Abstract
Recent in vitro and in vivo studies have reported fluid shear stress-induced increases in endothelial layer hydraulic conductivity (Lp) that are mediated by an increased production of nitric oxide (NO). Other recent studies have shown that NO induction by shear stress is mediated by the glycocalyx that decorates the surface of endothelial cells. Here we find that a selective depletion of the major components of the glycocalyx with enzymes can block the shear stress-induced response of Lp. Heparinase and hyaluronidase block shear-induced increases in Lp, which is consistent with their effects on NO production. But chondroitinase, which does not suppress shear-induced NO production, also inhibits shear-induced Lp. A further surprise is that treatment with the general proteolytic enzyme pronase does not suppress the shear Lp response. We also find that heparinase does not alter baseline Lp significantly, whereas chondroitinase, hyaluronidase, and pronase increase it significantly.
Keywords: endothelium, nitric oxide, shear stress
fluid shear stress-mediated regulation of endothelial hydraulic conductivity (Lp) was first demonstrated in vitro by Sill et al. (25) using bovine aortic endothelial cell (BAEC) monolayers cultured on porous, polycarbonate substrates. They observed a 3.76-fold increase in Lp after 3 h of exposure to steady shear stress of 20 dyn/cm2 that could be readily reversed by an exposure to dibutyrl cAMP, demonstrating that the shear-induced increase was not due to endothelial denudation. A subsequent study using the same BAEC model showed that the Lp response to steady shear stress is mediated by shear-induced nitric oxide (NO) since the shear response of Lp could be completely blocked by a preincubation of the BAEC monolayer with a NO synthase (NOS) inhibitor (3). Bovine retinal microvascular endothelial cells in vitro also displayed a marked increase in Lp in response to 20 dyn/cm2 shear stress that could be completely blocked by a NOS inhibitor (14).
Similar phenomena have been observed in vivo. Lever et al. (15) found that the Lp of rabbit carotid arteries in an ex vivo flow loop increased significantly after 20 min of exposure to a step change in shear stress of about 1 dyn/cm2. Williams (33) measured Lp after step changes in shear stress in arterioles, capillaries, and venules of the frog mesentery using the modified Landis technique. The response of the vessels was graded across the capillary bed, with arteriolar capillaries demonstrating no response, true capillaries a moderate response, and venular capillaries a strong response (5-fold increase) to a step change in shear stress. Most recently, Kim et al. (13) observed that changes in Lp were positively correlated with the magnitude of acute changes in shear stress in autoperfused microvessels in rat mesenteric tissue. The effect was greater in capillaries compared with terminal arterioles and could be eliminated by superfusion with a NOS inhibitor.
As indicated above, several studies, both in vitro and in vivo, have shown that the shear-induced increase in endothelial Lp is stimulated by shear-induced NO. It appears that shear-induced NO from endothelial cells is a mechanotransduction event that is mediated by the cell surface glycocalyx (GCX). Proteoglycans are major constituents of the GCX that are composed of core proteins having either a transmembrane linkage (syndecans) or a membrane linkage (glypicans) with extracellular domains that are covalently linked to glycosaminoglycans, the most prominent of which are heparan sulfate (HS), chondroitin sulfate (CS), and hyaluronic acid (HA). The structure of the GCX is reviewed in much greater detail in Tarbell and Pahakis (27) and Weinbaum et al. (30). A study in canine femoral arteries showed that the selective depletion of HA with the enzyme hyaluronidase blocked a flow-induced NO production (19), whereas an in vitro study in BAECs observed that the depletion of HS with heparinase had the same effect (10). A more extensive recent investigation in BAECs confirmed that the depletion of HA and HS blocked a shear-induced NO production, but a similar depletion of CS (with chondroitinase) had no effect (20).
