Abstract
Exposure to ambient air pollution has been associated with increases in blood pressure. We have previously demonstrated activation of the Rho/Rho kinase pathway in experimental hypertension in rats. In this investigation, we evaluated the effects of particulate matter of <2.5 μm (PM2.5) exposure on cardiovascular responses and remodeling and tested the effect of Rho kinase inhibition on these effects. C57BL/6 mice were exposed to concentrated ambient PM2.5 or filtered air for 12 wk followed by a 14-day ANG II infusion in conjunction with fasudil, a Rho kinase antagonist, or placebo treatment. Blood pressure was monitored, followed by analysis of vascular function and ventricular remodeling indexes. PM2.5 exposure potentiated ANG II-induced hypertension, and this effect was abolished by fasudil treatment. Cardiac and vascular RhoA activation was enhanced by PM2.5 exposure along with increased expression of the guanine exchange factors (GEFs) PDZ-RhoGEF and p115 RhoGEF in PM2.5-exposed mice. Parallel with increased RhoA activation, PM2.5 exposure increased ANG II-induced cardiac hypertrophy and collagen deposition, with these increases being normalized by fasudil treatment. In conclusion, PM2.5 potentiates cardiac remodeling in response to ANG II through RhoA/Rho kinase-dependent mechanisms. These findings have implications for the chronic cardiovascular health effects of air pollution.
Keywords: hypertension, vasoconstrictors, angiotensin II
ambient air pollution mediated by fine particulate matter (PM) of <2.5 μm in aerodynamic diameter (PM2.5) has been associated with adverse cardiovascular outcomes (8, 31, 39, 49). One important mechanism is the elevation in blood pressure (BP) that may occur within hours to days after exposure to high concentrations of PM2.5 (6, 16, 21, 52). Recent studies (21, 58) have shown that commonly encountered levels of airborne pollutants can result in a prohypertensive response in humans that may be exaggerated in predisposed individuals. This potentiating effect of inhaled particulates has been noted with other chronic conditions or risk factors such as atherosclerosis, diabetes, and postmenopausal status (31, 40, 49). Although the precise mechanisms remain elusive, there is increasing evidence that PM2.5 exposure results in rapid changes in the vasculature (7, 32). We (51) have previously shown that PM2.5 exposure results in the activation of RhoA/Rho-kinase (ROCK) through ROS pathways and hypothesized that this important signaling cascade may mediate at least some of the prohypertensive effects of PM2.5. Given the important role of RhoA/ROCK in a multitude of processes, including the regulation of smooth muscle tone, cellular migration, and hypertrophy (36, 47), in the present study we examined the effect of PM2.5 exposure on vascular function and cardiac remodeling and tested the effects of ROCK antagonism.
MATERIALS AND METHODS
All experimental procedures were approved by the Committees on Use and Care of Animals of New York University and The Ohio State University.
Animals and PM2.5 Exposure Protocol
Male C57BL/6 mice (8 wk old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal exposure and the monitoring of exposure atmosphere and ambient aerosol were performed as previously described (49, 51) using a versatile aerosol concentration enrichment system. Briefly, 52 mice were exposed to concentrated PM2.5 or filtered air (FA) in a mobile trailer at The Ohio State University campus of Columbus, OH (“Ohio's air pollution exposure system for the interrogation of systemic effects”). FA-exposed mice received an identical protocol with the exception of a high-efficiency particulate air filter positioned in the inlet valve position to remove PM2.5 in the filtered air stream. The exposure protocol was composed of 10× ambient concentration for 6 h/day, 5 days/wk, for a total of 12 wk from May to August 2008. The exposure was followed by an osmotic minipump implantation for the infusion of angiotensin II (ANG II) or vehicle. This was followed by treatment with fasudil or vehicle 24 h later. The concentration of PM2.5 in ambient air and in the chambers was monitored in filters that retained PM using an oscillating microbalance (Tapered-Element Oscillating Microbalance, model 1400, Rupprecht and Patashnick, East Greenbush, NY) to weigh PM.
Analysis of PM2.5 Concentration and Composition in the Exposure Chamber
To calculate exposure mass concentrations of PM2.5 in the exposure chambers, samples were collected on Teflon filters (Gelman Teflo, 37 mm, 0.2-mm pore, Gelman Sciences, Ann Arbor, MI) and weighed before and after sampling in a temperature- and humidity-controlled weighing room using an oscillating microbalance (Tapered-Element Oscillating Microbalance, model 1400, Rupprecht and Patashnick). Weight gains were used to calculate exposure concentrations. The analysis of PM2.5 composition was performed by RTI following compendium Environmental Protection Agency method IO-3.3 [“Determination of metals in ambient particulate matter using X-ray fluorescence (XRF) spectroscopy (http://www.epa.gov/ttnamti1/files/ambient/inorganic/mthd-3-3.pdf)]. The Thermo Quant'X XRF system was used (Thermo Fisher Scientific, Waltham, MA).
ANG II Infusion and Fasudil Treatment
At the end of the 12-wk exposure, mice received an osmotic pump (Alza, Mountain View, CA) containing ANG II (0.75 mg/kg/day ANG II in 0.15 mol/l NaCl and 0.01 N acetic acid) or vehicle for a duration of 14 days. This dose of ANG II provides a plasma concentration similar to that reported in patients with renovascular hypertension and was based on prior publications (19, 41). Fasudil (1 mg·kg−1·day−1) or placebo (0.9% NaCl) was started the day after ANG II and was administered intraperitoneally for 13 days. A previous study (47) has shown that fasudil is a inhibitor of ROCK.
