Abstract
Stem cell therapy for myocardial tissue repair is limited by the poor survival of transplanted cells, possibly because of inadequate supply of oxygen and nutrients. The purpose of this study was to assess the oxygenation level and functional recovery after allogenic transplantation of mesenchymal stem cells (MSC) in a rat model of myocardial infarction (MI). Myocardial oxygen tension (Po2) was measured by electron paramagnetic resonance oximetry using an implantable oxygen-sensing spin probe (OxySpin). MSCs incubated with OxySpins showed substantial uptake of the probe without affecting its oxygen sensitivity or calibration. The cells internalized with OxySpins were able to differentiate into osteogenic, adipogenic, cardiomyocyte, and endothelial cell lineages. The labeled cells tested positive for CD44 and CD29 markers and negative for the hematopoietic markers CD14 and CD45. For the in vivo studies, MI was induced in rats by permanently ligating the left anterior descending coronary artery. MSCs with OxySpins were transplanted in the infarct region of hearts. A significant increase in Po2 was observed in the MSC group compared with the untreated MI group (18.1 ± 2.6 vs. 13.0 ± 1.8 mmHg, n = 4, P < 0.05) at 4 wk after transplantation. Echocardiography showed a significant improvement in ejection fraction and fraction shortening, which inversely correlated with the magnitude of fibrosis in the treated hearts. The cell-transplanted hearts also showed an increase in vascular endothelial growth factor level and capillary density in the infarct region. The study established our ability to measure and correlate changes in myocardial tissue oxygenation with cardiac function in infarcted rat hearts treated with MSCs.
Keywords: myocardial infarction, oxygen-sensing spin probe, electron paramagnetic resonance oximetry
myocardial ischemia causes oxygen deprivation and induces an acute inflammatory response, leading to apoptosis of cardiomyocytes. Several revascularization treatments are used in the clinic to restore blood flow and oxygen supply to the affected region to prevent or minimize the occurrence of irreversible tissue damage. In the absence or failure of such acute treatments, myocardial infarction (MI) will occur, eventually leading to scar tissue formation and cardiac dysfunction, including ventricular remodeling and arrhythmias. In such extreme situations, stem cell therapy (cardiomyoplasty) is being pursued as a potential treatment to replace the lost cardiomyocytes and revascularize the ischemic tissue (9, 11, 20, 22). However, cell therapy to regenerate the damaged cardiac tissue is faced with a number of challenges, including the right choice of cell type and treatment conditions. One of the impediments to a successful cardiomyoplasty has been our inability to determine the optimal oxygen concentration necessary for the survival and engraftment of transplanted cells before they can provide functional benefits. Furthermore, the choice of stem cell type and the specific oxygen requirements of the cells to ensure survival in the hostile ischemic environment need to be determined.
To monitor oxygen concentration (Po2) in the myocardium subjected to stem cell therapy, a noninvasive technique capable of providing accurate and reliable measurements of Po2 over a period of time in a live animal is required. The paucity of such capability has severely hampered our ability to investigate the role of myocardial oxygenation in stem cell therapy. Electron paramagnetic resonance (EPR) oximetry is a recently established technology for noninvasive and repeated measurements of myocardial oxygenation in small animal models of cardiac stem cell therapy (18, 33). The principle of this method is based on the effect of molecular oxygen on the paramagnetic property of lithium naphthalocyanine-based microcrystals [oxygen-sensing spin probe (OxySpin)] (25). The oxygen-induced change in the paramagnetic property is detected using EPR spectroscopy, which is similar to magnetic resonance imaging (MRI), but uses a very low magnetic field. The OxySpin crystals are nontoxic, biocompatible, and stable in tissues for long periods of time, several months or longer (33). We have recently reported the applicability of this technology for real-time monitoring of Po2 in a murine model of acute MI where we measured the change in oxygenation for 4 wk postimplantation after transplantation of skeletal myoblasts in the infarct region (18, 33).
Mesenchymal stem cells (MSC) have recently been shown to be promising and widely applicable to cellular cardiomyoplasty (2, 27). MSCs are multipotent cells found in various types of tissues, including bone marrow, umbilical cord, adipose tissue, and dental pulp (3, 7, 17, 21, 23, 26). MSCs have the potential to differentiate into cells of vascular lineages, including smooth muscle and endothelial cells. MSCs have the capacity to differentiate into cardiomyocyte-like cells in vitro (1), although it has been observed to be a rare event in in vivo studies (2, 8, 19, 31). The effectiveness of MSC-based stem cell therapy has been attributed more to the paracrine-signaling properties of these cells, rather than to their differentiation abilities (12, 30). Although MSCs have been shown to have several advantages, a recent report cautioned the potential risks of whole bone marrow cells and in particular MSCs to treat nonhematopoietic disorders (5). This observation necessitates further studies to optimize conditions for in vitro preparation of MSCs before use in clinical studies.
A growing body of literature on MSC-based therapy for MI suggests that understanding the importance of the tissue microenvironment and how it may be manipulated is critical to realize effective therapeutic potential (6, 14, 32). Oxygen concentration is one of the vital components within the microenvironment. Oxygen plays a significant role in the control and regulation of many physiological, metabolic, and signaling pathways involved in cellular engraftment and host tissue regeneration. We, therefore, sought to build upon our previous experiences using transplantable cells and oxygen-sensing EPR probe to measure this important parameter in stem cell therapy. Specifically, the goal of this study was to determine the cardiac tissue oxygen concentration in a rat model of acute MI treated with MSC. To achieve this goal, we performed in vitro characterization of OxySpin and evaluated the effect of the OxySpin probe on the proliferation and differentiation characteristics of the cells. These experiments were necessary to ensure that MSC phenotype and differentiation potential remained unchanged after exposure to or uptake of the probe. Cultured MSCs with OxySpin probes were then transplanted into infarcted rat hearts, and the in vivo tissue Po2 was monitored using EPR oximetry. The Po2 data were correlated with the functional data and infarct size at 4 wk after transplantation.
MATERIALS AND METHODS
MSCs
Cryopreserved primary rat MSCs, isolated from the bone marrow of adult Fisher 344 rats, were procured from Chemicon (Billerica, MA). The cells were characterized by the supplier to be positive for CD29 (integrin β1) and CD54. The primary cells were thawed and cultured using Dulbecco's modified Eagles medium (DMEM + GlutaMAX-1 low glucose 1×) containing 10% heat-inactivated FBS and penicillin/streptomycin (GIBCO). Accutase (Chemicon), a cell-detachment solution containing proteolytic and collagenolytic enzymes, was used for separation of adherent cells. MSCs of passage five or less were used for experiments. The cells were grown at 37°C in a humidified environment at 5% CO2 in air.
