Abstract
Patients with hypothyroidism are at a higher risk for coronary vascular disease. Patients with diabetes and related vascular complications also have an increased incidence of low thyroid function. While thyroid hormones (THs) may be key regulators of a healthy vasculature, potential undesirable side effects hinder their use in the treatment of vascular disorders. TH analogs such as 3,5-diiodothyropropionic acid (DITPA) may provide a safer treatment option. However, the relative potency of DITPA on vascular growth, cardiac function, and metabolism is poorly understood. We hypothesized that the vascular growth-promoting effects of DITPA can be obtained with a minimum effect on cardiac function. Thyroidectomized Sprague-Dawley rats were given slow-release pellets with either thyroxine (T4, 2.7 or 5.2 mg) or DITPA (80 mg) for 6 wk and were compared with placebo. Heart mass, body mass, body temperature, serum THs, cardiac function (echocardiograms and hemodynamics), and myocardial arteriolar density were determined. Hypothyroidism led to reductions in cardiac function, heart mass, body temperature, and myocardial arterioles. High-dose T4 prevented arteriolar loss and the development of hypothyroidism. Low-dose T4 partially prevented the reduction in cardiac function but had minimal effects on arteriolar loss. In contrast, DITPA treatment prevented myocardial arteriolar loss but not the progression of hypothyroid-induced changes in cardiac function. The results suggested that DITPA can promote a healthy vasculature independently from its thyroid-related metabolic effects. Drugs in this class may provide new therapeutic options for patients with vascular disease.
Keywords: hypothyroidism, myocardial arterioles, vascular disease
hypothyroidism has been linked to a variety of cardiovascular diseases, such as myocardial infarction (9), coronary atherosclerosis (5), and congestive heart failure (2). Thyroid hormone (TH) supplementation can increase cardiac contractility (16), promote cardiac hypertrophy (7), and induce vasodilatation (22). Tomanek et al. (29) have shown that THs are proangiogenic and can stimulate angiogenesis and arteriolar growth in normal hearts. We have shown that TH treatment of rats during an induction of hypothyroidism prevents the associated loss of arterioles (15). These beneficial effects of THs make them potentially attractive for the use of treating various cardiovascular diseases. However, concerns have been raised about the potential undesirable adverse effects such as increased heart rate and metabolism.
It has been shown that the TH analog 3,5-diiodothyropropionic acid (DITPA) has inotropic selectivity (23) due partly to the enhanced release of sarcoplasmic reticulum calcium (11) while at the same time not increasing heart rate. DITPA can also induce angiogenesis and arteriolar growth in postinfarcted myocardium (31, 35) and increase the protein expression of vascular endothelial growth factor (VEGF)164, VEGF188, basic fibroblast growth factor (bFGF or FGF-2), angiopoietin-1, and Tie-2 (32). Our previous studies also demonstrated that DITPA administration increased myocardial blood flow in BIO-TO2 cardiomyopathic hamsters (13) and prevented the loss of brain blood vessels in rats as they developed hypothyroidism (25). However, phase-2 clinical trials conducted by the Department of Veterans Affairs and Titan Pharmaceuticals, in the management of patients with New York Health Association class III and IV heart failure, were terminated recently due to body mass loss, although this occurred in overweight rather than normal-weight individuals (clinicaltrials.gov).
Although clinical trials for DITPA in the treatment of end-stage heart failure have been terminated, the proangiogenic properties of DITPA or other analogs remain an attractive direction for new drug development, particularly for coronary atherosclerosis and peripheral vascular diseases. In vivo animal studies examining the proangiogenesis effects of DITPA were done by Tomanek and colleagues (31, 35) using the myocardial infarction model. We have also shown that hypothyroidism induced by either propylthiouracil (PTU) or surgical thyroidectomy leads to a dramatic reduction in myocardial arterioles within 6 wk and can be prevented by thyroxine (T4) supplementation (15, 28). However, the ability of DITPA to preserve a healthy vasculature in myocardium during developing hypothyroidism is unclear. Additionally, it is not clear whether thyroid analogs such as DITPA can promote healthy coronary vasculature at doses that lead to minimal or reduced effects on cardiac function and metabolism. If so, drugs in this class may provide important new therapies for patients with ischemic heart disease or diabetic vasculopathy. In this study, we used a surgical thyroidectomy in rats to induce hypothyroidism and supplemented with either T4 or DITPA for 6 wk to examine the ability of DITPA to prevent the vascular loss and reduction of heart function.
