Abstract
Cholera toxin B subunit conjugated to saporin (SAP, a ribosomal inactivating protein that binds to and inactivates ribosomes) was injected in both stellate ganglia to evaluate the physiological response to targeted ablation of cardiac sympathetic neurons. Resting cardiac sympathetic activity (cardiac sympathetic tonus), exercise-induced sympathetic activity (heart rate responses to graded exercise), and reflex sympathetic activity (heart rate responses to graded doses of sodium nitroprusside, SNP) were determined in 18 adult conscious Sprague-Dawley male rats. Rats were randomly divided into the following three groups (n = 6/group): 1) control (no injection), 2) bilateral stellate ganglia injection of unconjugated cholera toxin B (CTB), and 3) bilateral stellate ganglia injection of cholera toxin B conjugated to SAP (CTB-SAP). CTB-SAP rats, compared with control and CTB rats, had reduced cardiac sympathetic tonus and reduced heart rate responses to graded exercise and graded doses of SNP. Furthermore, the number of stained neurons in the stellate ganglia and spinal cord (segments T1–T4) was reduced in CTB-SAP rats. Thus CTB-SAP retrogradely transported from the stellate ganglia is effective at ablating cardiac sympathetic neurons and reducing resting, exercise, and reflex sympathetic activity. Additional studies are required to further characterize the physiological responses to this procedure as well as determine if this new approach is safe and efficacious for the treatment of conditions associated with excess sympathetic activity (e.g., autonomic dysreflexia, hypertension, heart failure, and ventricular arrhythmias).
Keywords: cholera toxin B subunit, stellate ganglion, neurotoxin, saporin
cardiovascular diseases (e.g., hypertension, stroke, heart failure, coronary heart disease) are the leading causes of death for both men and women in the United States. Excessive sympathetic activity is responsible for, and/or contributes to, the morbidity and mortality associated with these life-threatening disorders. Accordingly, efforts to reduce sympathetic activity are the first-line therapy for most, if not all, cardiovascular diseases. However, despite favorable effects, adverse complications (because of generalized sympathoinhibition, e.g., fatigue, impotence) limit compliance to these treatments.
Thoracic sympathectomy (stellate ganglion together with second and third thoracic ganglia ablation) partially overcomes these limitations and is an established minimally invasive procedure for sympathetic blockade in patients with hyperhidrosis, facial flushing, intractable angina, and ventricular tachyarrhythmias. The potential complications associated with this procedure include Horner's syndrome, paraesthesia, compensatory hyperhidrosis, and residual flushing as well as deaths in the individuals undergoing the procedure for intractable angina (32). These complications severely limit patient satisfaction with the procedure. However, targeted ablation of cardiac sympathetic neurons may reduce the incidence of these complications as well as maintain afferent function.
Saporin (SAP) is a ribosomal inactivating protein that binds to and inactivates ribosomes, disabling the cell's protein synthetic machinery and causing the cell to die over a period of hours to days through apoptotic mechanisms (2, 25, 30). SAP can be linked to molecules that allow targeting of the toxin to a precisely defined population of cells (19). For example, SAP can be linked to retrogradely transported molecules like cholera toxin B subunit (CTB) that binds to specific membrane components that are differentially expressed on nerve cells. This provides a means for targeting SAP to a subset of neurons that could not previously be selectively ablated. Specifically, SAP conjugated to CTB, which binds to GM1 gangliosides located in the plasma membranes of sympathetic preganglionic neurons (SPNs), could be used to ablate SPNs by injecting the compound in the stellate ganglia.
Therefore, we tested the hypothesis that bilateral stellate ganglia injection of SAP conjugated to CTB reduces resting cardiac sympathetic activity (cardiac sympathetic tonus), exercise-induced sympathetic activity (heart rate responses to graded exercise), and reflex sympathetic activity [heart rate responses to graded doses of sodium nitroprusside (SNP)]. To test this hypothesis, 18 adult Sprague-Dawley male rats were randomly divided into the following three groups (n = 6/group): 1) control (no injection), 2) bilateral stellate ganglia injection of unconjugated CTB and 3) bilateral stellate ganglia injection of cholera toxin B conjugated to SAP (CTB-SAP). Specifically, we injected CTB-SAP bilaterally in the stellate ganglia and compared its physiological and neurotoxic effects on cardiac sympathetic neurons with that of unconjugated CTB and no stellate injections. We studied conscious, chronically instrumented rats to negate the confounding effects of anesthetic agents and surgical trauma.
MATERIALS AND METHODS
Surgical Procedures
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (14). Eighteen adult Sprague-Dawley male rats were randomly divided into the following groups (n = 6/group): 1) control (no injection), 2) bilateral stellate ganglia injection of CTB, and 3) bilateral stellate ganglia injection of CTB-SAP.
All surgical procedures were performed using aseptic surgical techniques. Rats were anesthetized with pentobarbital sodium (50 mg/kg ip), atropinized (0.05 mg/kg ip), intubated, and prepared for aseptic surgery. Supplemental doses of pentobarbital sodium (10–20 mg/kg ip) were administered if the rat regained the blink reflex or responded during the surgical procedures.
