Abstract
Microvascular development is often perceived to result from a balance of positive and negative factors that impact signaling for proliferation and survival. The survival signaling that results from hypoxia-induced VEGF-A has been well established, but the factors that antagonize this signaling have been poorly studied. As endogenous inhibitors of angiogenesis, thrombospondins (TSPs) are likely candidates to affect survival signaling. Here we report that TSP1 antagonized microvascular survival to retinal hyperoxia, and Akt signaling in both the retina and in cultured endothelial cells. TSP1 expression is correlated with the association of the CD36 receptor with Src versus Fyn. In the presence of TSP1, CD36 is coprecipitated with Fyn as previously shown by others. However, in the absence of TSP1, there is a preferential association with Src. We now demonstrate that these Src family kinases play an important role in modulating microvascular survival in response to TSP1 by crossing tsp1−/− mice to the src−/− and fyn−/− mice and testing the survival of retinal blood vessels in hyperoxia. We find that tsp1−/−, fyn−/−, and double-mutant tsp1−/−/fyn−/− mice have a similar enhancement of capillary survival in oxygen, whereas in a tsp−/− background, the loss of only one allele of src restores the balance in survival and apoptosis to that of wild-type mice. Taken together, we hypothesize that TSP1 antagonizes VEGF-driven Akt survival signaling in part through the recruitment of Fyn to membrane domains containing CD36, but when TSP1 is absent, an opposing Src recruitment contributes to VEGF-driven Akt phosphorylation and capillary survival.
Keywords: vascular endothelial growth factor
thrombospondins (TSPs) are secreted, multidomain macromolecules that act as regulators of cell interactions in vertebrates. The TSP family consists of five members (TSP1–4, and cartilage oligomeric matrix protein COMP or TSP5). TSP1 and TSP2 have been the most extensively studied members of this family and share similar domains and overall structure and interact with many of the same cell-surface receptors (1, 4, 21). TSP1 is a matricellular, calcium-binding protein that regulates adhesion/migration, cytoskeletal organization, and apoptosis (8). TSP1 is also a naturally occurring inhibitor of angiogenesis that limits vessel density in normal tissues and blocks pathological blood vessel development (18). The inhibition of angiogenesis and the induction of apoptosis by TSP1 have been linked to CD36 receptor binding, followed by the activation of signaling pathways that include p59fyn, caspase-3-like proteases, and p38 mitogen-activated protein kinases (20). Whereas TSP1 protein expression is low in most adult tissues, it is upregulated at sites of tissue remodeling, including wound healing and neoplasia (22). In contrast to the negative role of TSP1 in the suppression of endothelial functions, TSP1 supports the migration and proliferation of vascular smooth muscle cells (23). TSP1 may also inhibit angiogenesis by decreasing the association of vascular endothelial growth factor (VEGF) with its receptor, VEGFR2, by decreasing the levels of active matrix metalloproteinase-9, which can release VEGF from the extracellular matrix (31). Both TSP1 and TSP2 can also inhibit endothelial cell proliferation by the inhibition of cell-cycle progression and the induction of cell death, but the mechanisms responsible for the inhibition of cell-cycle progression are different from those leading to cell death and involve an interaction with the VLDL receptor (26).
Our investigation of the function of TSP1 and TSP2 in physiological angiogenesis has used the developing murine retina as a model system. Mice are born blind and acquire vision as a consequence of postnatal retinal development, a process dependent on angiogenesis. Because of the highly reproducible kinetics of retinal angiogenesis and its planar architecture, the murine retina is particularly valuable for investigating normal developmental angiogenesis and the effects of environmental variables on that process. Previous studies of TSP1 function in the developing retina have shown it to play a role in modulating normal vascular patterning and pathological angiogenesis (41, 42). We have used the developing retina to interrogate the interplay between VEGF-A and TSP1 and TSP2 in regulating the remodeling process, which is dependent on VEGF-A-induced survival signaling (2). VEGF-A is a key angiogenic factor that is exquisitely sensitive to oxygen levels: hypoxia increases VEGF transcription, translation, and mRNA stability (12, 33, 36). One advantage of the developing retina as a model is the ability of ambient oxygen to control VEGF-A expression by retinal astrocytes and, as a consequence, control vascular expansion and regression (27, 35, 37). The exposure of neonatal mouse pups to hyperoxia downregulates endogenous VEGF-A expression, leading to the apoptosis of endothelial cells in immature vessels not yet coated with pericytes. The injection of multiple members of the VEGF family can rescue these vessels from regression (3, 40). We have recently shown that this oxygen-driven regression is regulated by a loss of Akt signaling within the developing retina (38). In this study, we investigated the role of TSP1 and TSP2 in this oxygen-driven vascular regression and Akt signaling in the developing retina. Our data support the hypothesis that TSP1 can modulate developmental microvascular remodeling in the retina by antagonizing the Akt signaling provided by VEGF-A. Furthermore, we provide evidence that both Fyn and Src signaling downstream of the TSP receptor, CD36, play a role in this process, with Fyn being the mediator of TSP1/CD36 negative functions and Src providing a positive signaling that stimulates microvascular survival.