MATERIALS AND METHODS
Animal model and study design.
Thyroidectomized rats used in this study were purchased from Charles River (Wilmington, MA). Thirty-two male Sprague-Dawley rats were thyroidectomized at the age of 10.5 wk. Twelve days after surgery, they were randomly and evenly assigned to four groups (n = 8) and treated with subcutaneous T4 pellets (two doses, 2.7 and 5.2 mg), DITPA pellets (80 mg; DITPA was kindly provided by Dr. Eugene Morkin, University of Arizona), and placebo pellets. All pellets were prepared at 60-day release rates (Innovative Research of America, Sarasota, FL) and implanted in the neck area. Eight age- and sex-matched rats served as controls. All animals were exposed to a 12-h:12-h light-dark cycle and given standard rat chow and water ad libitum. After a 6-wk treatment, echocardiographic and hemodynamic measurements were collected. Changes in myocardial arteriolar density were quantified morphometrically. Serum was collected for total triiodothyronine (T3) and T4 assays. All procedures in this study were approved by the University of South Dakota Animal Care and Use Committee and followed institutional guidelines for animals.
Echocardiography and hemodynamics.
Echocardiography was performed using a VisualSonics Vevo 660 high-resolution imaging system with a 25-MHz RMV-710 transducer (Toronto, ON, Canada) as reported by our group previously (15). In brief, animals were anesthetized with isoflurane, and M-mode images were obtained from the short axis of the left ventricle (LV) at the level of the papillary muscles and used to measure LV internal dimensions (LVID) and wall thickness. Fractional shortening (FS) was calculated by the formula: %FS = [(LVIDd − LVIDs)/LVIDd] × 100, where d indicates diastole and s indicates systole. After echocardiograms were completed, hemodynamic measurements were performed by cannulation with a Millar (Houston, TX) ultraminiature pressure transducer catheter into the LV as described previously (15). Measurements were recorded and processed electronically by a MPVS-400 pressure-volume unit (Millar, Houston, TX).
Quantification of arterioles.
A determination of myocardial arteriolar density was performed as we have reported previously (15). Briefly, mouse anti-α-smooth muscle actin Cy3-conjugated monoclonal antibody (Sigma, St. Louis, MO) was used to label arterioles. Arteriolar length density (LD, average length of arterioles/unit myocyte volume) was calculated based on the following formula: LD (in mm/mm3) = ∑(a/b)/M, where a and b are the maximum and minimum external arteriolar diameters, respectively, and M is the area of myocytes (1).
Measurements of T3 and T4 serum levels.
Blood samples were collected and separated into serum aliquots. T3 and T4 were measured with solid-phase competitive ELISA kits according to the manufacturer's protocol (T3 kit, Bio-quant, San Diego, CA; and T4 kit, Diagnostic Systems, Webster, TX).
Statistical analyses.
One-way ANOVA models were used for all responses. An inverse transformation on maximal rate of pressure rise (+dP/dt) and a natural log transformation on heart weight, heart weight-to-body weight ratio, and heart rate were applied for analysis. Nonparametric one-way ANOVA (Kruskal-Wallis) analyses were performed on measurements of serum T3 and T4. Diagnostics were conducted to verify the assumptions before applying the models. A post hoc Dunnett's test was used to compare all treatment groups with the control group. Statistical analyses were performed using JMP version 7 (SAS Institute, Cary, NC), and significance was accepted at P < 0.05.
RESULTS
Physical data.