Radiotelemetry implantation.
After anesthesia was induced, a telemetry device (pressure, temperature, and electrocardiogram; Data Sciences International PhysioTel C50-PXT) was implanted in all rats as previously described (7, 33), and a catheter was placed in the intraperitoneal space for the infusion of fluids and drugs. Specifically, the transmitter body, which contains the thermistor, and the intraperitoneal catheter were placed in the intraperitoneal space through a ventral abdominal approach. The catheter was exteriorized on the dorsal aspect of the neck. The pressure sensor of the telemetry device, located within the tip of a catheter, was inserted in the descending aorta for continuous, nontethered recording of pulsatile arterial blood pressure. The electrical leads from the telemetry device were placed in a modified lead II configuration by placing the negative electrode slightly to the right of the manubrium and the positive electrode at the anterior axillary line along the fifth intercostal space. A minimum of 1 wk was allowed for recovery and for the animals to regain their presurgical weight. During the recovery period, the rats were handled, weighed, and acclimatized to the laboratory, treadmill, and investigators.
Stellate ganglia injections.
After the recovery period, the animals were anesthetized as described above, and the stellate ganglia were approached (one at a time) via a ventral thoracotomy through the first intercostal space. Specifically, a 2-cm midline incision was made, and the left and subsequently the right pectoral and intercostal muscles were partially dissected (∼5 mm) between the first and second rib to obtain access to the left and subsequently the right stellate ganglion (RSG), which are localized dorsal to the subclavian artery and vein. CTB (4 μl, 1 mg CTB dissolved in 200 μl of distilled water) or 4 μl of CTB conjugated with SAP (2.5 mg/ml) were mixed with 1 μl of 3% Evan's blue dye. The purpose of the Evan's blue dye was to visualize the injectate (CTB and SAP are colorless) assuring localization within the ganglia. All injections were confined within the stellate ganglia. The CTB/Evan's blue dye or CTB-SAP/Evan's blue dye solutions were mineral oil pressure-injected in the right and left stellate ganglia using a glass micropipette. A minimum of 1 wk was allowed for recovery and for the animals to regain their presurgical weight.
Experimental Procedures
Resting cardiac sympathetic activity (cardiac sympathetic tonus).
After the recovery period, conscious, unrestrained rats were studied in their home cages (∼13,350 cm3) for all experiments. On the day of the experiment, rats were brought in the laboratory and allowed to adapt to the environment for ∼1 h to ensure stable hemodynamic conditions. After the stabilization period, beat-by-beat, steady-state hemodynamic variables were recorded over 10–15 s. Subsequently, the heart rate and arterial pressure responses to cardiac autonomic sympathetic and parasympathetic blockade (β1-adrenergic and muscarinic-cholinergic receptor blockade) were determined. Drug doses for the sympathetic and parasympathetic antagonists were calculated relative to the animal's body weight on each experimental day. Cardiac muscarinic-cholinergic receptor blockade was achieved by infusion of the nonspecific muscarinic-cholinergic receptor antagonist atropine methyl bromide [methylatropine (MA) 3 mg/kg] through the intraperitoneal catheter. Because the heart rate response to MA reached its peak in 10–15 min, this time interval was standardized before the heart rate measurement. Cardiac β1-adrenergic receptor blockade was achieved by infusion of the specific β1-adrenergic receptor antagonist metoprolol (MT, 10 mg/kg) in the intraperitoneal catheter. MT was infused 15 min after MA, and again the heart rate response was measured after 15 min. The entire data collection took ∼2 h. At the end of the experiment, the rats were returned to their housing facilities. Sympathetic tonus was calculated as HRM − HRI, where HRM is heart rate after muscarinic-cholinergic receptor blockade and HRI is heart rate after β1-adrenergic receptor blockade.
Reflex sympathetic activity.
On an alternate day (at least 48 h apart), the rats were brought in the laboratory and allowed to adapt to the environment for ∼1 h to ensure stable hemodynamic conditions. After the stabilization period, beat-by-beat, steady-state hemodynamic variables were recorded over 10–15 s. Subsequently, four doses (0.05, 0.20, 0.50, and 0.80 mg/kg) of SNP (in random order) were injected through the intraperitoneal catheter. At least 10–15 min were allowed between doses to allow hemodynamic parameters to return to baseline levels. The mean peak changes in mean arterial pressure and heart rate in response to bolus injections of SNP were measured. The entire data collection took ∼2 h. The total volume delivered was ∼2 ml.
Exercise-induced sympathetic activity.
On an alternate day (at least 48 h apart), the rats were brought in the laboratory and allowed to adapt to the environment for ∼1 h to ensure stable hemodynamic conditions. After the stabilization period, beat-by-beat, steady-state hemodynamic variables were recorded over 10–15 s. Subsequently, each rat ran continuously, without aversive stimuli on a motorized treadmill at 5, 10, 15, and 20 m/min on a 10% grade for ∼3 min at each workload. The steady-state arterial pressure and heart rate responses were recorded during the 3rd min at workloads 5–15 m/min. However, all animals required gentle coaxing, (i.e., tapping on the hindquarters) during the highest workload. When rats failed to complete the entire 3 min at the highest workload, data were recorded at the point when the rats resisted the coaxing. By using these relatively low workloads with no aversive stimuli and providing training sessions, we feel we are truly studying a response to exercise rather than a response to stress. A cross-over design, resting, reflex, and exercise protocols, was used to prevent an order effect.