BP Monitoring
Systolic BP (SBP) was measured using a computerized noninvasive tail-cuff manometry system (Visitech IITC model 129 System, Visitech Systems, Apex, NC). To avoid procedure-induced anxiety, mice were trained for 5 consecutive days before the experimental procedure, and fasudil was administrated 1 h earlier. The first 10 of 20 BP values recorded with each measurement were disregarded, and the remaining 10 values were collected and averaged for analysis of each mouse. BP was recorded daily after ANG II or placebo infusion for duration of 14 days.
Myograph Experiments
Myograph experiments were performed as previously described (49). Briefly, at the end of the study period, the aorta was removed, and 2-mm thoracic aortic rings were mounted in individual organ chambers. Rings were subjected to graded doses of the vasoconstrictor phenylephrine (PE; 10−9–10−5 mol/l). To examine functional RhoA/ROCK activity in the aorta, aortic rings were precontracted by PE (10−6.5 mol/l) with fasudil added in an cumulative manner. The degree of relaxation was taken as an indication of Rho/ROCK activity.
RhoA Activity Assays
We measured RhoA activity using two approaches. In the first approach, we measured the translocation of RhoA from the cytosol to membrane; in the second approach, we measured RhoA activity based on the binding of “active” Rho-GTP.
RhoA translocation assay.
Cardiac tissue was homogenized in cold homogenization buffer [containing (in mM) 00 Tris·HCl (pH 7.4), 1 EGTA, 1 EDTA, 1 PMSF, and 1 Na3VO4]. The homogenate was centrifuged at 100,000 g and 4°C for 20 min. The supernatant (cytosolic fraction) was collected, and the pellet (membrane fraction) was resuspended in homogenization buffer containing 1% Triton X-100. Protein concentrations were determined with a bicinchoninic acid kit (Pierce). RhoA migration was determined by Western blot analysis. Briefly, equal amounts of proteins were separated by SDS-PAGE and subsequently transferred to a nitrocellulose membrane. The membrane was then incubated with monoclonal Rho antibody. Finally, membranes were incubated with a horseradish peroxidase-linked secondary antibody and visualized with an enhanced chemiluminescence kit (Amersham).
RhoA activity.
RhoA-GTP levels in the aorta were determined with the G-LISA RhoA activation assay kit (Cytoskeleton, Denver, CO) according to the manufacturer's instructions. The RhoA G-LISA kit contains a Rho-GTP-binding protein linked to the wells of a 96-well plate. Active GTP-bound RhoA in the tissue lysates binds to the wells, whereas inactive GDP-bound RhoA is removed during washing steps.
Quantitative Real-Time PCR
Total RNA was prepared from heart tissue with a RNeasy Mini kit (Qiagen, Valencia, CA). cDNA was synthesized by a Transcriptor First-Strand cDNA Synthesis Kit (Roche, Indianapolis, IN). Quantitative real-time PCR was performed using the iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Sequences of primers used for real-time PCR in this study are shown in Table 1.
Table 1.
Primers used for real-time quantitative RT-PCR in this study
| Primer | Forward Oligonucleotides | Reverse Oligonucleotides |
|---|---|---|
| GEF-H1 | 5′-GAGGCGCAAGAACTTGGTAG-3′ | 5′-CCATCTCGGCTTTCTGTCTC-3′ |
| p115RhoGEF | 5′-CAGCTCTGAGAATGGCACTG-3′ | 5′-AGGATCGCTTCAGGGACAG-3′ |
| PDZ-RhoGEF | 5′-GGGGTCCTACCCTGAAGAAG-3′ | 5′-TTCCCAGGCCTCACAGTATC-3′ |
| l-Arginine | 5′-AAGTTAGAGCCCCGTGTCCT-3′ | 5′-CAGCTGCCAGAAGGAAGAAC-3′ |
| ROCK-I | 5′-TGGTGAAACACCAGAAGGAGCTGA-3′ | 5′-TTGCCCGCAACTGCTCAATATCAC-3′ |
| ROCK-II | 5′-TAGCGCCCTGCAAAGTTTAT-3′ | 5′-CCGAATGGACTGGTTTTGTT-3′ |
| Guanine dissociation inhibitor | 5′-GCCCTGTTTTGTCTTTGCTC-3′ | 5′-TCCATACTGAGGGCAAGTCC-3′ |
| Atrial natriuretic peptide | 5′-ATTGACAGGATTGGAGCCCAGAGT-3′ | 5′-TGACACACCACAAGGGCTTAGGAT-3′ |
| α-Tubulin | 5′-TGTGTCTTCCATCACTGCTTCCCT-3′ | 5′-AGCAGGCATTGGTGATCTCTGCTA-3′ |
| Collagen type I | 5′-TTCTCCTGGCAAAGACGGACTCAA-3′ | 5′-AGGAAGCTGAAGTCATAACCGCCA-3′ |
| Collagen type III | 5′-AGCTTTGTGCAAAGTGGAACCTGG-3′ | 5′-CAAGGTGGCTGCATCCCAATTCAT-3′ |
| Osteopontin | 5′-TCAGCTGGATGAACCAAGTCTGGA-3′ | 5′-ACTAGCTTGTCCTTGTGGCTGTGA-3′ |
| Transforming growth factor-β | 5′-GTGCGGCAGCTGTACATTGACTTT-3′ | 5′-TGTACTGTGTGTCCAGGCTCCAAA-3′ |
| MMP-1 | 5′-TTACGGCTCATGAACTGGGTCACT-3′ | 5′-GGATGTGGTGTTGTTGCACCTGTT-3′ |
| MMP-2 | 5′-TCTGGTGCTCCACCACATACAACT-3′ | 5′-CTGCATTGCCACCCATGGTAAACA-3′ |
| MMP-3 | 5′-AGCTGAGGACTTTCCAGGTGTTGA-3′ | 5′-ACACAGGATGCCTTCCTTGGATCT-3′ |
| MMP-9 | 5′-TGAACAAGGTGGACCATGAGGTGA-3′ | 5′-TAGAGACTTGCACTGCACGGTTGA-3′ |
| GAPDH | 5′-TGCATCCTGCACCACCAACTGCTT-3′ | 5′-ACAGCCTTGGCAGCACCAGTGGAT-3′ |
GEF, guaninine nucleotide exchange factor; ROCK, Rho-kinase; MMP, matrix metalloproteinase.