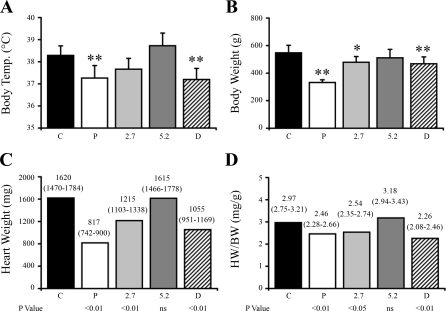
Twelve days after surgery, the rats were supplemented with two different doses of T4 or DITPA for 6 wk as indicated in materials and methods. The body temperature was taken at the time of the terminal experiments. The rats treated with placebo had a significantly lower body temperature, and whereas both T4 treatment doses prevented the decline in body temperature, DITPA was unable to preserve normal body metabolism as evidenced by a significant decline in body temperature compared with the control group (Fig. 1A). Placebo rats failed to gain weight, whereas weight gain was largely restored in all treatment groups, although the 2.7 mg T4 and DITPA groups remained significantly lower than controls (Fig. 1B). Rats treated with placebo, low-dose T4 (2.7 mg), and DITPA pellets showed significant cardiac atrophy compared with the controls. Heart weight in the 5.2 mg T4 group was the same as controls (Fig. 1C). After heart weight was normalized with body weight, this trend was the same with only the 5.2 mg T4 treatment group having a normal heart weight-to-body weight ratio (Fig. 1D).
Fig. 1.
Physical changes at terminal experiments. A: body temperature (Temp). B: body weight. C: heart weight. D: heart weight-to-body weight ratio (HW/BW). Thyroidectomized rats treated with placebo (P) pellets and thyroid hormone analog 3,5-diiodothyropropionic acid [DITPA (D); 80 mg/60-day release pellets] are shown. Thyroidectomized rats treated with 2 different doses of thyroxine (T4) pellets are labeled as 2.7 and 5.2 (in mg/60-day release). C, control. Data in A and B are means ± SD. Values in C and D are expressed as means (95% confidence interval). **P < 0.01 vs. control; *P < 0.05 vs. control. NS, nonsignificant.
Cardiac function.
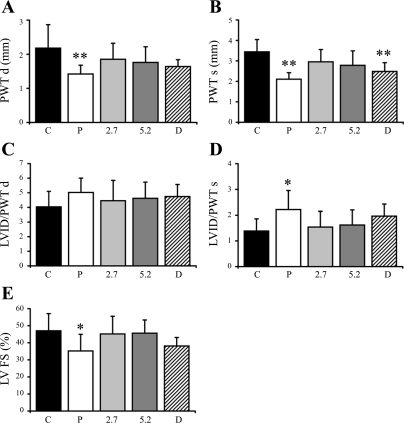
Cardiac dimensions collected by echocardiography in terminal experiments are reported in Fig. 2. All animals were kept under light anesthesia (0.5–1% isoflurane) to reduce the effects of anesthesia on cardiac function and dimensional measurements. In general, changes in systolic and diastolic posterior wall thickness (PWT) confirmed cardiac atrophy in hypothyroidism induced by thyroidectomy (placebo group), with a limited restoration of these parameters in the DITPA-treated group (P < 0.01 vs. control in PWTs; nonsignificant vs. control in PWTd) but normalized in both T4-treated groups (Fig. 2, A and B). Since the hearts in the placebo group were only half the size of controls, comparing the ratio of LVID to PWT in systole and diastole may allow a better comparison of relative cardiac shape. With these comparisons, only the increase in systolic LVID-to-PWT ratio in placebos reached significance (Fig. 2, C and D). FS, an indication of cardiac function, was impaired significantly in hypothyroid rats with limited improvement in the DITPA-treated group, although statistically there is no difference compared with controls. Both T4 doses prevented the impairment of cardiac function (Fig. 2E).
Fig. 2.
Echocardiographic data. PWTd, posterior wall thickness in diastole (A); PWTs, posterior wall thickness in systole (B); LVID/PWTd, ratio of left ventricle (LV) internal dimension to posterior wall thickness in diastole (C); LVID/PWTs, ratio of LV internal dimension to posterior wall thickness in systole (D). FS, fractional shortening (E). Values are means ± SD. **P < 0.01 vs. control; *P < 0.05 vs. control.