OxySpin
Microcrystalline particulates of lithium 5,9,14,18,23,27,32,36-octa-n-butoxy-2,3-naphthalocyanine (LiNc-BuO) were used as OxySpin (25). The OxySpin is paramagnetic and can be detected by EPR spectroscopy. Approximately 10 mg of LiNc-BuO in 500 μl of medium containing 5 mg of BSA were sonicated for a total of 7.5 min (using five cycles of 30 s sonication, followed by 1 min cooling on ice) to get sub-micron-sized (270 ± 120 nm) particulates using a probe sonicator (22.5 kHz; Sonic Dismembrator, model 100; Fischer Scientific). The OxySpin was calibrated by measuring its EPR line width after exposure to different concentrations of oxygen using premixed oxygen and nitrogen gases of known composition (25). A small amount of OxySpin (∼10 μg) sealed in a 0.8-mm-diameter gas-permeable Teflon tube (Zeus Industrial Products, Orangeburg, SC) was inserted in a 3-mm quartz EPR tube to perform the measurements. The oxygen-induced line broadening (change in peak-to-peak width) of the signal was used to measure extracellular oxygen concentration. A linear variation of line width was observed as a function of Po2 in the range of 0–300 mmHg.
Uptake of OxySpin Particulates by MSCs
MSCs were incubated with three different doses of sonicated OxySpin particulates (50, 100, or 200 μg/ml of culture medium). Cellular uptake of OxySpin was monitored using EPR spectroscopy after 24, 48, and 72 h of incubation time. Except for the confocal studies, for all further in vitro experiments, the cells were labeled for 48 h using a dose of 100 μg/ml OxySpin. After incubation, the cells were washed three times with Dulbecco's PBS (GIBCO) to remove the uninternalized particulates. The cells were detached using Accutase, collected, and suspended for analysis or transplantation. For in vivo studies, 10 mg of LiNc-BuO in 500 μl of medium containing 5 mg BSA were mixed briefly by sonicating for 30 s before transplantation in the heart.
Z-stack Localization Studies of OxySpin in MSCs
MSCs at passage 3 were cultured on sterile cover slips in six-well plates at a seed density of 3×104 cells/dish in the presence of 100 μg/ml of OxySpin for 72 h. The additional time was necessary to maximize internalization of the probes to study localization of the particulates after uptake. The cells attached to cover slips were washed with 1× PBS and fixed with 4% paraformaldehyde for 10 min at room temperature and blocked for 30 min with 1% BSA in 0.01% TBS-Tween. The cells were stained with 5 μM DiI (Invitrogen/Molecular Probes) to identify the membrane and 10 μM Draq5 (Alexis) to identify the nucleus. The cover slips with cells were then fixed to a glass slide with mounting medium (Gel Mount Aqueous mounting medium) and viewed using a confocal fluorescence microscope (LSM 510; Zeiss, Thornwood, NY). Images were overlaid using LSM Image Browser software to generate a merged image for each specimen. Z-stacks were created by obtaining images at 0.37-μm intervals for control cells and at 0.57-μm intervals for the labeled cells.
Cytotoxicity Studies
Cell viability.
MSCs were cultured in the presence of OxySpin (100 μg/ml) for 24, 48, 72, and 96 h. The viability of the cells was assessed using both an automated cell counter (NucleoCounter; New Brunswick Scientific, Edison, NJ) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The cell counter technique uses propidium iodide, which binds to cellular nuclei. Depending upon sample preparation, the counts provide the total number of cells and number of nonviable cells, from which the number of viable cells can be calculated. The cells were seeded at 1×105, 7×104, 5×104, and 3×104 in six-well plates for 24-, 48-, 72-, and 96-h studies, respectively. All experiments were performed in at least two parallels and repeated three times.
Cell survival was also evaluated with MTT. Dark blue formazan crystals insoluble in aqueous solutions are formed when mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring of the component. Cells were seeded and incubated in 5% CO2 at 37°C using 8 × 103 cells/well for 24- and 48-h labeling, and 6 × 103 cells/well for the 72- and 96-h labeling. On removing the labeling medium after incubation, 100 μl of MTT reagent (5 mg/ml) was added, and the cells were incubated for 4 h at 37°C. Acidified isopropanol (100 μl) was used to dissolve the formazan crystals formed. Absorbance at 570 nm was measured by an automated plate reader (AD340; Beckman Coulter). All assays were performed in at least three parallels and repeated four times.
Membrane integrity of the cells after internalization of the probe was examined using Trypan blue exclusion assay. After incubation, the cells were washed, detached using Accutase, and treated with Trypan blue to assess viability. The number of live and dead cells was counted using a hemocytometer. Cytotoxicity was also determined by measuring the amount of lactate dehydrogenase (LDH) released in the cell culture medium using an LDH assay kit according to the procedure provided by the manufacturer (Sigma). After incubation, an aliquot of the medium was taken and assayed for LDH activity using a Varian (model Cary 50) spectrophotometer.
Cell proliferation assay.
The effect of OxySpin on the proliferation of MSCs was evaluated by bromodeoxyuridine (BrdU) incorporation assay according to the manufacturer's protocol (Roche). Briefly, sonicated OxySpins (100 μg/ml) were added to cells at 60–75% confluence grown in 96-well plates and incubated for 48 h. BrdU (10 μM) was added to each well and incubated overnight. The incorporated BrdU was detected using a BrdU-specific monoclonal antibody conjugated with peroxidase (1:100 dilution) followed by incubation with the substrate solution (tetramethylbenzidine). The peroxidase reaction was stopped by the addition of 25 μl of 1 M sulfuric acid. Light absorbance at 450 nm (reference wavelength: 690 nm) was measured within 5 min using a microplate reader (model AD 340; Beckmann Coulter). All experiments were performed with at least three parallels and repeated three times.
