Abstract
Cell therapy with endothelial progenitor cells (EPCs) is an emerging therapeutic option to promote angiogenesis or endothelial repair. Although the release of angiogenic paracrine factors is known to contribute to their therapeutic effect, little is known about their release of proinflammatory factors and expression of proinflammatory adhesion molecules. “Early” EPCs and “late” EPCs were isolated from human peripheral blood and their release of chemokines and thromboinflammatory mediators as well as their expression of the proinflammatory adhesion molecules was assessed at baseline and with stimulation. The effect of simvastatin on monocyte chemoattractant protein-1 (MCP-1) secretion by late EPCs from patients with vascular disease was also evaluated. All groups of EPCs released chemokines and thromboinflammatory mediators. Early EPCs primarily released thromboinflammatory mediators such as tissue factor (0.5 ± 0.1 ng/million cells, P < 0.05), whereas adult late EPCs primarily released chemokines such as MCP-1 (287 ± 98 ng/million cells, P < 0.05). Stimulation with tumor necrosis factor (TNF)-α augmented the expression of proinflammatory adhesion molecules and paracrine factors by all EPC subtypes. The release of MCP-1 by late EPCs was markedly reduced by simvastatin treatment of the cells. All EPC subtypes expressed proinflammatory paracrine factors and adhesion molecules involved in atherosclerosis. Future clinical studies should therefore not only assess the efficacy of EPCs but also monitor inflammatory activation following EPC transplantation in patients. Pharmacological modulation of EPCs before and after transplantation may represent a novel approach to improve their safety.
Keywords: endothelial progenitor cells, inflammation, cardiovascular cell therapy
cardiovascular cell therapy using stem and progenitor cells is a promising novel approach to repair and regenerate cardiovascular cells in patients with cardiovascular disease. Seminal preclinical studies demonstrated that adult human blood contained “endothelial progenitor cells” (EPCs) which were able to markedly augment neovascularization when transplanted in immune deficient rodents with chronic ischemia (2, 16). Although it is known that impaired endothelial function is a prognostic marker for cardiovascular disease (13), multiple studies have now shown that reduced native circulating EPC numbers are not only correlated with reduced endothelial function (15) but also with cardiovascular disease (9, 28), reduced collateral formation (17), and worse long-term cardiovascular outcomes in patients (31). This suggests that replenishment or supplementation of native EPCs could potentially improve endothelial function and cardiovascular outcomes. However, recent larger, controlled clinical studies transplanting EPCs (3) and other bone marrow mononuclear cells (21) in patients with myocardial infarction have shown rather limited or even no benefits in overall left ventricular systolic function when compared with the control groups (1).
There is a tremendous diversity in methods and cells that have been employed to study the effects of “EPCs” on cardiovascular function (3, 11, 12, 14–16, 25, 29, 30, 32), and there is a clear need for appropriately defining cells, both for a better mechanistic understanding and for developing clinical therapies (18). There are at least two distinct types of EPCs with apparently distinct properties (11, 12, 14, 15, 25, 29, 32). The first type are referred to as cultured angiogenic cells (CACs) or “early” EPCs because they are obtained from the short-term culture (4–7 days) of circulating adult peripheral blood mononuclear cells and are derived from a myeloid lineage. The second type of EPCs are referred to as late or “out-growth” EPCs since they are obtained from the long-term culture (2–4 wk) of mononuclear cells. These are found to a much greater extent in umbilical cord blood than adult human blood, form functional blood vessels, do not express myeloid markers, and form highly proliferative endothelial colonies derived from single cells (32). An example for the physiological and clinical significance of distinguishing between EPC subtypes is highlighted by the fact that late EPC numbers are higher in patients with coronary disease (12) than control subjects, whereas early EPCs are lower (15). Interestingly, both EPC types appear to use the release of paracrine factors as a key modality by which EPCs enhance angiogenesis (14, 25, 26, 30).
Even though the angiogenic efficacy of early and late EPCs is well-established, less is known about their potential for harm. A recent in vivo animal study has shown that transplantation of EPCs could potentially promote atherogenesis (10), and concerns have been raised about the balance between safety and efficacy of cardiovascular cell therapy using EPCs or stem/progenitor cells (5, 23). Because inflammatory activation is a critical component of atherogenesis and endothelial dysfunction (6, 20), we studied the release of proinflammatory mediators and expression of surface adhesion molecules in distinct EPC subtypes. We are able to demonstrate that all studied EPC subtypes secrete proinflammatory mediators and can also upregulate proinflammatory adhesion molecules upon stimulation in a manner similar to that of mature endothelial cells.
METHODS
Isolation and culture of early and late EPCs.
