Abstract
Adventitial fibroblasts have previously been proposed to be a major constituent of the neointima following coronary balloon angioplasty. The present study utilized the bromodeoxyuridine (BrdU) pulse-chase technique to track adventitial fibroblast migration early after balloon injury in swine. BrdU (30 mg/kg), a marker of proliferating cells, was given intravenously 1 or 2 days after balloon angioplasty. For each time point, one animal was euthanized 24 h after injection to identify the location of the proliferating cells, while a second animal was euthanized 25 days after angioplasty to determine whether the proliferating cells migrated to form the neointima. Our results demonstrate that BrdU-positive cells were located primarily in the adventitia with all three time points 24 h after balloon angioplasty. Furthermore, when BrdU was injected on day 1 or 2 only 0.65 ± 0.17% and 1.7 ± 0.64%, respectively, of neointimal cells were BrdU positive on day 25. In conclusion, these results demonstrate a negligible contribution of coronary adventitial fibroblasts to neointima formation following coronary balloon angioplasty, supporting the concept that the neointima is primarily of smooth muscle cell origin.
Keywords: smooth muscle, migration, adventitia
restenosis is the sequela of percutaneous transluminal coronary angioplasty (PTCA) both with and without stenting. During restenosis, cells migrate from the vascular wall to form the neointima (NI). Recent elegant studies using carotid xenografts from green fluorescent protein (GFP) transgenic mice have shown that the NI is developed from cells within the surrounding vascular wall (1); however, the phenotype and origin of these precursor cells remain controversial. Attempts to conclusively identify the origin and phenotype of neointimal precursors have been greatly hampered by the lack of specific cell phenotype markers, especially a selective marker for adventitial fibroblasts. Initially, smooth muscle cells (SMC) from the media were proposed to migrate to form the NI after PTCA (3, 6), while adventitial fibroblasts (AFB) were considered a quiescent cell type that did not contribute to vascular pathologies. However, recent studies have provided evidence that AFB become activated early both in the atherosclerotic process (25) and after balloon angioplasty (10, 13, 16, 17, 19) and subsequently migrate and proliferate to form the NI in both rodents (8, 11, 12, 20) and pigs (15, 18).
Rather than phenotype-selective gene expression markers, these studies have relied on exogenous labeling of target cells. For example, in the rat carotid injury model selective labeling of adventitial cells with LacZ or GFP was used to track adventitial migration into the NI after injury (8, 11, 12, 20). Fluorescent labeling of AFB was attempted in pig coronary arteries (23); however, the fluorescent marker did not selectively label the adventitia but also labeled the perivascular adipose tissue and myocardium, making this technique unsuitable to track AFB migration. In swine coronary PTCA, the bromodeoxyuridine (BrdU) pulse-chase method is the most applicable technique to date for tracking of AFB migration. The BrdU pulse-chase method is a technique that exploits the proliferative response of vascular cells following a balloon coronary injury. BrdU is injected into the animal after PTCA and is taken up by proliferating cells. Once incorporated into the cell it acts as a permanent marker, and migration can be tracked based on the relative distribution of BrdU labeling at early and late time points. Using this methodology, two independent laboratories have concluded that 86% (18) and 43% (15) of neointimal cells are of AFB origin, respectively. However, the methodology employed by one laboratory (18) elicits questions pertaining to the manner in which the BrdU was administered. The authors administered BrdU both intramuscularly and intravenously. When given intravenously BrdU quickly enters the circulatory system and is quickly degraded within 60 min (7), thus creating a finite period over which proliferating cells are labeled during the pulse. However, when administered intramuscularly BrdU is released over a greater time period, thus possibly affecting the interpretation of results.
The purpose of the present study was to determine whether AFB contributes to formation of the NI following PTCA in pig coronary arteries by utilizing an intravenous-only BrdU pulse-chase method. Our results demonstrate that <2% of neointimal cells are of adventitial origin, supporting the concept that SMC are the major constituent of the NI following balloon angioplasty in porcine coronary arteries (2).
MATERIALS AND METHODS
Animal procurement and husbandry.