MATERIALS AND METHODS
Animal and genotyping by PCR.
All animal studies were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center prior to their conduct. Mice used for these studies were on FVB/N, SV129, and FVB/SV129 mixed backgrounds. The retinal regression studies and Western blots shown in Figs. 1–5 were performed on an FVB/N inbred background. Because the src−/− and fyn−/− mice were on a SV129 background, we used tsp1−/− mice on the SV129 background for the retinal studies and breeding shown in Fig. 6. For breeding src−/− mice, we were unable to get enough tsp−/−/src−/− mice on the SV129 background and thus used a mixed background of tsp1−/− FVB/N X src−/− or fyn−/− SV129 to generate the mice for the Western blot analysis shown in Fig. 6E. The wild-type controls for this blot were a mixture of retinas from wild-type siblings of the tsp1−/−/src−/− and siblings of the tsp−/−/fyn−/− mice. PCR genotyping for tsp1 and tsp2 was performed using three primers for tsp1 and four primers for tsp2. The primer sequences for the target genes are shown in Table 1. PCR amplification was performed using the following conditions: 40 cycles at 94°C for 0.5 min, 65°C for 1 min, 72°C for 2 min, followed by a final cycle at 72°C for 5 min (Table 1).
Fig. 1.
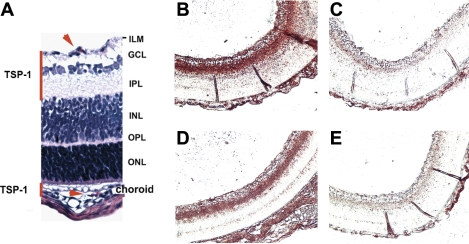
Thrombospondin (TSP) 1 is expressed in vascular compartments of the developing neonatal retina. A: histology of the different retinal layers with red lines indicating regions of TSP1 deposition, and red arrows pointing to erythrocytes contained in capillaries. TSP1 staining in retina of wild-type (B), tsp1−/− (C), tsp2−/− (D), and tsp1−/−/tsp2−/− (E) mice is shown. ILM, inner limiting membrane; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Fig. 5.
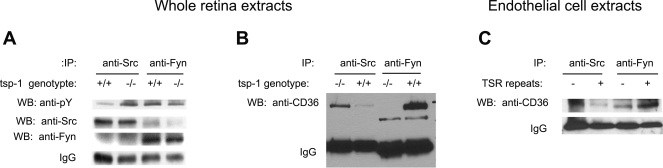
Src and Fyn phosphorylation and CD36 coimmunoprecipitation are modulated by TSP1. A: immunoprecipitation (IP) with antibodies to mouse Src and Fyn from whole retina lysates were blotted for phospho-tyrosine and total Src and Fyn. B: immunoprecipitation of whole retina extracts with antibodies to Src and Fyn were blotted with anti-CD36 antibody. C: immunoprecipitation of human dermal endothelial cell extracts with antibodies to Src and Fyn before or after 3TSR treatment and were blotted for CD36. WB, Western blot; PY, phosphotyrosine.
Fig. 6.
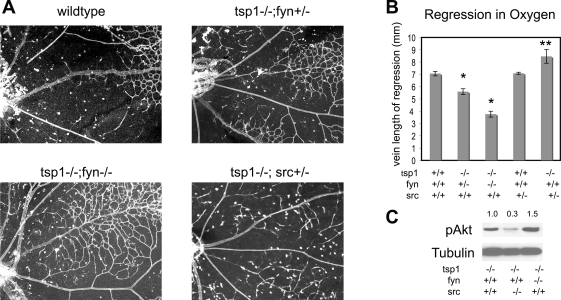
Survival in hyperoxia in tsp1−/− retina is not affected by loss of Fyn but blocked by loss of Src. A: representative examples of whole mount lectin staining of retinas following 24 h of oxygen from P7 to P8. The wild-type shown here is a fyn+/− littermate to the tsp1−/−/fyn +/−. B: quantification of vein length from optic disc to capillary plexus (distance of regression along the vein) is shown for related genotypes. N = 5–9 per sample. The *P < 0.001, significant differences from wild-type; tsp1−/−/fyn +/− was also significantly different from tsp1−/−/fyn −/−. **P < 0.001, significant differences from tsp−/− mice. C: a representative Western blot is shown probed for pAkt (S473) and α-tubulin expression using protein lysates from P8 retinas of tsp1−/−, tsp−/−/src−/−, and tsp1−/−/fyn−/− mice. The densitometry of pAkt bands normalized to tubulin is listed above the blot, and genotypes are shown below the blot (n > 4).
Table 1.