In the present study we investigated the hypothesis that the GCX is the mechanotransduction element that mediates shear-induced increases in endothelial Lp. We used the same BAEC monolayer preparation previously employed to study the shear effects on Lp by Chang et al. (3) and the same GCX-degrading enzymes employed by Pahakis et al. (20) in their investigation of shear-induced NO production. We also examined the influence of GCX-degrading enzymes on the baseline Lp values of BAEC monolayers. Heparinase and hyaluronidase block the shear-induced increases in Lp, consistent with their effects on NO production. But to our surprise, chondroitinase also inhibits the shear-induced increases in Lp.
MATERIALS AND METHODS
Chemicals and materials.
The following chemicals were obtained from Sigma-Aldrich Chemical (St. Louis, MO): bovine serum albumin (BSA, 30% solution), Eagles's minimum essential medium (MEM), phenol red-free MEM, penicillin-streptomycin solution, l-glutamine, trypsin-EDTA solution, HEPES, sodium bicarbonate, heparin (sodium salt, grade I-A, 181 United States Pharmacopaeia U/mg), fibronectin, heparinum heparinase III, Proteus vulgaris chondroitinase ABC, Streptomyces hyalurolyticus hyaluronidase, pronase E from Streptomyces griseus, and (6R)-5,6,7,8-tetrahydrobiopterin. Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Transwell polyester filters (24.5 mm diameter, and 0.4-μm pore size) were purchased from Costar (Cambridge, MA).
BAEC culture and insert preparation.
BAECs were purchased from VEC Technologies (Rensselaer, NY) and grown in T-75 flasks with 10% FBS-MEM. The flasks were kept at 37°C in 5% CO2-95% room air. Upon reaching confluency (3 to 4 days), the cells were split for continuing maintenance of the cell line. The model for the endothelium was developed by plating BAECs at a density of 1.20 × 105 cells/cm2 on Transwell polyester filters with 0.4-μm pores that were previously coated with fibronectin. The filters were incubated in 5% CO2-95% room air at 37°C with 10% FBS-MEM. The shear-permeability experiments were run 6–8 days after plating, once the cells reached total confluency, but were free from overgrowth. Cells were used from passages 5 to 8.
Enzyme treatments.
Before the water flux measurement, the monolayers were incubated for 2 h with one of the following enzymes: Flavobacterium heparinum heparinase III (15 mU/ml), P. vulgaris chondroitinase ABC (12 mU/ml), or S. hyalurolyticus hyaluronidase (1.5 U/ml). All of these enzyme concentrations are in sigma units, and the time of incubation is in agreement with our previous study of shear-induced NO production (20). The enzymes were used to degrade HS, CS, and HA, respectively. In addition, a set of monolayers was incubated for 1 min before the Lp measurements with the nonspecific protease pronase E (0.1 mg/ml). This is the same dose and the same time of exposure employed by Adamson (1) and Williams (31). It should be noted that all of the shear experiments used media that was enriched with (6R)-5,6,7,8-tetrahydrobiopterin (60 μM), a cofactor for endothelial NOS (eNOS) activity (23, 24) that was used in our study on the role of the GCX in shear-induced NO (20).
Determination of water flux across the endothelium.
The measurement of water flow across the endothelial monolayer was performed with an apparatus developed in our laboratory (25) that was kept inside a Plexiglas box and maintained at 37°C. The seeded filters were placed inside a chamber to form a luminal (top) compartment and an abluminal (bottom) compartment separated only by the BAEC monolayer. The abluminal compartment was connected to a reservoir via Tygon and borosilicate glass tubing. The vertical displacement of the reservoir with respect to the liquid covering the cells allowed us to apply a hydrostatic pressure differential across the monolayer. When a 10-cmH2O differential pressure was applied, the volumetric flow rate (Jv) was measured by tracking the position of a bubble that was inserted into the calibrated borosilicate glass tube. The Lp was calculated from the following relationship: Lp = (Jv/A)/ΔP, where A is the BAEC monolayer area and ΔP is the pressure differential across the monolayer. After 60 min of applied pressure differential to drive the water flux, a baseline Lp was established, and a defined shear stress was then applied to the endothelial monolayer using a rotating disk separated by a distance h (500 μm) from the monolayer surface. The rotating disk generated a fluid shear stress distribution on the monolayer surface defined by τ = μ × ω × r/h, where μ is the viscosity of the media, ω is the rotational speed, and r is the radial distance from the center of the disk. The parameters were adjusted to achieve a maximum steady shear stress of 20 dyn/cm2 at the edge of the disk, and this is the value that is reported in the table and figures. The average shear stress over the entire filter area is two-thirds of the maximum. During the entire experiment, the luminal compartment and the reservoir were supplied with gas (5% CO2-95% room air) to maintain the experimental media at the physiological pH of 7.4. The experimental media for all of the Lp measurements was MEM supplemented with 1% BSA. The Lp values were recorded for 4 h.
