Abstract
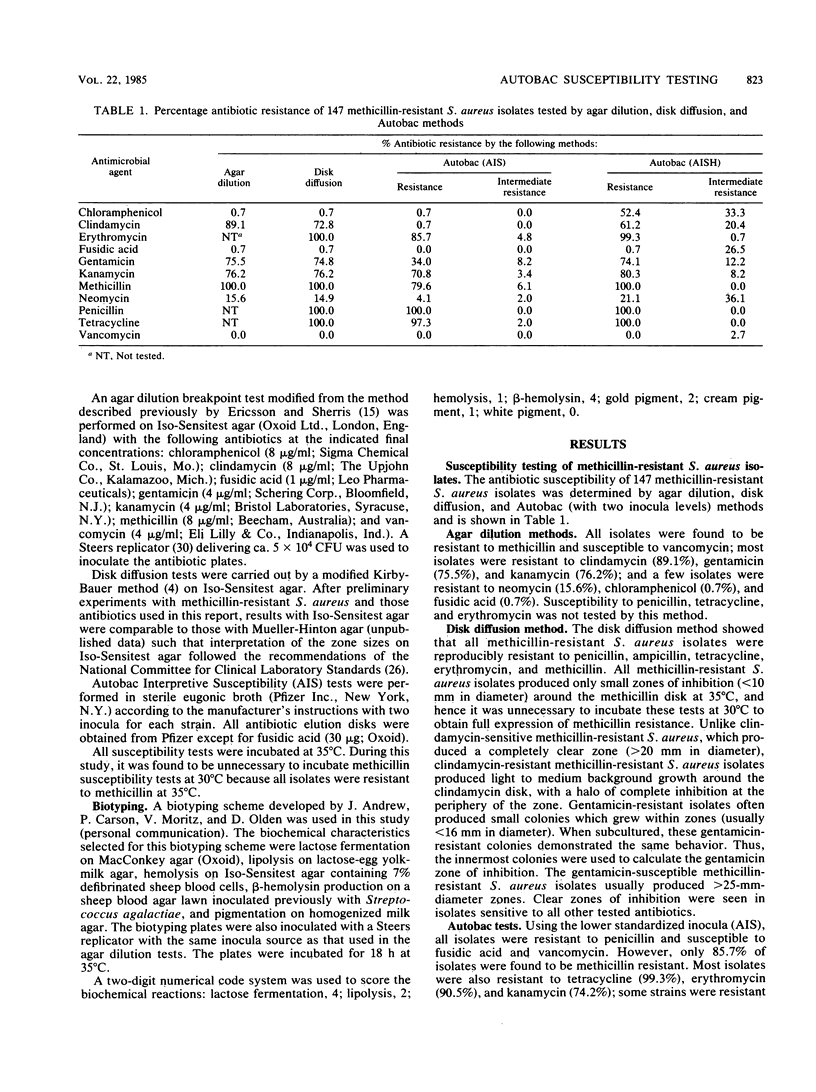
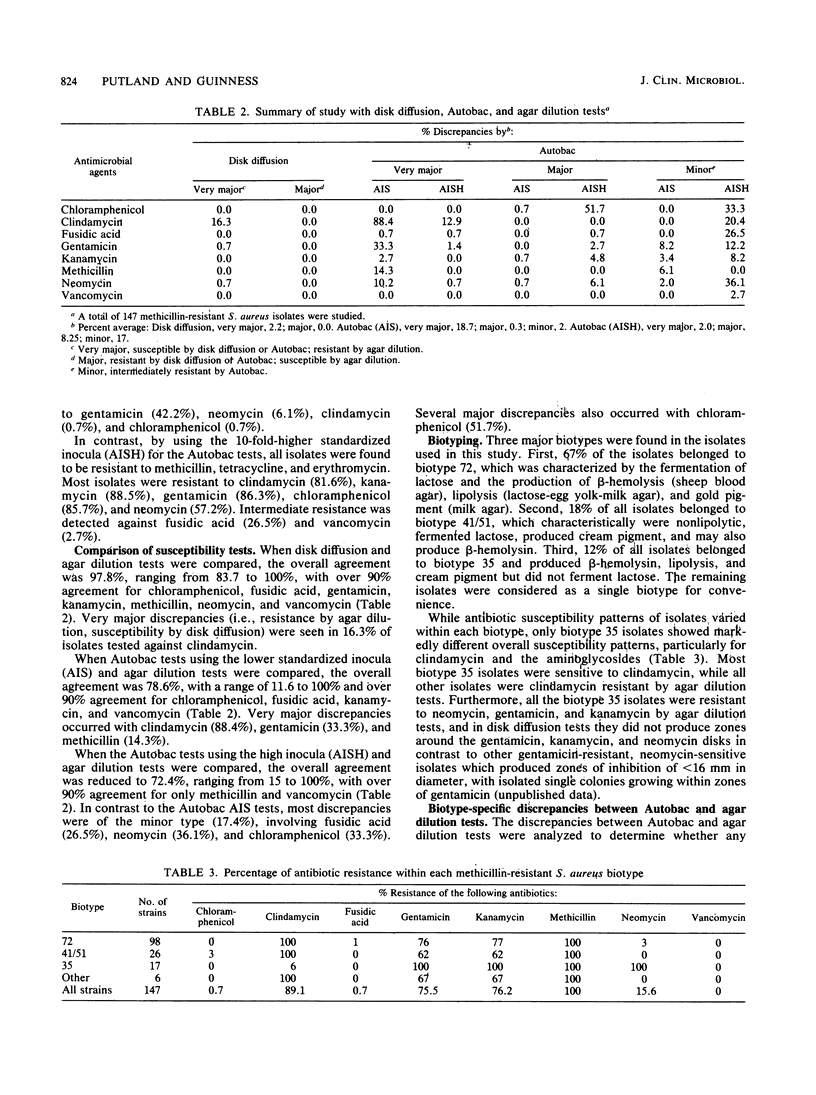
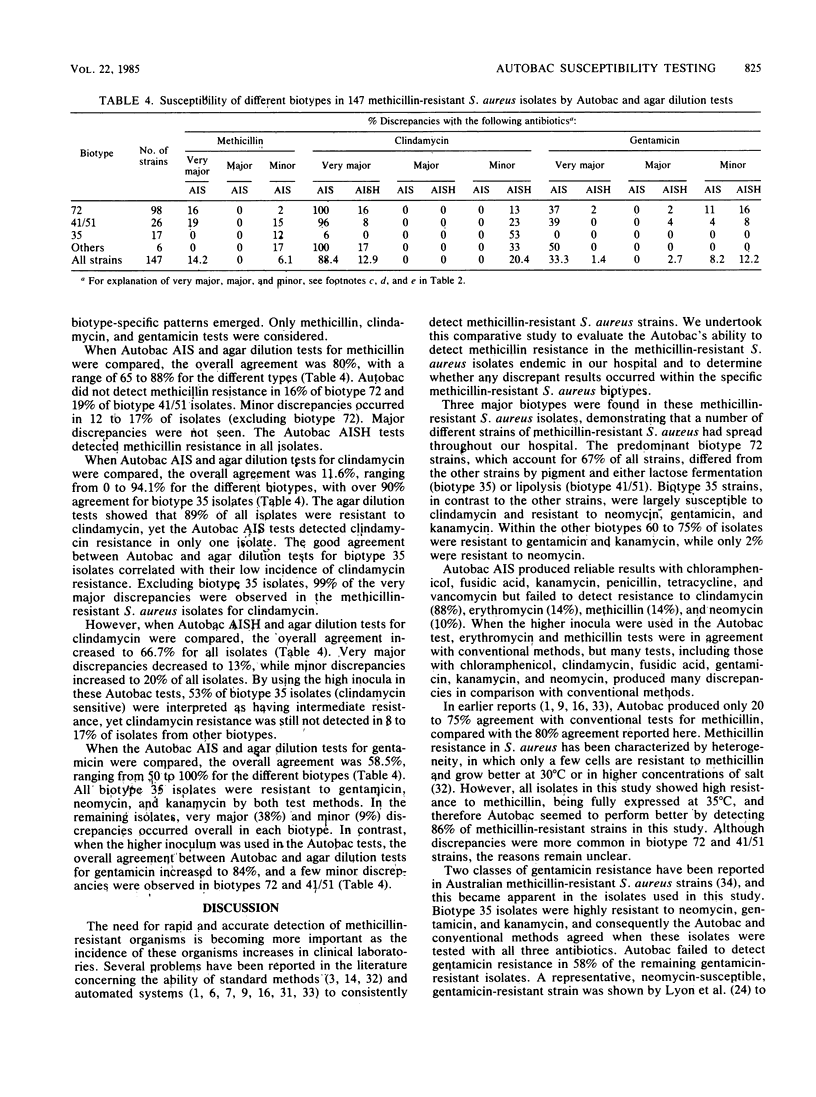
Semiautomated rapid broth elution (Autobac Multi-Test System; General Diagnostics, Div. Warner-Lambert Co., Morris Plains, N.J.) and disk diffusion tests were compared with an agar dilution breakpoint method to determine the antibiotic susceptibility of 147 methicillin-resistant Staphylococcus aureus isolates from our hospital. Although the disk diffusion method, in general, correlated well with the agar dilution tests, the overall agreement of the Autobac tests with agar dilution tests was only 79%, with many very major discrepancies occurring with clindamycin (88%), gentamicin (33%), and methicillin (15%). When we used a 10-fold higher inoculum for the Autobac tests, all isolates were shown to be resistant to methicillin, but significant numbers of major and minor discrepancies occurred with chloramphenicol, fusidic acid, and neomycin. The majority of isolates were shown to belong to three biotypes, distinguishable by lactose fermentation, lipolysis, hemolysis, and pigment production. The antibiotic susceptibility profile of one biotype was found to be markedly different from those of the other biotypes and contained a high incidence of clindamycin susceptibility and neomycin, gentamicin, and kanamycin resistance. In contrast, the other two biotypes had a high incidence of clindamycin, gentamicin, and kanamycin resistance and neomycin susceptibility and accounted for most of the very major discrepancies in the clindamycin and aminoglycoside tests. In these methicillin-resistant S. aureus strains, discrepancies possibly may arise from partial expression of methicillin resistance, dissociated or inducible clindamycin resistance, and instability of gentamicin resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Janney A., Sanders C. V., Marier R. L. Interlaboratory variation of antibiograms of methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains with conventional and commercial testing systems. J Clin Microbiol. 