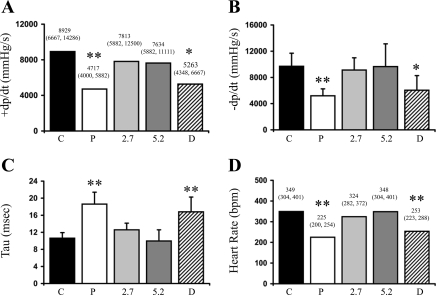
Hemodynamic data (Fig. 3) confirmed the deterioration of cardiac function in the placebo group, as evidenced by a 53% reduction in +dP/dt, a 54% reduction in the maximal rate of pressure decline (−dP/dt), and a 75% increase in the time constant of LV isovolumic relaxation (τ), compared with controls. Each of these parameters was restored in the T4 treatment groups. However, DITPA treatment did not prevent the deterioration in either +dP/dt or −dP/dt. As expected, hypothyroidism in the placebo group led to a significant decrease in heart rate, with normalization by both T4 doses. DITPA treatment did not prevent the onset of hypothyroid-induced bradycardia.
Fig. 3.
Hemodynamics data. +dP/dt, maximal rate of pressure rise; −dP/dt, maximal rate of pressure decline; τ, time constant of LV isovolumic relaxation; bpm, beats/min. Values in A and D are expressed as means (95% confidence interval). Data in B and C are means ± SD. **P < 0.01 vs. control; *P < 0.05 vs. control.
Myocardial arterioles.
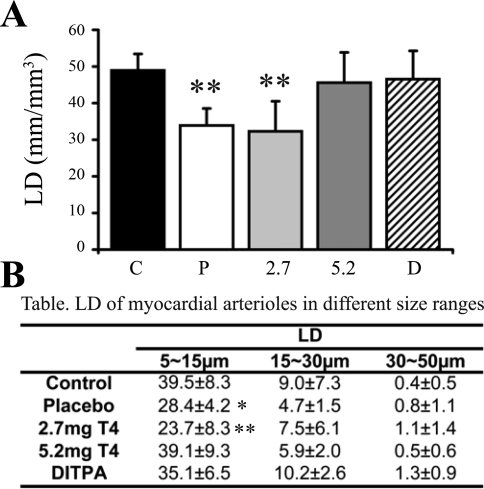
Arteriolar LD in the placebo group was reduced by 31%, and a similar reduction in numerical density was also observed (31%, data not shown) versus that in controls (Fig. 4A). This change was due exclusively to arteriolar loss in the smaller arteriolar size range (5–15 μm). Larger sized arterioles ranging from 15 to 30 and from 30 to 50 μm were not affected (Fig. 4B). Low-dose T4 (2.7 mg) did not prevent arteriolar loss. However, the higher dose of T4 (5.2 mg) prevented any changes in arteriolar LD. Importantly, DITPA prevented the reduction in arteriolar LD (Fig. 4) despite being ineffective in preventing a reduced body temperature and cardiac dysfunction.
Fig. 4.
A and B: arteriolar length density (LD) in myocardium. Values are means ± SD. **P < 0.01 vs. control; *P < 0.05 vs. control.
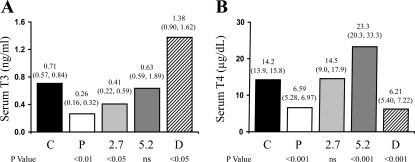
TH serum levels.
THs in whole serum were detected by ELISA assays. As shown in Fig. 5, serum T3 and T4 were significantly decreased in the placebo group. Low-dose T4 treatment normalized serum T4 levels but was unable to restore serum T3 levels to normal. High-dose T4 normalized serum T3 levels but led to significantly higher serum T4 levels. As previously reported, DITPA has a strong affinity to T3 receptors but not T4 receptors (23) and it can also be detected by free T3 assays (13). Consequently, T3 was elevated by DITPA but T4 was reduced to the same extent as in the placebo group (Fig. 5).
Fig. 5.
Serum triiodothyronine (T3; A) and T4 (B) levels. Values are expressed as median (25th and 75th percentiles).
