Abstract
Activation of the G protein Gs results in increases in cAMP, a necessary step in the pathway for ATP release from rabbit and human erythrocytes. In all cells, the level of cAMP is the product of its synthesis by adenylyl cyclase and its hydrolysis by phosphodiesterases (PDEs). Both iloprost (Ilo), a PGI2 analog, and isoproterenol (Iso), a β-agonist, stimulate receptor-mediated increases in cAMP in rabbit and human erythrocytes. However, the specific PDEs associated with each of these signaling pathways in the erythrocyte have not been fully characterized. Previously, we reported that PDE3B is present in rabbit and human erythrocyte membranes and that PDE3 inhibitors potentiate Ilo-induced increases in cAMP. Here we report that inhibitors of either PDE2 or PDE4, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) and rolipram, respectively, potentiate Iso-induced increases in cAMP in rabbit and human erythrocytes. Importantly, these inhibitors had no effect on cAMP increases associated with the incubation of erythrocytes with Ilo. In addition, we establish, for the first time, the presence of PDE2A protein in rabbit and human erythrocyte membranes. Finally, we determined that preincubation of human erythrocytes with EHNA and rolipram together potentiate Iso-induced ATP release, whereas preincubation with cilostazol enhances Ilo-induced release of ATP. These results are consistent with the hypothesis that, in rabbit and human erythrocytes, Ilo-induced increases in cAMP and ATP release are regulated by PDE3, whereas those associated with Iso are regulated by the activities of both PDE2 and PDE4. These studies demonstrate that PDE activity in these cells is localized to specific signaling pathways.
Keywords: red blood cell, adenosine 3′, 5′-cyclic monophosphate, adenosine 5′-trisphophate, rolipram, β-adrenergic receptors
the role of the erythrocyte in the transport of oxygen to meet the metabolic needs of all tissues is well established. In addition to this transport function, it has been shown that when erythrocytes are exposed to mechanical deformation, reduced oxygen tension, and some pharmacological stimuli, they release ATP (5, 24, 35, 37, 39). This erythrocyte-derived ATP can contribute to the control of vascular resistance via activation of purinergic receptors on the endothelium, resulting in the local release of vasodilators such as nitric oxide, prostacyclin (PGI2), and hyperpolarizing factors (10, 38). Two well-characterized pharmacological stimuli for ATP release from mammalian erythrocytes are β-receptor agonists and PGI2 analogs acting via the β-adrenergic receptor (β2AR) and PGI2 receptor (IPR), respectively (25, 31, 36, 44). Importantly, activation of these G protein-coupled receptors is associated with increases in intracellular cAMP (35), a requirement for ATP release from human and rabbit erythrocytes (39).
A signal transduction pathway has been described that relates pharmacological stimuli to ATP release from erythrocytes (25). The proposed pathway includes the heterotrimeric G protein, Gs, adenylyl cyclase (AC), protein kinase A, and the cystic fibrosis transmembrane conductance regulator. Activation of Gs stimulates AC activity and, consequently, increases cAMP synthesis in rabbit and human erythrocytes (25). However, the regulation of cAMP levels by the activity of phosphodiesterases (PDEs) has not been fully characterized in these cells.
It is well established that cAMP is a second messenger in a large number of intracellular signaling pathways (11, 47). Thus local levels of cAMP in a cell must be tightly regulated. Within the erythrocyte, regulation of the concentration of this second messenger is a critical control point for modulation of ATP release in response to pharmacological stimuli. In all cells, intracellular cAMP levels are the result of the balance between cAMP synthesis by ACs and its hydrolysis by PDEs. Erythrocytes possess PDE activity; however, the individual PDE isoforms present and their association with any signaling pathway are less well defined. Previously, we reported that, in rabbit and human erythrocytes, PDE3 modulates increases in cAMP associated with IPR activation (13). However, the PDE(s) involved in the regulation of increases in cAMP associated with activation of the β2AR in these cells has not been determined.
In nonerythroid cells, it is well accepted that cAMP generated in response to specific receptor stimulation is compartmentalized and that the PDEs that regulate the increases in cAMP aid in conferring selectivity to the associated cell response (18, 40). Here we investigate the hypothesis that the PDEs responsible for the regulation of cAMP increases associated with activation of the IPR and the β2AR in erythrocytes are distinct.
In the present study we focused on PDE2, -3, and -4 because PDE3 activity has been linked with the IPR, whereas the activity of PDE2 and PDE4 has been shown to be associated with β2AR in other cell types (8, 12, 22, 28, 32, 46). We wished to confirm that PDE3 activity is associated with the hydrolysis of cAMP generated in response to activation of the IPR and that this PDE does not modulate cAMP increases resulting from activation of the β2AR. In addition, we wished to demonstrate that PDE2 and PDE4 activities contribute to the regulation of cAMP generated in response to activation of the β2AR but not the IPR. We sought to establish, for the first time, that PDE2 protein is present in rabbit and human erythrocyte membranes. Finally, we demonstrate that inhibitors of the selective PDEs that regulate the increases in cAMP in the β2AR and IPR signaling pathways augment ATP release in response to receptor activation.
METHODS
Isolation of erythrocytes.