1983 Nov;18(5):1226–1236. doi: 10.1128/jcm.18.5.1226-1236.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Mayhall C. G. Comparison of epidemiological markers used in the investigation of an outbreak of methicillin-resistant Staphylococcus aureus infections. J Clin Microbiol. 1983 Aug;18(2):395–399. doi: 10.1128/jcm.18.2.395-399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Badal R. E. Reliability of the microdilution technic for detection of methicillin-resistant strains of staphylococcus aureus. Am J Clin Pathol. 1977 May;67(5):489–495. doi: 10.1093/ajcp/67.5.489. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Boyce J. M., White R. L., Bonner M. C., Lockwood W. R. Reliability of the MS-2 system in detecting methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1982 Feb;15(2):220–225. doi: 10.1128/jcm.15.2.220-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G. [Staphylococcus strains naturally resistant to methicillin and 5-methyl-3-phenyl-4-iso-oxazolyl-penicillin]. Ann Inst Pasteur (Paris) 1962 Aug;103:222–230. [PubMed] [Google Scholar]
- Carlson J. R., Conley F. E., Cahall D. L. Methicillin-resistant Staphylococcus aureus susceptibility testing with Abbott MS-2 system. Antimicrob Agents Chemother. 1982 Apr;21(4):676–677. doi: 10.1128/aac.21.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary T. J., Maurer D. Methicillin-resistant Staphylococcus aureus susceptibility testing by an automated system, Autobac I. Antimicrob Agents Chemother. 1978 May;13(5):837–841. doi: 10.1128/aac.13.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Wong E. S., Falkow S. Common R-plasmids in Staphylococcus aureus and Staphylococcus epidermidis during a nosocomial Staphylococcus aureus outbreak. Antimicrob Agents Chemother. 1982 Feb;21(2):210–215. doi: 10.1128/aac.21.