The primer sequences for genotyping of tsp1 and tsp2
| Genes/Primers | Sequences |
|---|---|
| tsp1 | |
| 228MGR | 5′-GAGTTTGCTTGTGGTGAACGCTCAG-3′ |
| 229MGF | 5′-AGGGCTATGTGGAATTAATATCGG-3′ |
| 213NeoMGF | 5′-TGCTGTCCATCTGCACGAGACTAG-3′ |
| tsp2 | |
| 512TSP2GA | 5′-CTGGTGACCACGTCAAGGACACTTCAT-3′ |
| 513TSP2GB | 5′-ATGCACCTTTGGCCACGTACATCCTGC-3′ |
| 584t2in4 | 5′-GGAGAAGAATTAGGGAGGCTTAGG-3′ |
| 447Neo | 5′-GATCAGCAGCCTCTGTTCACATAC-3′ |
TSP, thrombospondin.
Whole mount immunofluorescence and immunohistochemistry.
For whole mount preparations, the eyes were fixed with 10% formalin at 4°C from 2 h to overnight. Dissection of the retina was done by carefully removing the cornea, lens, and sclera. After being washed three times with phosphate-buffered saline (PBS) for 15 min and blocked with PBS containing 0.5% Triton X-100, 0.02% azide, PBS, and 10% goat serum for 1–3 h, the eyes were incubated by shaking at 4°C overnight with Bandeiraea simplicifolia BS-1, and isolectin-FITC (Sigma) was added to the blocking buffer. The retinas were washed four to five times for 1 h each with PBS, flattened with four incisions, and mounted under coverslips. The flat-mounted retinas were viewed with conventional fluorescence microscopy. Images were captured using a Leica DC200 digital camera and software. For immunohistochemistry, mouse eyes were fixed in 4% paraformaldehyde prepared in PBS overnight, paraffin embedded, and sectioned at 5 μm thickness. The sections were deparaffinized using xylene, blocked with 1% BSA in PBS, and incubated overnight with chicken IgY anti-TSP1 (1:50). On the following day, the slides were washed in PBS (4×) for 15 min each followed by anti-IgY-horseradish peroxidase (1:100) and developed with 3-amino-9-ethyl carbazole (Sigma) as directed by the manufacturer.
Quantification of mRNA expression.
Retinas were separated in diethyl pyrocarbonate-PBS. Total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was synthesized by TaqMan reverse transcription reagents with random primers (PE Applied Biosystems). For the quantification of mRNA expression, real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (PE Applied Biosystems). Primers were designed using the primer design software Primer Express 2.0 (PE Applied Biosystems). The forward and reverse primers of each pair are located on different exons. The primer sequences for the target genes are shown in Table 2. cDNA samples were mixed with primers and SYBR Master Mix (PE Applied Biosystems) in a total volume of 25 μl. The same cDNA was used for PCR to ensure consistency in the reverse transcription efficiency, and primers were tested for similar efficiencies using 10-fold dilutions of control cDNA from cells that contained a high expression of each. PCR was conducted using the following parameters: 95°C for 10 min, and 40 cycles at 95°C for 15 s, and 60°C for 1 min. PCR reactions were performed in 96-well optical reaction plates (Applied Biosystems). Quantitative real-time PCR was normalized to the copies of β-actin mRNA from the same sample. To compare the relative levels of mRNAs, the following equation was used: fold change (ΔFC) = 2−ΔCT, with the change of critical threshold (ΔCT) calculated as the difference in CT values between TSP2 and β-actin and TSP1 and β-actin. Acquired data were analyzed by Sequence Detector software (PE Applied Biosystems).
Table 2.
The quantitative PCR primer sequences for amplification of mouse cDNA
| Target Genes | Forward Primer | Reverse Primer |
|---|---|---|
| tsp-1 | 5′-TGGCCAGCGTTGCCA-3′ | 5′-TCTGCAGCACCCCCTGAA-3′ |
| tsp-2 | 5′-GCATAGGGCCAAGAGCTTCTG-3′ | 5′-CCGGTTAATGTTGCTGATGCT-3′ |
| vegf-a | 5′-AAGGAGAGCAGAAGTCCCATGA-3′ | 5′-CTCAATTGGACGGCAGTAGCT-3′ |
| ve-cadherin | 5′-GGCCCTGGACAGACTGCA-3′ | 5′-CCTTCGTGGAGGAGCTGATC-3′ |
| β-actin | 5′-AACCGTGAAAAGATGACCCAGAT-3′ | 5′-CACAGCCTGGATGGCTACGT-3′ |
VE-cadherin, vascular endothelial cadherin.
Hyperoxic exposure of mice and quantification of retina capillary regression.
Seven-day-old pups with mothers were used for exposure to 70% oxygen. The oxygen concentration varied <2%. During the exposure, food and water were provided ad libitum. To quantify capillary regression, we used three methods: 1) distance of capillary loss from the optic disc along major venules, 2) area of capillary loss, and 3) mRNA levels of vascular endothelial (VE)-cadherin in the whole retina as a representation of vascular content after hyperoxia.
Endothelial cell culture.