Two experiments were always run side by side. One endothelial monolayer was untreated, whereas the companion monolayer was treated with an enzyme. These pairs of monolayers were always plated together from the same flask and grown the same number of days in the same media. This procedure allowed us to minimize the effects associated with cell variability. In presenting the results, therefore, we have a separate set of controls for each enzyme.
NO determination.
NO was determined under static and shear conditions by exactly the same methods described in Pahakis et al. (20) using the fluorometric assay described by Misko et al. (18) with accuracy down to 10 nmol/l to detect nitrite, the major stable metabolite of NO. Cumulative concentrations are reported that account for sample dilutions and evaporation.
Statistical analysis.
Lp values are presented as means ± SE. Tests for statistical significance were conducted using the two-way ANOVA function (time and treatment) from Minitab software and a post hoc analysis using the Tukey method with P < 0.05 considered as significant.
RESULTS
Table 1 displays the baseline values for Lp 60 min after the application of a 10-cmH2O pressure differential. The control values of the baseline Lp are within the normal ranges that have been reported for BAECs in previous studies (2, 3, 5, 25) and are within a factor of two of the values reported for in vivo studies in frog mesenteric capillaries (31, 32). For comparison to mammalian values, for example, Rumbaut et al. (22) found that the mean Lp for venules from the mesentery of Sprague-Dawley rats was 2.3 × 10−7 cm·s−1·cmH2O−1. Tedgui and Lever (28) estimated Lp values of rabbit aortic endothelium as 1.2 × 10−7 cm·s−1·cmH2O−1. Our average Lp values are higher but of the same order of magnitude as these in vivo values. The heparinase III treatment increased the baseline Lp by 25% (not significant), whereas the hyaluronidase treatment led to a 97% increase; the chondroitinase treatment led to a 81% increase, and the pronase treatment led to a 91% increase (all significant).
Table 1.
Average baseline Lp and SE for the different enzymatic treatments
| n | Baseline Lp, cm·s−1·cmH2O−1 | SE | |
|---|---|---|---|
| Control | 4 | 2.51×10−7 | 2.83×10−8 |
| Heparinase III | 4 | 3.15×10−7 | 4.36×10−8 |
| Control | 5 | 4.26×10−7 | 2.67×10−8 |
| Hyaluronidase | 5 | 8.39×10−7 | 9.48×10−8 |
| Control | 16 | 4.81×10−7 | 4.56×10−8 |
| Chondroitinase | 14 | 8.92×10−7 | 6.12×10−8 |
| Control | 4 | 4.74×10−7 | 8.67×10−8 |
| Pronase | 5 | 9.05×10−7 | 11.8×10−8 |
Hyaluronidase, chondroitinase, and pronase significantly increased the hydraulic conductivity (Lp) compared with controls (P < 0.05). Heparinase III did not alter baseline Lp significantly (P = 0.25). n, Number of runs.