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collopy B. T., Dalton M. F., Wright C., Mullany C. Comparison of the clinical significance of methicillin-resistant and methicillin-sensitive Staphylococcus aureus isolations. Med J Aust. 1984 Feb 18;140(4):211–214. doi: 10.5694/j.1326-5377.1984.tb103997.x. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Kollisch N. R., Hsieh C. R., Connolly M. G., Jr, McCabe W. R. Vancomycin treatment of bacteremia caused by oxacillin-resistant Staphylococcus aureus: comparison with beta-lactam antibiotic treatment of bacteremia caused by oxacillin-sensitive Staphylococcus aureus. J Infect Dis. 1983 Jan;147(1):137–143. doi: 10.1093/infdis/147.1.137. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Barry A. L., O'Toole R., Sherris J. C. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl Microbiol. 1972 Aug;24(2):240–247. doi: 10.1128/am.24.2.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell G. R., Guinness M. D. Australian evaluation of Autobac I with suggested interpretive and technical modifications. Antimicrob Agents Chemother. 1979 Sep;16(3):255–261. doi: 10.1128/aac.16.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P. The erythromycin group of antibiotics. Br Med J. 1957 Jul 13;2(5036):57–63. doi: 10.1136/bmj.2.5036.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieble H. G., Krause S. L., Pappas S. A., DiCostanzo M. B. The prevalence of high-level methicillin resistance in multiply resistant hospital staphylococci. Medicine (Baltimore) 1981 Jan;60(1):62–69. doi: 10.1097/00005792-198101000-00006. [DOI] [PubMed] [Google Scholar]
- Kayser F. H. Methicillin-resistant staphylococci 1965-75. Lancet. 1975 Oct 4;2(7936):650–653. doi: 10.1016/s0140-6736(75)90129-4. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Stokes A. Studies on recently isolated cultures of methicillin-resistant Staphylococcus aureus. J Gen Microbiol. 1979 Oct;114(2):329–339. doi: 10.1099/00221287-114-2-329. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Iuorio J. L., May J. W., Skurray R. A. Molecular epidemiology of multiresistant Staphylococcus aureus in Australian hospitals. J Med Microbiol. 