DISCUSSION
The major finding from this study is that the TH analog DITPA can promote a healthy coronary vasculature independently from its thyroid effects on cardiac function. This is an important finding because it raises the possibility that drugs in this class can be used to safely treat vascular impairment in patients with heart disease. Potential patient groups who may benefit from thyroid analogs with proangiogenic properties and minimal thyroid metabolic effects encompass a large segment of the population with heart disease. Not only would this include patients with mild hypothyroidism who are at higher risk for coronary vascular disease (3, 4) but also patients with diabetes who are prone to vasculopathy. It is worth noting here that patients with diabetes have a much higher incidence of low thyroid function than the general population (10). Our recent animal study also suggests that serum hormone levels in mild hypothyroidism may underestimate the incidence of thyroid deficiency at the tissue level (15). Importantly, we simply do not know how many individuals with vascular disease may have deficient TH levels in key target organs such as the heart and brain.
In this study, we compared the ability of an inadequate T4 dose (mild hypothyroidism), a higher T4 dose, and a therapeutic dose of DITPA to prevent arteriolar loss from hypothyroidism developing as a result of surgical thyroidectomy. The results confirmed that hypothyroidism induced by surgical thyroidectomy led to decreased body temperature, cardiac atrophy, reduced cardiac function, and significant myocardial loss of arterioles as previously reported by our group (15). The higher dose of T4 prevented a deterioration of cardiac function and a loss of myocardial arterioles and restored body temperature, body weight, and heart rate to normal. The low dose of T4 largely prevented the reduction in body temperature and almost restored cardiac function but had minimal effects on the development of cardiac atrophy and the loss of myocardial arterioles. In contrast, DITPA treatment prevented arteriolar loss but was unable to prevent the decline in body temperature or hemodynamic function and had only minimal effects on echo parameters of function or LV chamber geometry. This may seem counterintuitive based on reports typically showing an association between changes in cardiac function and vascular density in pathological overloading conditions such as ischemia (17) or hypertrophy (27). However, the induction of hypothyroidism represents the opposite situation with vascular unloading and loss of vessels. The molecular mechanism by which DITPA maintained the coronary vascular density despite the progression to hypothyroidism is not clear. However, we recently observed that cotreatment of rats with PTU and the coronary vasodilator, dipyridimole, prevented the loss of vessels but not the progression to hypothyroidism (Y. Liu and A. M. Gerdes, unpublished observation). So, the current result with DITPA is not surprising.
Many studies have shown that both THs and DITPA can stimulate angiogenesis in normal animals (30, 32) and myocardial infarction models (31, 35). However, the relative potency of the proangiogenic properties of DITPA and thyroid cardiac functional and metabolic properties are poorly understood. In this study, hypothyroidism alone reduced myocardial arteriolar density by 31% and a 6-wk DITPA treatment largely prevented this loss of blood vessels. Our previous study in BIO-TO2 cardiomyopathic hamsters showed that DITPA treatment restored impaired resting and maximal myocardial blood flow to normal (13). Although arteriolar changes were not assessed in that study, the current study suggests that improved microvascular density was likely responsible. Others have also shown that DITPA can directly induce the proliferation of endothelial cells and the relaxation of vascular smooth muscle cells (26). Using a chick chorioallantoic membrane model, Mousa et al. (21) reported that DITPA increased blood vessel spouting and branches to a similar extent as THs and that this effect was mediated through the membrane receptor integrin αVβ3 with downstream activation of the MAPK pathway. It has been demonstrated by Tomanek's group that DITPA-promoted angiogenesis is associated with increased bFGF, VEGF, angiopoietin, and Tie-2 (32). Spooner et al. (26) also showed that DITPA led to vasorelaxation after myocardial infarction through endothelial nitric oxide and β-adrenergic pathways. The current study suggests that the vasorelaxation-nitric oxide effect of DITPA can prevent vessel loss at a dose having minimal effects on myocyte function. This suggests that the effects of DITPA on vessel growth and myocyte function may be mediated through different signaling pathways or cell-specific differences in receptor subtypes or affinity. Further studies are needed to elucidate the molecular signaling mechanisms associated with angiogenesis from THs and analogs, such as DITPA, particularly in mammalian systems.