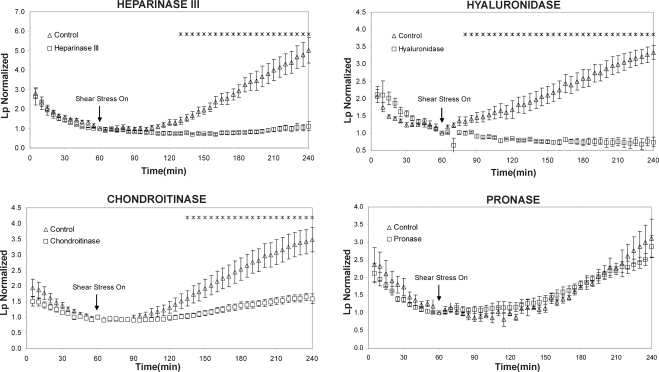
Figure 1 shows the shear stress responses of Lp for each of the selective enzyme treatments for glycosaminoglycan components compared with the companion control responses without treatment. The Lp values are normalized by the control values provided in Table 1, so that the normalized Lp value is 1.0 at 60 min for all cases. All of the experiments showed the characteristic “sealing” behavior in which Lp decreased continuously to its baseline value during the first 60 min of exposure to the pressure differential. This behavior has been observed in all previous experiments with BAECs (3, 25) and in other cell types in vitro (3) as well as in vivo (13). The sealing effect was not affected significantly by any of the enzyme pretreatments, as the control and treatment curves cannot be distinguished during the first 60 min.
Fig. 1.
Effect of different enzyme treatments on bovine aortic endothelial cell (BAEC) hydraulic conductivity (Lp) response to shear stress. At time 0 min, a hydrostatic pressure differential of 10 cmH2O was applied to the monolayers to drive water flow across them, and a baseline level was established after 1 h. The Lp values were normalized to the baseline level for each monolayer. At time 60 min, shear stress of 20 dyn/cm2 was applied and Lp recorded for 3 h. The monolayers that were treated with heparinase III (n = 5), hyaluronidase (n = 4), and chondroitinase (n = 16) displayed a significant attenuation of the shear-stress effect on Lp, whereas the pronase-treated monolayers (n = 6) did not. Inhibition of the shear response by chondroitinase was not as great as by heparinase or hyaluronidase. *P < 0.05, significant difference between treated and control monolayers. n, Number of runs.
The most important results displayed in Fig. 1 are the observations that pretreatments with heparinase III and hyaluronidase completely blocked the shear-induced increase in Lp, whereas chondroitinase pretreatment significantly inhibited the shear Lp response, but pronase had no significant effect on the response. These observations indicate that the GCX mediates shear-induced changes in endothelial Lp and suggest that the mechanism involves a shear-induced NO production since it was previously shown that heparinase and hyaluronidase block a shear-induced NO production in BAECs (20). The mechanism is subtle because heparinase blocks shear-induced Lp while having no significant influence on baseline Lp, and pronase, which has a significant effect on baseline Lp, does not affect shear-induced Lp.
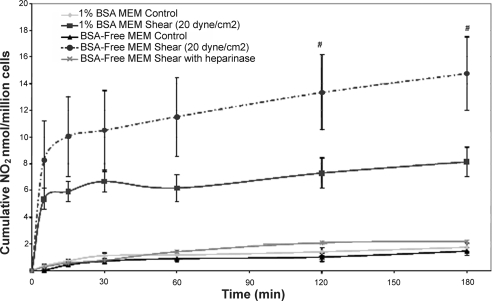
To explore these nuances further, we measured NO production in response to shear stress in media lacking protein (BSA-free MEM), because several previous studies have shown that protein-free media leads to large increases in Lp, up to 10-fold (7, 12). Figure 2 shows the characteristic NO response to shear stress in 1% BSA-MEM media as has been described previously (10). Also shown is the surprising enhancement of NO production in response to shear stress in BSA-free MEM media and the complete suppression of the shear response in this media when the monolayer was pretreated with heparinase. Our earlier studies (10, 20) showed that in media containing 1% BSA-MEM, heparinase completely blocked the shear response as well.
Fig. 2.
Cumulative nitric oxide concentration vs. time for BAECs under static and shear conditions for 1% BSA media, BSA-free media, and heparinase-treated monolayers in BSA-free media. Shear was applied at time 0 (n = 9). #P < 0.05 vs. 1% BSA shear. n, Number of runs.