Rabbits were anesthetized with ketamine (12.5 mg/kg) and xylazine (1.5 mg/kg) intramuscularly, followed by pentobarbital sodium (10 mg/kg) administered via a cannula placed in an ear vein. A catheter was placed in a carotid artery, and heparin (500 units) was administered. After 10 min, the animals were exsanguinated. Immediately after collection of blood, erythrocytes were isolated by centrifugation at 500 g at 4°C for 10 min. The supernatant and buffy coat were removed by aspiration. Packed erythrocytes were resuspended and washed three times in a physiological salt solution containing (in mM) 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 21.0 Tris-base, and 5.5 dextrose with 0.5% bovine serum albumin (pH adjusted to 7.4). Erythrocytes were prepared on the day of use. Human blood was obtained by venipuncture using a syringe containing heparin (500 units). Immediately after collection of blood, erythrocytes were prepared as described above. Healthy human blood was obtained from volunteers on the day each study was done; all volunteers gave written, informed consent. Protocols for blood collection were approved by the appropriate St. Louis University committees.
Preparation of erythrocyte membranes.
Washed erythrocytes were diluted 1:100 with ice-cold lysis buffer containing 5 mM Tris·HCl and 2 mM EDTA (pH 7.4) and stirred at 4°C for 20 min. The lysate was centrifuged at 23,300 g for 15 min at 4°C. The supernatant was removed and discarded. The pellet containing the erythrocyte membranes was resuspended and centrifuged two additional times with ice-cold buffer. Membrane protein concentrations were determined using the BCA Protein Assay (Pierce). The final pellet was frozen at −80°C.
Western blot analysis.
Purified erythrocyte membranes were solubilized in SDS buffer containing 0.277 M SDS, 60% glycerol, 0.25 M Tris·HCl (pH 6.8), 0.004% bromophenol blue, and 0.400 M dithiothreitol, boiled, loaded onto a precast gel (Pierce), and subjected to electrophoresis. The proteins were transferred to a polyvinylidene difluoride membrane in buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. Membranes were blocked overnight with 5% nonfat dry milk in PBS containing 0.1% Tween 20 and were then immunoblotted with a monoclonal mouse antibody directed against amino acids 869-912 in the N terminus of PDE2A (Affinity Bioreagents) or with a polyclonal goat antibody directed to an internal region of PDE2A (Santa Cruz). The membranes were then incubated with an appropriate secondary antibody in 1% nonfat dry milk, and the proteins that were identified were visualized using enhanced chemiluminesence (Pierce).
Incubation of erythrocytes with pharmacological agents.
Washed erythrocytes were diluted to a 50% hematocrit (1 ml) and were preincubated with a PDE inhibitor or its vehicle for 30 min. The inhibitors studied were vinpocetine, a selective PDE1 inhibitor (Sigma- Aldrich); 3-isobutyl-1-methylxanthine (IBMX), a nonselective PDE inhibitor (Sigma-Aldrich); rolipram, a selective PDE4 inhibitor (Tocris); erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA), a selective PDE2 inhibitor (Biomol); and cilostazol, a selective PDE3 inhibitor (Sigma-Aldrich). The concentrations of inhibitors were chosen based on the IC50 values of each inhibitor in other cell types (4, 16, 17, 21). Importantly, these concentrations had either little or no effect on baseline cAMP levels. The vehicle for vinpocetine, rolipram, and cilostazol was N′,N-dimethylformamide (Sigma-Aldrich), and the vehicle for EHNA, isoproterenol (Iso; Sigma-Aldrich), and iloprost (Ilo; Cayman) was saline. Erythrocytes were then incubated with either Iso (1 μM; 30 min) or Ilo (1 μM; 15 min) in the absence or presence of the various PDE inhibitors. The reaction was stopped with the addition of 4 ml ice-cold acid ethanol containing 1 mM HCl. For measurement of ATP, washed erythrocytes were diluted to a 20% hematocrit (3 ml) and were preincubated with a PDE inhibitor or its vehicle for 30 min. Erythrocytes were then incubated with either Iso (1 μM; 5, 10, and 15 min) or Ilo (10 nM; 5, 10, and 15 min) in the absence or presence of the various PDE inhibitors.
Measurement of cAMP.
The erythrocyte-ethanol mixture was centrifuged at 14,000 g for 10 min at 4°C. The supernatant was removed and stored overnight at −20°C. Samples were centrifuged a second time at 3,700 g for 10 min at 4°C. The supernatant was again removed and dried under vacuum centrifugation. Concentrations of cAMP were determined by EIA (GE Healthcare).
Measurement of ATP.
ATP was measured using the luciferin-luciferase technique (41). A 200-μl sample of an erythrocyte suspension (0.04% hematocrit) was injected into a cuvette containing 100 μl of 10 mg/ml crude firefly tail extract (Sigma) and 100 μl of a 10 mg/ml distilled water (FLE 250; Sigma). The light emitted was detected using a luminometer (TD 20/20; Turner Designs). A standard curve was obtained for each experiment. Cell counts were obtained from the suspension of erythrocytes, and amounts of ATP measured were normalized to 4 × 108 cells/ml.
Measurement of total intracellular ATP.
A known number of erythrocytes were lysed in distilled water at room temperature. ATP in the lysate, diluted 8,000-fold, was measured using the ATP assay. The values were normalized to the ATP concentration per erythrocyte.
Measurement of hemoglobin.
Erythrocyte suspensions used to measure ATP were centrifuged at 500 g for 10 min at 4°C. The amount of hemoglobin present in the supernatant was determined by measurement of absorbance at 405 nm (oxyhemoglobin). Studies in which free hemoglobin was detected were not included.
Data analysis.
Statistical significance was determined using ANOVA. In the event that the F-ratio indicated a change had occurred, a Fisher's LSD test was performed to identify individual differences. Results were reported as means ± SE.
RESULTS
Effect of the nonselective PDE inhibitor IBMX on Ilo- and Iso-induced increases in cAMP.