1984 Feb;17(1):79–89. doi: 10.1099/00222615-17-1-79. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Analysis of plasmids in nosocomial strains of multiple-antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):817–826. doi: 10.1128/aac.23.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock J. E., Jr, Marsik F. J., Wenzel R. P. Methicillin-resistant Staphylococcus aureus: introduction and spread within a hospital. Ann Intern Med. 1980 Oct;93(4):526–532. doi: 10.7326/0003-4819-93-4-526. [DOI] [PubMed] [Google Scholar]
- Rountree P. M., Vickery A. M. Further observations on methicillin-resistant staphylococci. Med J Aust. 1973 May 26;1(21):1030–1034. doi: 10.5694/j.1326-5377.1973.tb110903.x. [DOI] [PubMed] [Google Scholar]
- Selkon J. B., Stokes E. R., Ingham H. R. The role of an isolation unit in the control of hospital infection with methicillin-resistant staphylococci. J Hosp Infect. 1980 Mar;1(1):41–46. doi: 10.1016/0195-6701(80)90030-4. [DOI] [PubMed] [Google Scholar]
- Stotler R. W., Meyer M. C. Detection of oxacillin-resistant staphylococci by the AutoMicrobic system. J Clin Microbiol. 1983 Nov;18(5):1205–1211. doi: 10.1128/jcm.18.5.1205-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Caruthers J. Q., Baker C. N. Effect of temperature on the in vitro susceptibility of Staphylococcus aureus to penicillinase-resistant penicillins. Antimicrob Agents Chemother. 1973 Sep;4(3):263–269. doi: 10.1128/aac.4.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Grubb W. B., Ashdown N. Gentamicin resistance in methicillin-resistant Staphylococcus aureus. Pathology. 1983 Apr;15(2):169–174. doi: 10.3109/00313028309084707. [DOI] [PubMed] [Google Scholar]
- Vickery A. M., Beard-Pegler M. A., Rountree P. M. Strain differentiation in methicillin-resistant Staphylococcus aureus. Pathology. 1983 Jul;15(3):235–240. doi: 10.3109/00313028309083499. [DOI] [PubMed] [Google Scholar]



