular Ca2+ and Na+ concentrations by facilitating ICa and slowing the late sodium current (42, 46). These H2O2-induced changes of intracellular ions may subsequently alter the ESAC. Furthermore, the GSAC for multiple ions may also alter the ESAC with time during stretch. We have tested the SAC effects on EAD induction by varying ESAC from −20 to 0 mV. In the presence of 100 μM H2O2, EADs and automaticity were facilitated by setting the ESAC to 0 mV, but attenuated by setting the ESAC to −20 mV (n = 6). The representative traces were shown in Fig. 4A. For this myocyte, a relatively low value of GSAC (2.4 nS) was applied for 10 s after the end of 1-Hz pacing. This GSAC caused the largest depolarization at 0-mV ESAC and the least depolarization at −20-mV ESAC. This low value of GSAC did not induce automaticity at −20- or −10-mV ESAC. However, sustained automaticity was successfully induced at 0-mV ESAC. The effects of ESAC on EAD induction are likely a result of altered cell depolarization level. Indeed, in a total of six rat ventricular myocytes, application of 4.0-nS GSAC induced 17.6 ± 0.7 mV depolarization for the −10 mV-ESAC, whereas this GSAC induced a 13.3 ± 0.9 mV depolarization for the −20-mV ESAC and a 23.3 ± 1.0 mV depolarization for the 0-mV ESAC (Fig. 4B, P < 0.05). In addition to the depolarization level, setting ESAC to a higher level also shifts the membrane potential toward the ICa reactivation window, which further facilitates EAD induction.
Fig. 4.
The effect of SAC reversal potential (ESAC) on GSAC-induced cell depolarization and automaticity in rat ventricular myocytes. A: at a relatively low value of GSAC (2.4 nS), increasing ESAC levels from −20 to 0 mV significantly increased the GSAC-induced cell depolarization. The sustained automaticity was successfully induced by the GSAC at ESAC = 0 mV, but not at the ESAC value of −10 or −20 mV. B: bar graph showing the cell depolarization induced by 2.4-nS GSAC at the ESAC level of −20, −10, and 0 mV (n = 6).
Increasing pacing frequency promotes EAD development.
We have tested the frequency-dependent effect on APD by using 3-Hz stimulation, alternating with the 1-Hz stimulation, in both control and H2O2 solutions. Representative results are shown in Fig. 5A, in which we have superimposed responses to GSAC = 2 nS at 1-Hz (solid line) or 3-Hz (dotted line) stimulation in the control solution (top) and after 10-min exposure to 100 μM H2O2 (bottom). Application of GSAC in control solution produced a depolarization on which the repetitive APs were superimposed. APs labeled a occurred just before GSAC was turned on; those labeled d occurred after the GSAC has been turned off; and those labeled b and c occurred progressively during the GSAC application. After 10 min in H2O2 solution, the cell produced EADs during GSAC application only when stimulated at 3-Hz but not at 1-Hz rate. Figure 5B shows the same data for the period just before and after turning on the GSAC to show the progressive changes in AP shapes.
Fig. 5.
A: superimposed recordings from an isolated rat ventricular cell stimulated at 1 Hz (solid lines) and 3 Hz (dotted lines) before (top) and after 10 min exposure to 100 μM H2O2 (bottom), with GSAC (2 nS) activation period indicated by double-headed arrows. EADs occurred in H2O2 solution only at 3-Hz stimulation during this low GSAC application. B: a faster time scale illustrating the changes in APs (replotted from A).
In control solution, the 1- and 3-Hz APs are nearly superimposed when GSAC was turned off (Fig. 5B, top, labeled a at 6 s), but, while the GSAC was turned on (from 6.5 to 11.5 s), the APs at 3 Hz had longer duration than those at 1 Hz. For the same cell in the 100 μM H2O2 solution, APs at 1 and 3 Hz had longer APD than in control solution, but are not different from each other (1 vs. 3 Hz) when the GSAC was off (Fig. 5B, bottom, labeled a). However, when the GSAC was turned on, APs at 3 Hz had significantly longer APD than those at 1 Hz and increased their APD progressively to form EADs (bottom, labeled b and c), while the APs at 1 Hz did not form EADs. In summary of these findings, fast pacing did not significantly alter APD in rat ventricular myocytes in control and in 100 μM H2O2 solution. However, when SAC was activated, fast pacing significantly prolonged APD in myocytes for both solutions and facilitated EAD development in myocytes exposed to 100 μM H2O2.