Perfusion, tissue processing, and immunocytochemistry.
After completion of the studies (∼3 wk), the rats were deeply anesthetized with pentobarbital sodium (100 mg/kg), injected with heparin (1,000 IU), and flushed transcardially with oxygenated tissue culture medium (catalogue no. D-8900; Sigma, St. Louis, MO). Perfusions were done with 4% formaldehyde in 0.1 M phosphate buffer, pH 7.4. Rats were perfused transcardially with 500 ml of tissue culture medium and then 1 liter of fixative. Spinal cords and stellate ganglia from all rats were removed and postfixed intact in formaldehyde for 3 days at room temperature on a shaker. After postfixation, the thoracic spinal cords segments (T1–T4) were removed. The rostral edge of each dorsal root entry zone was taken as the rostral boundary for each segment. Blocks of thoracic segments T1–T4 were obtained because SPNs projecting to the stellate ganglia are concentrated in these segments (44). Spinal cords and ganglia were cryoprotected in increasing concentrations of sucrose (10, 20, and 30%), embedded in optimal-cutting temperature, and sectioned on a cryostat.
The stellate ganglia and dorsal root ganglia (T1–T4) were sectioned at 10-μm intervals and stained with cresyl violet. The cardiac sympathetic postganglionic cell bodies, on every 16th section, were counted and measured with the aid of a ×40 objective and MicroBrightField Neurolucida software interfaced with a BH-2 Olympus microscope. Only neurons with distinct, prominent nucleoli were counted. Based on a study by Jones (15), no correction for split nucleoli is necessary in 10-μm sections.
The spinal cords blocks (T1–T4) were sectioned horizontally at 10-μm intervals. The sections were rinsed three times (10 min each) in Tris phosphate-buffered saline (pH 7.4) with 0.05% thimerosal (TPBS) and then blocked in 10% heat-inactivated normal horse serum (NHS; Invitrogen) in TPBS for 1 h. Sections were incubated overnight at 4°C in goat anti-CTB antiserum (1:10,000; List Biologicals) in TPBS containing 5% NHS. After being rinsed three times (10 min each), sections were incubated with biotinylated donkey anti-goat antiserum (1:500; Jackson Laboratories) in TPBS with 5% NHS for 2 h at room temperature. After being rinsed, sections were incubated with Avidin-Biotin Complex (ABC Elite kit; Vector Laboratories) according to the manufacturer's instructions. CTB-immunoreactive SPNs were revealed by a nickel-intensified diaminobenzidine reaction. Neuronal cell bodies, which appeared black, were counted and measured in every 10th spinal cord section.
Data analysis.
All recordings were sampled at 2 kHz, and the data were expressed as means ± SE. All data were the average of every beat during the last 10–15 s of the period. A one-factor ANOVA with post hoc Holm-Sidak method was used to compare sympathetic tonus and resting mean arterial pressure and heart rate in the control, CTB, and CTB-SAP groups (Fig. 1 and Table 1). A two-factor ANOVA with repeated measures on one factor was used to compare mean arterial blood pressure and heart rate at rest and during exercise in the control, CTB, and CTB-SAP groups (Fig. 2). A two-factor ANOVA with post hoc Holm-Sidak method was used to compare the change in mean arterial pressure and heart rate in response to SNP in the three groups (Fig. 3). Finally, a Student's paired t-test was used to compare neuronal number and soma area between the CTB and CTB-SAP groups (Figs. 4 and 5).
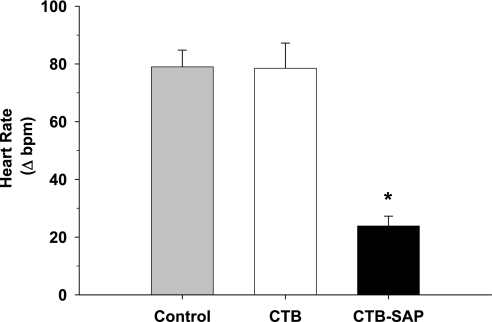
Fig. 1.
Resting cardiac sympathetic activity (i.e., cardiac sympathetic tonus) in the control group, group with bilateral stellate ganglia injection of unconjugated cholera toxin B (CTB), and group with bilateral stellate ganglia injection of cholera toxin B conjugated to saporin (CTB-SAP). There was no difference in sympathetic tonus between the control and CTB group. However, sympathetic tonus was significantly lower in the CTB-SAP group. *P < 0.05, CTB-SAP vs. control and CTB.
Table 1.
Resting MAP and HR in noninjected rats (control) and rats that had CTB or CTB-SAP injected in both stellate ganglia
| Control | CTB | CTB-SAP | |
|---|---|---|---|
| MAP | 110±2 | 116±2 | 111±1 |
| HR | 351±7 | 364±10 | 328±6*† |
Values are means ± SE. CTB, bilateral stellate ganglia injection of unconjugated cholera toxin B; CTB-SAP, bilateral stellate ganglia injection of CTB conjugated to saporin; MAP, mean arterial pressure; HR, heart rate. P ≤ 0.05, CTB vs. CTB-SAP (*) and control vs. CTB-SAP (†).