Oxygen Consumption Measurements
The effect of OxySpin on cellular mitochondrial function was assessed by measuring the cellular oxygen consumption using EPR spectroscopy. Labeled MSCs were taken in a capillary tube (∼30 μl), and then both ends of the tube were sealed. EPR measurements were performed using a Bruker X-band (9.8 GHz) spectrometer (Bruker Instruments, Karlshrue, Germany). In control experiments, OxySpin (10 μg) was added externally to MSCs in suspension, and the oxygen consumption was measured. EPR spectra were acquired until most of the oxygen enclosed in the capillary tube was consumed by the cells, which correspondingly decreases the line width of the probe. The accurate measure of line width was attained using custom-developed data acquisition software. The oxygen consumption rates (OCR) were determined from Po2 data as a function of time. The following expression was used to calculate the OCR and expressed as nanomoles per minute per 1 × 106 cells: OCR = mα, where m is the slope of the Po2 curve (in mmHg/min) and α is the solubility of oxygen in water (1.59 nmol/mmHg at 22°C).
In Vitro Differentiation Assay for MSCs
Adipogenesis.
MSCs were induced to differentiate into adipocytes using Chemicon's MSC adipogenesis kit. The cells were labeled with 100 μg/ml of OxySpin for 48 h. The protocol involved a 21-day-long process (as specified in the manual) that started after the cells reached 100% confluence. The factors that were used toward initiating the formation of fat cells were a combination of steroidal and nonsteroidal chemicals, including dexamethasone (10 mM), 3-isobutyl 1-methylxanthine (0.5 M), insulin (10 mg/ml), and indomethacin (10 mM). Mature adipocytes form lipid vacuoles were stained with Oil Red O staining. The nuclei were stained using hematoxylin.
Osteogenesis.
Osteogenic differentiation was detected by the deposits of calcium caused by osteoblasts. The osteogenesis kit was obtained from Chemicon. The cells were grown in 24-well plates in low-glucose medium and labeled with 100 μg/ml of OxySpin for 48 h. The induction of osteogenesis started once the cells reached 100% confluence. The plates were pretreated with collagen type I and vitronectin to promote osteogenesis. Reagents that were used for the 14- to 17-day process (as specified in the manual) included dexamethasone (1 mM), ascorbic acid 2-phosphate (0.1 mM), glycerol 2-phosphate solution (1 M), and l-glutamine (100×). Alizarin red staining was used to stain the deposition of calcium orange-red.
Cardiomyogenesis.
MSCs of passage 2 on reaching a confluency of 70–75% were labeled by incubation with OxySpin for 48 h. The labeled cells were then seeded in 60-mm petri dishes at a cell density of 7.5×104/ml and cultured for 1 wk in DMEM + GlutaMAX-1 low glucose 1× containing 5% heat-inactivated FBS and penicillin/streptomycin. After 1 wk, cells were treated with 10 μM 5-azacytidine (Sigma-Aldrich) for 24 h. The cells were then washed with PBS (1×) and replaced with DMEM + GlutaMAX-1 low glucose 1× medium containing 5% FBS. Expression of cardiomyocyte markers was examined by immunocytochemistry between 2 and 3 wk after treatment.
Endothelial differentiation.
MSCs of passage 2 on reaching a confluency of 70–75% were labeled by incubation with OxySpin for 48 h. The labeled cells were then seeded in 60-mm petri dishes at 5.5×104/ml and grown in complete Clonetics Cambrex EGM-2 medium (Lonza, NJ). Expression of endothelial cell markers was examined by immunocytochemistry after 3 wk of culture.
Immunocytochemistry.
Labeled and control cells grown in 60-mm petri dishes and six-well plates were fixed with 2% paraformaldehyde for 5 min. The cell membrane was permeabilized with 0.5% Triton X (1× PBS) for 15 min followed by 30 min of incubation with normal goat serum (Jackson ImmunoResearch). After being washed with PBS (1×), the cells were incubated overnight with the primary antibodies. Cardiomyocyte specific antibodies used were mouse monoclonal heavy chain cardiac myosin antibody (1:50; Abcam) and monoclonal anti-mouse connexin-43 (1:250; Chemicon). The antibodies specific to endothelial lineage used were monoclonal anti-mouse platelet/endothelial cell adhesion molecule-1 (CD31) (1:50; Chemicon) and rabbit anti-human von Willebrand Factor (1:1,000; Chemicon). Secondary antibodies conjugated with Alexa Fluor 488 goat anti-mouse (Invitrogen), Alexa Fluor 594 goat anti-mouse (Invitrogen), and Alexa Fluor 488 goat anti-rabbit (Invitrogen) were used to visualize the expression of primary antibodies. The nucleus of the cells was stained with hard set mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories).
Characterization of MSC Phenotype
MSCs of the second and fifth passages were analyzed using flow cytometry to characterize the phenotype of cells labeled with OxySpin (100 μg/ml for 48 h). The cells (1×106) were incubated with antibody or isotype control for 45 min on ice. Cell aliquots were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibody against CD44 (Chemicon), CD14 (Chemicon), CD29 (integrin β1; Biolegend), and CD45 (BD Pharmingen). Aliquots of cells were also stained with respective isotype controls, IgG-conjugated to FITC or PE. Flow data were acquired using a FACS Calibur (BD) and analyzed using CellQuest software (BD).
Induction of MI and Cell Transplantation In Vivo
Fisher-344 rats were used in the study. All of the procedures were performed with the approval of the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, Revised 1996). MI was created by permanently occluding the left anterior descending coronary artery (LAD). An oblique 12-mm incision was made 8 mm away from the left sternal border toward the left armpit. The chest cavity was opened with scissors by a small incision (10 mm long) at the level of the third or fourth intercostal space, 2–3 mm from the left sternal border. The LAD was visualized as a pulsating bright red spike running through the midst of the heart wall from underneath the left atrium toward the apex. The LAD was ligated 1–2 mm below the tip of the left auricle using a tapered needle and a 6-0 polypropylene ligature passed underneath the LAD, and a double knot was made to occlude the LAD. Occlusion was confirmed by a sudden change in color (pale) of the anterior wall of the left ventricle (LV). Electrocardiogram changes were recorded, and ST elevation was observed after LAD ligation. The chest cavity was closed by bringing together the third and fourth ribs with one 4-0 polypropylene silk suture. The layers of muscle and skin were closed with a 4-0 polypropylene suture, and the rats were allowed to recover under warm light.
Multiple injections of MSCs (a total of 5×105 cells in 100 μl) with OxySpin were given in the infarct and peri-infarct regions of the hearts 30 min after LAD ligation (MI + MSC group). The control (no LAD ligation, non-MI) group of animals received OxySpin only (in 100 μl). The MI group (LAD ligated) received OxySpin only. The chest cavity was closed after transplantation of the stem cells. Oxygen measurements were performed immediately and then 4 wk after MI using in vivo EPR oximetry.