Collagen Staining
Masson's trichrome staining and picrosirius red staining were used for the histochemical determination of collagen expression in heart tissues. Four successive sections were collected on the same slide, and at least 10 sections from 3 consecutive slides per area per mouse were examined. Each image was digitized with a digital camera and analyzed under a research microscope (Zeiss Axioskop with Spot I digital camera, Jena, Germany) with NIH Image software (version 1.61, http://rsb.info.nih.gov/nih-image). Results are expressed as percentages of the total selected area. All analyses were performed blindly without knowledge of the origin of the samples.
Picrosirius red staining.
Frozen heart cross sections were fixed with ice-cold methanol for 5 min and washed twice in PBS. Sections were stained with picrosirius red (0.1% Sirius red in a saturated aqueous solution of picric acid, Sigma Chemical, St. Louis, MO) for 1 h and washed in acidified water (0.5% acetic acid). Sections were then dehydrated and mounted in a resinous medium. Type I/III collagen appears red on light microscopy, and collagen I appears yellow, whereas collagen III appears red on polarizing microscopy.
Masson's trichrome staining.
Frozen heart cross sections were fixed with acetone. Sections were then stained with Masson's trichrome stain. Collagen appears blue on light microscopy.
Gelatin Zymography for Matrix Metalloproteinase Activity
Gelatin zymography was performed to determine gelatinolytic activities of matrix metalloproteinase (MMP)-2 and MMP-9 as previously described (42). Myocardial protein (40 μg) was treated with sampling buffer (0.5 mol/l Tris·HCl, glycerol, 10% SDS, and 0.1% bromphenol blue) in a final solution of 20 μl. SDS-PAGE was performed using a 10% polyacrylamid gel containing 0.1% gelatin at 125 V for 60 min. SDS was removed with Triton X-100 for 60 min, and the gel was incubated in a developing buffer (Tris base, Tris·HCl, NaCl, CaCl2, Brij-35, and ZnCl2) overnight. Gels were stained for 3 h with 0.5% Coomassie G250 and destained for 60 min in 7% acetic acid and 35% methanol. Gelatinolytic activities were detected as a clear band against a black background (in arbitrary units/cm) and analyzed as relative optical densities.
Morphometric Analyses of Cardiac Hypertrophy
Frozen heart cross sections were stained with hematoxylin and eosin. Four successive sections were collected on the same slide, and at least 10 sections from 3 consecutive slides per area per mouse were examined. Each image was digitized with a digital camera and analyzed under a research microscope (Zeiss Axioskop with Spot I digital camera, Jena, Germany) with NIH Image software (version 1.61). Nuclear and cytosolic areas were quantified, respectively, and the results are expressed as ratios of nuclear to cytosolic areas. All analyses were performed blindly without knowledge of the origin of the samples.
Data Analyses
All data are expressed as means ± SD unless otherwise specified. Differences among groups were tested by one-way ANOVA and Boneferroni's post hoc test. In addition, the interaction between FA and PM was analyzed by two-way ANOVA using Graphpad Prizm software (version 4). P values of <0.05 were considered statistically significant.
RESULTS
PM2.5 Concentrations during the Study Period
Whole body exposure to PM2.5 was made using a versatile aerosol concentration enrichment system as previously described (11, 30). The mean daily ambient PM2.5 concentration at the study site was 6.5 ± 5.5 μg/m3, whereas the mean concentration inside the PM2.5 chamber was 74.3 μg/m3. During the exposure time period, the outdoor mean temperature was 27.4 ± 6.3°C (median 28.5°C), and the mean outdoor humidity was 65.8 ± 22.2% (median 61.5%). Because mice were exposed 6 h/day, 5 days/wk, the equivalent PM2.5 concentration to which mice were exposed to in the chamber “normalized” over the 12-wk period was 11.4 μg/m3 after taking into account nonexposed time and weekends, which is well within the annual average PM2.5 National Ambient Air Quality Standard of 15.0 μg/m3 [United States Environmental Protection Agency (51a)]. XRF elemental analysis revealed higher concentrations of a range of metals. Of note, in the transition metal category, levels of both Fe and Zn in the transition metals were disproportionately high (Supplemental Figs. 1 and 2).1
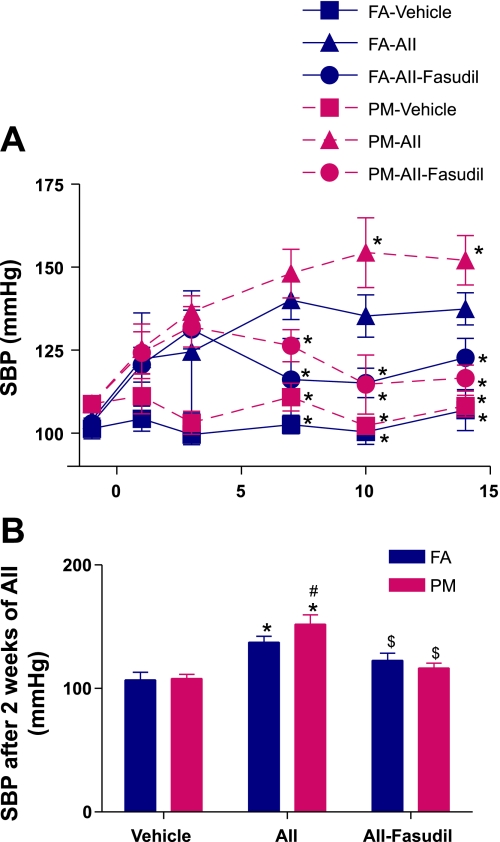
Fig. 1.