Early EPCs were isolated as previously published (25) by culturing human mononuclear cells. These had been obtained after centrifugation of buffy coat preparations from anonymous donors of a blood bank over a Ficoll gradient (25). The mononuclear cells were cultured in the presence of endothelial growth media EGM2-MV, which is endothelial basal medium EBM-2 (Lonza, Walkersville, MD) supplemented with EGM-2-MV-SingleQuots (Lonza) containing vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-I, epidermal growth factor, and 5% FBS. One million mononuclear cells per square centimeter were plated on fibronectin-coated tissue culture T-75 flasks (BD Biosciences). As previously published, the mononuclear cells were cultured for 4 days. Next, the flasks were washed two times with PBS to remove the nonadherent cells while the remaining adherent cells have both endothelial and myeloid characteristics (25, 30).
Late EPCs were isolated from either human adult blood or umbilical cord blood by culturing mononuclear cells in endothelial basal medium EBM-2 (Lonza) supplemented with EGM-2 SingleQuots (Lonza) and an additional 10% FBS. Next, 5 × 106 mononuclear cells/cm2 were plated on collagen-coated tissue culture flasks (BD Biosciences). After 1 day of culture, nonadherent cells were discarded, and the media were replaced daily for the next 2–4 wk. Subsequently, endothelial colonies appeared (on average 1 colony/107 or 108 plated mononuclear cells). The colonies were replated on new plates, and highly proliferative endothelial cells grew out from these colonies, which then formed confluent monolayers (32, 33). To confirm the endothelial phenotype, adherent cells were incubated with DiI-labeled acetylated low density lipoprotein (LDL; Molecular Probes-Invitrogen, Carlsbad, CA) for 4 h, and, after fixation, they were incubated with the fluorescein isothiocyanate-labeled endothelial surface stain Ulex europeaus lectin 1 (Biomeda, Foster City, CA) for 1 h, as well as the nuclear 4′,6-diamidino-2-phenylindole stain (Molecular Probes-Invitrogen) for 15 min (25).
The key differences in the commonly used methods to isolate early and late EPCs are different culture times of the mononuclear cells (4 days for early EPCs vs. 2–4 wk for late EPCs), different flask coatings (fibronectin for early EPCs vs. collagen for late EPCs), and different FBS concentrations (5% for early EPCs vs. total of 12% for late EPCs) (25, 30, 32).
The mononuclear cells from which the EPCs were isolated were obtained from anonymous blood bank donors or volunteer subjects, and the study was approved by the Human Subjects Institutional Review Board of Indiana University. The enrolled volunteer subjects provided written consent.
To compare the vulnerability of EPCs with that of mature endothelial cells, we obtained mature human aortic endothelial cells from Lonza and cultured them according to manufacturer instructions.
Flow cytometry.
Phenotypic differences between EPC subtypes and the effects of inflammatory stimulation on surface markers were confirmed with flow cytometry. Cells were detached by 2 mM EDTA and then incubated with fluorescent antibodies against the myeloid marker CD45 (BD Biosciences, San Jose, CA) and the endothelial marker CD31 [platelet endothelial cell adhesion molecule (PECAM); BD Biosciences] to confirm the origin of these cells. Cells were also labeled with antibodies for the inflammatory endothelial surface marker CD54 and CD106 [intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1, respectively; BD Biosciences] to assess the response to tumor necrosis factor (TNF)-α stimulation. To control for nonspecific antibody binding, all cells were also labeled with a fluorescent negative isotype control antibody. Samples were analyzed on a Becton-Dickinson FACSCalibur instrument. The data are presented as fluorescence overlay graphs of positive markers and negative isotype controls, as well as quantitative analysis of mean fluorescence.
Paracrine activity assay.
To measure the paracrine activity of all EPCs, cells were placed in basal medium (without serum or potentially contaminating supplemental growth factors), and samples were collected after 24 h. Because early EPCs typically do not proliferate and do not survive long term, we assessed their paracrine function 4 days after the mononuclear cells had been isolated and cultured. On the other hand, late EPCs had to be isolated by culturing the mononuclear cells for at least 2–4 wk after which the colonies appeared. The cells were then further cultured and passaged multiple times, and the paracrine activity assessment was performed by switching the cells to the same basal medium we used for the early EPCs. Levels of chemokines and thromboinflammatory mediators in media samples were measured by the Pierce Biotechnology Assay Service, using SearchLight Proteome Arrays (Pierce Biotechnology, Woburn, MA). The SearchLight Proteome Array is a quantitative multiplexed sandwich enzyme-linked immunosorbent assay (ELISA) containing up to 12 different capture antibodies spotted on the bottom of a 96-well polystyrene microtiter plate. Each antibody captures specific protein present in the standards and samples added to the plate. The bound proteins are then detected with a biotinylated detection antibody, followed by the addition of streptavidin-horseradish peroxidase (HRP) and, last, SuperSignal ELISA Femto Chemiluminescent substrate. The luminescent signal produced from the HRP-catalyzed oxidation of the substrate is measured by imaging the plate using the SearchLight Imaging System, which is a cooled charge-coupled device camera. The data are then analyzed using ArrayVision customized software. The amount of luminescent signal produced is proportional to the amount of each protein present in the original standard or sample. Concentrations are extrapolated off a standard curve. The number of adherent cells was also quantified, and the data are expressed as secreted nanograms per 106 cells for every paracrine factor over a 24-h period. To measure whether inflammatory stimulation with the cytokine TNF-α could augment the release of paracrine activity, cells were exposed to 10 ng/ml TNF-α during the 24-h period in the EBM-2 medium. Change in paracrine activity is shown as a change in the release of the paracrine factor [monocyte chemoattractant protein-1 (MCP-1)] in nanograms per 106 cells. All results shown reflect n = 3 or greater.