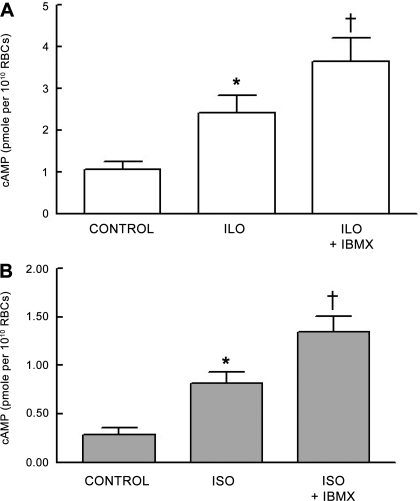
To determine the effect of the nonselective inhibition of PDE activity on Ilo- and Iso-induced increases in cAMP, rabbit erythrocytes were incubated with each agonist in the absence or presence of IBMX (10 μM). This concentration of IBMX had no effect on baseline cAMP levels. Incubation of rabbit erythrocytes with either Ilo (1 μM; n = 7) or Iso (1 μM; n = 7) in the absence of IBMX resulted in a 141 ± 16% and 172 ± 34% increase in cAMP, respectively. Pretreatment of erythrocytes with IBMX potentiated the increases in cAMP associated with both Ilo (n = 7; P < 0.01; Fig. 1A) and Iso (n = 7; P < 0.01; Fig. 1B) administration. These data demonstrate that PDE activity is present in rabbit erythrocytes and regulates increases in cAMP associated with agonist-induced activation of the IPR and β2AR.
Fig. 1.
A: effect of 3-isobutyl-1-methylxanthine (IBMX; 10 μM) on iloprost (Ilo; 1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 7). Erythrocytes were incubated with IBMX for 30 min before addition of Ilo for 15 min. B: effect of IBMX (10 μM) on isoproterenol (Iso; 1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 7). Erythrocytes were incubated with IBMX for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.01). RBC, red blood cell.
Effect of the selective PDE1 inhibitor vinpocetine on Ilo- and Iso-induced increases in cAMP.
To establish that PDE1 is not responsible for the hydrolysis of cAMP generated by the activation of the IPR and β2AR, rabbit erythrocytes were incubated with either Ilo (1 μM; n = 8) or Iso (1 μM; n = 4) in the absence or presence of the selective PDE1 inhibitor vinpocetine (30 μM). Vinpocetine, at the concentration used, had no effect on basal cAMP levels or on either the Iso- or Ilo-induced increases in cAMP (Table 1). The results are consistent with the hypothesis that PDE1 activity is not associated with the regulation of either Ilo- or Iso-induced increases in cAMP in rabbit erythrocytes.
Table 1.
Effect of 30 μM vinpocetine on 1 μM iloprost- and 1 μM isoproterenol-induced increases in cAMP in rabbit erythrocytes
| Group |
cAMP, pmol per 1010 red blood cells |
||
|---|---|---|---|
| Baseline | Agonist alone | Agonist + vinpocetine | |
| Iloprost | 0.50±0.13 | 1.3±0.25* | 1.24±0.18* |
| Isoproterenol | 0.35±0.12 | 1.3±0.22* | 1.20±0.27* |
Values are means ± SE; n = 8 in iloprost group and n = 4 in isoproterenol group.
Different from baseline, P < 0.05.
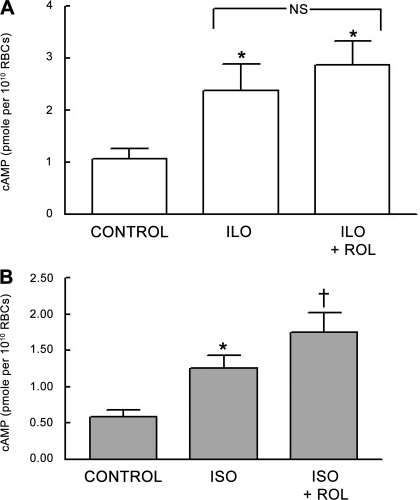
Effect of the selective PDE3 inhibitor cilostazol on Ilo- and Iso-induced increases in cAMP.
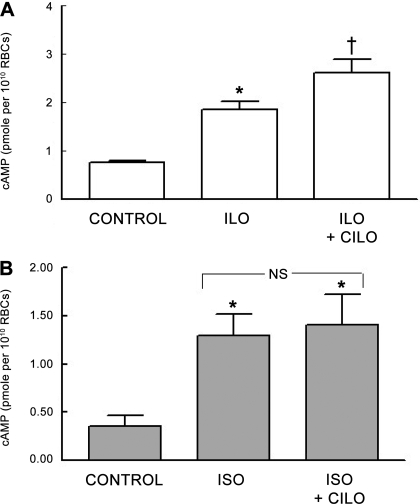
Previously, we identified PDE3B as a component of both rabbit and human erythrocyte membranes. The selective PDE3 inhibitor cilostazol (Cilo) potentiated the Ilo-induced increases in cAMP in these cells (13). Therefore, to determine whether PDE3 is also responsible for the modulation of Iso-induced increases in cAMP in rabbit erythrocytes, cells were incubated with Iso (1 μM; n = 7) or Ilo (1 μM; n = 7) in the absence and presence of Cilo (10 μM). This concentration of Cilo did produce a small but significant increase in baseline levels of cAMP from 0.80 ± 0.04 to 0.91 ± 0.04 pmol/1010 erythrocytes (P < 0.05). In contrast with the results with vinpocetine, Ilo-induced increases in cAMP were augmented by Cilo (P < 0.01; Fig. 2A), whereas Cilo had no effect on the Iso-induced increases in cAMP (Fig. 2B). These data suggest that, in rabbit erythrocytes, PDE3 is involved with the regulation of Ilo-induced increased in cAMP but not those associated with the activation of the β2AR.