DISCUSSION
Shear-induced alterations in endothelial Lp appear to be a fundamental physiological response to acute changes in flow that have been observed in many endothelial cell types in vitro (3, 25, 27) and in several preparations either in vivo or in intact vessels (31, 32, 34). Several studies have shown that this response is mediated by shear-induced NO release (3, 11, 14), and recent investigations have indicated that shear NO induction is mediated by the GCX (10, 20). The present study provides evidence that it is indeed the GCX that mediates the mechanotransduction events relating the mechanical force of shear stress to the physiological response of Lp. The GCX has also been implicated in cell alignment and proliferation (35) and in maintaining the integrity of arteries (29).
It should be noted that shear stress-induced Lp responses that have been observed using in vivo and ex vivo models (13, 15, 33) are inherently based on endothelium that have been acclimated to shear stress, whereas the in vitro endothelial layers of the present study were not exposed to shear stress before experiencing a step increase at the beginning of the experiment. That both the in vivo and in vitro systems exhibit an increase in Lp in response to an increase in shear stress suggests either that a change in shear stress, whether from zero (in vitro) or a homeostatic value (in vivo), initiates an endothelial remodeling process that is characterized by an increase in Lp as endothelial junctions remodel or that endothelial mechanotransduction leading to an Lp (NO) increase proceeds independent of remodeling. It has been suggested elsewhere (27), but not proven, that glypican core proteins of the GCX mediate the NO response to shear stress, whereas syndecan core proteins mediate the remodeling response. This would imply that the NO (Lp) response to shear stress is uncoupled from the remodeling response. Additional studies will be required to test this hypothesis.
The rotating disk device employed in these experiments induces a radial gradient in shear stress across the endothelial monolayer, and an earlier study using the same apparatus showed that BAEC Lp has a threshold of sensitivity to (maximum) shear stress of 1 dyn/cm2 and an increasing response up to 20 dyn/cm2. This implies that there could be a radial gradient in Lp across the monolayer and that we are sampling an average response over the entire surface.
The enzyme treatments for heparinase, hyaluronidase, and chondroitinase employed in this study, including the concentrations and times of exposure, were identical to those previously described by Florian et al. (10) and Pahakis et al. (20).Those studies showed that about 43% of the fluorescence associated with an antibody (HS) or a specific lectin (CS) was removed by the treatment. The HA removal, determined by ELISA quantification of the HA released into the media, was 62%. Pahakis et al. (20) also showed that no enzyme removed more than 5% of a nontarget component, indicating the specificity of the enzymes. Furthermore, they demonstrated that none of the enzyme treatments affected agonist-induced NO release (histamine or bradykinin), indicating that the NO production apparatus of the cells was not impaired by the enzyme treatments.
The effects of enzyme treatments on endothelial Lp have been investigated in only a few previous studies. Parameswaran et al. (21) applied hyaluronidase to rabbit mesentery and found that Lp increased by 72%. This is of the same order as the 97% increase that we observed (Table 1). Dull et al. (8) used a heparinase-III treatment on lung microvascular endothelial cells in vitro and observed a 144% increase in Lp. These changes after heparinase treatment are much higher than the 25% increase in Lp observed in the present study (Table 1). However, Dull et al. (8) used 15 mU/ml in international units (personal communication, R. O. Dull), and we used 15 mU/ml in sigma units. Therefore, their concentration was much higher since 1 international unit equals 600 sigma units. Chondroitinase, which increased Lp by 81% (Table 1), has not been investigated for its effect on Lp in any previous studies. The general proteolytic enzyme pronase at a concentration of 0.1 mg/ml induced a 91% increase in baseline Lp. This was somewhat lower than the range observed by Williams (31) (160%) and Adamson (1) (145%), both using 0.1 mg/ml pronase in frog mesenteric capillaries. It therefore appears that our baseline data on enzyme influences on Lp are consistent with the limited data available in the literature. Even higher increases in baseline Lp (up to 900%) have been observed in preparations using protein-free media, but without any enzyme treatment (7, 12).