Measurement of Myocardial Po2 by EPR Oximetry
Measurements of myocardial oxygenation were performed noninvasively using an L-band in vivo EPR spectrometer (Magnettech) equipped with automatic coupling and tuning controls for measurements in beating hearts. Rats, under anesthesia (2% isoflurane in room air), were placed in a right lateral position with their chest close to the loop of the surface-coil resonator. EPR spectra were acquired as single 30-s scans. The instrument settings were as follows: incident microwave power, 4 mW; modulation amplitude, 180 mG; modulation frequency, 100 kHz; and receiver time constant, 0.2 s. The peak-to-peak width of the EPR spectrum was used to calculate Po2 using a standard calibration curve.
Echocardiography
The rats were kept under isoflurane (2% in air) anesthesia. Short-axis two-dimensional echocardiography images were obtained from rats orientated on a heating pad in a left lateral decubitus or supine position. LV parameters were obtained from M-mode interrogation in a short-axis view. LV posterior wall thickness, LV internal diastolic diameter (LVIDd), and LV internal systolic diameter (LVIDs) were calculated from the echo data. LV percent fractional shortening (LV%FS) and LV ejection fraction (LVEF) were calculated as follows: LV%FS = (LVIDd − LVIDs)/LVIDd × 100; and LVEF = [(LVIDd)3 − (LVIDs)3]/ (LVIDd)3 × 100. All echocardiography measurements were averaged from at least three separate cardiac cycles of four animals per group.
Assessment of Fibrosis
Rats were killed 4 wk after MI, and the hearts were recovered, washed with cold PBS, and fixed in formalin. After 12 h, the heart sections were embedded in paraffin, and LV cross sections from apex, mid-LV, and base were stained with Masson-Trichrome. Images of left ventricular area (LVA) of each slide were taken using a Nikon model C-PS (20× objective) stereomicroscope equipped with a Spot Insight camera (Diagnostic Instruments). Fibrosis and total LV area of each image were measured using the MetaVue image analysis software (Molecular Devices, Downingtown, PA). The percentage of the fibrosis was calculated as (fibrosis area/total LVA) × 100 using four animals per group.
Immunohistochemical Staining of Cardiac Tissue for α-smooth muscle actin and VEGF Expression
Hearts were fixed in formalin and embedded in paraffin. Sections (6 μm) were cut and used for Masson-trichrome staining for fibrosis. For immunofluorescence staining, the fixed tissue sections were serially rehydrated in 100, 95, and 80% ethanol after deparaffinization with xylene. Slides were kept in steam for 30 min and then washed in PBS (pH 7.4) three times for 5 min each. The tissue sections were then incubated with 2% goat serum and 5% BSA in PBS to reduce nonspecific binding. The sections were then incubated for 4 h with mouse anti-α-smooth muscle actin (α-SMA) or anti-vascular endothelial growth factor (VEGF). The sections were then incubated with appropriate anti-mouse secondary antibodies (1:1,000 dilution) conjugated to Texas red (α-SMA) or FITC (VEGF). Nuclei were counterstained with hardest DAPI (Vector Laboratories). The tissue slides were visualized using an inverted Nikon fluorescence microscope. Separate sections were also stained without primary antibodies to examine nonspecific binding. Blood vessels staining positive for α-SMA were counted in both infarct and peri-infarct regions of the heart.
Data Analysis
The statistical significance of the results was evaluated using ANOVA and a Student's t-test. The values were expressed as means ± SD. A P value of <0.05 was considered significant.
RESULTS
Endocytosis of OxySpins by MSCs.
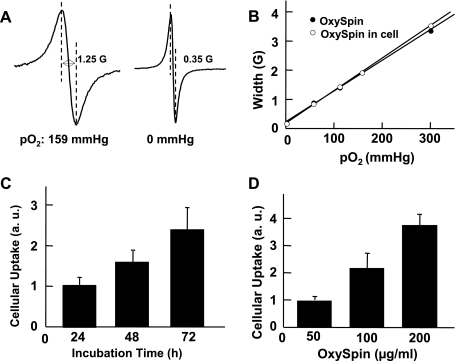
Incubation of MSCs (at 70% confluence) with OxySpins resulted in substantial endocytosis of the probe by the cells. After incubation, the extracellular OxySpins were removed by repeated washings. The cells showed an intense EPR spectrum that was highly sensitive to oxygen concentration in the medium (Fig. 1A), confirming the presence of intracellular OxySpins. The peak-to-peak width of the spectrum showed a linear variation with Po2 that was not significantly different from that of uninternalized OxySpins, suggesting that the oxygen-sensing property (calibration) of the probe was not altered upon internalization in cells (Fig. 1B). The cellular uptake was monitored by measuring EPR intensity, which is a measure of the OxySpins internalized by the cells, by varying time period of incubation to 24, 48, and 72 h (Fig. 1C). The results showed a time-dependent increase in the uptake of OxySpins. Similarly, the dose effect of OxySpins incubated with MSCs was studied in the range of 50–200 μg/ml (Fig. 1D). Approximately a twofold increase in spin density was observed on doubling the concentration of OxySpin, whereas a 1.5-fold increase was observed on doubling of incubation time. The mean value of uptake was ∼3 × 1010 spins/cell (at 100 μg/ml dosage and 48 h incubation time).
Fig. 1.
Characterization of oxygen-sensing spin probe (OxySpins) internalized in mesenchymal stem cells (MSCs). A: electron paramagnetic resonance (EPR) spectra obtained from a suspension of OxySpin-labeled MSCs equilibrated with room air (159 mmHg) or 0% (0 mmHg) oxygen. B: effect of equilibrated oxygen concentration on the line width of OxySpin alone or OxySpin internalized in MSCs. In both cases, the EPR line width was linearly proportional to Po2, and there was no significant difference between the sensitivity of line width to Po2. C: cellular uptake of OxySpin by MSCs at a concentration of 100 μg/ml of medium showed a linear increase with increasing period of incubation. D: cellular uptake of OxySpin by MSCs for an incubation period of 48 h showed a linear increase with increasing dosage of OxySpin. Data were obtained from 3 independent experiments and expressed as means ± SD. AU, arbitrary units.