A: time course of mean systolic blood pressure (SBP) during ANG II (AII) infusion. n = 8–12 animals/group. FA, filtered air; PM, particular matter [of <2.5 μm in diameter (PM2.5)] *P < 0.05 vs. the FA-ANG II group. B: mean SBP after 2 wk of ANG II. n = 8–12 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
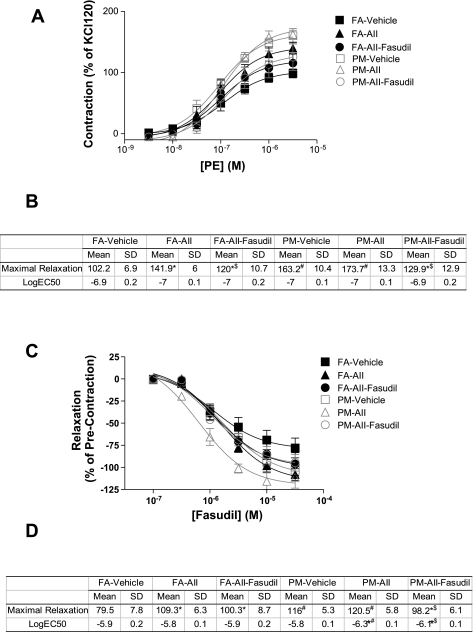
Fig. 2.
PM2.5 exposure enhances aortic response to phenylephrine (PE) through activation of RhoA/Rho-kinase (ROCK). A and B: aortic rings were mounted in myograph chambers. After equilibration, responses to PE were analyzed. n = 6 animals/group. *P < 0.05 vs. the same exposure infused with vehicle; #P < 0.05 vs. FA infused with the same drug; $P < 0.05 vs. the same exposure infused with ANG II. C and D: aortic rings were precontracted by PE (10−7.5 M), and fasudil was then added in an accumulative manner. n = 6 animals/group. *P < 0.05 vs. the same exposure infused with vehicle; #P < 0.05 vs. FA infused with the same drug; $P < 0.05 vs. the same exposure infused with ANG II.
BP Changes
There were no significant differences in mean SBP after 12 wk of PM2.5 exposure (101.4 ± 11.8 vs. 108.8 ± 7.4 mmHg for FA and PM, respectively, n = 12, P = 0.079). At the end of this exposure period, mice were randomized to receive vehicle, ANG II, or ANG II plus fasudil. No significant differences were noted between vehicle-treated PM2.5 and FA mice during the 14 days (Fig. 1, A and B). However, when ANG II was infused in conjunction with PM2.5, differences in SBP emerged after 1 wk of ANG II infusion (Fig. 1, A and B). Fasudil treatment decreased SBP to a greater degree in PM2.5 compared with FA mice (Fig. 1, A and B).
Vascular Reactivity
Figure 2, A and B, shows the responses of thoracic aortic rings to the α-adrenergic agonist PE. PM2.5 exposure potentiated maximal aortic contractile responses to PE in control animals, but, importantly, the increase in maximal constriction was highest in the group that received ANG II and was exposed to PM2.5. Fasudil treatment reduced maximal constriction in both FA and PM groups but to a higher level in PM animals. There were no changes in EC50 values in any of the groups. PM2.5 exposure increased relaxation to the ROCK inhibitor fasudil compared with FA + vehicle and FA+ ANG II-treated mice, respectively (Fig. 2, C and D). Notably, fasudil treatment decreased the aortic sensitivity to fasudil.
Cardiac Remodeling With PM2.5
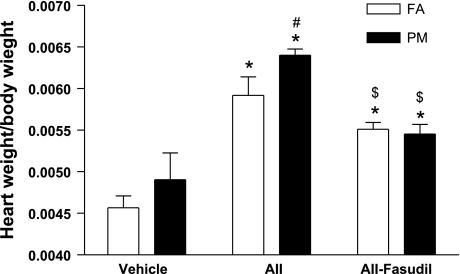
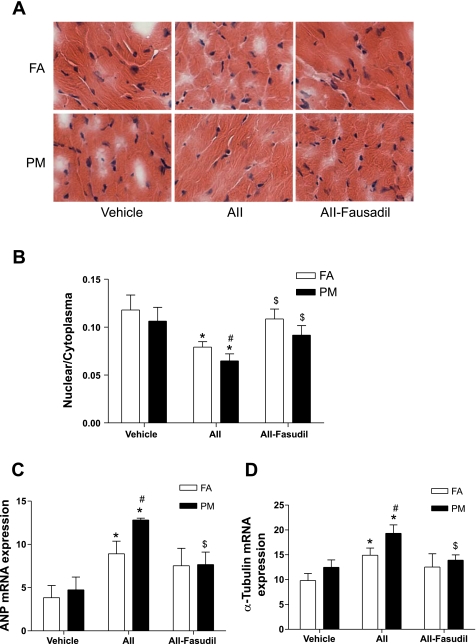
Figure 3 shows that compared with vehicle-treated FA mice, cardiac mass in vehicle-treated PM2.5 mice increased but did not attain statistical significance. Cardiac mass in the PM2.5 + ANG II group was significantly higher (Fig. 3). Fasudil treatment significantly attenuated the increase in cardiac mass in PM2.5 + ANG II mice and eliminated the difference between the two groups (Fig. 3). We assessed hypertrophy at the cellular level by measuring the nuclear-to-cytosol ratio of cardiomyocytes and quantified the expression of cardiac hypertrophy markers atrial natriuretic peptide (ANP) and α-tubulin by real-time PCR. Figure 4, A and B, shows that PM2.5 exposure did not significantly affect the nuclear-to-cytosol ratio of cardiomyocytes in vehicle-treated mice but significantly decreased the nuclear-to-cytosol ratio of cardiomyocytes in ANG II-treated mice. This effect of PM2.5 exposure was abolished by fasudil. Consistent with the morphometric analyses, PM2.5 exposure increased the mRNA expression of ANP and α-tubulin in ANG II-treated mice, with this effect also being reversed by fasudil treatment (Fig. 4, C and D).