It was somewhat surprising to observe that heparinase, which removed as much antibody-associated fluorescence as chondroitinase (20), had a much smaller effect on baseline Lp than chondroitinase (Table 1). The change in fluorescence intensity, however, is not equivalent to the change in mass because of possible differences in the binding affinities of the antibodies, and it may be that more CS was removed than HS. CS also resides closer to the plasma membrane than HS (26), and this may be significant. But we really do not know enough about the detailed structure of the GCX to explain the contributions of individual components to the overall hydraulic resistance.
It is striking to note in Fig. 1 that none of the enzymes affected the transient behavior of Lp during the initial 60-min sealing period. This was also observed by Dull et al. (8) using lung microvascular endothelial cells treated with heparinase III. These results imply that sealing is not mediated by the GCX. DeMaio et al. (4) using BAECs, showed that sealing involves the recruitment along microtubules of the protein zonula occludens-1 to the tight junction regions of the cell. When the BAEC monolayers were subjected to the same elevation in pressure (10 cmH2O) without any differential pressure to drive transmural flow, the recruitment of zonula occludens-1 to the junctions was blocked (DeMaio, unpublished data, 2004; available upon request from J. M. Tarbell). This indicates that it is the flow through the intercellular junctions induced by the pressure differential and not the elevation of pressure per se that drives the sealing process. Tarbell et al. (26) estimated the fluid wall shear stress in the intercellular junctions associated with normal transmural flow to be on the order of 25–50 dyn/cm2, the same magnitude as that of flowing blood on the walls of endothelial cells (17). Thus it may be this intercellular junction shear stress acting in a region of the junction that is not covered by GCX that drives the sealing phenomenon. It is generally believed that the GCX extends into the entrance of the interendothelial junction but does not line the entire junction (36).
The data in Fig. 1 for the control cases show shear-induced increases in Lp after 3 h of exposure to a maximum of 20 dyn/cm2, ranging between 3.5× and 5.0× the 60-min baseline value. Even though this range is consistent with the shear-induced responses observed in previous studies (3, 25), for the purpose of comparison in the present study, we used monolayers that had been cultured and plated from the same flask for each set of control and enzyme-treated cells. It is evident that pretreatment with either heparinase III or hyaluronidase completely blocks the response, whereas chondroitinase also inhibits the response significantly, but not completely. A previous study employing the same cell type, shear stress magnitude, and enzyme treatments showed that the significant shear-induced increase in NO production over 3 h was blocked by heparinase III and hyaluronidase, but not chondroitinase. Earlier studies have shown that shear-induced increases in Lp are mediated by NO (3, 13, 14). Taken together, these studies strongly suggest that a shear-induced increase in endothelial Lp is mediated by the GCX serving as a mechanotransducer for NO production.
This interpretation of the data is not, on the surface, supported by the observation that chondroitinase inhibits the shear Lp response (Fig. 1) while having no influence on the shear NO response (20). This seeming contradiction could be the result of an effect of the enzyme on a pathway downstream of NO or in parallel with NO that influences the Lp. For example, it is has been observed that a reduction of CS can upregulate cAMP activity (6), and it is well known that an elevation of cAMP reduces endothelial Lp (25). CS proteoglycans and CD44 that contains CS interact with cytoskeletal elements including microtubules (16) that are known to mediate the movement of tight junction proteins away from intercellular junctions (6). Additional studies will be required to assess these possibilities.
Unlike our observations with heparinase, hyaluronidase, and chondroitinase, Williams (31), using pronase in frog mesenteric capillaries at a concentration (0.1 mg/ml) that greatly increased baseline Lp (160%), found that this treatment actually led to an increase in the sensitivity of Lp to step increases in shear stress. This suggests that there is something fundamentally different about the mechanotransduction process when the GCX is highly disrupted. To pursue this hypothesis, we also treated our cells with pronase at the same dose and exposure time as Williams (31), and although we did not alter the baseline Lp as much as Williams (91% vs. 160%), we observed that the shear Lp response was not attenuated as it was for all other enzymes (Fig. 1). Unfortunately, it was not possible to increase the pronase dose because it had been shown by Adamson (1) and Chang et al. (3) in frog mesenteric capillaries and BAECs, respectively, that at only a slightly higher dose (0.125 vs. 0.100 mg/ml), there was a complete breakdown of the endothelial transport barrier.