Human dermal microvascular endothelial cells were isolated from neonatal foreskin as previously described (30). The use of human tissue was approved by the Beth Israel Deaconess Medical Center Institutional Review Board. Cells were grown to confluence, placed in 2% serum overnight, and pretreated with a recombinant protein that contains all three type 1 repeats of TSP1, designated 3TSR (200 μg/ml), for 35 min before stimulation with 50 ng/ml of VEGF-A165. The production of 3TSR, which contains amino acids 361 to 530, has been described previously (24). Cells were lysed after 10 min in radioimmunoprotection assay (RIPA) lysis buffer with protease and phosphatase inhibitors.
Analysis of signaling.
Cell pellets and freshly dissected retinas were homogenized with RIPA buffer [IPA-Boston Bioproducts, Worchester, MA; consisting of 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.5% Na+ deoxycholate, and 0.1% SDS] containing a protease inhibitor cocktail (Sigma) on ice. Immunoprecipitations and blotting were done using standard methods and repeated at least three times to ensure reproducibility. The primary antibodies used for Western blot analysis in this study were typically used at 1:1,000 dilution and included anti-phosphorylated (p)Akt S473 (Cell Signaling), anti-total Akt1/2 (Santa Cruz), anti-β-actin (Sigma), anti-CD36 (Cayman Chemical), anti-mouse Src (Clone GD11, Upstate), anti-Fyn (Biosource, now Invitrogen, Carlsbad, CA), and anti-phosphotyrosine (BD Transduction, San Jose, CA).
RESULTS
TSP1 protein is abundant in the regions of the developing retina that are vascularized.
Immature retinal blood vessels are sensitive to elevated oxygen levels due to the loss of VEGF-A that occurs in hyperoxia. This condition leads to the apoptosis of vessels throughout the retina but is most pronounced near the optic disk (2, 3). We have previously linked this vascular regression to the loss of Akt signaling, presumably in response to the loss of VEGF-A, a potent activator of endothelial Akt signaling (38). We surmised that, as endogenous inhibitors of angiogenesis, TSPs might be part of the balance of factors in the retina that can antagonize Akt survival signaling. We investigated the expression of TSP1 and TSP2 mRNAs by RT-PCR and found both to be present in wild-type retinas (data not shown). In Fig. 1A, a diagram of the retina is shown, with the two regions that contain blood vessels marked with red lines. The blood vessels in the ganglion cell layer and inner nuclear layer are the ones that develop postnatally and whose survival in rodents can be regulated by a hyperoxia-induced downregulation of VEGF in the first 2 wk of postnatal development. TSP1 protein accumulated in the inner plexiform layer, which is largely cell-free, suggesting that the TSP1 protein is secreted into the extracellular matrix and does not remain cell associated (Fig. 1B). Whereas we could not surmise from these studies which cells are the source of secreted TSP1, previous studies have reported that astrocytes, which are also found in this region of the retina, make TSP1 (9). Controls for the specificity of this antibody included the tsp1−/− and tsp2−/− retinas. TSP1 was found in wild-type and tsp2−/− retinas but not in tsp1−/− or tsp1−/−/tsp2−/− retinas (Fig. 1, B–E).
TSP1 expression antagonizes capillary survival in the retina.
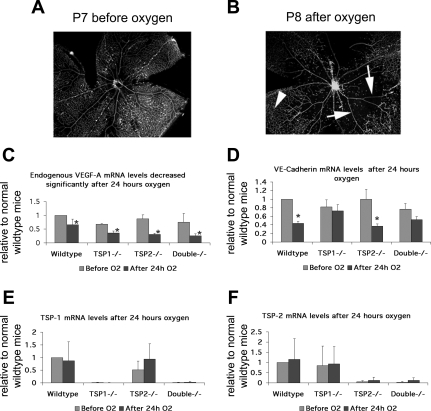
To explore the roles of TSP1 and TSP2 in microvascular survival in vivo, we placed neonatal pups in 70% oxygen-30% room air for 24 h starting at postnatal day 7 (P7). At P7, the microvasculature is nearly complete in the most superficial layer (Fig. 2A), but the loss of VEGF-A due to the hyperoxia withdraws a major survival signal and leads to a loss of the preexisting blood vessels in a pattern that is most pronounced near the central optic disc (Fig. 2B). We observed that VEGF-A levels were significantly reduced by hyperoxia in all genotypes. However, we could observe no significant differences in the oxygen-induced reductions between different genotypes (Fig. 2C). To examine not just the vessels close to the optic disc but also to take an average of endothelial cell numbers within the whole retina, we relied on a quantitative measure of vascular burden obtained through real-time RT-PCR for VE-cadherin. Because VE-cadherin is only expressed by endothelial cells in the retina, its mRNA has been used as a way to average the vasculature from all regions of the retina (38). mRNA levels of VE-cadherin in wild-type and tsp2−/− retinas after hyperoxia decreased significantly compared with mRNA levels of VE-cadherin in tsp1−/− and tsp1−/−/tsp2−/− retinas after hyperoxia (Fig. 2D). Thus the loss of tsp1 expression protected blood vessels from a regression in hyperoxia. In this genetic background, no significant differences in mRNA levels of VE-cadherin or visible differences in capillary density were detected at P7 before the administration of oxygen. We also measured the levels of tsp1 and tsp2 mRNAs and did not find significant differences after hyperoxia (Fig. 2, E and F).