Ten domestic juvenile male farm pigs (∼34 kg) were obtained from the University of Missouri (South Farm-Columbia, MO) and housed in the laboratory animal center located on campus. Pigs were fed a normal chow diet (Purina Lab Mini-pig Chow), and water was available ad libitum. Animals were on a 12:12-h light-dark cycle. All protocols were approved by the University of Missouri's Animal Care and Use Committee.
Surgery.
All swine received 325 mg of aspirin the day before, the day of, and the day after surgery, with a maintenance dose of 162 mg given for the duration of the study. Animals were anesthetized with an intramuscular injection of 5 mg/kg Telazol, 2.2 mg/kg xylazine, and 0.5 mg/kg atropine. Intubation was performed, and anesthesia was maintained with 2% isoflurane (AErrane; Baxter) and 1 l/min oxygen by means of mechanical ventilation (Mallard Medical). Presurgical Excede (5 mg/kg) was given intramuscularly. An ear catheter was placed to administer fluids and heparin. Vital signs were monitored throughout the procedure. The right femoral artery was exposed, and a 7-F inducer was inserted into the artery. Heparin was given immediately after femoral access (300 U/kg) and subsequently every hour (100 U/kg). A 6-F guide catheter was guided up the aorta and into the left ostium, and the left circumflex artery (LCX) was selectively engaged. Contrast dye (Visipaque 320 mgI/ml; Amersham Health) was injected and visualized with fluoroscopy. Arterial diameter was determined by quantitative angiography (Infimed). A 1.3–1.4:1.0 overinflation was utilized and calculated from the angiography. A standard balloon catheter (Maverick, Boston Scientific), 15–20 mm in length, was inserted into the LCX and inflated to the target pressure for 30 s three times, with a 1-min break between inflations. After the balloon injury an externalized jugular catheter was placed to inject BrdU (Sigma, St. Louis, MO). Briefly, an autoclaved catheter (Saint-Gobain Tygon tubing, formulation S-54-HL, Fisher Scientific, ID = 0.050 in., OD = 0.090 in.) was placed into the right jugular vein just cranial to the right atrium in the vena cava. The catheter was tunneled with a custom-made trocar and externalized on the dorsal aspect of the neck on the midline. Rimadyl (3 mg/kg) and buprenorphine (0.01 ml/kg) were given postsurgically. Animals were euthanized either 2, 3, or 25 days after injury under anesthesia and the heart was surgically removed, as approved by the Panel on Euthanasia of the American Veterinary Medical Association.
BrdU pulse-chase protocol.
BrdU was administered to label and track proliferating cells. Three BrdU injection protocols were utilized in this study. Two injections (i.e., pulses), at a concentration of 30 mg/kg dissolved in lactated Ringer solution (30 mg/ml), were given 12 h apart on day 1 or 2 after angioplasty. Subsequently, an animal from each group was killed 24 h after injection per pulse (day 2 or 3, respectively) to determine the initial distribution of the BrdU-positive proliferating cells. A second animal for each injection per pulse time point was killed 25 days after angioplasty (day 25) to determine localization of BrdU-positive cells relative to the NI. All injections were given intravenously, except in the final protocol, where an initial intramuscular injection was followed by an intravenous injection.
Immunohistochemistry.
After surgical removal of the heart, the arteries were carefully dissected out, embedded in paraffin, and sectioned (5 μm). Sections were deparaffinized and stained manually with Tris buffer and water wash after each step. To inhibit background staining, avidin-biotin blocking solution (Vector SP-2001, Vector Laboratories, Burlingame, CA) was utilized and 3% hydrogen peroxide was used to quench endogenous peroxidase activity. To inhibit nonspecific protein binding, nonserum protein block (Dako, Carpinteria, CA) was applied. Primary antibodies, BrdU (1:200, Zymed, San Francisco, CA), α-smooth muscle actin (α-SMA) (1:200, Dako), and smooth muscle myosin heavy chain (SMMHC) (1:800, Biomedical Technologies, Stoughton, MA) were incubated overnight at 4°C. The BrdU staining was visualized after a 5-min exposure to diaminobenzidine (Dako). α-SMA was visualized with the Dakocytomation LSAB system (Dako) and the Vector VIP chromagen (Vector Laboratories). Slides were counterstained with Vector methyl green (Vector Laboratories) to view the nuclei.