To examine further the behavior of endothelial mechanotransduction under conditions where the GCX is highly disrupted, we conducted experiments in protein-free media. We did not pursue shear Lp experiments in this media because of the extreme elevation of baseline Lp (900%). However, since we have found in BAECs that shear-induced NO production mediates the shear Lp response (3), we measured the NO response to shear under conditions where baseline Lp was most highly disturbed—in protein-free media. Consistent with the observations of Williams (31), we found that shear-induced NO, which mediates an Lp increase, was significantly enhanced in protein-free media (Fig. 2). But, when the cells were pretreated with heparinase at the same concentration and time of exposure as in the experiments of Fig. 1 and then sheared in protein-free media, the shear-induced NO production was completely inhibited.
The basic observations presented in Figs. 1 and 2 support a mechanism involving shear-induced NO, mediated by the GCX, that enhances Lp. It is noteworthy that glypicans, which contain HS, are linked to caveolae where eNOS resides (27). It is also important to realize that HA binds to its CD44 receptor which is localized in caveolae (27), thus providing a link between HA and shear-induced Lp (NO) that is blocked when HA is depleted. Several studies have implicated a role for platelet endothelial cell adhesion molecule 1 (PECAM-1) in the shear-stress induction of eNOS and NO (e.g., Ref. 9). The relationship between the GCX and PECAM-1 is not clearly established, but Tarbell and Pahakis (27) speculated that the GCX may serve to sense fluid shear stress and then distribute the associated force to the intercellular junctions where PECAM-1 resides, using the cytoskeleton that is linked to both the GCX and PECAM-1 to transmit the force.
The observation of Williams (31) that pronase treatment, which increased Lp by 160%, actually leads to an enhanced sensitivity of Lp to shear stress is consistent with our observation that the sensitivity of NO production to shear stress is enhanced when the GCX is highly disrupted in protein-free media (Fig. 2) and is not inconsistent with our observation that pronase treatment, which was less disruptive to the GCX (increased Lp by 91%), did not suppress the shear Lp response. Even though the GCX is highly disrupted in protein-free media, as suggested by the highly elevated Lp (900%; Ref. 17), it appears that HS still plays a central role in the mechanotransduction of shear stress since shear-induced NO is completely blocked by heparinase in this media (Fig. 2), like it is in 1% BSA media, as shown in an earlier study by our group (10). This is still consistent with a glypican-caveolae-eNOS mechanism since these connections would be expected to remain intact even when the GCX has been diminished by a lack of protein interactions.
The final, and most difficult, observation to account for in terms of a glypican-caveolae-eNOS mechanism is the observation of Williams (31) that pronase treatment led to an enhanced sensitivity of Lp to shear stress and our observation that pronase treatment did not suppress the shear Lp sensitivity. It may be that the short exposure to the enzyme (1 min) was not sufficient to degrade the glypicans that reside deep in the GCX in caveolae and that fluid shear stress, not dissipated by a degraded GCX (30), was able to penetrate to the level of the caveolae. Future studies will be required to resolve these issues.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-57093.