Fig. 3.
Effect of PM2.5 exposure on cardiac mass. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
Fig. 4.
PM2.5 exposure enhances ANG II-induced cardiomyocyte hypertrophy. A: slides of heart tissue were stained by hematoxylin and eosin. B: results are expressed as the ratio of nuclear to cytosolic size. C and D: mRNA expression of the cardiomyocyte hypertrophy markers atrial natriuretic peptide (ANP; C) and α-tubulin (D) were analyzed by real-time RT-PCR. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
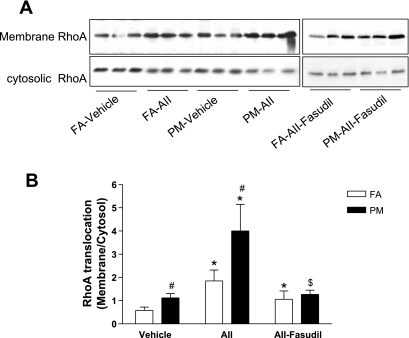
Cardiac and Vascular RhoA Activity
Figure 5 shows that PM2.5 exposure increased RhoA translocation to the membrane in vehicle-treated mice compared with FA. ANG II alone had a significant effect on RhoA activation. However, in conjunction with PM2.5, there was a marked increase in RhoA translocation in PM2.5 + ANG II mice. Fasudil treatment decreased these increases in RhoA translocation and to a higher level in PM2.5-exposed mice. In separate experiments, we also evaluated Rho activity in the aorta to confirm that these effects of PM2.5 extended to the vasculature, as previously demonstrated by us (50). PM2.5 in conjunction with ANG II increased Rho activity 139.3 ± 9.4% compared with the FA + ANG II group (100 ± 18.6%).
Fig. 5.
PM2.5 exposure increases cardiac RhoA translocation. A: membrane and cytosolic RhoA levels were analyzed by Western blot. B: results are expressed as the ratio of membrane RhoA to cytosolic RhoA. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
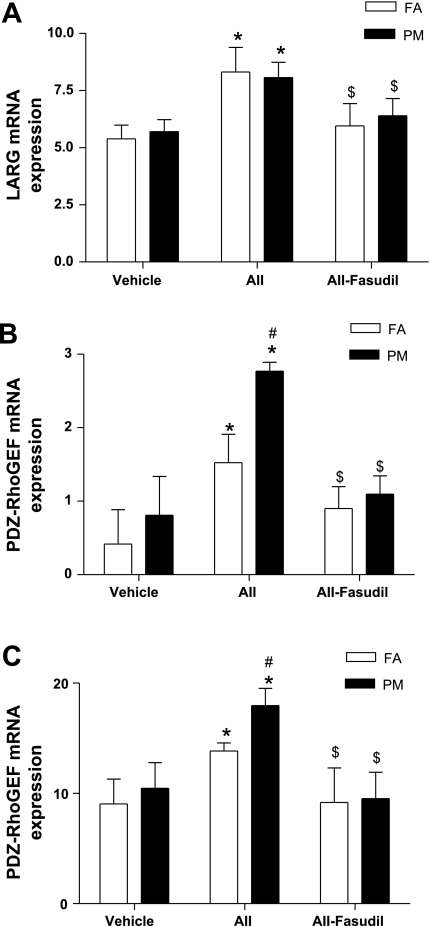
Expression of Guanine Nucleotide Exchange Factors With PM2.5 Exposure
Since RhoA activity was increased by PM2.5 exposure, we next analyzed the expression of genes that may play a role in RhoA activation. PM2.5 alone did not significantly increase mRNA expression of any of the guanine nucleotide exchange factors (GEFs). In contrast, mRNA for l-arginine, PDZ-RhoGEF, and p115-RhoGEF (Fig. 6), but not GEF-H1 (data not shown), was increased in the FA + ANG II group compared with the FA + vehicle group. mRNA expression of PDZ-RhoGEF and p115-RhoGEF but not l-arginine was increased in PM2.5 + ANG II-treated mice (Fig. 6). These increased expression levels of Rho-GEFs in the FA + ANG II and PM + ANG II groups were markedly inhibited by fasudil treatment (Fig. 6). Neither ANG II nor PM2.5 exposure affected the mRNA expression of guanine dissociation inhibitor or the expression of ROCK-I and ROCK-II (data not shown). Protein expression of RhoA was also analyzed by Western blot analysis. No significant difference between groups were observed (data not shown). Results in the aorta mirrored the responses seen in the myocardium (data not shown).
Fig. 6.