BrdU quantification.
Slides were photographed with an Olympus BX60 photomicroscope. All quantification was performed with ImagePro software. Figure 1 demonstrates the areas that were examined for BrdU-positive cells. Analysis of the early time points (days 2 and 3) was performed on the adventitia adjacent (AA) to the medial tear, the adventitia opposite (AO) the medial tear, as well as the media (M) at the dissected end (Fig. 1A). Analysis of the late time point (25 days) was assessed in the AA and AO as well as the NI (Fig. 1B). Multiple regions of interest (NI = 3, AA = 3, AO = 1) in each section were counted in triplicate and expressed as a mean percentage (BrdU-positive cells/total cells × 100). Both ends of the medial tear were counted and averaged together.
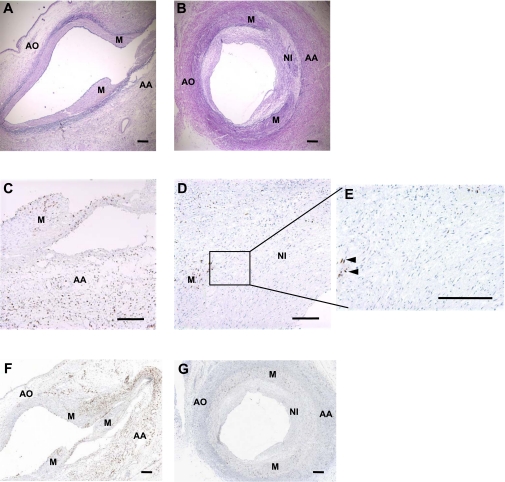
Fig. 1.
Immunohistochemistry of coronary arteries following balloon angioplasty at 2 and 25 days with intravenous bromodeoxyuridine (BrdU) administration on day 1. A and B: photomicrographs of sections from 2 (n = 3) or 25 (n = 4) days after angioplasty, respectively, stained with Verhoeff-van Gieson (VVG) to visualize the elastic fibers. The regions demarcated are the regions assessed for BrdU-positive cells. AA, adventitia adjacent to medial tear; AO, adventitia opposite medial tear; M, media at dissected ends; NI, neointima. C and D: photomicrographs of sections from 2 or 25 days after angioplasty, respectively, stained for BrdU (brown). BrdU was administered intravenously twice on day 1 after the injury for these sections. Day 2 demonstrates that the BrdU is mainly localized in the adventitia; on day 25 <1% of BrdU-positive cells were located in the neointima. E: increased magnification (×20) of the 25-day neointima. Note the absence of BrdU staining (see Figs. 2E and 5E for reference). F and G: ×4 images stained for BrdU (brown) at 2 or 25 days after angioplasty, respectively. Horizontal bars = 100 μm.
Statistical analysis.
All data are presented as means ± SE. A two-way ANOVA was utilized to compare BrdU labeling in the M and AA on days 2 and 3 with Sigma Stat 16.0. Statistical significance was set at P < 0.05.
RESULTS
Intravenous BrdU administration.
To determine the origin of BrdU-positive labeled cells early after injury, BrdU incorporation into proliferating cells was examined 24 h after injection 1 or 2 days after angioplasty. Fewer than 1% of cells were BrdU positive in the noninjured arteries, with no significant differences between the media and adventitia (data not shown). Proliferating cells were abundant in the adventitia 1 and 2 days after angioplasty. On days 2 and 3 after angioplasty, 22 ± 5.5% and 29 ± 4.8% of cells in the AA and 10 ± 3.8% and 27 ± 5.0% of cells in the AO were BrdU positive, respectively (Fig. 1, C and F, Fig. 2, C and F, and Fig. 3). Medial cells showed a significantly lower proliferative activity compared with the AA, with only 7 ± 2.2% and 12 ± 4.8% of medial cells identified as BrdU positive at these same time points (Fig. 1, C and F, Fig. 2, C and F, and Fig. 3; main effect of vascular region, M vs. AA, P < 0.05). Together, these results show that there are greater numbers of BrdU-positive, proliferating cells in the adventitia than in the media early after injury.