Fig. 2.
A: effect of cilostazol (Cilo; 10 μM) on Ilo (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 7). Erythrocytes were incubated with Cilo for 30 min before addition of Ilo for 15 min. B: effect of Cilo (10 μM) on Iso (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 4). Erythrocytes were incubated with Cilo for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.01). NS, not statistically different.
Effect of the selective PDE2 inhibitor EHNA on Ilo- and Iso-induced increases in cAMP.
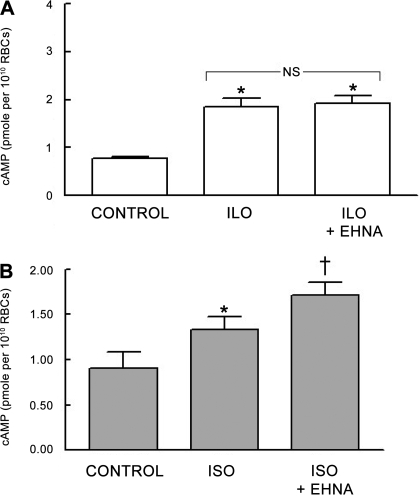
To determine whether PDE2 activity regulates increases in cAMP stimulated by either Iso or Ilo, rabbit erythrocytes were incubated with these agonists in the absence or presence of the selective PDE2 inhibitor EHNA (30 μM). Like Cilo, EHNA at the concentration used did produce a small but significant increase in baseline levels of cAMP from 0.80 ± 0.04 to 1.18 ± 0.06 pmol/1010 erythrocytes (P < 0.01). However, EHNA potentiated cAMP increases produced by Iso (n = 8; P < 0.01; Fig. 3A) but had no effect on Ilo-induced increases in cAMP (n = 7; Fig. 3B). These results are consistent with the conclusion that PDE2 activity modulates increases in cAMP produced by Iso but not Ilo in rabbit erythrocytes.
Fig. 3.
A: effect of erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA; 30 μM) on Ilo (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 7). Erythrocytes were incubated with EHNA for 30 min before addition of Ilo for 15 min. B: effect of EHNA (30 μM) on Iso (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 8). Erythrocytes were incubated with EHNA for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.01).
Identification of PDE2 as a component of rabbit and human erythrocyte membranes.
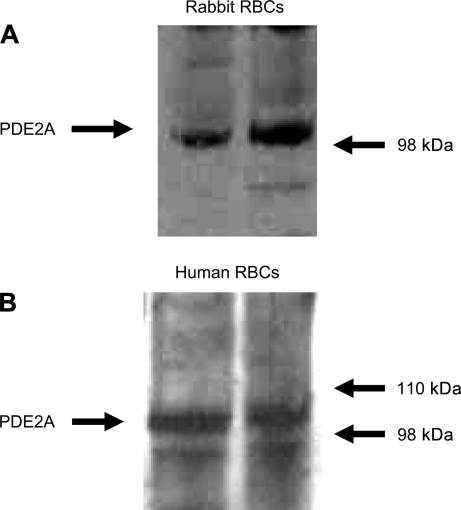
Although the above studies provide strong support for the hypothesis that PDE2 activity regulates increases in cAMP associated with activation of the β2AR, they do not establish that PDE2 protein is present in erythrocytes. There are no prior reports that PDE2 is a component of erythrocyte membranes. However, in other cell types, an isoform of PDE2, PDE2A, is found in association with the cell membrane (4, 30). Therefore, to determine whether PDE2A protein is present in membranes of rabbit and human erythrocytes, cell membranes were isolated and probed by Western blot analysis with a monoclonal antibody directed against amino acids 869-912 in the N terminus of PDE2A or with a polyclonal antibody directed to an internal epitope of PDE2A. Both antibodies identified a band with a predicted molecular mass of ∼105 kDa (Fig. 4, A and B) in rabbit and human erythrocytes.
Fig. 4.
A: identification of PDE2A in rabbit erythrocyte membranes. Rabbit erythrocyte membranes were probed with a monoclonal antibody generated against amino acids 869-912 in the N terminus of human PDE2A (representative of 7 individual membrane preparations). B: identification of PDE2A in human erythrocyte membranes. Human erythrocyte membranes were probed with a polyclonal antibody directed to an internal region of human PDE2A (representative of 8 individual membrane preparations).
Effect of the selective PDE4 inhibitor rolipram on Ilo- and Iso-induced increases in cAMP.
In addition to PDE2, PDE4 activity has been associated with the β2AR in cardiac myocytes (8, 46). Therefore, to determine whether PDE4 activity also contributes to the regulation of Iso-induced increases in cAMP in mammalian erythrocytes, rabbit erythrocytes were preincubated with the selective PDE4 inhibitor rolipram (20 μM) before the addition of either Iso (1 μM; n = 9) or Ilo (1 μM; n = 7). Rolipram, at this concentration, had no effect on basal cAMP levels but potentiated Iso-induced increases in cAMP (P < 0.05; Fig. 5A). Importantly, rolipram had no effect on Ilo-induced increases in cAMP (Fig. 5B). This finding, coupled with the finding that an inhibitor of PDE2 activity potentiates Iso-induced increases in cAMP, is consistent with the hypothesis that both PDE2 and PDE4 contribute to the regulation of cAMP associated with the activation of the β2AR in rabbit erythrocytes.
Fig. 5.