Fig. 2.
Exposure of neonatal pups to 70% oxygen alters mRNA expression of vegf-a and vascular endothelial (ve) cadherin, but not of tsp1 or tsp2. Blood vessels are shown on flat mounting of whole wild-type retinas after fluorescent labeling with lectin: postnatal day 7 (P7) retinas have capillary coverage of the retina surface before oxygen (A), but after 24 h of oxygen (B), central capillaries surrounding the optic nerve have regressed leaving behind a circular capillary free zone with only the large arterioles and venules remaining (note arrows to large vessels and arrowhead to remaining capillaries). C: VEGF-A mRNA levels were reduced by hyperoxia significantly (*P < 0.01) in all genotypes, but there was no significant difference in VEGF-A reduction between genotypes (P > 0.05). D: ve-cadherin mRNA levels after hyperoxia differed between retinas without tsp1, and those with wild-type levels of TSP1 (*P < 0.0001), although no significant differences were detected before oxygen (P > 0.05) between any of the genotypes. There was no significant difference in tsp1 (E) or tsp2 (F) mRNA levels after oxygen (P > 0.05).
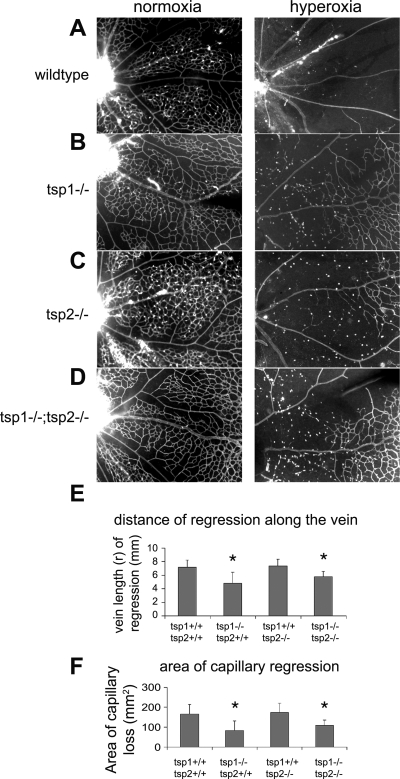
In addition to measuring VE-cadherin mRNA levels as an indication of vascular response to oxygen, we also used whole mount staining with lectin to see the extent of capillary regression from the optic disc. To aid in the visibility of the fluorescently labeled blood vessels, we have photographed a segment of the tsp1−/− and tsp2−/− retinas at a higher magnification compared with the low magnification images used to illustrate the effects of oxygen in Fig. 2, A and B. Figure 3 shows the hyperoxia-induced loss of capillaries near the optic disk, which is positioned on the left of each image. Figure 3, A–D, shows retinal blood vessels at the start of hyperoxia treatment (left) and after hyperoxia treatment (right). Consistent with our observations that TSP1 was the dominant family member expressed in retina at this stage, we observed significant alterations in retinal regression from hyperoxia in tsp1−/− mice. It was clear that the capillary loss in wild-type (Fig. 3A) and tsp2−/− (Fig. 3C) mice induced by hyperoxia was greater than the capillary loss of tsp1−/− (Fig. 3B) and tsp1−/−/tsp2−/− mice (Fig. 3D). One way to quantify the regression is to measure the distance along the veins (not arterioles) where the many capillary branches have been lost. The veins were identified by their wider girth and numerous branches to the capillary network. Venules alternate with arterioles around the optic disc. Arterioles have fewer branches, thus a measuring of the distance of regression along arterioles is less precise. With the use of statistical analysis, the distance of regression from the optic disc along the vein of tsp1−/− and tsp1−/−/tsp2−/− mice induced by hyperoxia was significantly shorter than that in wild-type and tsp2−/− retinas (n = 5) (Fig. 3E). Although Fig. 3C visually appears to have a mild change from wild-type, these changes were not strong enough to be captured in a statistically significant manner, and, hence, we do not deem them of major importance. Similarly, when we calculate the cleared area, which is nearly circular, using (πr2), the area of capillary regression surrounding the optic disc of tsp1−/− and tsp1−/−/tsp2−/− mice was also significantly smaller than that of wild-type and tsp2−/− mice (Fig. 3F).
Fig. 3.
TSP1 impacts the regression of vessels in oxygen in the neonatal retina. Quantification of regression for genotypes represented in the following: wild-type (A), tsp1−/− (B), tsp2−/− (C), and tsp1−/−/tsp2−/− (D) before (left) and after (right) 24 h of breathing 70% oxygen-30% room air. E: distance of capillary regression is the radius (r), measured along the vein from optic disc to the remaining capillaries (n = 5 retinas). F: area (πr2) of capillary regression. *P < 0.0001.