Localization of OxySpins in MSCs
The cellular localization of OxySpins in MSCs was determined using confocal microscopy. Z-stacks of the MSCs fixed on cover slips were obtained at 0.37- and 0.57-μm intervals for the control and labeled cells, respectively. Figure 2 shows some representative stack images of the labeled cells. The images showed no observable differences on the spindle-shaped morphology or the overall phenotype upon labeling. The OxySpins were observed to be distributed in all slices of the image stack and were primarily localized around the nuclear membrane.
Fig. 2.
Representative Z-stack of OxySpin localization in MSCs. The cell membrane was stained using DiI (yellow), and the nucleus was labeled with Draq5 (red). A: confocal image of unlabeled MSCs. B–D: confocal images of MSCs labeled with OxySpin (100 μg/ml for 72 h). Arrows point to the dark crystalline OxySpins localized around the nuclear membrane inside the cytoplasm.
Cytotoxicity of OxySpins on MSCs
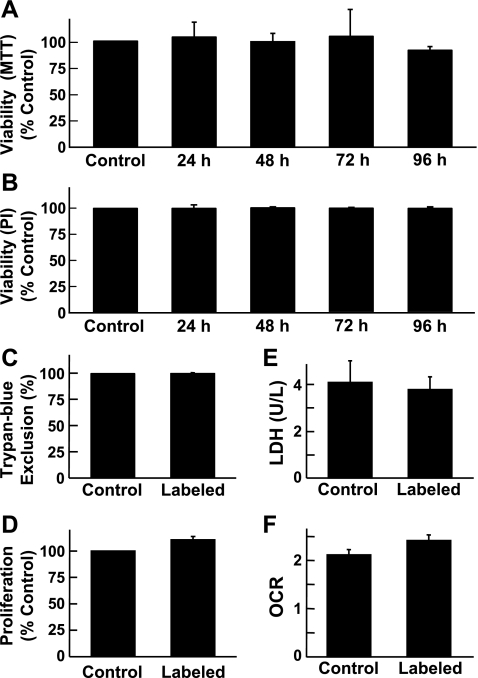
The cells were examined for possible cytotoxicity induced by uptake and exposure to the OxySpins. Standard cytotoxicity tests, including MTT assay for mitochondrial viability, Trypan blue dye exclusion assay for cellular membrane integrity, propidium iodide-based cell viability, BrdU assay for cell proliferation, LDH assay for membrane damage, and oxygen consumption assay for mitochondria metabolism/respiration showed no significant effect on MSC viability (Fig. 3).
Fig. 3.
Effect of OxySpin on cell viability and proliferation. MSCs were incubated with OxySpin (100 μg/ml) for 24, 48, 72, or 96 h to determine mitochondrial viability measured by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (A) and nuclear viability measured by propidium-iodide (PI) staining (B). MSCs were incubated with OxySpin (100 μg/ml) for 48 h to determine membrane integrity measured by Trypan blue exclusion assay (C), cell proliferation measured by bromodeoxyuridine (BrdU) incorporation assay (D), membrane damage measured by lactate dehydrogenase (LDH) leak in cell culture supernatants (E), and cellular respiration (metabolism) measured as oxygen consumption rate (OCR, nmol/min per 1 × 106 cells; F). In A–D, data are expressed as percent of controls, which did not include OxySpins. In all, data represent means ± SD obtained from 3 independent experiments. Overall, the results show that OxySpin has no significant effect on the viability or proliferation of MSCs.
Effect of OxySpin on Differentiation Potential of MSCs
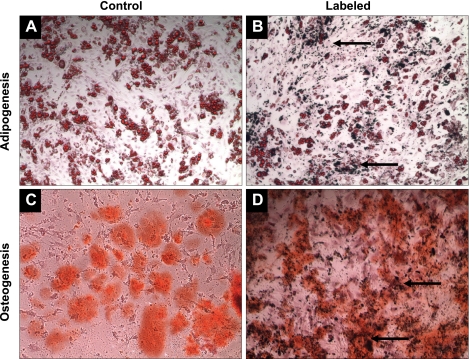
MSCs are multipotent stem cells that have the ability to differentiate into cell types of various lineages. To determine the effect of OxySpin exposure and uptake on the multilineage differentiation capability of MSCs, the cells were induced to differentiate to adipogenic, osteogenic, cardiomyogenic, and endothelial lineages. Figure 4 shows the differentiation of MSCs into adipocytes and osteocytes. Figure 5 shows the differentiation of MSCs into cardiomyocytes (positive staining for myosin heavy chain and connexin-43) and endothelial cells (positive staining for CD31 and von Willebrand markers). The results clearly established that the differentiation potential of MSCs was not altered because of uptake of OxySpin.
Fig. 4.
Effect of OxySpin on the differentiation of MSCs in osteogenic and adipogenic lineages. A and B are unlabeled and labeled MSCs induced to differentiate into adipocytes, respectively. Lipid vacuoles were formed after the 21-day process of induction, which was stained red by the Oil O red stain. C and D are unlabeled and labeled MSCs induced to differentiate into osteoblasts. Alizarin red stained the calcium deposition occurring in the process orange red. The arrows point to the OxySpin deposition in the cells. The results demonstrate that differentiation of MSCs into bone cells or fat cells was not affected by OxySpin.
Fig. 5.
Effect of OxySpin on the differentiation of MSCs into cardiac and endothelial lineages. A: MSCs unlabeled (control) and labeled with OxySpin were induced to differentiate into cardiomyocyte-like cells using 5-azacytidine. Immunofluorescence staining for cardiac markers heavy chain (HC) cardiac myosin (green) and connexin-43 (red), superimposed on 4′,6-diamidino-2-phenylindole (DAPI) staining for nucleus (blue). B: expressions of endothelial markers CD31 (green) and von Willebrand Factor (vWF; red) are shown along with DAPI staining for nucleus (blue) in unlabeled (control) and labeled MSCs after 3 wk of culture in complete endothelial medium.