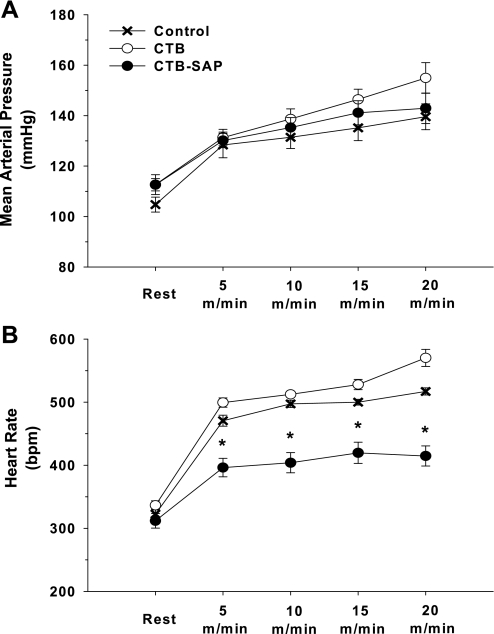
Fig. 2.
Exercise-induced sympathetic activity [i.e., mean arterial pressure (A) and heart rate (B)] at rest and during graded treadmill exercise (5, 10, 15 and 20 m/min) in the control, CTB, and CTB-SAP groups. There were no differences in mean arterial pressure during graded exercise between the three groups. Similarly, there was no difference in the heart rate response during graded exercise between the control and CTB group; however, the heart rate response was significantly lower in the CTB-SAP group. bpm, Beats/min. *P < 0.05, CTB-SAP vs. control and CTB.
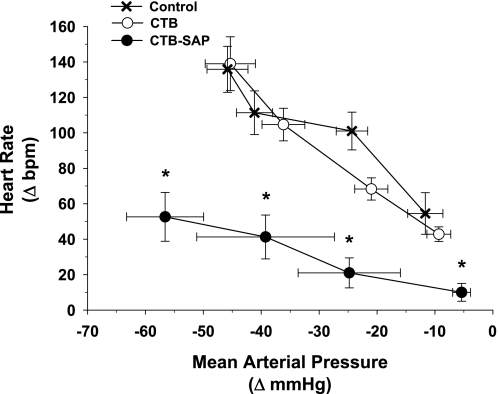
Fig. 3.
Reflex sympathetic activity [i.e., the mean arterial pressure and heart rate responses to increasing doses of sodium nitroprusside (0.05, 0.20, 0.50, and 0.80 mg/kg)] in the control, CTB, and CTB-SAP groups. There were no differences in the mean arterial pressure and heart rate responses to graded doses of sodium nitroprusside between the control and CTB groups. However, the reflex heart rate responses to sodium nitroprusside were significantly lower in the CTB-SAP group. *P < 0.05, CTB-SAP vs. control and CTB.
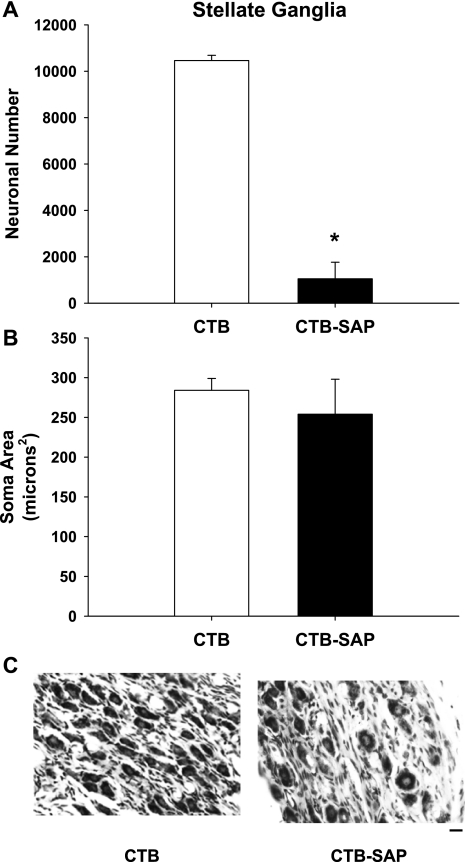
Fig. 4.