A: effect of rolipram (Rol; 20 μM) on Ilo (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 7). Erythrocytes were incubated with Rol for 30 min before stimulation with Ilo for 15 min. B: effect of Rol (20 μM) on Iso (1 μM)-induced increases in cAMP in rabbit erythrocytes (n = 9). Erythrocytes were incubated with Rol for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.05).
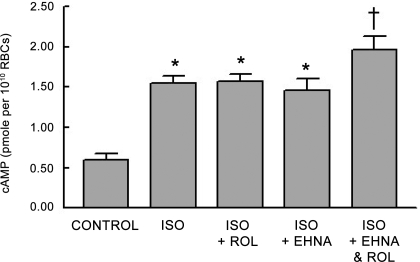
Effect of the combination of rolipram and EHNA on the Iso-induced increases in cAMP.
As reported above, we determined that inhibitors of the activity of either PDE2 or PDE4 result in increases in cAMP associated with the incubation of rabbit erythrocytes with Iso. This result, coupled with reports that both PDE2 and PDE4 are associated with the β2AR signaling pathway in other cells (22, 23, 46), suggests that both PDEs participate in the regulation of increases in cAMP associated with the activation of the β2AR in rabbit erythrocytes. Therefore, to determine whether PDE2 and PDE4 act synergistically to hydrolyze increased levels of cAMP associated with the activation of the β2AR in erythrocytes, we incubated rabbit erythrocytes with the combination of EHNA (10 μM) and rolipram (10 μM) before the addition of Iso. The concentrations of the inhibitors used in these studies had no effect on Iso-induced increases in cAMP when administered alone. Moreover, the combination of inhibitors had no effect on basal cAMP levels. However, incubation of rabbit erythrocytes with this combination potentiated Iso-induced increases in cAMP (n = 9; P < 0.05; Fig. 6). This finding suggests that Iso-induced increases in cAMP are regulated by the activity of both PDE2 and PDE4 in rabbit erythrocytes.
Fig. 6.
Effect of Rol (10 μM; n = 10) and EHNA (10 μM; n = 8) alone and in combination (n = 9) on Iso (1 μM)-induced increases in cAMP in rabbit erythrocytes. Erythrocytes were incubated with inhibitors for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.01).
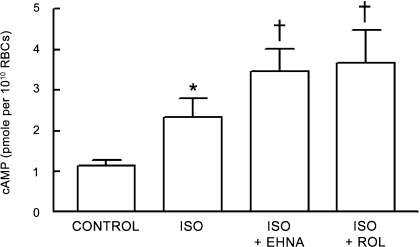
Effect of selective inhibitors of PDE2 and PDE4 on Iso-induced increases in cAMP in human erythrocytes.
To determine whether the inhibition of either PDE2 or PDE4 would potentiate the Iso-induced increases in cAMP in human erythrocytes, cells were preincubated with either EHNA (30 μM; n = 7) or rolipram (20 μM; n = 7) before the addition of Iso (1 μM). Incubation of erythrocytes with either inhibitor potentiated the Iso-induced increases in cAMP (P < 0.01; Fig. 7). These results demonstrate that increases in cAMP associated with the activation of the β2AR in human erythrocytes are regulated by PDE2 and -4, as is the case in rabbit erythrocytes.
Fig. 7.
Effect of EHNA (30 μM) or Rol (20 μM) on Iso (1 μM)-induced increases in cAMP in human erythrocytes (n = 7). Erythrocytes were incubated with either Rol or EHNA for 30 min before addition of Iso for 30 min. Values are means ± SE. *Different from respective control (P < 0.05); †different from all other values (P < 0.01).
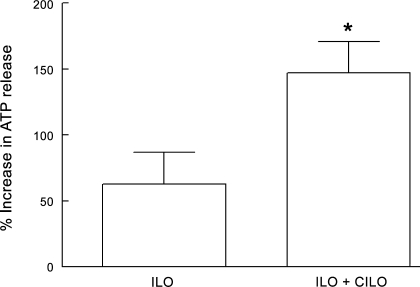
Effect of the selective PDE3 inhibitor Cilo on Ilo-induced ATP release from human erythrocytes.
We reported previously that incubation of rabbit and human erythrocytes with Ilo (1 μM) stimulates ATP release (36). Recently, we reported that pretreatment of erythrocytes with the PDE3 inhibitor Cilo potentiates Ilo-induced increases in cAMP in both rabbit and human erythrocytes (13). Since increases in cAMP are a requisite for the release of ATP from erythrocytes (39), we investigated whether an inhibitor of PDE3 would enhance the release of ATP from erythrocytes incubated with Ilo. Cells were incubated with Ilo (10 nM) in the absence and presence of Cilo (30 μM). Pretreatment with Cilo had no effect on baseline ATP levels (6 ± 1 nM per 4 × 108 erythrocytes without Cilo and 5 ± 1 nM per 4 × 108 erythrocytes in the presence of Cilo). However, Cilo potentiated the release of ATP induced by Ilo (Fig. 8).
Fig. 8.
Effect of Cilo (30 μM) on Ilo (10 nM)-induced increases in ATP release from human erythrocytes (n = 9). Erythrocytes were incubated with Cilo for 30 min before addition of Ilo. Values are means ± SE. *Different from Ilo alone (P < 0.01).
Effect of the selective PDE2 inhibitor EHNA or the selective PDE4 inhibitor rolipram alone or in combination on Iso-induced ATP release from human erythrocytes.