Effect of OxySpin on Cell Surface Antigen Profile of MSCs
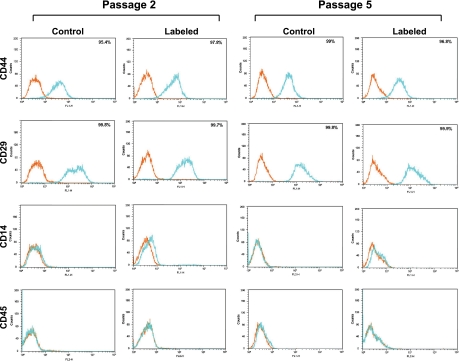
MSCs are nonhematopoietic stem cells characterized by specific cell surface antigens that are not expressed in most hematopoietic stem cells. We used flow cytometry to determine whether or not the presence of OxySpin would affect the cell surface antigen profile of MSCs. The cells were studied at passages 2 and 5. Antigen expression was observed to be positive for CD44 and CD29 and negative for the hematopoietic markers CD14 and CD45 (Fig. 6). The expression of these markers was ≥95% for the positive markers and ≤2% for the negative markers in both the control and labeled MSCs.
Fig. 6.
Representative cell surface antigen expression profile of MSCs at 2nd and 5th passages. Flow cytometry profiles of unlabeled (control) and OxySpin-labeled MSCs are shown with isotype control staining in orange and specific antibody staining in blue traces. Analysis of the data showed that the MSCs were <2% positive for CD45 and CD14 and express cell surface antigens CD29 (integrin β1) and CD44.
Improvement of Cardiac Function Upon MSC Transplantation in MI Hearts
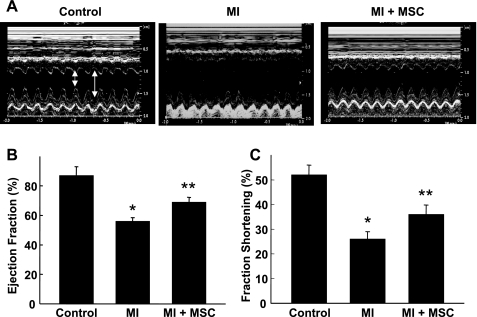
M-mode echocardiography was used to determine the effect of transplanted MSCs in the functional recovery of infarct hearts at 4 wk after therapy (Fig. 7). LVEF was significantly decreased in the MI group compared with control. The LVEF was significantly improved in the MSC-treated group (MI + MSC) compared with the MI group. Similarly, LV fraction shortening was significantly decreased in the MI group compared with control, whereas the decrease was significantly attenuated in the MI + MSC group.
Fig. 7.
Recovery of cardiac function at 4 wk after MSC transplantation. Echocardiography was performed in noninfarct (control), infarct [myocardial infarction (MI)], and infarct hearts treated with MSCs (MI + MSC). Representative recordings of M-mode echocardiogram (A), left ventricle (LV) ejection fraction (B), and fraction shortening (C) are shown. Small arrow, systole; large arrow, diastole. Results are expressed as means ± SD (n = 4 animals/group). *P < 0.01 vs. control. **P < 0.05 vs. MI. Results show that treatment of MI by MSCs improved functional recovery.
Recovery of Myocardial Po2 in MSC-transplanted Hearts
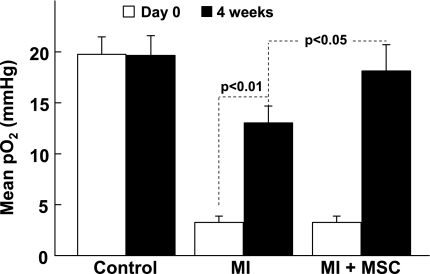
The measurements of myocardial tissue Po2 in the infarct region of hearts were performed immediately after transplantation of MSCs (day 0) and at 4 wk after MI (Fig. 8). The mean baseline Po2 in the noninfarct control hearts was 19.7 ± 1.4 mmHg. After 4 wk, the Po2 value in the noninfarct control heart was 19.6 ± 1.6 mmHg, indicating that the Po2 levels did not change significantly over the 4-wk period in the control group. A marked decrease of myocardial Po2 was observed in the MI group (3.0 ± 0.7 mmHg). There was no significant difference between the Po2 values of MI and MI + MSC groups on day 0. However, the MI + MSC group showed a significantly higher Po2 compared with the MI group (18.1 ± 2.6 vs. 13.0 ± 1.8 mmHg) at 4 wk after MSC transplantation. Interestingly, the MI group also showed a significant increase in Po2 at 4 wk compared with MI at day 0.
Fig. 8.
Myocardial Po2 at the site of cell transplantation. Myocardial Po2 values were measured using in vivo EPR oximetry. Myocardial Po2 measured at the therapeutic site on day 0 and then at 4 wk after transplantation of MSCs. No MI was induced in the control group. Values are expressed as means ± SD (n = 4 animals/group). The MI group shows a significant recovery of Po2 at 4 wk. At 4 wk, the recovery of Po2 in the MI + MSC group was significantly higher than the MI group. The results indicate that treatment of MI in the ischemic heart by MSCs improved tissue oxygenation.
Reduction of Fibrosis in MSC-transplanted Hearts
Rats were killed 4 wk after MI, and heart sections were embedded in paraffin. LV cross sections were stained with Masson-Trichrome for determination of fibrosis. The fibrotic area was significantly reduced in the MI + MSC group compared with the MI group (Fig. 9).
Fig. 9.
Tissue fibrosis at 4 wk after MSC transplantation. A: sections of hearts stained with Masson-Trichrome dye. B: percentage of fibrosis in hearts at 4 wk after transplantation. Data are expressed as means ± SD. *P < 0.05 vs. MI group (n = 4 animals/per group). Hearts treated with MSCs exhibited a significant reduction in fibrosis compared with the MI group.
Angiogenesis Induced by MSCs in Infarct Tissue
The transplanted MSCs in the infarct region induced an increase in the expression of VEGF and α-SMA, indicating an increase in new vascular structure formation (Fig. 10). The vessel density and the VEGF intensity were significantly higher in the MI + MSC group compared with the MI group.
Fig. 10.
Vascular endothelial growth factor (VEGF) expression and vessel density 4 wk after MSC transplantation. A: cryosections of myocardial tissue from MI and MI + MSC group were immunolabeled with antibodies against VEGF (green) and α-smooth muscle actin (α-SMA, red). B: quantification of the VEGF expression and vessel density. Data show a significant increase in VEGF expression and vasculature in the MSC-treated group compared with the MI group.
DISCUSSION
The present study demonstrated the feasibility of using MSC with OxySpin for noninvasive and repeated measurements of oxygen concentration following myocardial stem cell therapy using EPR oximetry. The OxySpin showed no apparent effect on the viability, metabolism, and proliferation of the cells. Most importantly, the labeled MSCs retained their ability to differentiate. Transplantation of MSCs to infarcted rat hearts resulted in a significant increase in myocardial oxygenation and recovery of cardiac function.