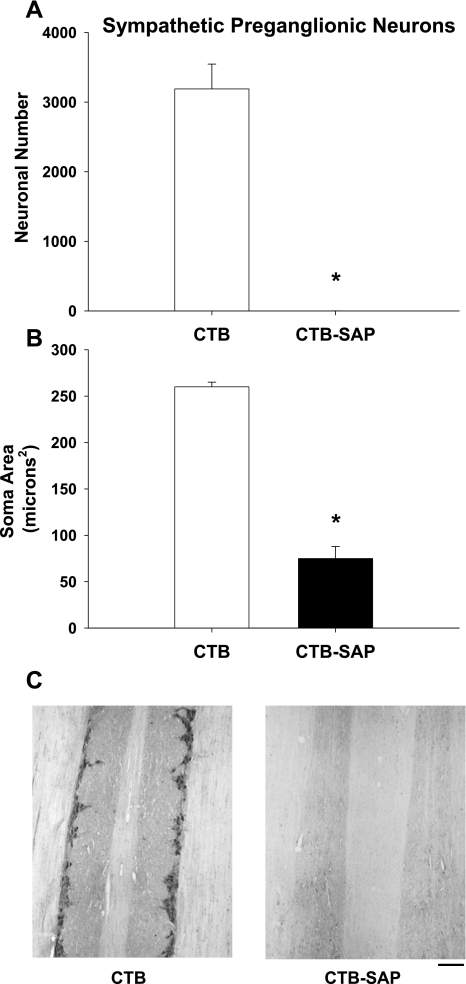
Neuronal number (A), soma area (B) and cresyl violet-stained neurons (C) of the stellate ganglia from rats that had CTB or CTB-SAP injected in both stellate ganglia. Counts of stellate neurons in the CTB-SAP group showed a significant reduction in the number of neurons compared with the CTB group (A). These results are consistent with C showing many stellate neurons in the CTB group and few neurons in the CTB-SAP group. However, mean soma area (B) was not different between the two groups. Scale bar = 15 μm. *P < 0.05, CTB vs. CTB-SAP.
Fig. 5.
Neuronal number (A), soma area (B), and CTB-immunoreactive neurons of the T1–T4 spinal cord (C) from rats that had CTB or CTB-SAP injected in both stellate ganglia. A similar number of CTB-immunoreactive SPNs were found on both sides of the intermediolateral cell column of the T1–T4 spinal cord; however, virtually all of the CTB-immunoreactive SPNs were eliminated in the CTB-SAP group (A and C). Similarly, mean soma area of SPNs was decreased in the CTB-SAP group compared with the CTB group (B). Scale bar = 250 μm. *P < 0.05, CTB vs. CTB-SAP.
RESULTS
Resting arterial pressure and heart rate on the three experimental days were averaged for each group and presented in Table 1. Mean arterial pressure was not different between the control, CTB, and CTB-SAP groups. Similarly, heart rate was not different between the control and CTB groups. However, heart rate was significantly lower in the CTB-SAP group compared with the control and CTB groups.
Figure 1 presents cardiac sympathetic tonus in the control, CTB, and CTB-SAP groups. There were no differences in sympathetic tonus between the control and CTB groups (79 ± 6 vs. 78 ± 9 beats/min, respectively). However, sympathetic tonus was significantly lower in the CTB-SAP group (24 ± 3 beats/min), indirectly documenting a lower level of cardiac sympathetic activity at rest.
Figure 2 presents exercise-induced sympathetic activity [i.e., mean arterial pressure (A) and heart rate (B)] at rest and during graded treadmill exercise (5, 10, 15 and 20 m/min) in the control, CTB, and CTB-SAP groups. There were no differences in mean arterial pressure during graded exercise between the three groups. However, the heart rate response to graded exercise was significantly lower in the CTB-SAP group compared with the control and CTB groups. Rats ran without aversive stimuli; however, all animals required gentle coaxing (i.e., tapping on the hindquarters) during the highest workload. When rats failed to complete the entire 3 min at the highest workload, data were recorded at the point when the rats resisted the coaxing. The average running duration during the highest workload was 2.3 ± 0.3, 1.0 ± 0.3, and 1.8 ± 0.5 min for the Control, CTB, and CTB-SAP groups, respectively.
Figure 3 presents reflex sympathetic activity [i.e., the mean arterial pressure and heart rate responses to increasing doses of SNP (0.05, 0.20, 0.50, and 0.80 mg/kg)] in the control, CTB, and CTB-SAP groups. There were no differences in the mean arterial pressure response to SNP between the three groups. Furthermore, the reflex increase in heart rate was not different between the control and CTB groups; however, the reflex heart rate response was significantly lower in the CTB-SAP group compared with the control and CTB groups.
Figure 4 presents neuronal number (A), soma area (B), and cresyl violet-stained stellate neurons (C) from rats that had CTB or CTB-SAP injected in both stellate ganglia. Counts of stellate neurons in the CTB-SAP group showed a significant reduction in the number of neurons compared with the CTB group (1,051 ± 713 vs. 10,463 ± 222, respectively). These results are consistent with Fig. 4C showing many stellate neurons in the CTB group and few neurons in the CTB-SAP group. However, of the 700 neurons analyzed, the soma area (Fig. 4B) was not different between the two groups (CTB: 284.03 ± 15.47 vs. CTB-SAP: 254.28 ± 44.2 μm2).
Figure 5 presents neuronal number (A), soma area (B), and CTB-immunoreactive neurons of the T1–T4 spinal cord (C) from rats that had CTB or CTB-SAP injected in both stellate ganglia. A similar number of CTB-immunoreactive SPNs were found on both sides of the intermediolateral cell column of the T1–T4 spinal cord (Fig. 5, A and C). However, virtually all of the CTB-immunoreactive SPNs were eliminated in the CTB-SAP group (Fig. 5, A and C). Similarly, mean soma size of SPNs was decreased (−85%) in the CTB-SAP group compared with the CTB group (Fig. 5B). Control slides (without primary antibody) did not have labeled cells.