Reports of the effects of DITPA on LV systolic and diastolic function have been inconsistent. Mahaffey et al. (17) and Pennock et al. (24) showed that DITPA alone or the combination of DITPA and captopril improved both systolic and diastolic function by significantly increasing +dP/dt and −dP/dt values and reducing end-diastolic pressure. However, Spooner et al. (26) reported that DITPA alone did not alter the hemodynamics significantly with the only effect being a decrease in +dP/dt. Morkin et al. (20) showed DITPA improved diastolic function with systolic cardiac function unchanged. As shown here in rats, a previous study in our laboratory showed no detectible hemodynamic changes in cardiomyopathic hamsters after treatment with DITPA, which was also administered by subcutaneous pellets (13). The discrepancies between these studies may be related to differences in the type of experimental model, dosing, modes of administration, or animal species. The model used in the current study was thyroidectomy with slow-release DITPA pellets for 6 wk. This is the first animal model to examine the ability of DITPA to prevent the deterioration of cardiovascular function with the induction of hypothyroidism. Most previous studies used post-myocardial infarction animal models with a subcutaneous injection of DITPA for 2 to 3 wk or heart failure patients with orally administered DITPA in clinical trials. In summary, the effects of DITPA on LV function remain inconclusive.
Reports on the proangiogenic properties of DITPA, however, are clear, and the current study indicates that this important effect can be obtained at lower doses that do not stimulate changes in cardiac function. During a review of the DITPA clinical trial (“A TH to fight heart failure: Phase II trial”; clinicaltrials.gov) at the 2008 Heart Failure Society of America meeting, it was noted that the selected DITPA dose was poorly tolerated and may have been too high. Rather than targeting cardiac function, new clinical studies with thyroid analogs may consider the induction of important biological effects, such as the restoration or maintenance of normal microcirculation, since it appears that these effects can be obtained at lower, better tolerated doses. Indeed, key features of other beneficial cardiovascular drugs such as β-blockers and angiotensin-converting enzyme inhibitors are their biological effects, which improved ventricular remodeling and long-term outcome.
Thyroid analogs, such as DITPA, may provide attractive treatment options for coronary atherosclerosis and peripheral vascular diseases such as that occurring in diabetes mellitus (DM). DM affects 135 million people worldwide, and many of these patients have macro- and microangiopathy in multiple organs (34). Endothelial function is impaired due to the chronic state of hyperglycemia in these patients, and the macro- and microcirculatory dysfunction is considered one of the major causes of morbidity and mortality in patients with DM (18). For example, there are ∼82,000 foot amputations in the United States each year as a result of DM (cdc.gov). Therapeutic angiogenesis has been discussed recently as a novel approach to treat DM patients (12). In consideration of the strong proangiogenic property of DITPA with limited thyroid metabolic effects, further clinical studies in this area may prove fruitful. It has also been shown by Morkin et al. (19) that DITPA can lower cholesterol and improve cardiac performance without affecting heart rate. Patients with DM usually have a disturbed blood lipids profile and have a high incidence of coronary atherosclerosis and heart failure.
The determination of two different T4 doses in this study was based on a previous report from our laboratory and adjusted by body weight (15). The goal in selecting the T4 doses was to have a dose that normalized serum TH levels and cardiac function and another lower dose that produced mild hypothyroidism (subclinical hypothyroidism). A dosing study of DITPA pellets was also done previously in BIO-TO2 cardiomyopathic hamsters (13). The dose used in that study and the current study was equivalent to ∼3.8 mg·kg−1·day−1. The previous study showed minimal effects of DITPA on cardiac function but restoration of impaired blood flow to normal in BIO-TO2 hamsters (13). Based on this information, we correctly predicted that the selected dose of DITPA in this study would prevent vascular loss during the progression to hypothyroidism but would not prevent the development of cardiac functional and metabolic signs of hypothyroidism.