PM2.5 exposure increases the mRNA expression of guanine nucleotide exchange factors (GEFs) l-arginine (LARG; A), PDZ-RhoGEF (B), and p115RhoGEF (C). Results are fold increases over GAPDH. Total RNA was prepared from heart tissue, and the mRNA expression of the indicated genes was analyzed by real-time RT-PCR. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with ANG II or fasudil; $P < 0.05 vs. the same exposure in animals infused with ANG II.
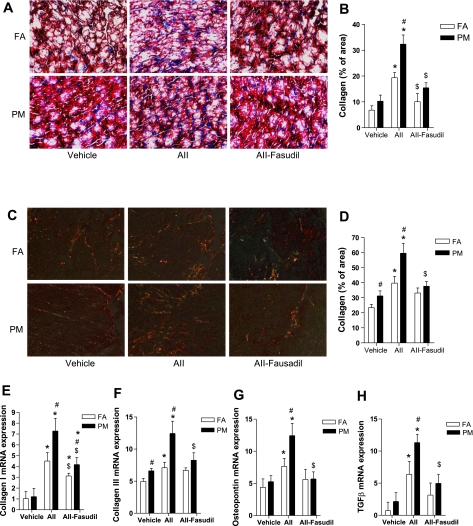
Cardiac Collagen Deposition
To investigate the role of PM2.5 exposure in cardiac collagen deposition, Masson's trichrome staining was performed. Figure 7, A and B, shows that PM2.5 exposure significantly enhanced cardiac collagen deposition in ANG II-treated mice, with fasudil treatment reducing the increase in collagen deposition. These results were confirmed by picrosirius red staining (Fig. 7, C and D). Some markers of cardiac fibrosis were also analyzed by real-time PCR. Figure 7, E–H, shows the effects on collagen I/III, transforming growth factor (TGF)-β, and osteopontin. The latter have been shown to play a key role in promoting myocardial fibrosis. PM2.5 alone had an effect on collagen III expression but not on other markers. PM2.5 exposure, however, enhanced ANG II-induced mRNA expression of collagen I, collagen III, TGF-β, and osteopontin. Fasudil treatment abolished these additive effects of PM2.5 exposure (Fig. 7, E–H).
Fig. 7.
PM2.5 exposure enhances ANG II-induced cardiac collagen deposition. A and B: representative micrographs (A) and summary of data (B) analyzed by Masson's trichrome staining. C and D: representative micrographs (C) and summary of data (D) analyzed by picrosirius red staining. E–H: mRNA expression of cardiac collagen deposition-related genes analyzed by real-time RT-PCR and expressed as fold increases over GAPDH. TGF, transforming growth factor. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
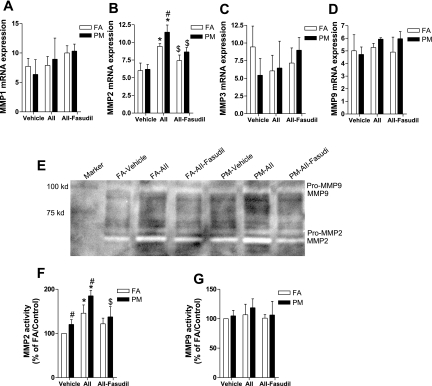
Cardiac Expression and Activity of MMPs
Studies (5, 9, 13, 29, 46) have shown that ANG II induces cardiac remodeling through an upregulation of MMP activity. We examined the expression of MMPs in cardiac tissue by real-time PCR and evaluated MMP activity by zymography. Figure 8, A–D, shows that ANG II increases the mRNA expression level of MMP-2, with PM2.5 exposure further enhancing this increased expression of MMP-2. In addition to its effect on enhancement of mRNA expression of MMP-2, PM2.5 exposure increased activated lower-molecular-weight forms of MMP-2, as shown by zymography (Fig. 8, E and F).
Fig. 8.
PM2.5 exposure increases cardiac matrix metalloproteinase (MMP)-2 expression and activation. A–D: total mRNA was isolated from cardiac tissue, and mRNA expression levels of MMP-1 (A), MMP-2 (B), MMP-3 (C), and MMP-9 (D) were measured by real-time PCR and expressed as fold increases over GAPDH. E–G: activity of MMP-2 and MMP-9 in cardiac tissue analyzed by zymography. A representative picture (E) and the activity summaries of MMP-2 (F) and MMP-9 (G) are shown. n = 6 animals/group. *P < 0.05 vs. the same exposure in animals infused with vehicle; #P < 0.05 vs. FA exposure in animals infused with the same drug; $P < 0.05 vs. the same exposure in animals infused with ANG II.
DISCUSSION
In this study, we demonstrated an important effect of PM2.5 preexposure on BP and cardiovascular remodeling in a model of experimental hypertension. PM2.5 activated RhoA in the aorta and myocardium with resultant cardiac fibrosis and the activation of matrix-degrading enzymes. These changes were prevented by the ROCK antagonist fasudil. RhoA activation may represent an important pathway through which PM2.5 mediates its effects.
In a prior publication (50), we demonstrated an important effect of PM2.5 exposure on BP in a rat model of hypertension. Consistent with our previous study, our results in mice demonstrate that, parallel with an increase in vasoconstrictor tone, short-term PM2.5 exposure potentiates hypertension through a RhoA/ROCK-dependent mechanism, as evidenced by the normalization of BP by the ROCK inhibitor fasudil. The RhoA/ROCK pathway is involved in the regulation of a variety of fundamental cell functions (28). Several studies (20, 36, 37) have demonstrated a critical role for this pathway in the regulation of systemic, pulmonary, and mesenteric vascular tone. Abnormal activation of the RhoA/ROCK pathway has been implicated in the pathogenesis of hypertension by Ca2+ sensitization of the contractile apparatus (28, 35, 43). Our results strongly implicate RhoA/ROCK as playing a pathophysiological role in PM2.5-mediated BP effects and cardiovascular remodeling (21, 52, 58).