We have previously characterized the uptake of the probe using a number of different cell lines, including smooth muscle cells, ovarian cancer cells, and skeletal myoblasts (4, 24, 33). We observed that the probe could be easily internalized by endocytosis and remained nontoxic to the cells. In the present study, MSCs showed an uptake of ∼3 × 1010 spins/cell at 100 μg/ml dosage after 48 h of incubation time. The results suggested that the magnitude of labeling of MSCs with OxySpins is comparable to that of skeletal myoblasts (33). Confocal microscopy clearly showed the presence of the nanoprobes around the perinuclear membrane. It should be noted that micron-sized superparamagnetic iron oxide and nanoscale, ultrasmall superparamagnetic iron oxide particles used in MRI applications have likewise been reported to localize around the nuclear membrane (34).
Multiple viability tests and proliferation assays showed no significant reduction in cellular viability and no difference in the proliferation rate were observed after labeling of the MSCs with OxySpin particulates. The MTT assay, which is a measure of mitochondrial viability, revealed that the labeling had no significant effect on mitochondrial function. The integrity of mitochondrial function was also evident from the oxygen consumption (respiration) data, which did not significantly change between the labeled and unlabeled control cells. This would imply that, even if labeled MSCs are transplanted to the ischemic region of infarcted myocardium, changes in Po2 reported by the oxygen-sensitive probes are because of external factors and not due to chemical reactions between the OxySpin and molecular oxygen.
The uptake of OxySpin by MSC through endocytosis and its effect on the viability and stem cell characteristics of MSCs were thoroughly examined in the in vitro studies, where the cells were coincubated with the probe in culture. However, we do not know whether a similar particulate uptake will occur when the probe is mixed with the cells and injected in the infarcted tissue. The tests have confirmed that, if particulates are engulfed by MSC after delivery to the in vivo environment or during the short period of time during which the cells and OxySpin are mixed before implantation, the oxygen-sensing capability of the spin probe is preserved. Furthermore, exposure to the probe showed no apparent effect on the viability, metabolism, or proliferation of the MSCs.
MSC are a precursor to a number of mature or terminally differentiated cell types. This characteristic, multilineage potential must be preserved upon cellular uptake or labeling of MSCs with OxySpins to maintain their usefulness for stem cell-based therapeutics. Although considered inert, the possibility was recognized that phagocytic uptake of the OxySpin crystals could induce alterations in phenotype, as determined by membrane surface markers, or their differentiation potential. CD44 and CD29 (integrin β1) are cell surface markers that are commonly used to identify MSCs. An increase in CD44 expression by MSCs has been shown to be an indication of their migratory phenotype (35), whereas CD29 (integrin β1) is an integral membrane protein whose expression is important for MSC migration to the infarct region of the heart (15). CD14, a macrophage marker, and the hematopoietic surface marker CD45 are commonly used as negative markers for identifying the MSC phenotype. In these studies, both the unlabeled control and OxySpin-laden cells from second and fifth passage samples stained positive for both CD44 and CD29 expression and were negative for CD45 and CD14 expression. This evidence suggests that expression of two of the most important MSC cell surface markers, with respect to cellular migration to the infarct tissue, is preserved following uptake and retention of the OxySpins.
We have also shown in the present study that MSCs in culture could be induced to differentiate into osteocytes or adipocytes after labeling with OxySpin. More importantly, MSCs labeled with OxySpin retained their potential to differentiate into cardiomyocyte-like cells and endothelial cells which could promote cardiac tissue regeneration in vivo. Although it is not feasible to evaluate the possibility of differentiation along every possible cell line that has been reported for MSCs, it clearly shows that their multilineage potential is preserved after uptake of the OxySpin probes. While recent evidence indicates that paracrine-signaling effects of MSC transplantation are the primary reason for therapeutic efficacy (10, 12, 30), the possibility remains that, under appropriate circumstances, MSCs may be capable of differentiation to cardiomyocyte-like cells in vivo (16, 19). If so, future studies may demonstrate that MSCs transplanted to the ischemic myocardium may be critical on both fronts, through paracrine-signaling effects and the attenuation of ventricular wall thinning and cardiac remodeling through differentiation into cardiac lineage cells. Regardless of the mechanism, the survival and retention of a sufficient number of transplanted MSCs in the ischemic myocardium is crucial for stem cell therapy to realize its full potential in cardiac applications. Because the levels of tissue oxygenation in the infarct and peri-infarct regions may play a fundamental role, there is a need for a reliable method to monitor tissue oxygenation during therapy.
From our in vivo studies using a rat model of MI, hearts treated with MSCs exhibited significant functional recovery and a reduction in infarct size. These results are in concordance with previous studies by other laboratories (13, 28, 29). However, in our study, we used EPR oximetry to measure the recovery of myocardial oxygenation at the therapeutic site in the heart. At the end of the 4-wk study period, there was a significant improvement in the tissue Po2 of the MSC-treated group compared with the untreated MI group. The recovery in Po2 in the MSC-treated rat hearts approached normal (preinfarct) values at the end of the study. This would imply that additional oxygen supply has been made available to the infarct tissue region, most likely through angiogenesis.
The functional parameters of the heart showed substantial improvement following MSC transplantation. The ejection fraction and fraction shortening parameters, which are the measures of myocardial contractility, were found to increase, whereas the amount of fibrotic scar tissue in the infarcted region was attenuated after cell therapy. An increase in the VEGF expression was observed along with increased vessel density in the MI + MSC groups indicated by the expression of α-SMA in the infarct region. The improved oxygenation in the infarct heart can be correlated with the enhancement in cardiac function of the MSC-treated hearts as a result of enhanced angiogenesis and neovasculature.
In our previous study using a mouse model of acute MI, we had used skeletal myoblasts labeled (internalized) with OxySpin and monitored changes in oxygenation for up to 4 wk (18). In the present work using rat hearts, we deliberately chose to use MSCs mixed with OxySpin particulates to increase the signal-to-noise ratio of the acquired EPR signal. This change was viewed as necessary because of the larger size of the experimental animals, rats vs. mice. Although not internalized, the probe particulates were expected to be retained in the region of interest, as could be verified by the EPR signal obtained 4 wk after implantation.