DISCUSSION
In this study, we tested the hypothesis that bilateral stellate ganglia injection of SAP reduces resting cardiac sympathetic activity (cardiac sympathetic tonus), exercise-induced sympathetic activity (heart rate responses to graded exercise), and reflex sympathetic activity (heart rate responses to graded doses of SNP). The major findings of this study include: 1) CTB-SAP rats had reduced cardiac sympathetic tonus (Fig. 1), reduced heart rate responses to graded exercise (Fig. 2), and reduced reflex heart rate responses to SNP (Fig. 3) and 2) associated with the reduced resting, exercise-induced and reflex-induced sympathetic activity was a reduction in the number and/or area of stellate postganglionic neurons and cardiac SPNs (Figs. 4 and 5).
It is important to note that the measures of sympathetic tonus provide only an indirect indication of resting cardiac sympathetic activity. However, sympathetic tonus represents the effect of the sympathetic nervous system on the heart without the influence of the opposing limb (parasympathetic) of the autonomic nervous system. By using sympathetic tonus, investigators are also able to account for the difference in intrinsic heart rate. Finally, the heart rate responses to autonomic blockers (using procedures identical to the procedures used in this study) are significantly associated with heart rate at rest, during exercise, and after exercise (4, 5).
We performed bilateral stellate injections in this study. It has been known for decades that the sympathetic control of heart rate is primary exerted by the RSG (31, 38). Accordingly, cardiac sympathetic tonus, the reflex heart rate response to reductions in arterial pressure induced by SNP, and the heart rate responses during graded dynamic exercise were lower in CTB-SAP rats. This is an important consideration because several long-term cohort studies have found resting heart rate to be a risk factor for mortality from coronary heart disease, cardiovascular diseases, cancer, or all causes (8, 10–13, 16, 28, 45, 46). Specifically, high heart rates predict cardiovascular morbidity and mortality in the healthy population, in hypertensive patients, and in those with coronary heart disease or heart failure (1). Thus selectively reducing heart rate may reduce mortality and cardiovascular events. However, this theory requires additional investigation because existing agents that lower heart rate [with the exception of ivabradine, a pacemaker current, I(f), or “funny” channel inhibitor], such as β-adrenergic receptor antagonists and nondihydropyridine calcium channel blockers, have other cardiovascular actions that confound the conclusions (9, 49).
It has also been known for decades that the left stellate ganglion (LSG) is primarily responsible for ventricular arrhythmic events (38, 39, 41). The response to ischemia is complex and involves the cardio-cardiac sympathetic reflex with the efferent limb mainly through the RSG and the afferent limb mainly through left afferent fibers (21–23). Specifically, cardiac sympathetic afferents are excited by ischemia and elicit a cardio-cardiac sympathetic reflex (3, 22) that plays a major role in the genesis of ventricular arrhythmias (35). The cardio-cardiac sympathetic reflex, in addition to mediating cardiac sympathoexcitation, inhibits cardiac vagal efferent activity (20, 26). Left sympathectomy reduces the influence of the cardio-cardiac sympathetic reflex and protects the heart during ischemia. However, very high heart rates may overcome the protective effects of removal of the LSG (39). For these reasons, we studied the effect of bilateral SPN ablation.
These data may be clinically relevant because the mechanisms mediating several forms of life-threatening ventricular tachycardia involve sympathetic activity induced catecholamine activation of cAMP-dependent protein kinase A, which phosphorylates several key calcium regulatory proteins and increases intracellular calcium. Excessive calcium release during sympathetic activation may generate depolarizing membrane currents, which lead to delayed afterdepolarizations and ventricular arrhythmias (17, 47). Importantly, left stellate ganglionectomy increases the threshold for ventricular fibrillation (37) and increases ventricular refractoriness (42). In addition, left stellate ganglionectomy reduces arrhythmic events in high-risks patients with myocardial infarction (43), young adults with catecholaminergic polymorphic ventricular tachycardia (48), and individuals with long-QT syndrome who were not protected by full-dose β-adrenergic receptor antagonists therapy (36). Thus sympathetic activity is a critical factor for many forms of life-threatening ventricular tachycardia.
Accordingly, β-adrenergic receptor antagonists are the first-line therapy for symptomatic ventricular tachycardia (18, 29). Similarly, calcium channel antagonists (e.g., verapamil) may be added to the treatment regimen for patients with ventricular tachycardia. However, despite their favorable effects, adverse complications limit compliance to these medications. For example, the Medical Research Council trial (23a) on hypertensive patients documented that, for every myocardial infarction or stroke prevented by β-adrenergic receptor antagonists, three patients withdrew from the study secondary to impotence and another seven withdrew because of fatigue (24).