Fig. 2.
Immunohistochemistry of coronary arteries after balloon angioplasty at 3 and 25 days with intravenous BrdU administration on day 2. A and B: photomicrographs of sections from 3 (n = 4) or 25 (n = 4) days after angioplasty, respectively, stained with VVG to visualize the elastic fibers. C and D: photomicrographs of sections from 3 or 25 days after angioplasty, respectively, stained for BrdU (brown) when BrdU was administered intravenously twice on day 2 after the injury. Day 3 shows that BrdU is mainly localized in the adventitia; on day 25 sparse amounts (<2%) of BrdU cells are localized in the neointima. E: increased magnification (×20) of the 25-day neointima; representative BrdU-positive stained cells are indicated by arrowheads. F and G: ×4 images stained for BrdU (brown) at 3 or 25 days after angioplasty, respectively. Horizontal bars = 100 μm.
Fig. 3.
Percentage of BrdU-labeled cells in the media and adventitia on days 2 and 3. Bar graph represents % of BrdU-labeled cells by vascular region on days 2 and 3, 24 h after intravenous (IV) BrdU injection on days 1 and 2, respectively. Compared with the media, BrdU-positive cells were greater in the AA and AO at 3 days and in the AA at 2 days after angioplasty. *P < 0.05; †P = 0.06.
To determine whether these proliferating adventitial cells contribute to subsequent NI formation, BrdU incorporation into the NI was determined 25 days after angioplasty. On day 25, the NI showed negligible BrdU-positive cells (0.65 ± 0.17% and 1.7 ± 0.64% when BrdU was given on day 1 or 2, respectively; Fig. 1, D, E, and G, Fig. 2, D, E, and G, and Fig. 4). Proliferating adventitial cells noted early after injury remained localized to the adventitia, both adjacent and away from the medial rupture (AA 4.2 ± 1.6% and 20.9 ± 4.0%, AO 3.6 ± 1.9% and 17.7 ± 6.7% of cells when BrdU was given on day 1 or 2 after angioplasty, respectively). Furthermore, few BrdU cells (<2%) occupied the NI when BrdU was administered intravenously 12 h apart on days 3, 4, 5, and 6 with BrdU localization assessed on day 25 for each time point (data not shown). These results collectively demonstrate that few cells proliferating from day 2 to day 7 after angioplasty migrate to form the NI.
Fig. 4.
Percentage of BrdU-positive cells that migrated to form the neointima (25 days) after balloon injury. In all injection protocols, <2% of the cells in the neointima (NI) were BrdU positive. Similar to early after injury (Fig. 3), BrdU-positive cells remained primarily distributed in the adventitia. *P < 0.05; †P = 0.06 vs. NI. IM, intramuscular.
Combined intramuscular and intravenous BrdU administration 1 day after injury.
Our results are in stark contrast to Shi et al. (18), who reported that 86% of neointimal cells were BrdU positive and were of adventitial origin. A notable difference between our study and the study by Shi et al. (18) was the route of administration of BrdU. Therefore, we reproduced the protocol of Shi et al. (18), consisting of two BrdU injections on day 1 after injury, with the first BrdU injection administered intramuscularly and the second intravenously (30 mg/kg). Using this protocol, on day 25 after injury we found that only 1.2% of neointimal cells were BrdU labeled. Moreover, <2% of cells were BrdU positive in the AA and AO (Fig. 4 and Fig. 5, D, E, G). Examination of BrdU labeling on day 2 after injury demonstrated that 15% of cells in the AA and AO were BrdU positive, while only 4% of medial cells were BrdU positive (Fig. 5, C and F). Thus the lack of BrdU incorporation in the NI with the protocol of Shi et al. (18) was not due to a lack of adventitial BrdU incorporation. These data further support our finding of negligible BrdU-positive cells migrating from the adventitia to form the NI (Fig. 4).