In summary, we have demonstrated that MSCs can be used with OxySpin for monitoring of oxygen concentration in animal models of stem cell therapy in the MI heart. The OxySpins had no effect on the viability, metabolism, and proliferation of MSCs. Furthermore, the stem cell characteristics of MSCs, including their ability to differentiate into cells of osteogenic, adipogenic, and more importantly the cardiac and endothelial lineages, were preserved in the labeled cells. Implantation of MSCs in MI hearts resulted in increased myocardial oxygenation, which positively correlated with the recovery of cardiac function. The present study established our ability to monitor changes in oxygen concentration in myocardial stem cell therapy using in vivo EPR oximetry.
GRANTS
This work was supported by American Heart Association Predoctoral Fellowship GRT00007883 (S. M. Chacko) and National Institutes of Health R01 EB-006153 (P. Kuppusamy). The development of electron paramagnetic resonance oximetry was supported by National Institutes of Health Grant R01 EB-004031 (P. Kuppusamy).
REFERENCES
- 1.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Charokopos N, Kalogeridis A, Kouzi-Koliakou K, Kyriakopoulou I, Klonizakis I, Papakonstantinou C. Cardiomyogenic potential of human adult bone marrow mesenchymal stem cells in vitro. Thorac Cardiovasc Surg 56: 77–82, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Atsma DE, Fibbe WE, Rabelink TJ. Opportunities and challenges for mesenchymal stem cell-mediated heart repair. Curr Opin Lipidol 18: 645–649, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barachini S, Trombi L, Danti S, D'Alessandro D, Battolla B, Legitimo A, Nesti C, Mucci I, MDA, Cascone MG, Lazzeri L, Mattii L, Consolini R, Petrini M. Morpho-functional characterization of human mesenchymal stem cells from umbilical cord blood for potential applications in regenerative medicine. Stem Cells Dev 18: 293–305, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bratasz A, Pandian RP, Deng Y, Petryakov S, Grecula JC, Gupta N, Kuppusamy P. In vivo imaging of changes in tumor oxygenation during growth and after treatment. Magn Reson Med 57: 950–959, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 22: 1943–1956, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cheng PH, Snyder B, Fillos D, Ibegbu CC, Huang AH, Chan AW. Postnatal stem/progenitor cells derived from the dental pulp of adult chimpanzee (Abstract). BMC Cell Biol 9: 20, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christoforou N, Gearhart JD. Stem cells and their potential in cell-based cardiac therapies. Prog Cardiovasc Dis 49: 396–413, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Collins SD, Baffour R, Waksman R. Cell therapy in myocardial infarction. Cardiovasc Revasc Med 8: 43–51, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Dai W, Hale SL, Kloner RA. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen Med 2: 63–68, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Germani A, Di Rocco G, Limana F, Martelli F, Capogrossi MC. Molecular mechanisms of cardiomyocyte regeneration and therapeutic outlook. Trends Mol Med 13: 125–133, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J 20: 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Grauss RW, Winter EM, van Tuyn J, Pijnappels DA, Steijn RV, Hogers B, van der Geest RJ, de Vries AA, Steendijk P, van der Laarse A, Gittenberger-de Groot AC, Schalij MJ, Atsma DE. Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 293: H2438–H2447, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate (Abstract). Sci STKE 2005: pe37, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell 18: 2873–2882, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y, Dong A, Du Y, Huang X, Wang J, Lei X, Zheng X. Homing and differentiation of mesenchymal stem cells delivered intravenously to ischemic myocardium in vivo: a time-series study. Pflugers Arch 453: 43–52, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kermani AJ, Fathi F, Mowla SJ. Characterization and genetic manipulation of human umbilical cord vein mesenchymal stem cells: potential application in cell-based gene therapy. Rejuvenation Res 11: 379–386, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Kutala VK, Vikram DS, Wisel S, Chacko SM, Kuppusamy ML, Mohan IK, Zweier JL, Kwiatkowski P, Kuppusamy P. Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol 293: H2129–H2139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruglyakov PV, Sokolova IB, Zin'kova NN, Viide SK, Aleksandrov GV, Petrov NS, Polyntsev DG. In vitro and in vivo differentiation of mesenchymal stem cells in the cardiomyocyte direction. Bull Exp Biol Med 142: 503–506, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Lyon A, Harding S. The potential of cardiac stem cell therapy for heart failure. Curr Opin Pharmacol 7: 164–170, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Mageed AS, Pietryga DW, DeHeer DH, West RA. Isolation of large numbers of mesenchymal stem cells from the washings of bone marrow collection bags: characterization of fresh mesenchymal stem cells. Transplantation 83: 1019–1026, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Mazhari R, Hare JM. Advances in cell-based therapy for structural heart disease. Prog Cardiovasc Dis 49: 387–395, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol 123: 702–711, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Pandian RP, Kutala VK, Parinandi NL, Zweier JL, Kuppusamy P. Measurement of oxygen consumption in mouse aortic endothelial cells using a microparticulate oximetry probe. Arch Biochem Biophys 420: 169–175, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med 35: 1138–1148, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 25: 818–827, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Schuleri KH, Boyle AJ, Hare JM. Mesenchymal stem cells for cardiac regenerative therapy. Handb Exp Pharmacol 180: 195–218, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Shyu KG, Wang BW, Hung HF, Chang CC, Shih DT. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J Biomed Sci 13: 47–58, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg 30: 353–361, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80: 229–236, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Tomita Y, Makino S, Hakuno D, Hattan N, Kimura K, Miyoshi S, Murata M, Ieda M, Fukuda K. Application of mesenchymal stem cell-derived cardiomyocytes as bio-pacemakers: current status and problems to be solved. Med Biol Eng Comput 45: 209–220, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 29: 74–82, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Wisel S, Chacko SM, Kuppusamy ML, Pandian RP, Khan M, Kutala VK, Burry RW, Sun B, Kwiatkowski P, Kuppusamy P. Labeling of skeletal myoblasts with a novel oxygen-sensing spin probe for noninvasive monitoring of in situ oxygenation and cell therapy in heart. Am J Physiol Heart Circ Physiol 292: H1254–H1261, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, van den Bos EJ, Wielopolski PA, de Jong-Popijus M, Duncker DJ, Krestin GP. High-resolution magnetic resonance imaging of iron-labeled myoblasts using a standard 1.5-T clinical scanner. Magma 17: 201–209, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 24: 928–935, 2006. [DOI] [PubMed] [Google Scholar]