The TH and DITPA treatments were initiated 12 days after thyroidectomy. The status of the animals right before the treatment has not been addressed in thyroidectomized rats, but we have recently completed a temporal study examining the arteriolar changes after initiating PTU (1-, 3-, and 6-wk time points). In that study, we did not observe a significant loss of arterioles until 3 wk after beginning the treatment. Arteriolar density in 7-day PTU-treated rats was identical to controls (Y. Liu and AM Gerdes, unpublished observation). We suspect the small, but insignificant, reduction in arterioles between controls and DITPA-treated thyroidectomized rats may reflect a small amount of arteriolar loss between surgery and the initiation of treatment. However, based on our unpublished observations in rats treated with PTU for 7 days, we do not think the delay adversely affects the outcome and conclusions of the experiment.
TH analogs are being developed and tested in an effort to identify compounds that promote desirable effects of THs while minimizing undesirable effects. Potential therapeutic applications of thyroid analogs were reviewed recently by Brenta et al. (8). Minor modifications in structure may alter the efficiency of thyroid analog uptake (6), TH receptor binding (14), or recruitment of coactivators or corepressors (33). Our previous study and the current one indicate that DITPA can be administered at a dose which promotes a healthy vasculature in the brain and heart with limited metabolic effects (25). It would be helpful to determine the angiogenic properties of other available thyroid analogs to better understand their potential therapeutic utility. In the meantime, more clinical studies with DITPA may be warranted.
GRANTS
This study was supported by the National Center for Research Resources Grant P20-RR-017662 and by the South Dakota 2010 Initiative Research Centers Program.
Acknowledgments
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1.Adair TH, Wells ML, Hang J, Montani JP. A stereological method for estimating length density of the arterial vascular system. Am J Physiol Heart Circ Physiol 266: H1434–H1438, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid 12: 511–515, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Asvold BO, Bjoro T, Nilsen TI, Gunnell D, Vatten LJ. Thyrotropin levels and risk of fatal coronary heart disease: the HUNT study. Arch Intern Med 168: 855–860, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol 156: 181–186, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Auer J, Berent R, Weber T, Lassnig E, Eber B. Thyroid function is associated with presence and severity of coronary atherosclerosis. Clin Cardiol 26: 569–573, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow JW, Raggatt LE, Lim CF, Kolliniatis E, Topliss DJ, Stockigt JR. The thyroid hormone analogue SKF-94901 and iodothyronine binding sites in mammalian tissues: differences in cytoplasmic binding between liver and heart. Acta Endocrinol (Copenh) 124: 37–44, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Breisch EA, White FC, Hammond HK, Flynn S, Bloor CM. Myocardial characteristics of thyroxine stimulated hypertrophy. A structural and functional study. Basic Res Cardiol 84: 345–358, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Brenta G, Danzi S, Klein I. Potential therapeutic applications of thyroid hormone analogs. Nat Clin Pract Endocrinol Metab 3: 632–640, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Franklyn JA, Gammage MD, Ramsden DB, Sheppard MC. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond) 67: 585–590, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Gilani BB, MacGillivray MH, Voorhess ML, Mills BJ, Riley WJ, MacLaren NK. Thyroid hormone abnormalities at diagnosis of insulin-dependent diabetes mellitus in children. J Pediatr 105: 218–222, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Hoit BD, Pawloski-Dahm CM, Shao Y, Gabel M, Walsh RA. The effects of a thyroid hormone analog on left ventricular performance and contractile and calcium cycling proteins in the baboon. Proc Assoc Am Physicians 109: 136–145, 1997. [PubMed] [Google Scholar]