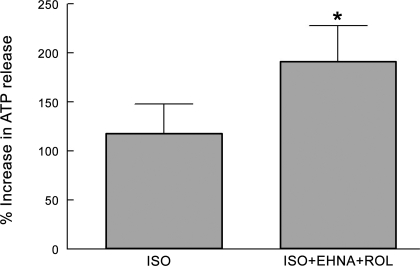
We have shown that incubation of rabbit erythrocytes with Iso (10 μM) stimulates ATP release from these cells (25). Here we demonstrate that inhibitors of either PDE2 (EHNA) or PDE4 (rolipram) potentiate Iso-induced increases in cAMP in both rabbit and human erythrocytes (Figs. 3B, 5B, and 7). In addition, we demonstrate that, when administered together at concentrations that alone have no effect on cAMP accumulation, EHNA and rolipram act synergistically to augment Iso-induced increases in cAMP (Fig. 6). Therefore, to determine whether inhibition of either PDE2 or PDE4 would enhance ATP release in human erythrocytes, cells were incubated with Iso (1 μM) in the absence and presence of either rolipram (20 μM) or EHNA (30 μM). Pretreatment with either inhibitor alone had no effect on baseline ATP levels or on Iso-induced ATP release. However, since both PDE2 and PDE4 were shown to modulate Iso-induced increases in cAMP, when human erythrocytes were incubated with Iso (1 μM) in the presence of the combination of EHNA (30 μM) and rolipram (20 μM), Iso-induced ATP release was augmented (Fig. 9). Baseline levels of ATP were 7.7 ± 0.8 nM per 4 × 108 erythrocytes without rolipram and EHNA and 6.5 ± 0.9 nM per 4 × 108 erythrocytes in the presence of both inhibitors.
Fig. 9.
Effect of EHNA (30 μM) and Rol (20 μM) on Iso (1 μM)-induced increases in ATP release from human erythrocytes (n = 18). Erythrocytes were incubated with EHNA and Rol for 30 min before addition of Iso. Values are means ± SE. *Different from Iso alone (P < 0.01).
DISCUSSION
Rabbit and human erythrocytes possess PDE activity that is associated with the hydrolysis of cAMP (1). This conclusion was reached based on the finding that incubation of erythrocytes with the nonselective PDE inhibitor IBMX results in increases in cAMP. However, the specific PDEs present in mammalian erythrocytes were not defined and nor was their activity associated with any discrete signaling pathway. Recently, we reported that PDE3B is present in rabbit and human erythrocyte membranes and that this PDE is responsible for the hydrolysis of cAMP generated by incubation of erythrocytes with the active PGI2 analog Ilo (13, 36). In the present study, we demonstrate pharmacologically that PDE2 and PDE4 activities are also present in rabbit and human erythrocytes and establish for the first time that the PDE2 protein is a component of the membranes of these cells. In addition, we determined that increases in cAMP produced by the β-agonist Iso are regulated by the activity of PDE2 and -4 in both rabbit and human erythrocytes.
PDEs are ubiquitously expressed in mammalian tissues, and because of their ability to hydrolyze cyclic nucleotides, they play a major role in the regulation of many signal transduction pathways. Currently, 11 distinct PDE enzyme families have been characterized based on gene product, biochemical properties, mode of regulation, sensitivity to pharmacological agents, and their specificity for hydrolysis of cAMP, cGMP, or, in some cases, both cyclic nucleotides (4, 17, 18).
The presence of differentially regulated PDE isoforms within cells that are associated with discrete signaling pathways allows for subcellular regulation of cAMP levels. This tight regulation of local cAMP concentration within a cell permits the precise regulation of cellular responses to external stimuli (7, 15, 26, 34). In nonerythroid cells, distinct PDEs are associated with G protein-coupled receptor signaling pathways and thereby contribute to the compartmentalization of cAMP signaling (34, 42, 45). For example, there is evidence for involvement of PDE2 and -4 in the regulation of increases in cAMP resulting from activation of the β2AR in cardiac myocytes (8, 22, 23, 46). In contrast, increases in cAMP associated with the activation of the IPR were reported to be regulated by the activity of PDE3 in platelets and the pulmonary vasculature (12, 20, 28, 32). A functional role for PDE3 in rabbit and human erythrocytes was demonstrated by the finding that selective inhibitors of PDE3 augmented increases in cAMP associated with the agonist-induced activation of the IPR in these cells (13). Here we confirm that finding (Fig. 2) in rabbit erythrocytes and establish that selective inhibitors of the activity of PDE2 and -4 do not augment increases in cAMP associated with activation of the IPR in these cells (Figs. 3 and 5).
The only other PDE activity previously reported to be present in mammalian erythrocytes is that of PDE1 (27). However, this PDE was reported to hydrolyze cGMP, and the effect of PDE1 activity on cAMP levels in erythrocytes was not investigated. Importantly, PDE1 activity was not reported to be associated with any signaling pathway (27). Previously, we reported that the selective inhibitor of PDE1 activity vinpocetine did not potentiate increases in cAMP associated with the activation of the IPR in human erythrocytes (13). Here we demonstrate that vinpocetine has no effect on cAMP increases resulting from receptor-mediated activation of either the IPR or the β2AR signaling pathways in rabbit erythrocytes (Table 1). These results suggest that increases in cAMP produced by activation of the IPR or β2AR are regulated by PDEs other than PDE1 in both rabbit and human erythrocytes.
In addition to PDE1 and -3, we have determined for the first time that PDE2 is a component of the membranes of rabbit and human erythrocytes (Fig. 4). EHNA, a selective inhibitor of PDE2, did not augment increases in cAMP associated with receptor-mediated activation of the IPR in rabbit erythrocytes (Fig. 3A). Importantly, EHNA did potentiate increases in cAMP associated with incubation of both rabbit and human erythrocytes with the β2AR agonist Iso (Figs. 3B and 7). Taken together, these results provide support for the hypothesis that PDE2 participates in the regulation of increases in cAMP associated with β2AR activation but not with increases in cAMP associated with the IPR signaling pathway in rabbit and human erythrocytes.