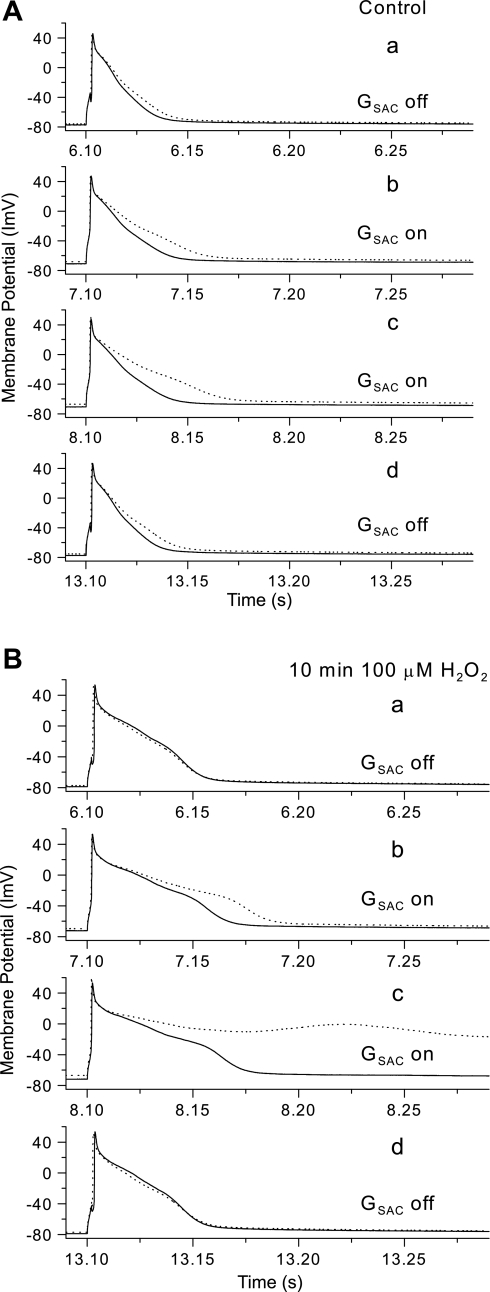
These changes in AP waveforms are shown more clearly in Fig. 6, in which we have superimposed the APs at 3-Hz (dotted lines) with those at 1-Hz rate (solid lines) with the four vertical panels illustrating the APs at the four time points indicated by a, b, c, and d of Fig. 5A. For Fig. 6A (control solution), the fast stimulation slightly prolonged APD at GSAC off, but dramatically prolonged APD at GSAC on (b and c), and this effect was completely reversed after the GSAC was turned off (d).
Fig. 6.
APs recorded in control solution (A) and after 10 min exposure to H2O2 (B). Panels from top to bottom illustrate APs labeled a, b, c, and d in Fig. 5A at 1-Hz (solid lines) and at 3-Hz stimulation (dotted lines).
For the same cell after 10-min exposure to H2O2 (Fig. 6B), the APD was prolonged before the GSAC is turned on (compared with the control solution), but the further effect on APD of the fast pacing was absent. While the GSAC was turned on (panels b and c), there was further lengthening of APD at 1-Hz stimulation and a very pronounced further lengthening of APD at 3 Hz, leading to an EAD in panel c. Similar to the control solution, the effect of GSAC is completely reversed after the GSAC has been turned off (panel d).
These data are summarized in Table 2. GSAC significantly depolarized the RMP at both pacing frequencies. In both control and H2O2 solution, increasing pacing frequency tended to increase APD90, but the increase was significant only when GSAC was applied.
Table 2.