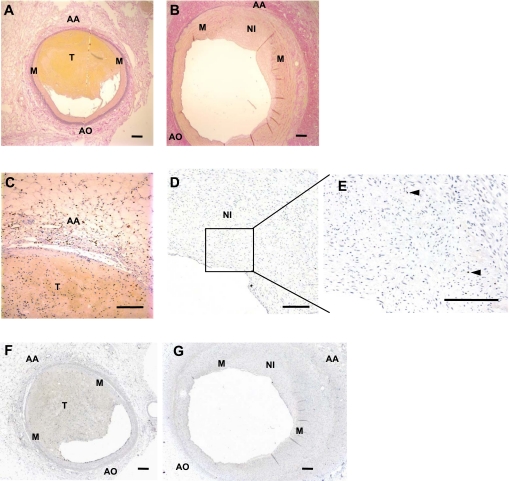
Fig. 5.
Immunohistochemistry of coronary arteries after balloon angioplasty at 2 and 25 days with intramuscular and intravenous BrdU injections on day 1. A and B: photomicrographs of sections from 2 (n = 1) or 25 (n = 1) days after injury, respectively, stained with VVG to visualize the elastic fibers. C and D: photomicrographs of sections from day 2 or day 25, respectively, stained for BrdU when BrdU was given intramuscularly and intravenously on day 1. Staining on day 2 shows that most of the BrdU cells are found in the adventitia, whereas day 25 demonstrates ∼1% of BrdU cells in the neointima. E: enlarged image (×20) of the neointima on day 25; representative BrdU-positive stained cells are indicated by arrowheads. F and G: photomicrographs of sections (×4) stained for BrdU (brown) at 2 or 25 days after angioplasty, respectively. T, Thrombus. Horizontal bars = 100 μm.
Identification of myofibroblasts.
To determine whether the <2% of BrdU-positive cells in the NI were myofibroblasts, sections from each time point were double stained for BrdU and α-SMA (Fig. 6A). Figure 6B demonstrates that only 0.1% and 1.9% of the neointimal cells were BrdU/α-SMA double stained when the BrdU injection was given on day 1 or 2, respectively. Similarly, when the Shi et al. (18) protocol was used only 0.2% of cells were BrdU/α-SMA positive (Fig. 6B). These results demonstrate that <2% of the NI is composed of adventitia-derived myofibroblasts.
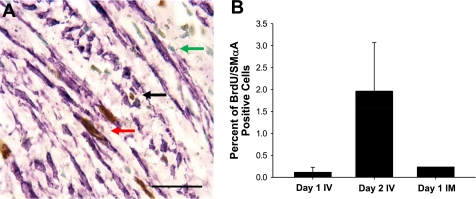
Fig. 6.
BrdU and α-smooth muscle actin (α-SMA) double staining to determine whether the BrdU-positive cells in the neointima were myofibroblasts. A: representative image demonstrating a single labeled BrdU cell (black arrow) and a BrdU/α-SMA double-labeled cell (red arrow). Green staining is a nuclear stain (green arrow). Horizontal bar = 100 μm. B: % of neointimal BrdU-positive cells that also expressed α-SMA for all injection protocols.
Identification of smooth muscle cells.
Our BrdU pulse-chase results revealed that <2% of BrdU-positive neointimal cells could have originated from the adventitia, suggesting that the NI is primarily composed of SMC. To determine whether the NI is composed of SMC, sections were stained with SMMHC, a specific marker of differentiated SMC. The results reveal a uniform staining of SMMHC across the NI and M; however, no staining was observed in the adventitia other than around the vasa vasorum (Fig. 7).
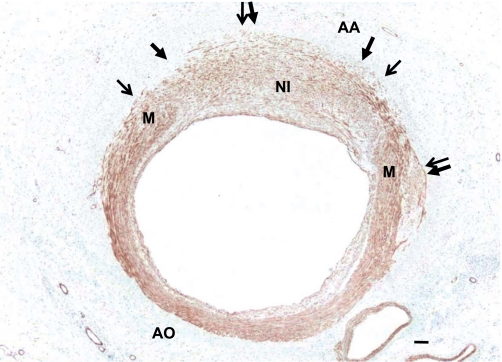
Fig. 7.
Neointimal cells express smooth muscle myosin heavy chain (SMMHC), a marker of differentiated smooth muscle cells. Slides stained with SMMHC demonstrate a uniform expression in the NI 25 days after angioplasty (n = 3). Arrows denote the external elastic lamina. M, media adjacent to neointima. Horizontal bar = 25 μm.