TSP1 expression alters endogenous Akt phosphorylation in the retina and endothelial Akt phosphorylation in culture.
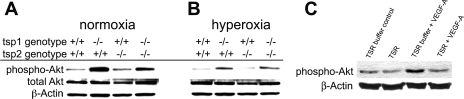
Because we have previously found that Akt signaling is rate limiting for capillary regression in oxygen (38), we investigated whether changes in Akt signaling might contribute to the altered capillary regression in tsp1−/− retinas. We used Western blot analysis of dissected whole retinal lysates to compare the levels of phosphorylated Akt in the retinas of the various genotypes before and after oxygen. Figure 4A shows that the pAkt levels of tsp1−/− and tsp1−/−/tsp2−/− retinas were significantly increased compared with wild-type and tsp2−/− mice before hyperoxia, and similar results were obtained in hyperoxia. pAkt levels after hyperoxia were significantly (P = 0.0041) decreased compared with before hyperoxia in all genotypes. A comparison of the changes in pAkt before and after oxygen showed no significant differences between tsp1−/−/tsp2−/− and wild-type mice. pAkt levels in tsp1−/− retinas were already higher before oxygen treatment and, similar to the wild-type retinas, also decreased after hyperoxia. VEGF-A has been shown to be decreased by hyperoxia in the retina and the administration of VEGF-A to the vitreous before hyperoxia protects retinal capillaries from regression (2). While the signaling changes in vivo are most important, we wanted to test more directly whether TSP1 could antagonize VEGF-induced Akt signaling in a more controlled environment. To do this, we used primary microvascular endothelial cells pretreated with a recombinant form of the active fragment of TSP1, the three type 1 repeats (3TSR), which have been shown to mediate apoptosis of endothelial cells via binding to CD36 (10, 14). We then stimulated the cells with VEGF-A and measured Akt phosphorylation with a phosphospecific antibody. Under these circumstances, VEGF-A-mediated induction of pAkt was blunted in the presence of 3TSR but not in control buffer (Fig. 4C). Thus, in addition to the previously described TSP1-CD36-fyn-p38 apoptotic signal (20), we found that 3TSR could also antagonize VEGF-induced Akt signaling.
Fig. 4.
TSP1 reduces steady-state levels of Akt phosphorylation in retina. Phosphorylated (p)Akt levels before (A) and (B) after 24 h of breathing 70% oxygen are shown. pAkt levels in tsp1−/− and tsp1−/−/tsp2−/− retinas were significantly increased compared with wild-type and tsp2−/− retinas before hyperoxia, and similar relative changes were observed after hyperoxia, though pAkt levels were reduced in all genotypes by oxygen. C: 3 type 1 repeats of TSP1 (3TSR) blocked VEGF-A-induced Akt phosphorylation in primary dermal endothelial cells.
TSP1 presence and antagonism of survival signaling corresponds to an opposing interaction of CD36 with Fyn versus Src.
We investigated the relationship between CD36 and Fyn and Src signaling with TSP1 expression and Akt activation. We observed that Fyn was more phosphorylated in wild-type retinas than in tsp1−/− retinas, and, surprisingly, Src was more highly phosphorylated in tsp1−/− retinas (Fig. 5A). This suggested that Fyn and Src might play antagonistic roles in response to TSP1 signaling through CD36. To test whether their interaction with CD36 was similarly regulated, we used whole retinal lysates to coimmunoprecipitate Src and Fyn with CD36. In wild-type retinas, we observed a strong association of Fyn with CD36, similar to previous studies (20). However, in tsp1−/− retinas, we observed a preferential association between Src and CD36 (Fig. 5B). This was complemented by immunoprecipitations in cultured endothelial cells treated with 3TSR, though in this model the differences are less pronounced presumably due to the background from endogenous expression of TSP1 in endothelial cells (Fig. 5C).