Targeted, bilateral stellate ganglia injection of SAP may overcome these issues because bilateral stellate ganglia injection of SAP selectively reduces the major source of norepinephrine release to the heart. Importantly, bilateral stellate ganglia injection of SAP is a preganglionic denervation, which suggests that there would be no reinnervation (48). Furthermore, bilateral stellate ganglia injection of SAP does not completely eliminate catecholamines to the heart, which suggests that postdenervation supersensitivity would be reduced (40). This procedure may also avoid limitations of medical therapy such as incomplete compliance due to the potential for anhydrosis, Horner's syndrome, and compensatory hyperhidrosis.
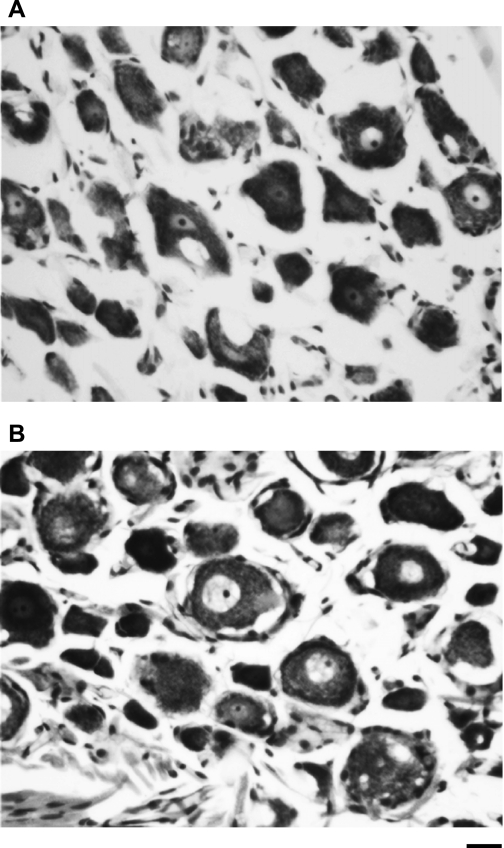
It is important to note that thoracic sympathectomy (stellate ganglion ablation) is markedly different form targeted ablation of cardiac sympathetic pre- and postganglionic fibers. Stellectomy ablates fibers passing through the ganglia disrupting afferent signals as well as efferent fibers ascending and descending within the sympathetic chain. For example, fibers traveling to the superior cervical ganglia are disrupted, resulting in Horner's syndrome, and afferent fibers are disrupted, resulting in paraesthesia and the absence of cardiac pain. In this context, rats with bilateral stellate ganglia injections of SAP did not have Horner's syndrome, and neurons within the dorsal root ganglia were not affected (Fig. 6), suggesting that afferent pathways remained functional. However, additional studies will be required to further characterize this new procedure.
Fig. 6.
Cresyl violet-stained neurons of the dorsal root ganglia from rats that had CTB (A) or CTB-SAP (B) injected in both stellate ganglia. It is clear that CTB-SAP did not alter neurons within the dorsal root ganglia. Scale bar = 25.4 μm.
In addition, stellectomy largely prevents norepinephrine release in the heart (27, 34, 37, 49); that is, there is no way to grade the level of sympathetic denervation. In sharp contrast, with stellate injections of SAP, it may be possible to adjust the dosage to partially denervate the heart to desirable levels. Finally, this new method provides an additional technique to selectively denervate sympathetic control of the heart, providing options for susceptible individuals.
Conclusion
The results of this study show that CTB-SAP retrogradely transported from the stellate ganglia is effective at ablating SPNs innervating the heart and reducing resting, exercise, and reflex sympathetic activity. Because CTB binds to GM1 ganglioside, CTB-SAP is likely to provide an efficient means of eliminating central neurons that express this molecule in their membranes, including, not only SPNs, but also motor neurons, bulbospinal neurons, and sensory neurons with myelinated axons (19). Additional studies are required to further characterize the physiological responses to this procedure and determine if this new approach is safe and efficacious for the treatment of conditions associated with excess sympathetic activity (e.g., autonomic dysreflexia, hypertension, heart failure, and ventricular arrhythmias).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-88615.
Acknowledgments
We thank Adrienne Jordan for expert technical assistance.
REFERENCES
- 1.Bohm M, Reil JC. Perspectives of I(f) inhibition by ivabradine in cardiology. Drugs 67, Suppl 2: 43–49, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer 68: 349–355, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Casati R, Lombardi F, Malliani A. Afferent sympathetic unmyelinated fibres with left ventricular endings in cats. J Physiol 292: 135–148, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler MP, DiCarlo SE. Sinoaortic denervation prevents postexercise reductions in arterial pressure and cardiac sympathetic tonus. Am J Physiol Heart Circ Physiol 273: H2738–H2745, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Chandler MP, DiCarlo SE. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol Regul Integr Comp Physiol 274: R510–R516, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 112: 736–749, 1980. [DOI] [PubMed] [Google Scholar]
- 9.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 372: 817–821, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Gann PH, Daviglus ML, Dyer AR, Stamler J. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epidemiol Biomarkers Prev 4: 611–616, 1995. [PubMed] [Google Scholar]
- 11.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J 125: 1148–1154, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J 121: 172–177, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association detection project in industry. Am J Epidemiol 149: 853–862, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Laboratory Animals Resources Commission of Life Science. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Research Council, National Academy Press, 1996.