Percent change in AP parameters with increased stimulation frequency
| 1 Hz, GSAC = 0 nS |
%Change From GSAC = 0 nS |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 Hz, GSAC = 2 nS | 3 Hz, GSAC = 0 nS | 3 Hz, GSAC = 2 nS | ||||||
| Control solution | ||||||||
| RMP, mV | −78.6±0.6 | −2.7±0.4* | −4.3±0.8 | −23.0±4.1* | ||||
| APD30, ms | 3.9±0.7 | −4.5±5.1 | 4.0±3.3 | −1.1±1.5 | ||||
| APD50, ms | 8.5±2.1 | −3.9±2.5 | 2.7±2.8 | 0.9±1.3 | ||||
| APD90, ms | 28.6±2.6 | 6.2±5.6* | 2.2±2.3 | 33.6±14.1* | ||||
| APA, mV | 98±25 | −12.6±17 | −0.4±1.2 | −0.6±2.0 | ||||
| 10-min Exposure to 100 μM H2O2 | ||||||||
| RMP, mV | −79.5±0.5 | −4.5±0.6† | −14.7±7.8 | −19.6±5.4† | ||||
| APD30, ms | 3.8±0.9 | −1.8±1.4 | −1.5±2.0 | −21±19.8 | ||||
| APD50, ms | 8.3±0.3 | −4.9±6.2 | −2.5±1.9 | 6.6±4.8 | ||||
| APD90, ms | 33.3±6.2 | 2.3±2.4 | 4.9±4.6 | 24±4.2† | ||||
| APA, mV | 103.0±3.8 | −8.7±6.1 | 11.5±12.3 | −1.1±2.5 | ||||
Values are means ± SE; n = 12.
P < 0.05 compared with 1 Hz for control solution with GSAC = 0 nS.
P < 0.05 compared with 1 Hz for 100 μM H2O2 with GSAC = 0 nS.
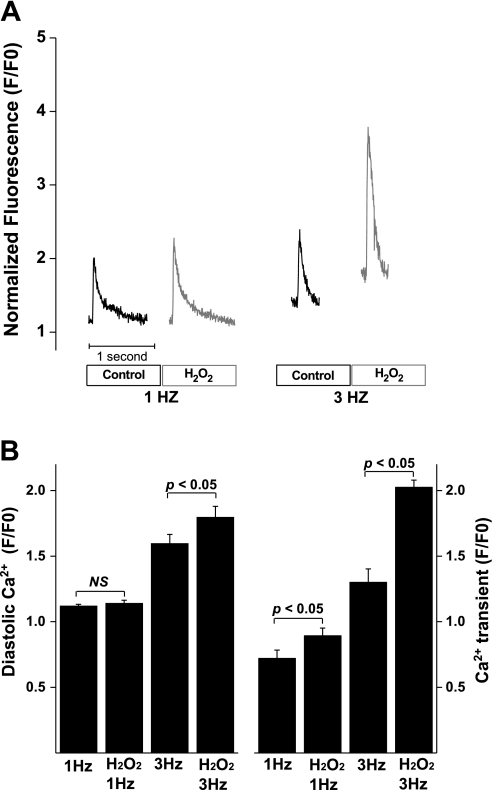
H2O2-induced increase in Ca2+ transient at different stimulation frequencies.
To explore the mechanisms for the fast pacing-induced facilitation of EADs in the presence of H2O2, we measured intracellular Ca2+ levels in rat ventricular myocytes at stimulation rate of 1 and 3 Hz. In a total of 10 myocytes, application of 100 μM H2O2 significantly increased Ca2+ transient at both 1- and 3-Hz stimulation frequencies, with a significantly larger increase at 3 Hz compared with 1 Hz. H2O2 did not change the diastolic Ca2+ levels at 1 Hz. However, it significantly increased the diastolic Ca2+ at 3-Hz stimulation. In other words, in the presence of 100 μM H2O2, fast pacing causes a significant Ca2+ load, in addition to a larger increase in Ca2+ transient (Fig. 7). The alteration of Ca2+ cycling at high stimulation frequency in the presence of 100 μM H2O2 likely contributes to the facilitation of SAC-induced EADs at fast-pacing rate.
Fig. 7.
H2O2-induced increase in diastolic Ca2+ and Ca2+ transient in rat ventricular myocytes. A: the representative Ca2+ transient traces in response to the application of H2O2 (100 μM) and the changes in stimulation frequencies (1 and 3 Hz). B: bar graph showing changes in the diastolic Ca2+ and Ca2+ transient in response to the application of H2O2 and changes in stimulation frequencies (n = 10). F/F0, ratio of fluorescence intensity to the resting fluorescence intensity.
DISCUSSION
Effects of model stretch on AP parameters.