It is well established that, in other cell types, PDE4 is also associated with the regulation of increases in cAMP produced by activation of the β2AR (8, 46). Here we used the selective PDE4 inhibitor rolipram to determine whether PDE4 activity also contributes to the regulation of the increases in cAMP associated with β2AR activation in rabbit and human erythrocytes. Rolipram potentiated the increases in cAMP associated with Iso-induced β2AR activation in erythrocytes of both species (Figs. 5B and 7). Importantly, in rabbit erythrocytes, rolipram had no effect on increases in cAMP associated with the incubation of these cells with the IPR agonist Ilo (Fig. 5A). In contrast with PDE3B and PDE2A, most PDE4 isoforms are located in the cytosol (2, 14, 26). The identification of a cytosolic isoform of PDE4 in erythrocytes is complicated by the presence of large amounts of hemoglobin in this cell. Although we do not report the presence of PDE4 protein in rabbit or human erythrocytes in this study, this does not diminish the importance of our finding that the selective inhibitor of PDE4 rolipram potentiated increases in cAMP associated with activation of the β2AR while having no effect on IPR-associated increases in cAMP in these erythrocytes (Fig. 5).
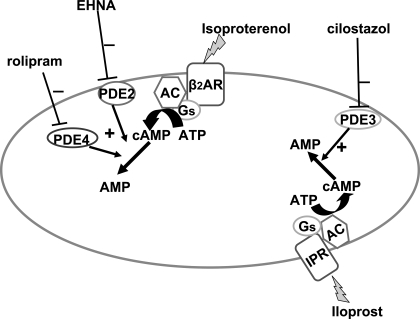
The results of studies with EHNA and rolipram provide strong evidence that PDE2 and PDE4 are both involved in the hydrolysis of cAMP generated in response to receptor-mediated activation of the β2AR in rabbit and human erythrocytes and not the IPR. Therefore, it was of interest to determine whether PDE2 and PDE4 act synergistically in the regulation of β2AR-mediated increases in cAMP in erythrocytes. When rabbit erythrocytes were incubated with concentrations of EHNA and rolipram that alone had no effect on Iso-induced increases in cAMP, we observed a significant augmentation of Iso-induced increases in cAMP (Fig. 6), suggesting that both PDEs are actively involved in the regulation of increases in cAMP generated in response to receptor-mediated activation of the β2AR in rabbit erythrocytes. The proposed relationships among PDE2, -3, and -4 and their association with the β2AR and IPR in rabbit and human erythrocytes are depicted in Fig. 10.
Fig. 10.
Model of PDE activity localized with the β2-adrenergic receptor (β2AR) and PGI2 receptor (IPR) in rabbit and human erythrocytes. Activation of the IPR leads to generation of cAMP in a restricted cAMP pool that is hydrolyzed by PDE3. In contrast, activation of the β2AR leads to generation of a distinct cAMP pool that is regulated by the activities of both PDE2 and PDE4. AC, adenylyl cyclase; Gs, heterotrimeric G protein.
In erythrocytes, receptor-mediated activation of either the Gs-coupled β2AR or IPR is associated with ATP release (25, 36). Here we demonstrate that in erythrocytes, PDE2 and PDE4 regulate increases in cAMP associated with activation of the β2AR, whereas PDE3 activity is associated with activation of the IPR. Since increases in cAMP are required for receptor-mediated ATP release from erythrocytes (39), we investigated the effect of inhibitors of PDE2, -3, and -4 on ATP release from human erythrocytes. We found that the selective PDE3 inhibitor Cilo potentiates Ilo-induced ATP release (Fig. 8). In contrast, although neither EHNA nor rolipram alone potentiated Iso-induced ATP release from human erythrocytes, pretreatment of erythrocytes with both PDE inhibitors did potentiate Iso-induced ATP release (Fig. 9). These data provide support for the hypothesis that these specific PDE isoforms are associated with individual signaling pathways in erythrocytes and participate in the regulation of receptor-mediated increases in cAMP and ATP release from these cells.
It is important to note that, at the concentrations used in these studies, the PDE inhibitors used are considered to be selective for the targeted PDE (17, 18, 43) and are based on published IC50 values (3, 4, 6). In addition, concentrations of IBMX (10 μM), vinpocetine (30 μM), and rolipram (20 μM) were chosen to have no effect on basal cAMP levels. Although EHNA (30 μM) and Cilo (10 μM) did produce small increases in baseline cAMP levels, these inhibitors had no effect on increases in cAMP in cells incubated with either Ilo or Iso, respectively (Figs. 2 and 3).
Under in vivo conditions, vessels are perfused with blood containing erythrocytes. It is well established that both Iso and prostacyclin analogs, including Ilo, produce vasodilation when administered therapeutically. Although each clearly has direct effects on vascular smooth muscle, we suggest that these agonists can additionally interact with receptors on erythrocytes leading to the release of ATP (25, 35, 36), a potent stimulus for the synthesis of endothelium-derived vasodilators. Therefore, it is possible that intravenously administered Iso or Ilo could also interact with circulating erythrocytes contributing to the associated vasodilation. In addition, since PDEs are now becoming important therapeutic targets in the treatment of pulmonary hypertension and peripheral vascular disease (17, 18, 43), identification of the individual PDEs that regulate cAMP accumulation and ATP release associated with activation of the β2AR and IPR signaling pathways in erythrocytes could provide new therapeutic targets for the development of drugs for the treatment of these conditions.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grants HL-64180 and HL-89094 and American Diabetes Association Grant RA-133.