DISCUSSION
Identification of the origin and phenotype of neointimal cells in both atherosclerosis and postangioplasty restenosis is vital to development of therapeutic interventions to alter the progression and composition of the NI in vasculoproliferative diseases. Studies to date have produced equivocal results, especially with regard to the role of adventitia-derived cells. Our data demonstrate that <2% of the neointimal cells were derived from proliferating adventitial cells early (1–7 days) after injury. Even this minimal contribution is likely an overestimation, because it assumes that all BrdU-positive cells in the NI originated exclusively from the adventitia. Given that there was minor, but detectable, BrdU labeling of medial cells early after injury, it is likely that the adventitial contribution is even less. The minor BrdU labeling also precluded determination of the relative adventitial and/or medial origin of the <2% BrdU-positive cells in the NI. Finally, <2% of neointimal cells were identified as myofibroblasts, as indicated by both BrdU and α-SMA labeling. Thus the present study fails to support a major role for migration and expansion of adventitia-derived cells in neointimal formation in a swine model of postangioplasty restenosis.
Our data contrast with earlier reports that proliferating AFB contribute significantly to neointimal formation following balloon injury in the rodent (8, 11, 12, 20) and pig (15, 18). The methodologies used for the rodent studies were designed to selectively label carotid adventitial cells with LacZ or GFP (4, 8, 11, 12, 20). This methodology cannot easily be adapted to a porcine coronary balloon injury model, in part because of the presence of periadventitial fat, removal of which would likely induce inflammation and consequent infiltration and labeling of cells other than adventitial cells. Reported attempts to fluorescently label the adventitia in a porcine coronary balloon injury model resulted in labeling only the myocardium and the surrounding adipose tissue but not the NI or adventitial layer (23). These results suggest either that the label was not incorporated into the adventitia or that AFB do not migrate to form the NI after balloon injury.
The most successful methodology to track AFB in the porcine coronary injury model is the BrdU pulse-chase technique, a technique adapted from an in vivo epithelial cell migration protocol (24). The principle behind the BrdU pulse-chase technique is to label proliferating cells at an early time point to localize the origin of proliferating cells and subsequently track labeled cells to determine if and to where the cells migrate. BrdU is a thymidine analog that is incorporated into the DNA of proliferating cells and rapidly cleared from the circulation (7). BrdU that is incorporated into the DNA becomes a persistent marker of the parent and daughter cells. However, an important limitation of this methodology is that the BrdU will incorporate into all proliferating cells, and thus labeling of specific phenotypes (e.g., AFB) cannot be performed.
Previously published reports in swine (15, 18) concluded that neointimal formation following coronary balloon angioplasty was largely due to invasion and expansion of AFB. Shi et al. (18) and Scott et al. (15) demonstrated that 86% and 43% of neointimal cells, respectively, were BrdU-positive myofibroblasts that migrated from the adventitia. These conclusions were based on the observation that most of the BrdU-positive cells between days 2 and 3 after angioplasty were located in the adventitia, with subsequent appearance in the NI. Our results partially support these findings that most of the BrdU-positive cells were localized in the adventitia early after injury (Figs. 1, 2, 3, 5). However, our findings are in direct contrast to those of Shi et al. (18) and Scott et al. (15) in that we found <2% of the NI was BrdU-positive myofibroblasts. Furthermore, in support of our findings, others have utilized a rabbit injury model with an intraperitoneal BrdU injection and demonstrated few BrdU-positive cells in the NI 21 days after injury (5). One methodological difference between these studies was the route of BrdU administration. Intravenous and intraperitoneal administration allow for BrdU to be taken up in proliferating cells and then quickly removed from the body because of the rapid degradation of BrdU (7), providing restricted temporal labeling of proliferating cells. However, Shi et al. (18) administered both intravenous and intramuscular BrdU. We speculated that the intramuscular BrdU may have been released more slowly, creating the potential to directly label proliferating cells in the NI, thus overestimating adventitial migration. However, this was not the case, because our results with the Shi et al. (18) protocol demonstrated that only ∼1% of the neointimal cells were BrdU positive (Figs. 4 and 5) compared with 86% by Shi et al. (18).