Stretch has been reported to depolarize RMP and to increase, decrease, or have a crossover effect on APD (37). The inconsistent effect on APD may reflect differences of stretching single myocytes, tissue, and intact hearts. Additionally, the different effects on APD may reflect the variety of experimental techniques used to stretch the myocardium and to record the electrical activity (3). Here, we show that incorporation of a SAC model into isolated rat ventricular myocytes resulted in depolarization of the membrane potential and increase in APD90. These results are consistent with a study by Zeng et al. (48), in which they demonstrated that stretch of isolated rat ventricular myocytes resulted in depolarization of RMP and an increase in both APD50 and APD90. Note that these experiments were done at room temperature, and the APD was quite long. Similarly, Kiseleva et al. (23) showed that stretch of a rat left ventricular slice resulted in an increase in APD90 and no change in APD50 or APD25, consistent with our findings (Table 1). The effects of myocardial stretch on APD may arise from the complex interactions between the SAC current and the intrinsic membrane currents (and channels) of the myocytes. Even if the GSAC were to be constant during stretch with a constant value of ESAC, the time dependence and polarity of the SAC current depend on the membrane potential of the myocyte. With a constant value of −10 mV for ESAC, the SAC current would be inward during diastole, but would change in sign as the membrane potential becoming positive to −10 mV, and would then reverse again in sign as the repolarization progresses to negative values, as illustrated in Fig. 3. The large variability in the repolarization process in ventricular myocytes of different species (and in different regions of the ventricle) may significantly alter the effects of a given SAC current. In addition, the ESAC also varies with species. Thus the role of SAC current in regulating myocyte depolarization and repolarization will be different for different species. Our SAC model provides opportunity of altering the GSAC, thereby changing the magnitude of the SAC current. This allows us to specifically study the effects of SAC current and eliminate the interplay of other substrates resulting from the real mechanical stretch. In addition, this SAC model can also be applied to mimic SAC currents in different species by accordingly setting the reversal potential to a relative level.
APD prolongation and EADs.
The mechanisms of EADs are complex with possible contributions of decreased outward current or increased inward current during the late plateau phase and with a significant contribution of ICa “window current” as part of the mechanism for the upstroke of the EAD (20). The sodium-calcium exchange current may also contribute to EAD development (38). The generation of EADs has long been recognized as a potential mechanism of the generation of extrasystoles, which may lead to reentrant activity (32).
Prolongation of APD has being regarded as a major mechanism for the increased abnormal activity, such as EAD and delayed afterdepolarization, and ventricular arrhythmias in heart failure (HF) (19). However, in vivo studies showed that inhibition of Ca2+/calmodulin-dependent protein kinase II (CaMKII) suppressed EADs in HF without shortening APD, indicating that APD prolongation is not a required mechanism for the induction of EADs (45). Instead, a CaMKII-related increase in ICa (41) is likely a mediator. Our results here showed that prolongation of APD by SAC activation itself did not produce EADs in rat ventricular myocytes in the control condition, suggesting that the APD prolongation itself may not be enough for EAD generation.
Previous studies have shown increases in ICa induced by acute exposure to H2O2 (5). At higher concentration levels (100–400 μM), H2O2 exposure induced EADs, in addition to a significant APD prolongation (42). It is important to note that these results were only observed with the perforated patch technique, but not with the “break-through” whole cell clamp, indicating that intracellular Ca2+ unbuffering is required. We have shown that the combination of stretch (created by a model SAC) and oxidative stress (approximated with low doses of H2O2) induced EADs in rat ventricular myocytes. Stretch alone was not sufficient to induce EADs at any value of GSAC. The addition of H2O2, which lengthened the APD at all repolarization levels, sensitized the cells to EADs in the presence of stretch conductance. This may be due to the ability of H2O2 to increase ICa, Ca2+ load, and Ca2+ transient.
The GSAC required for inducing EADs in H2O2 averaged 2.6 ± 0.4 nS (Table 1). For average RMP of −80 mV and ESAC of −10 mV, this conductance generates a current of −182 pA. Kamkin et al. (21) showed that stretching an isolated rat ventricular myocyte for 8 μm induced a current of −269 pA at holding potential of −45 mV. Thus the amount of stretch-activated current required to induce EADs in rat ventricular myocytes under 100 μM H2O2 from our SAC model can be reached by the physical stretch of rat ventricular myocytes.
Effects of pacing frequency.