REFERENCES
- 1.Adamson RH Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 428: 1–13, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancel LM, Fitting A, Tarbell JM. In vitro study of LDL transport under pressurized (convective) conditions. Am J Physiol Heart Circ Physiol 293: H126–H132, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chang YS, Yaccino JA, Lakshminarayanan S, Frangos JA, Tarbell JM. Shear-induced increase in hydraulic conductivity in endothelial cells is mediated by a nitric oxide-dependent mechanism. Arterioscler Thromb Vasc Biol 20: 35–42, 2000. [PubMed] [Google Scholar]
- 4.DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA. Shear stress regulates occludin content and phosphorylation. Am J Physiol Heart Circ Physiol 281: H105–H113, 2001. [DOI] [PubMed] [Google Scholar]
- 5.DeMaio L, Tarbell JM, Scaduto RC Jr, Gardner TW, Antonetti DA. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am J Physiol Heart Circ Physiol 286: H731–H741, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Dittmann J, Krischek C, Harisch G. Modulation of cAMP-dependent protein kinase by derivatives of chondroitin sulfate. Life Sci 60: PL201–PL206, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dull RO, Jo H, Sill H, Hollis TM, Tarbell JM. The effect of varying albumin concentration and hydrostatic pressure on hydraulic conductivity and albumin permeability of cultured endothelial monolayers. Microvasc Res 41: 390–407, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Dull RO, Mecham I, McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 292: L1452–L1458, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dusserre N, L'Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol 24: 1796–1802, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Hillsley MV, Tarbell JM. Oscillatory shear alters endothelial hydraulic conductivity and nitric oxide levels. Biochem Biophys Res Commun 293: 1466–1471, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol Heart Circ Physiol 248: H264–H273, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Kim MH, Harris NR, Tarbell JM. Regulation of capillary hydraulic conductivity in response to an acute change in shear. Am J Physiol Heart Circ Physiol 289: H2126–H2135, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Lakshminarayanan S, Gardner TW, Tarbell JM. Effect of shear stress on the hydraulic conductivity of cultured bovine retinal microvascular endothelial cell monolayers. Curr Eye Res 21: 944–951, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Lever MJ, Tarbell JM, Caro CG. The effect of luminal flow in rabbit carotid artery on transmural fluid transport. Exp Physiol 77: 553–563, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Lin XH, Dahlin-Huppe K, Stallcup WB. Interaction of the NG2 proteoglycan with the actin cytoskeleton. J Cell Biochem 63: 463–477, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Lipowsky HH Microvascular rheology and hemodynamics. Microcirculation 12: 5–15, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214: 11–16, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–H726, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parameswaran S, Brown LV, Ibbott GS, Lai-Fook SJ. Effect of concentration and hyaluronidase on albumin diffusion across rabbit mesentery. Microcirculation 6: 117–126, 1999. [PubMed] [Google Scholar]
- 22.Rumbaut RE, Wang J, Huxley VH. Differential effects of l-NAME on rat venular hydraulic conductivity. Am J Physiol Heart Circ Physiol 279: H2017–H2023, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113: 47–63, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10: 1115–1126, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Sill HW, Chang YS, Artman JR, Frangos JA, Hollis TM, Tarbell JM. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Physiol Heart Circ Physiol 268: H535–H543, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Tarbell JM, Demaio L, Zaw MM. Effect of pressure on hydraulic conductivity of endothelial monolayers: role of endothelial cleft shear stress. J Appl Physiol 87: 261–268, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med 259: 339–350, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Tedgui A, Lever MJ. Filtration through damaged and undamaged rabbit thoracic aorta. Am J Physiol Heart Circ Physiol 247: H784–H791, 1984. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290: H915–H920, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA 100: 7988–7995, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DA Change in shear stress (Deltatau)/hydraulic conductivity (Lp) relationship after pronase treatment of individual capillaries in situ. Microvasc Res 73: 48–57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DA Intact capillaries sensitive to rate, magnitude, and pattern of shear stress stimuli as assessed by hydraulic conductivity (Lp). Microvasc Res 66: 147–158, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Williams DA Network assessment of capillary hydraulic conductivity after abrupt changes in fluid shear stress. Microvasc Res 57: 107–117, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Williams DA A shear stress component to the modulation of capillary hydraulic conductivity (Lp). Microcirculation 3: 229–232, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Yao Y, Rabodzey A, Dewey CF Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293: H1023–H1030, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Adamson RH, Curry FR, Weinbaum S. A 1-D model to explore the effects of tissue loading and tissue concentration gradients in the revised Starling principle. Am J Physiol Heart Circ Physiol 291: H2950–H2964, 2006. [DOI] [PubMed] [Google Scholar]