To test whether Src and Fyn may have antagonistic functions in microvascular survival in response to TSP1 in vivo, we crossed the tsp1−/− mice to src+/− and fyn−/− mice and analyzed vascular regression along the vein after hyperoxia and pAkt expression in retinal lysates of untreated P8 mice. Representative images of key genotypes are shown in Fig. 6A. Because of the poor health associated with the src−/− mice, we obtained very few src−/− animals and they were runted, making an analysis of vascular distances from the optic disc within the retinal whole mounts unreliable. However, we were satisfied with substituting src+/− mice in the analysis of vascular regression since we could observe clear differences in the tsp−/− vasculature even after the loss of only one copy of the src gene. However, β-actin levels served as a good control to normalize for the smaller eye size in Western blot analyses (Fig. 6C). Figure 6B shows the quantification of the distance of regression along the veins for related genotypes. We observed significant differences between the wild-type and both tsp1−/−/fyn+/− and tsp1−/−/fyn−/− mice and between tsp1−/−/fyn+/− and tsp1−/−/fyn−/− mice (Fig. 6B, P < 0.001). We also observed significant differences between tsp−/− and tsp−/−src+/− mice (Fig. 6B, P < 0.001). When the tsp1+/+/fyn+/− animals are compared with the tsp1−/−/fyn+/−, the mice resemble the tsp1−/− mice (refer to Fig. 3), suggesting that the loss of one allele of fyn is not sufficient to inhibit vessel regression. However, comparing the loss of a second allele of fyn in the tsp1 null background (tsp1−/−/fyn−/−) resulted in a further protection from vessel regression and an increased Akt phosphorylation (Fig. 6, B and C). In contrast to that of fyn, the loss of one allele of src had no effect on the regression in the presence of TSP1, but when tsp1−/− mice were used as a starting point, the loss of only one allele was sufficient to inhibit the protection of capillaries conferred by tsp1 loss (Fig. 6B). Moreover, Western blot analysis shown in Fig. 6C on tsp1−/−/src−/− retinas showed a drastic reduction in Akt phosphorylation compared with that on tsp1−/− retinas. Thus, in the absence of TSP1, Src is rate limiting for capillary survival and the capillary density is paralleled by changes in pAkt expression. Taken together, our data are consistent with a proposed role of CD36:Fyn in mediating TSP1 antagonism of Akt signaling and promotion of apoptotic signaling, and the role of CD36:Src in promoting VEGF-driven Akt survival signaling in the absence of TSP1. We hypothesize that CD36:Src modulates intracellular signaling pathways that either cooperate with VEGF-VEGF receptor signaling or modulate that signaling in response to VEGF-A to impact decisions of survival and apoptosis during microvascular remodeling. Moreover, we find that TSP1 binding preferentially leads to CD36:Fyn over CD36:Src interactions, which have an overall negative effect on endothelial cell function in the retina.
DISCUSSION
It is believed that the pruning of microvessels and the recruitment of pericytes during remodeling of many developing vascular beds, such as the retina, account for the transition from a chaotic weblike organization to a more functional and organized vascular tree (3). There are many genes that have an impact in this maturation, and it is not uncommon for lethal knockouts of genes involved in angiogenesis to present with phenotypes that include failed microvascular remodeling. Nonetheless, very little is understood about the molecular signaling that regulates remodeling. We have previously shown that alterations in Akt signaling can change the final patterning of embryonic blood vessels and link Akt signaling to this natural remodeling process in the embryo and neonatal retina. We have modeled the survival signaling that participates in retinal microvascular remodeling with a transient exposure of the retina to hyperoxia (38). Based on the findings outlined in this study, we propose that in the developing retina, TSP1 to a greater extent than TSP2 plays the counterpart to VEGF in a ying-yang relationship that regulates both the proliferation of endothelial cells during initial microvascular formation, as well as survival signaling during subsequent remodeling. There is the possibility that TSP1, positioned in the extracellular matrix, can impact VEGF itself or VEGF binding to its receptors. However, some of our experiments on CD36 mediated downstream Fyn and Src signaling, and the genetic interactions between mice with loss of function of tsp1, src, and fyn, suggest that there is an impact of TSP1 to modulate endothelial cell Akt signaling rather than just VEGF binding. CD36 in concert with Src and Fyn may participate in cross talk with the VEGF receptors intracellularly.
Exposure of neonatal rodents to hyperoxia has been shown by in situ hybridization to downregulate VEGF-A mRNA production in retinal astrocytes (37). We have observed the same phenomenon by quantitative real-time RT-PCR. However, we did not find that the expression of TSP1 or TSP2 impacted the hyperoxia-induced reduction of VEGF-A. Therefore, we surmise that the decreased Akt signaling observed after hyperoxia in all genotypes (Fig. 2) is secondary to the hyperoxia-induced changes in VEGF and potentially other prosurvival genes and that TSP1 decreases Akt signaling via a mechanism independent of hyperoxia. We propose that TSP1 consistently reduces Akt signaling in response to survival factors, notably VEGF in our experiments. However, we cannot exclude a role for TSP1 in modulating Akt signals from other stimuli. In addition, the comparative impact of TSP1 versus TSP2 may not reflect differences in functional potential as much as yet unknown differences in expression levels or protein localization. Without better TSP2-specific antibodies, it is difficult to know where in the retina the TSP2 is localized.