Increasing pacing frequency from 1 to 3 Hz did not significantly increase APD in either control or H2O2 conditions. However, addition of GSAC, along with higher pacing rate, dramatically increased APD90 in both control and H2O2 conditions (Table 2). This fast-pacing-induced APD prolongation under the condition of SAC activation plays a role in facilitating EAD induction in acute oxidative stress. This pointed out a mechanism for the premature excitation-triggered EADs in stretched myocardium, such as in HF and other dilated cardiomyopathies. The mechanisms underlying the frequency-dependent APD prolongation in the presence of SAC activation is unclear. It is unlikely related to the frequency-dependent inhibition of Ito, because the prolongation is presented only for the late, but not the early, repolarization. Instead, an increase in the intracellular Ca2+ load at fast pacing during SAC activation (10) may contribute. Nevertheless, our data suggest that the SAC activation-associated, frequency-dependent APD prolongation plays an important role in facilitating EAD generation when the myocytes are exposed to H2O2, where the Ca2+ load and the transient are significantly elevated. This implicates SAC activation in premature beat triggered EADs in the condition of oxidative stress.
Limitations.
Our use of a computed current injected into an isolated myocyte to model the effects of SAC channels has advantages and disadvantages compared with physically stretching cells. Stretch-induced arrhythmias could depend on Ca2+ entry through SACs or a release of Ca2+ from internal stores in response to the SAC-induced depolarization. Mechanical deformation of ventricular myocytes induced an increase in intracellular Ca2+ that was sensitive to gadolinium, which blocks SACs (35). Additionally, intracellular buffering of Ca2+ abolished the effects of stretch in guinea pig ventricular myocytes (3). These findings suggest that Ca2+ may enter through the SACs. In contrast, a recent study showed that SAC currents in human atrial myocytes did not change with Ca2+-free external solution, suggesting that the current through SACs was not carried by Ca2+, but more likely by Na+ (22). In fact, the SAC has been found permeable to several ions, including K+, Na+, Ba2+, Ca2+, and Cl− (47). A possible interpretation is that the conductance for each of these ions may vary from species and tissue types. Although our model of SACs does not include actual exchange of ions across the cell membrane, it does reproduce changes in AP characteristics, consistent with data from physically stretched myocytes and tissue (21, 23, 48). Additionally, a similar SAC formulation was incorporated into guinea pig ventricular cell model and induced EADs in this model by SAC activation (31). Different from this study, here we used a SAC integrated into real rat ventricular myocytes rather than the model simulation, thus allowing pharmacological manipulations.
An increase in diastolic Ca2+ and Ca2+ transient amplitude has been observed in ventricular myocytes by fast pacing (2) or by exposure to H2O2 in a concentration-dependent manner (43). Our results show that fast pacing induced a significantly larger increase in diastolic Ca2+ and Ca2+ transient in rat ventricular myocytes in the presence of H2O2 than in control solution. The H2O2-mediated enhancement of diastolic Ca2+ and Ca2+ transient at fast rate is likely a contributor to SAC-induced abnormal impulses at high-pacing frequency in the presence of 100 μM H2O2. Although alterations in intracellular calcium homeostasis have been implicated in H2O2-induced injury, the mechanisms by which calcium homeostasis is altered remain unclear. It is possible that oxidant increases intracellular Ca2+ concentration by altering the activity of ion channels and/or transport proteins, either directly or through effects on other systems that modulate their activity, in addition to the mechanism of oxidant-induced cellular damage (34). Previous studies reported that H2O2 induces alterations of several ion channels and transporters, including Na current (42), L-type Ca current (14, 18), K currents (4), ryanodine receptors (1), sarcoplasmic reticulum Ca pump (27), and the Na/Ca exchanger (13, 16). Activation of several signaling pathways is involved in these alterations, most importantly the PKC (43) and the CaMKII (11, 46) pathways. These ionic alterations, in combination with the cellular injury caused by high-concentration H2O2, contribute significantly to the enhanced Ca2+ load, facilitating abnormal impulse induction (46). In this study, we used a low concentration of H2O2 (100 μM), a level that has been reported in human blood plasma (15). The changes in ionic currents, transporters, and the signaling pathways that alter intracellular calcium homeostasis by this low-level H2O2 have not been well documented. We did not observe EADs or automaticity triggered by this concentration of H2O2. EADs and sustained automaticity were induced only in the combination of H2O2 and SAC activation, implicating SAC activation in arrhythmogenesis under the condition of oxidative stress. However, as a limitation of this SAC model, we are unable to include stretch-induced changes in ion channels and transporters and the related signaling pathways. Furthermore, changes in cell length due to contraction were also not included. Cell shortening may decrease the open probability of SACs (26); thus our model system may overestimate the magnitude of the stretch current during systole.