Acknowledgments
We thank J. L. Sprague for inspiration.
REFERENCES
- 1.Babu CR, Azhar S, Krishna Murti CR. Loss of epinephrine stimulated synthesis of cyclic adenosine 3′:5′ monophosphate during maturation of rabbit and human reticulocytes. Med Biol 53: 148–155, 1975. [PubMed] [Google Scholar]
- 2.Baillie GS, Scott JD, Houslay MD. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett 579: 3264–3270, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA. Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Mol Pharmacol 47: 330–339, 1995. [PubMed] [Google Scholar]
- 4.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Butt E, Beltman J, Becker DE, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA. Characterization of cyclic nucleotide phosphodiesterases with cyclic AMP analogs: topology of the catalytic sites and comparison with other cyclic AMP-binding proteins. Mol Pharmacol 47: 340–347, 1995. [PubMed] [Google Scholar]
- 7.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 390: 88–91, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Devic E, Xiang Y, Gould D, Kobilka B. β-Adrenergic receptor subtype-specific signaling in cardiac myocytes from β1 and β2 adrenoceptor knockout mice. Mol Pharmacol 60: 577–583, 2001. [PubMed] [Google Scholar]
- 9.Dickinson NT, Jang EK, Haslam RJ. Activation of cGMP-stimulated phosphodiesterase by nitroprusside limits cAMP accumulation in human platelets: effects on platelet aggregation. Biochem J 323: 371–377, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellsworth ML Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fimia GM, Sassone-Corsi P. Cyclic AMP signaling. J Cell Sci 114: 1971–1972, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Fujitani K, Kambayashi J, Murata K, Yano Y, Shinozaki K, Yukawa M, Sakon M, Murata T, Kawasaki T, Shiba E, Mori T. Clinical evaluation on combined administration of oral prostacyclin analogue beraprost and phosphodiesterase inhibitor cilostazol. Pharmacol Res 31: 121–125, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol 295: H786–H793, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370: 1–18, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase A phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol 62: 971–974, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Lerner A, Epstein PM. Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem J 393: 21–41, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugnier C Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109: 366–398, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost 82: 407–411, 1999. [PubMed] [Google Scholar]
- 19.Manns JM, Brenna KJ, Colman RW, Sheth SB. Differential regulation of human platelet responses by cGMP inhibited and stimulated cAMP phosphodiesterases. Thromb Haemost 87: 873–879, 2002. [PubMed] [Google Scholar]
- 20.Maurice DH, Haslam RJ. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol 37: 671–681, 1990. [PubMed] [Google Scholar]
- 21.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol 64: 533–546, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98: 226–234, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Mongillo M, Zaccolo M. A complex phosphodiesterase system controls beta-adrenoceptor signalling in cardiomyocytes. Biochem Soc Trans 34: 510–511, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit 7: 669–674, 2001. [PubMed] [Google Scholar]
- 26.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298: 834–836, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Petrov V, Fagard R, Lijnen P. Human erythrocytes contain Ca2+, calmodulin-dependent cyclic nucleotide phosphodiesterase which is involved in the hydrolysis of cGMP. Methods Find Exp Clin Pharmacol 20: 387–393, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 288: L103–L115, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Rich TC, Xin W, Mehats C, Hassell KA, Piggott LA, Le X, Karpen JW, Conti M. Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells. Am J Physiol Cell Physiol 292: C319–C331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadhu K, Hensley K, Florio VA, Wolda SL. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J Histochem Cytochem 47: 895–906, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Sager G Receptor binding sites for beta-adrenergic ligands on human erythrocytes. Biochem Pharmacol 31: 99–104, 1982. [DOI] [PubMed] [Google Scholar]
- 32.Schermuly RT, Roehl A, Weissmann N, Ghofrani HA, Schudt C, Tenor H, Grimminger F, Seeger W, Walmrath D. Subthreshold doses of specific phosphodiesterase type 3 and 4 inhibitors enhance the pulmonary vasodilatory response to nebulized prostacyclin with improvement in gas exchange. J Pharmacol Exp Ther 292: 512–520, 2000. [PubMed] [Google Scholar]
- 33.Shakur Y, Fong M, Hensley J, Cone J, Movsesian MA, Kambayashi J, Yoshitake M, Liu Y. Comparison of the effects of cilostazol and milrinone on cAMP-PDE activity, intracellular cAMP and calcium in the heart. Cardiovasc Drugs Ther 16: 417–427, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J Biol Chem 274: 1588–1595, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep 57: 222–228, 2005. [PubMed] [Google Scholar]
- 36.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 15: 461–471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41: 751–773, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Strehler BL Bioluminescence assay: principles and practice. Methods Biochem Anal 16: 99–181, 1968. [DOI] [PubMed] [Google Scholar]
- 42.Tao J, Wang HY, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the beta2-adrenergic receptor. EMBO J 22: 6419–6429, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors—new opportunities for a long neglected target. Curr Top Med Chem 7: 421–436, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Willems C, Stel HV, van Aken WG, van Mourik JA. Binding and inactivation of prostacyclin (PGI2) by human erythrocytes. Br J Haematol 54: 43–52, 1983. [DOI] [PubMed] [Google Scholar]
- 45.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J 25: 2051–2061, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci USA 102: 909–914, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res 100: 1569–1578, 2007. [DOI] [PubMed] [Google Scholar]