An important finding in this study is that short-term PM2.5 exposure enhances ANG II-induced cardiac remodeling, as evidenced by the increase in cardiac mass, cardiomyocyte hypertrophy, and collagen deposition. While left ventricular remodeling may represent an adaptive response to pressure or volume overload to preserve cardiac function, an exuberant remodeling response predisposes to heart failure, arrhythmia, and sudden death. PM2.5 exposure increased cardiac RhoA activation, with reversal of these effects by fasudil, strongly indicating that RhoA/ROCK plays a significant role in mediating the effects of inhaled particulates on cardiac remodeling and BP. RhoA/ROCK has been demonstrated to be rapidly activated in response to various stimuli (3, 18, 23, 44). Overexpression of constitutively active RhoA or dominant negative mutants in cardiomyocytes modulates the expression of various genes and pathways involved in cardiac hypertrophy (3, 10, 24), whereas ROCK inhibition attenuates cardiac hypertrophy in hypertensive strains and in response to exogenous ANG II (44, 54). Recent studies (18, 23, 44, 54) have also suggested an important role for RhoA/ROCK in myocardial fibrosis. A characteristic consequence of inhibition of ROCK is an amelioration of left ventricular fibrosis, as has been previously demonstrated in these studies. A heterozygous deletional mutant of ROCK-I (predominantly expressed in the vasculature and heart) is characterized by reduced perivascular fibrosis (43). Consistent with a potential role for this pathway in cardiovascular disease, the same injurious stimuli such as ANG II and smoking, which are well known to trigger fibrosis, can trigger the activation of fibroblasts to a synthetic phenotype resulting in reparative fibrosis (38, 56). This process is initiated by TGF-β, which is typically produced in response to cellular damage and hormones such as ANg II (22, 38). TGF-β, in turn, stimulates the expression of genes that are characteristic of myofibroblasts, including smooth muscle actin, fibronectin, and osteopontin (25). We demonstrated an important effect of PM2.5 on both TGF-β and osteopontin, molecules that have been implicated as being obligatory in the activation of the myofibroblast and the transformation to a cell type actively involved in extracellular matrix synthesis (25, 57). Indeed, prior studies (22, 57) have suggested that both TGF-β and osteopontin are correlated with the transition to heart failure. While PM2.5 has been associated with a robust profibrotic response, these changes were not accompanied by parallel increases in collagenolytic enzymes such as MMP-1 and MMP-9. However, MMP-2 activity and expression were increased. The reasons for the preferential activation of MMP-2 in our work may be related to the influence of ROS on the activation of this enzyme, as previously demonstrated by us (42). However, we cannot exclude an effect of PM2.5 on MMP-1 activity, as has been demonstrated in pulmonary alveolar epithelial cells (1).
Our findings thus may have strong implications for the association between air pollution and congestive heart failure admissions and suggest a direct effect of PM2.5 on myocardial structure (6, 12, 53, 55). Such alterations could theoretically then increase myocardial stiffness, compliance, and diastolic filling properties of the left ventricle, resulting in a predisposition to diastolic heart failure.
How may PM2.5 mediate Rho activation? In our previous work (51), we demonstrated an important effect of PM2.5 in the activation of the NAD(P)H oxidase system and demonstrated that the ROS generated through this pathway in response to PM activates RhoA/ROCK. PM2.5 has previously been shown to regulate ROS generation through a NADPH oxidase-dependent mechanism in a variety of cell types and may represent an important upstream pathway by which PM2.5 may potentiate the effects of ANG II (4, 17, 27). Since ANG II is well known to regulate NADPH oxidases, this may well represent an important upstream pathway by which PM2.5 exerts additive effects on ANG II-mediated BP and cardiovascular remodeling (14, 41). In addition, PM2.5 may have direct effects on ANG II receptor subtype 1 signaling that could partly explain our findings. In a study with isolated pulmonary arterial rings and pulmonary arterial endothelial cells, Li et al. (26) demonstrated that ultrafine PM (SRM1648) at concentrations from 1 to 100 μg/ml could induce constriction in pulmonary artery rings and that this effect was blocked by losartan, an ANG II receptor subtype 1 antagonist. In their study (26), Zn, a significant component of the PM sample, could replicate these effects.
We believe that our data provides a mechanistic basis for the link between PM2.5 exposure and increases in BP and strongly implicates RhoA/Rho-kinase as playing a pathophysiologic role in the acute tonal responses and in the maladaptive remodeling response that sustains hypertension. The presence of high concentrations of transition metals in the inhaled particulates may argue for a role for reactive metals in potentiating free radical reactions. The locus of these interactions at the present time is unclear but there is intriguing information that inhaled particulates particularly those in the ultrafine range, may directly transgress into the circulation where it may mediate effects.(33, 34, 45) There is also interesting evidence of heightened oxidant stress with inhaled particulates in the myocardium mediated through the autonomic nervous system.(15) Regardless of the mechanism, the fact that inhaled particles induce these effects in the myocardium in hypertension has important implications for the cardiovascular effects of air pollution.
There are several limitations to our study, including the inability to distinguish the BP-dependent and -independent effects of RhoA/ROCK activation induced by PM2.5 exposure and whether the left ventricular changes are a cause of consequence of changes in systemic vascular resistance. These issues may be difficult to sort out in the context of an in vivo study as these ventricular-vascular adaptive responses are sometimes inseparable. Additionally, almost any drug that alters Rho/ROCK will also simultaneously affect BP. An additional limitation pertains to the use of pharmacological inhibitors of the ROCK pathway as these inhibitors may have nonspecific effects through targeting of other kinases, including PKC (48). Thus, some of our effects may relate to non-ROCK effects of fasudil. A final limitation pertains to the use of noninvasive BP monitoring used in this study. We exerted considerable care in the measurements with careful training of the mice.