Perspective.
Many forms of cardiomyopathies, including HF and cardiac infarction, where the myocardium is persistently stretched, predispose ventricular myocytes to a heightened susceptibility to EADs, an important trigger for arrhythmogenesis (28). A number of studies have highlighted the importance of SAC currents in the generation of EADs and arrhythmias (30). In this study, we showed that incorporation of a real-time simulation of SACs into an isolated rat ventricular myocyte induced EADs in the presence of low doses of H2O2, and that this effect is enhanced by increasing the stimulation rate. We conclude that SAC activation plays an important role in triggering EADs under oxidative stress. Furthermore, our data suggested that SAC activation is an important mediator for the premature beats-triggered (fast-pacing) abnormal impulses. These findings implicate SAC activation in promoting ventricular arrhythmias in cardiomyopathies with increased tension in ventricular wall and oxidative stress. This SAC-related mechanism may explain, at least in part, the clinical findings that reducing myocardial wall tension and oxygen demand by left ventricular offloading using intra-aortic balloon counterpulsation significantly reduced ventricular arrhythmias in patients with medically refractory ventricular arrhythmias (12).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grants (HL-088168, Y. Wang; HL-088488, M. B. Wagner; HL-077485, R. W. Joyner), American Health Assistance Foundation grant (H2007-019, Y. Wang), and the support from Emory Children's Healthcare of Atlanta (to Y. Wang) and Todd Franklin Cardiac Laboratory (to R. W. Joyner).
REFERENCES
- 1.Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun 249: 938–942, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Baartscheer A, Schumacher CA, Belterman CN, Coronel R, Fiolet JW. SR calcium handling and calcium after-transients in a rabbit model of heart failure. Cardiovasc Res 58: 99–108, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Belus A, White E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J Physiol 546: 501–509, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berube J, Caouette D, Daleau P. Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J Pharmacol Exp Ther 297: 96–102, 2001. [PubMed] [Google Scholar]
- 5.Chakraborti T, Ghosh SK, Michael JR, Batabyal SK, Chakraborti S. Targets of oxidative stress in cardiovascular system. Mol Cell Biochem 187: 1–10, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Cooper PJ, Lei M, Cheng LX, Kohl P. Selected contribution: axial stretch increases spontaneous pacemaker activity in rabbit isolated sinoatrial node cells. J Appl Physiol 89: 2099–2104, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dick DJ, Lab MJ. Mechanical modulation of stretch-induced premature ventricular beats: induction of mechanoelectric adaptation period. Cardiovasc Res 38: 181–191, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Duan J, Moffat MP. Potential cellular mechanisms of hydrogen peroxide-induced cardiac arrhythmias. J Cardiovasc Pharmacol 19: 593–601, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Duchen MR Mitochondria and Ca(2+) in cell physiology and pathophysiology. Cell Calcium 28: 339–348, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45: 38–54, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotopoulos GD, Mason MJ, Walker S, Jepson NS, Patel DJ, Mitchell AG, Ilsley CD, Paul VE. Stabilisation of medically refractory ventricular arrhythmia by intra-aortic balloon counterpulsation. Heart 82: 96–100, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldhaber JI, Ji S, Lamp ST, Weiss JN. Effects of exogenous free radicals on electromechanical function and metabolism in isolated rabbit and guinea pig ventricle. Implications for ischemia and reperfusion injury. J Clin Invest 83: 1800–1809, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo J, Giles WR, Ward CA. Effect of hydrogen peroxide on the membrane currents of sinoatrial node cells from rabbit heart. Am J Physiol Heart Circ Physiol 279: H992–H999, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett 486: 10–13, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hinata M, Matsuoka I, Iwamoto T, Watanabe Y, Kimura J. Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in guinea-pig ventricular myocytes. J Pharm Sci 103: 283–292, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol 154: 205–216, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Hudasek K, Brown ST, Fearon IM. H2O2 regulates recombinant Ca2+ channel alpha1C subunits but does not mediate their sensitivity to acute hypoxia. Biochem Biophys Res Commun 318: 135–141, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Janse MJ Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 61: 208–217, 2004. [DOI] [PubMed] [Google Scholar]