Our results also contradict those of Scott et al. (15), who demonstrated that 43% of cells in the NI were BrdU-positive cells from the adventitia. The only notable difference between their study and the present study is the sex of the animals used. Scott et al. used juvenile female domestic pigs, whereas we performed our studies in juvenile castrated male domestic pigs [Shi et al. (18) did not report the sex of their animals]. Although the juvenile age of the females (15) and the castrated status of the males in our study would diminish potential sex hormone effects, we cannot exclude potential sex differences with regard to the origin of neointimal cells after balloon angioplasty.
Our results and others (2, 4) support the notion that the NI is formed after balloon angioplasty primarily from migration and expansion of SMC from the adjacent media. De Leon (4) and others have shown in a rat carotid artery injury model that AFB do not migrate to form the NI, contrasting with other rodent carotid injury data (8, 11, 12, 20). Our results are also consistent with the conclusions of Christen et al. (2), who demonstrated that 30 days after PTCA in pig coronary arteries, 94% and 98% of neointimal cells expressed the smooth muscle differentiation markers smoothelin and SMMHC, respectively (21). The SMC origin of these cells was supported by evidence that adventitial and adventitia-derived cells from uninjured and injured arteries fail to express smoothelin or SMMHC 7 or 30 days after injury or in culture. Similarly, we also demonstrate a uniform staining of SMMHC (Fig. 7) across the NI to corroborate that the NI is primarily composed of SMC. Furthermore, Christen et al. concluded that ≤6% of the NI is composed of AFB, similar to our results that <2% of the NI is AFB.
Although our present data demonstrate that proliferating AFB do not abundantly migrate to form the NI, these proliferating cells in the adventitia may contribute significantly to arterial expansion and remodeling following balloon angioplasty. As early as 3 days after balloon angioplasty adventitial thickness increases, which is in part explained by the increased cellular density from proliferating AFB (19). Furthermore, it has been demonstrated that BrdU cells in the adventitia colocalize with procollagen type I within 3 days of the balloon angioplasty (17). In addition, activated adventitial cells likely act in a paracrine manner, producing growth factors and cytokines that influence vascular remodeling (23). Collectively these data suggest a role for proliferating AFB in vascular remodeling, including matrix production and adventitial expansion, after balloon angioplasty, rather than migration to the NI.
In conclusion, recent evidence has demonstrated that circulating progenitor cells do not contribute significantly to vascular lesion formation; rather, cells from the local vessel wall migrate to form the lesion (1). Although neointimal SMC originate from the vascular wall, there is controversy as to whether these cells are of adventitial or medial origin. Our results demonstrate that <2% of AFB migrate to form the NI after PTCA and that the NI is uniformly stained with SMMHC, a marker of SMC. These results, therefore, suggest that the NI is primarily composed of SMC originating from the media, not the adventitia. Furthermore, similar to our finding, Christen et al. (2) demonstrated that 94% and 98% of neointimal cells are positive for smoothelin and SMMHC, respectively, both of which are markers of differentiated SMC (21). Moreover, there is no evidence that fibroblasts transform into differentiated SMC expressing smoothelin (14). Collectively, these data and those of the present study demonstrate a negligible role for proliferating AFB in postangioplasty neointimal formation, rather supporting the concept of medial SMC migration and expansion as the dominant mechanism of neointimal formation in porcine coronary arteries after PTCA.
Limitations.
Although it was demonstrated that few BrdU-positive AFB migrate to form the NI, we cannot exclude the possibility that nonproliferating AFB migrate to the NI with subsequent proliferation at a time point after BrdU is cleared, and thus would not be detected as BrdU positive. However, BrdU labeling up to 6 days after injury failed to indicate substantial adventitial contribution. Thus our data clearly indicate that there is a negligible contribution of proliferating AFB to neointimal formation early (days) after injury as proposed by previous studies.