- 12.Khan TA, Sellke FW, Laham RJ. Gene therapy progress and prospects: therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther 10: 285–291, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Kuzman JA, Tang Y, Vogelsang KA, Said S, Anderson BE, Morkin E, Gerdes AM. Thyroid hormone analog, diiodothyropropionic acid (DITPA), exerts beneficial effects on chamber and cellular remodeling in cardiomyopathic hamsters. Can J Physiol Pharmacol 85: 311–318, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Leeson PD, Emmett JC, Shah VP, Showell GA, Novelli R, Prain HD, Benson MG, Ellis D, Pearce NJ, Underwood AH. Selective thyromimetics. Cardiac-sparing thyroid hormone analogues containing 3′-arylmethyl substituents. J Med Chem 32: 320–336, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Redetzke RA, Said S, Pottala JV, de Escobar GM, Gerdes AM. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am J Physiol Heart Circ Physiol 294: H2137–H2143, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Gerdes AM. Influence of hypothyroidism and the reversal of hypothyroidism on hemodynamics and cell size in the adult rat heart. J Mol Cell Cardiol 22: 1339–1348, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Mahaffey KW, Raya TE, Pennock GD, Morkin E, Goldman S. Left ventricular performance and remodeling in rabbits after myocardial infarction. Effects of a thyroid hormone analogue. Circulation 91: 794–801, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Meigs JB, Singer DE, Sullivan LM, Dukes KA, D'Agostino RB, Nathan DM, Wagner EH, Kaplan SH, Greenfield S. Metabolic control and prevalent cardiovascular disease in non-insulin-dependent diabetes mellitus (NIDDM): The NIDDM Patient Outcome Research Team. Am J Med 102: 38–47, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Morkin E, Ladenson P, Goldman S, Adamson C. Thyroid hormone analogs for treatment of hypercholesterolemia and heart failure: past, present and future prospects. J Mol Cell Cardiol 37: 1137–1146, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Morkin E, Pennock G, Spooner PH, Bahl JJ, Underhill Fox K, Goldman S. Pilot studies on the use of 3,5-diiodothyropropionic acid, a thyroid hormone analog, in the treatment of congestive heart failure. Cardiology 97: 218–225, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Mousa SA, O'Connor L, Davis FB, Davis PJ. Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated. Endocrinology 147: 1602–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ojamaa K, Balkman C, Klein IL. Acute effects of triiodothyronine on arterial smooth muscle cells. Ann Thorac Surg 56: S61–S66, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E. Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther 263: 163–169, 1992. [PubMed] [Google Scholar]
- 24.Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E. Combination treatment with captopril and the thyroid hormone analogue 3,5-diiodothyropropionic acid. A new approach to improving left ventricular performance in heart failure. Circulation 88: 1289–1298, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Schlenker EH, Hora M, Liu Y, Redetzke RA, Morkin E, Gerdes AM. Effects of thyroidectomy, T4, and DITPA replacement on brain blood vessel density in adult rats. Am J Physiol Regul Integr Comp Physiol 294: R1504–R1509, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Spooner PH, Thai HM, Goldman S, Gaballa MA. Thyroid hormone analog, DITPA, improves endothelial nitric oxide and beta-adrenergic mediated vasorelaxation after myocardial infarction. J Cardiovasc Pharmacol 44: 453–459, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol 22: 4803–4814, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 112: 3122–3130, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Tomanek RJ, Busch TL. Coordinated capillary and myocardial growth in response to thyroxine treatment. Anat Rec 251: 44–49, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Tomanek RJ, Doty MK, Sandra A. Early coronary angiogenesis in response to thyroxine: growth characteristics and upregulation of basic fibroblast growth factor. Circ Res 82: 587–593, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Tomanek RJ, Zimmerman MB, Suvarna PR, Morkin E, Pennock GD, Goldman S. A thyroid hormone analog stimulates angiogenesis in the post-infarcted rat heart. J Mol Cell Cardiol 30: 923–932, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zheng W, Christensen LP, Tomanek RJ. DITPA stimulates bFGF, VEGF, angiopoietin, and Tie-2 and facilitates coronary arteriolar growth. Am J Physiol Heart Circ Physiol 284: H613–H618, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Webb P Selective activators of thyroid hormone receptors. Expert Opin Investig Drugs 13: 489–500, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M, Egusa G, Okubo M, Yamakido M. Dissociation of microangiopathy and macroangiopathy in patients with type 2 diabetes. Diabetes Care 21: 1451–1454, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Weiss RM, Wang X, Zhou R, Arlen AM, Lei L, Lazartigues E, Tomanek RJ. DITPA stimulates arteriolar growth and modifies myocardial postinfarction remodeling. Am J Physiol Heart Circ Physiol 286: H1994–H2000, 2004. [DOI] [PubMed] [Google Scholar]