- 15.Jones R Split nucleoli as a source of error in nerve cell counts. Stain Technol 19: 91–95, 1937. [Google Scholar]
- 16.Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 113: 1489–1494, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Kontula K, Laitinen PJ, Lehtonen A, Toivonen L, Viitasalo M, Swan H. Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights. Cardiovasc Res 67: 379–387, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 91: 1512–1519, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Llewellyn-Smith IJ, Martin CL, Arnolda LF, Minson JB. Retrogradely transported CTB-saporin kills sympathetic preganglionic neurons. Neuroreport 10: 307–312, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Malliani A, Pagani M, Recordati G, Schwartz PJ. Spinal sympathetic reflexes elicited by increases in arterial blood pressure. Am J Physiol 220: 128–134, 1971. [DOI] [PubMed] [Google Scholar]
- 21.Malliani A, Parks M, Tuckett RP, Brown AM. Reflex increases in heart rate elicited by stimulation of afferent cardiac sympathetic nerve fibers in the cat. Circ Res 32: 9–14, 1973. [PubMed] [Google Scholar]
- 22.Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibres with atrial and ventricular endings. J Physiol 229: 457–469, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol 217: 703–709, 1969. [DOI] [PubMed] [Google Scholar]
- 23a.Medical Research Council Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. Br Med J 304: 405–412, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messerli FH, Grossman E. Beta-blocker therapy and depression. JAMA 288: 1845–1846, 2002. [PubMed] [Google Scholar]
- 25.Nayate A, Moore SA, Weiss RM, Taktakishvili O, Lin LH, Talman WT. Cardiac damage after lesions of the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol 296: R272–R279, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagani M, Schwartz PJ, Banks R, Lombardi F, Malliani A. Reflex responses of sympathetic preganglionic neurones initiated by different cardiovascular receptors in spinal animals. Brain Res 68: 215–225, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Pardini BJ, Lund DD, Schmid PG. Organization of the sympathetic postganglionic innervation of the rat heart. J Auton Nerv Syst 28: 193–201, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Persky V, Dyer AR, Leonas J, Stamler J, Berkson DM, Lindberg HA, Paul O, Shekelle RB, Lepper MH, Schoenberger JA. Heart rate: a risk factor for cancer? Am J Epidemiol 114: 477–487, 1981. [DOI] [PubMed] [Google Scholar]
- 29.Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 42: 863–870, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potts JT, Fong AY, Anguelov PI, Lee S, McGovern D, Grias I. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neuroscience 145: 1168–1181, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randall WC, Rohse WG. The augmentor action of the sympathetic cardiac nerves. Circ Res 4: 470–475, 1956. [DOI] [PubMed] [Google Scholar]
- 32.Rathinam S, Nanjaiah P, Sivalingam S, Rajesh PB. Excision of sympathetic ganglia and the rami communicantes with histological confirmation offers better early and late outcomes in Video assisted thoracoscopic sympathectomy (Abstract). J Cardiothorac Surg 3: 50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz PJ The rationale and the role of left stellectomy for the prevention of malignant arrhythmias. Ann NY Acad Sci 427: 199–221, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz PJ, Foreman RD, Stone HL, Brown AM. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am J Physiol 231: 923–928, 1976. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 109: 1826–1833, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz PJ, Snebold NG, Brown AM. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol 37: 1034–1040, 1976. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz PJ, Stone HL. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ Res 44: 637–645, 1979. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz PJ, Stone HL. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 62: 1256–1265, 1980. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz PJ, Stone HL. Left stellectomy and denervation supersensitivity in conscious dogs. Am J Cardiol 49: 1185–1190, 1982. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz PJ, Stone HL, Brown AM. Effects of unilateral stellate ganglion blockade on the arrhythmias associated with coronary occlusion. Am Heart J 92: 589–599, 1976. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz PJ, Verrier RL, Lown B. Effect of stellectomy and vagotomy on ventricular refractoriness in dogs. Circ Res 40: 536–540, 1977. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz P, Motolese M, Pollavini G, Lotto A, Ruberti U, Trazzi R, Bartorelli C, Zanchetti A. Prevention of Sudden Cardiac Death After a First Myocardial Infarction by Pharmacologic or Surgical Antiadrenergic Interventions. J Cardiovasc Electrophysiol 3: 2–16, 1992. [Google Scholar]
- 44.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988. [DOI] [PubMed] [Google Scholar]
- 45.Wannamethee G, Shaper AG. The association between heart rate and blood pressure, blood lipids and other cardiovascular risk factors. J Cardiovasc Risk 1: 223–230, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Wannamethee G, Shaper AG, Macfarlane PW. Heart rate, physical activity, and mortality from cancer and other noncardiovascular diseases. Am J Epidemiol 137: 735–748, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA 103: 511–518, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 358: 2024–2029, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimoto M, Wehrwein EA, Novotny M, Swain GM, Kreulen DL, Osborn JW. Effect of stellate ganglionectomy on basal cardiovascular function and responses to beta1-adrenoceptor blockade in the rat. Am J Physiol Heart Circ Physiol 295: H2447–H2454, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]