In conclusion, our results suggest that environmentally relevant, low concentrations of ambient PM2.5 exposure may have detrimental effects on the cardiovascular system, especially in conjunction with other predisposing conditions or risk factors. PM2.5 exposure alone has weak effects on measurable outcomes, but in conjunction with these additional factors may have a substantive impact in modulating outcomes such as BP and myocardial remodeling. Our findings have important implications for the association of PM2.5 with hypertension and heart failure and provide one putative mechanism by which PM2.5 may mediate its effects.
GRANTS
This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grants R01-ES-013406 and R01-ES-015146 (to S. Rajagopalan) and K01-ES-016588 to Dr. Sun. The whole body exposure was performed in facilities at New York University, which was supported by NIEHS Grants ES-00260 and ES-015495 and by Health Effects Institute Grant 4750-RFA05-1A/06-11 (to L. C. Chen).
Footnotes
Supplemental material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Amara N, Bachoual R, Desmard M, Golda S, Guichard C, Lanone S, Aubier M, Ogier-Denis E, Boczkowski J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 293: L170–L181, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Aoki H, Izumo S, Sadoshima J. Angiotensin II activates RhoA in cardiac myocytes: a critical role of RhoA in angiotensin II-induced premyofibril formation. Circ Res 82: 666–676, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Becher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, Schwarze PE. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal Toxicol 19: 645–655, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J 16, Suppl O: 107–109, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Brook RD Is air pollution a cause of cardiovascular disease? Updated review and controversies. Rev Environ Health 22: 115–137, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105: 1534–1536, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res 42: 415–423, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev 15: 2702–2719, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LC, Hwang JS. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. IV. Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhal Toxicol 17: 209–216, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295: 1127–1134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50: 212–218, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK NADPH oxidases: new regulators of old functions. Antioxidants Redox Signal 8: 1443–1445, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110: 749–755., 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrabi I, Rondeau V, Dartigues JF, Tessier JF, Filleul L. Effects of particulate air pollution on systolic blood pressure: a population-based approach. Environ Res 101: 89–93, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein upregulation at the blood-brain barrier. FASEB J 22: 2723–2733, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation 109: 2234–2239, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346: 1954–1962, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hilgers RH, Todd J Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens 25: 1687–1697, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health 91: 571–577, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118: 10–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, Kobayashi T, Matsuoka H. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res 55: 757–767, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kuwahara K, Saito Y, Nakagawa O, Kishimoto I, Harada M, Ogawa E, Miyamoto Y, Hamanaka I, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Tamura N, Ogawa Y, Nakao K. The effects of the selective ROCK inhibitor, Y27632, on ET-1-induced hypertrophic response in neonatal rat cardiac myocytes–possible involvement of Rho/ROCK pathway in cardiac muscle cell hypertrophy. FEBS Lett 452: 314–318, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Leask A TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74: 207–212, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Carter JD, Dailey LA, Huang YC. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect 113: 1009–1014, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Hyseni X, Carter JD, Soukup JM, Dailey LA, Huang YC. Pollutant particles enhanced H2O2 production from NAD(P)H oxidase and mitochondria in human pulmonary artery endothelial cells. Am J Physiol Cell Physiol 291: C357–C365, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98: 322–334, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328: 183–188, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol 17: 189–197, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356: 447–458, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112: 3930–3936, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation 105: 411–414, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med 166: 998–1004, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 99: 1426–1432, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290: C661–C668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension 51: 704–711, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56: 709–742, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Pope CA, 3rd Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 114: 2443–2448, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation 112: 2959–2965, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, Makino N. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol 35: 59–70, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, Radykewicz R, Stampfl A, Thorand B, Peters A. Cardiovascular effects of fine and ultrafine particles. J Aerosol Med 18: 1–22, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Senzaki H, Gluzband YA, Pak PH, Crow MT, Janicki JS, Kass DA. Synergistic exacerbation of diastolic stiffness from short-term tachycardia-induced cardiodepression and angiotensin II. Circ Res 82: 503–512, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Siomboing X, Gressier B, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC. Investigation of the inhibitory effects of HA-1077 and Y-32885 on the translocation of PKCbetaI, PKCbetaII and PKCzeta in human neutrophils. Mediators Inflamm 10: 315–321, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294: 3003–3010, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 28: 1760–1766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 28: 1760–1766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.United States Environmental Protection Agency. Federal Register Environmental Documents. Air Quality Criteria for Particulate Matter (online). http://www.epa.gov/EPA-AIR/2004/October/Day-29/a24232.htm [16 March 2009].
- 52.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 113: 1052–1055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D'Ippoliti D, Elosua R, Hormann A, Kulmala M, Lanki T, Lowel H, Pekkanen J, Picciotto S, Sunyer J, Forastiere F. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation 112: 3073–3079, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Wang YX, da Cunha V, Martin-McNulty B, Vincelette J, Li W, Choy DF, Halks-Miller M, Mahmoudi M, Schroeder M, Johns A, Light DR, Dole WP. Inhibition of Rho-kinase by fasudil attenuated angiotensin II-induced cardiac hypertrophy in apolipoprotein E deficient mice. Eur J Pharmacol 512: 215–222, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am J Cardiol 97: 404–408, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Wong LS, Martins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair Regen 12: 471–484, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Zahradka P Novel role for osteopontin in cardiac fibrosis. Circ Res 102: 270–272, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation 110: 2184–2189, 2004. [DOI] [PubMed] [Google Scholar]