CD36 and Src family kinases have been shown to associate with each other in platelets and endothelial cells (6, 17). The association of CD36 with Lyn is reportedly mediated by the lipids that comprise the Triton X-100-insoluble fraction (39). The data presented here indicate that TSP1 influences the organization of these cellular signaling networks. Whereas Fyn is coimmunoprecipitated with CD36 in wild-type retinas, Src preferentially immunoprecipitates with CD36 in tsp1−/− retinas. Since CD36 mediates the uptake of myristic acid, it can affect the myristolation and membrane localization of Src family kinases (19). Given that TSP1 inhibits this function of CD36, one would predict higher levels of myristolation in the tsp1−/− mice, which might influence the relative abundance of Src family kinases associated with the lipid raft fraction. Based on the work of many laboratories, it is likely that there is extensive cross talk and overlap in function between the receptors of TSP1. Hence, while we have identified a complex with CD36 that reflects differential Src and Fyn association, we can by no means conclude that this interaction is direct or exclusive to CD36. Other receptors such as integrins and tetraspanins may similarly respond to TSP1 and signal via Src and Fyn.
An interesting question is, How do Src family members elicit differential functions? The association of Src with VEGF functions has been seen in several models, but perhaps most relevant to our study is the finding that src but not fyn is selectively required for VEGF-induced permeability (11). Whether VEGF-induced permeability is at all related to TSP1 or CD36 has not been shown, but the Src/Fyn dichotomy may suggest that these functions are related. Like survival, VEGF-induced permeability has also been associated with Akt signaling through eNOS-dependent and eNOS-independent pathways (5, 13, 15, 28). Moreover, in some endothelial signaling pathways a direct association between Src and the p85 subunit of PI3K upstream of Akt activation has been observed (16). Whether or not the inhibition of VEGF-induced Akt signaling by TSP1 is via direct blockade of all VEGF signaling or a dampening of just Akt signaling is difficult to discern without a further elucidation of the molecular interactions involved.
The importance of CD36 to the function of TSPs has been extensively investigated, and the data suggest that there are both CD36-dependent and CD36-independent functions. Some studies have suggested that CD36 can interact with integrins and tetraspanins in the cell membrane of platelets, suggesting the possibility that TSP1 and integrins may also impact each other's signaling in endothelial cells (25). Moreover β1-integrins have been implicated in mediating TSP1 functions even in cells without CD36 (7, 32). In endothelial cells with CD36, however, discrete residues in the short CD36 cytoplasmic tail are critical for the angiogenic responses to TSP1 (29). Although studies have suggested that some of the antiangiogenic properties of TSP2 are also mediated through CD36, TSP2 does not appear to be a significant component of survival signaling in the developing retina, either due to differences between TSP1 and TSP2 in retinal biology or because TSP2 is expressed at lower levels than TSP1 (34).
GRANTS
This work was supported by National Institutes of Health Grants HL-71049 and CA-092644 (to L. E. Benjamin and J. Lawler).
REFERENCES
- 1.Adams JC Thrombospondin-1. Int J Biochem Cell Biol 29: 861–865, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1: 1024–1028, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol 19: 557–568, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA 102: 904–908, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull HA, Brickell PM, Dowd PM. Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett 351: 41–44, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem 279: 41734–41743, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol 19: 597–614, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120: 421–433, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 138: 707–717, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res 57: 1735–1742, 1997. [PubMed] [Google Scholar]
- 15.Hatakeyama T, Pappas PJ, Hobson RW 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem 278: 2118–2123, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA 88: 7844–7848, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iruela-Arispe ML, Luque A, Lee N. Thrombospondin modules and angiogenesis. Int J Biochem Cell Biol 36: 1070–1078, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 282: 15404–15415, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6: 41–48, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Lawler J The functions of thrombospondin-1 and -2. Curr Opin Cell Biol 12: 634–640, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Lawler J Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6: 1–12, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majack RA, Cook SC, Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci USA 83: 9050–9054, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao WM, Seng WL, Duquette M, Lawler P, Laus C, Lawler J. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res 61: 7830–7839, 2001. [PubMed] [Google Scholar]
- 25.Miao WM, Vasile E, Lane WS, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood 97: 1689–1696, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell 19: 563–571, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penn JS, Thum LA. Oxygen-induced retinopathy in the rat. Basic Life Sci 49: 1025–1028, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10: 159–170, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Primo L, Ferrandi C, Roca C, Marchio S, di Blasio L, Alessio M, Bussolino F. Identification of CD36 molecular features required for its in vitro angiostatic activity. FASEB J 19: 1713–1715, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Richard L, Velasco P, Detmar M. A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res 240: 1–6, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA 98: 12485–12490, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol 168: 643–653, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol 24: 27–34, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35: 101–111, 1994. [PubMed] [Google Scholar]
- 36.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol 18: 3112–3119, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15: 4738–4747, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci USA 102: 128–133, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorne RF, Law EG, Elith CA, Ralston KJ, Bates RC, Burns GF. The association between CD36 and Lyn protein tyrosine kinase is mediated by lipid. Biochem Biophys Res Commun 351: 51–56, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Upalakalin JN, Hemo I, Dehio C, Keshet E, Benjamin LE. Survival mechanisms of VEGF and PlGF during microvascular remodeling. Cold Spring Harb Symp Quant Biol 67: 181–187, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 228: 630–642, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn 235: 1908–1920, 2006. [DOI] [PubMed] [Google Scholar]