- 20.January CT, Moscucci A. Cellular mechanisms of early afterdepolarizations. Ann N Y Acad Sci 644: 23–32, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Kamkin A, Kiseleva I, Isenberg G. Stretch-activated currents in ventricular myocytes: amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc Res 48: 409–420, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Kamkin A, Kiseleva I, Wagner KD, Bohm J, Theres H, Gunther J, Scholz H. Characterization of stretch-activated ion currents in isolated atrial myocytes from human hearts. Pflügers Arch 446: 339–346, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kiseleva I, Kamkin A, Wagner KD, Theres H, Ladhoff A, Scholz H, Gunther J, Lab MJ. Mechanoelectric feedback after left ventricular infarction in rats. Cardiovasc Res 45: 370–378, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kohl P, Hunter P, Noble D. Stretch-induced changes in heart rate and rhythm: clinical observations, experiments and mathematical models. Prog Biophys Mol Biol 71: 91–138, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79: 943–956, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Lab MJ Mechanoelectric feedback (transduction) in heart: concepts and implications. Cardiovasc Res 32: 3–14, 1996. [PubMed] [Google Scholar]
- 27.Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca(2+)-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med 22: 37–47, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Nuss HB, Kaab S, Kass DA, Tomaselli GF, Marban E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol Heart Circ Physiol 277: H80–H91, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J 81: 3029–3051, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravens U Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol 82: 255–266, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Riemer TL, Sobie EA, Tung L. Stretch-induced changes in arrhythmogenesis and excitability in experimentally based heart cell models. Am J Physiol Heart Circ Physiol 275: H431–H442, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Roden DM Early after-depolarizations and torsade de pointes: implications for the control of cardiac arrhythmias by prolonging repolarization. Eur Heart J 14, Suppl H: 56–61, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki N, Mitsuiye T, Noma A. Effects of mechanical stretch on membrane currents of single ventricular myocytes of guinea-pig heart. Jpn J Physiol 42: 957–970, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd M, Bruening M, Auld AM, Barritt GJ. Effects of energy deprivation and hydrogen peroxide on contraction and myoplasmic free calcium concentrations in isolated myocardial muscle cells. Biochem Med Metab Biol 38: 195–204, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Sigurdson W, Ruknudin A, Sachs F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart: role of stretch-activated ion channels. Am J Physiol Heart Circ Physiol 262: H1110–H1115, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Song YD, Yang XC, Liu TF, Gu ZW. Nonselective cation current in rabbit ventricular myocytes. Methods Find Exp Clin Pharmacol 27: 377–383, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Taggart P, Lab M. Cardiac mechano-electric feedback and electrical restitution in humans. Prog Biophys Mol Biol 97: 452–460, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Volders PG, Kulcsar A, Vos MA, Sipido KR, Wellens HJ, Lazzara R, Szabo B. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc Res 34: 348–359, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Wagner MB, Kumar R, Joyner RW, Wang Y. Induced automaticity in isolated rat atrial cells by incorporation of a stretch-activated conductance. Pflugers Arch 2004. [DOI] [PubMed]
- 40.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation 113: 1849–1856, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, Sugianto J, Johnstone JL, Sun Y, Hill JA. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem 283: 25524–25532, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 500: 631–642, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward CA, Moffat MP. Role of protein kinase C in mediating effects of hydrogen peroxide in guinea-pig ventricular myocytes. J Mol Cell Cardiol 27: 1089–1097, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Wilders R, Kumar R, Joyner RW, Jongsma HJ, Verheijck EE, Golod D, van Ginneken AC, Goolsby WN. Action potential conduction between a ventricular cell model and an isolated ventricular cell. Biophys J 70: 281–295, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106: 1288–1293, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104: 79–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh TH, Herman P, Tsai MC, Tran Ba HP, Van den AT. A cationic nonselective stretch-activated channel in the Reissner's membrane of the guinea pig cochlea. Am J Physiol Cell Physiol 274: C566–C576, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Zeng T, Bett GC, Sachs F. Stretch-activated whole cell currents in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 278: H548–H557, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Zhang YH, Youm JB, Sung HK, Lee SH, Ryu SY, Ho WK, Earm YE. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J Physiol (Lond) 523: 607–619, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]