Another limitation is the age of the animals studied. We studied juvenile (3–4 mo) male swine, whereas most PTCA/stent placements would be performed in older human patients. SMC from older animals have enhanced proliferative and migratory properties that are associated with larger NI formation following injury (9, 22) and decreased apoptosis of NI cells. Collectively, these findings leave open the possibility that the etiology of lesion development, and thus the potential role of AFB, may be different in older individuals and warrants further investigation.
A final limitation is the use of balloon angioplasty, without stent deployment, on healthy, disease-free coronary arteries. The rationale for balloon angioplasty or stent placement in humans is to revascularize arteries occluded because of atherosclerotic lesion development or restenosis of previous PTCA. However, in our animals there was no preexisting lesion. Therefore, although the porcine coronary overstretch model closely mimics human postangioplasty restenosis (23), it may not fully recapitulate restenosis observed after PTCA of diseased vessels in humans. Finally, because stent placement is standard clinical practice in the majority of PTCA, the present findings should be interpreted with caution when generalizing to in stent restenosis.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-52490 and HL-071574.
Acknowledgments
We thank Jan Ivey for her surgical and technical assistance and Darla Tharp for critical reading of the manuscript.
REFERENCES
- 1.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol 26: 2696–2702, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Christen T, Verin V, Bochaton-Piallat ML, Popowski Y, Ramaekers F, Debruyne P, Camenzind E, van Eys G, Gabbiani G. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation 103: 882–888, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res 56: 139–145, 1985. [DOI] [PubMed] [Google Scholar]
- 4.De Leon H, Ollerenshaw JD, Griendling KK, Wilcox JN. Adventitial cells do not contribute to neointimal mass after balloon angioplasty of the rat common carotid artery. Circulation 104: 1591–1593, 2001. [PubMed] [Google Scholar]
- 5.Faggin E, Puato M, Zardo L, Franch R, Millino C, Sarinella F, Pauletto P, Sartore S, Chiavegato A. Smooth muscle-specific SM22 protein is expressed in the adventitial cells of balloon-injured rabbit carotid artery. Arterioscler Thromb Vasc Biol 19: 1393–1404, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest 89: 507–511, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriss JP, Revesz L. The distribution and fate of bromodeoxyuridine and bromodeoxycytidine in the mouse and rat. Cancer Res 22: 254–265, 1962. [PubMed] [Google Scholar]
- 8.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation 101: 1362–1365, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Cheng H, Lederer WJ, Froehlich J, Lakatta EG. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol 64: 1–11, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Malik N, Francis SE, Holt CM, Gunn J, Thomas GL, Shepherd L, Chamberlain J, Newman CMH, Cumberland DC, Crossman DC. Apoptosis and cell proliferation after porcine coronary angioplasty. Circulation 98: 1657–1665, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Mallawaarachchi CM, Weissberg PL, Siow RCM. Antagonism of platelet-derived growth factor by perivascular gene transfer attenuates adventitial cell migration after vascular injury: new tricks for old dogs? FASEB J 20: 1686–1688, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Mallawaarachchi CM, Weissberg PL, Siow RCM. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol 25: 1383–1387, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation 104: 2228–2235, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after balloon overstretch injury of porcine coronary arteries. Circulation 93: 2178–2187, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol 21: 739–745, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, O'Brien JE, Ala-Kokko L, Chung W, Mannion JD, Zalewski A. Origin of extracellular matrix synthesis during coronary repair. Circulation 95: 997–1006, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94: 1655–1664, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Pieniek M, Fard A, O'Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation 93: 340–348, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Siow RCM, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res 59: 212–221, 2003. [DOI] [PubMed] [Google Scholar]
- 21.van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol 134: 401–411, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez-Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, Fornoni A, Aitouche A, Pham SM. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg 40: 1199–1207, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox JN, Okamoto EI, Nakahara KI, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis: a role for circulating myofibroblast precursors? Ann NY Acad Sci 947: 68–92, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Wynford-Thomas D, Williams ED. Use of bromodeoxyuridine for cell kinetic studies in intact animals. Cell Tissue Kinet 19: 179–182, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, Ji J, Li L, Chen R, Hu W. Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem Biophys Res Commun 352: 681–688, 2007. [DOI] [PubMed] [Google Scholar]