The choice of assay factors was based on the following proinflammatory factors that may be involved in the atherogenic process: 1) Chemokines (6): MCP-1, interleukin (IL)-8, and regulated upon activation, normal T cell expressed and secreted (RANTES) and 2) thromboinflammatory factors (20): myeloperoxidase, tissue factor, and plasminogen activator inhibitor-1 (PAI-1). Statistical analysis was performed by ANOVA using GraphPad Prism software, and a value of P < 0.05 was considered statistically significant. After the ANOVA, a Tukey's post hoc test was performed to compare the paracrine activity of each EPC type with each other. The values for the overall ANOVA and the between group post hoc analysis are given in Figs. 1–6. All experiments were performed with n ≥ 3.
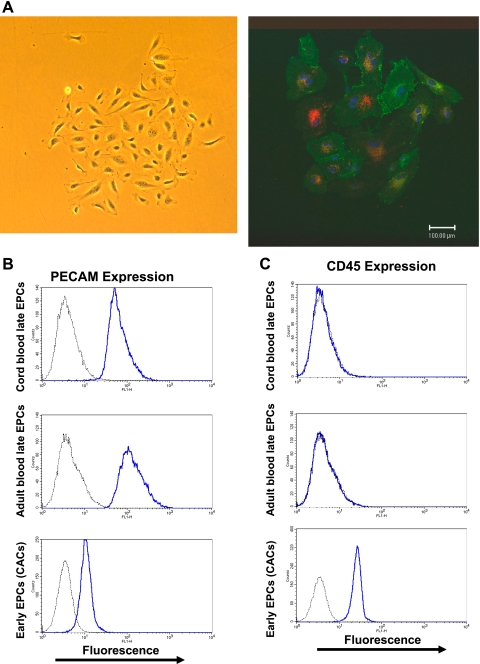
Fig. 1.
A: phase-contrast and fluorescent images of a cord blood-derived “late” endothelial progenitor cell (EPC) colony. Endothelial cells derived from the long-term culture of mononuclear cells (at least 2–4 wk) can form highly proliferative colonies derived from single circulating endothelial cells. Left: phase-contrast image of an EPC colony derived from umbilical cord blood mononuclear cells. Right: confocal microscopy image of an endothelial colony stained with the endothelial surface stain Ulex europaeus lectin (green = fluorescein isothiocyanate label), the nucleus stain 4′,6-diamidino-2-phenylindole (blue) as well as uptake of acetylated low density lipoprotein (red = DiI label). B and C: flow cytometry phenotyping of EPCs using the endothelial surface marker CD31 [platelet endothelial cell adhesion molecule (PECAM)] and the myeloid surface marker CD45. Late EPCs (or colony-forming EPCs) derived from the long-term culture (at least 3–4 wk) of circulating mononuclear cells from adult blood and umbilical cord blood are positive for the endothelial marker CD31 (PECAM) but not for the myeloid marker CD45 while “early” EPCs [or cultured carcinogenic cells (CACs)] derived from the short-term culture (4 days) of adult mononuclear cells also express the myeloid marker CD45. The negative isotype control antibody staining is shown in gray.
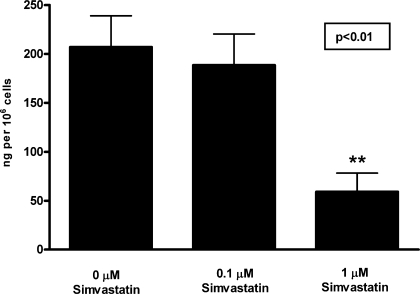
Fig. 6.
Secretion of the proinflammatory chemokine by EPCs treated with simvastatin. Adult blood-derived late EPCs were cultured with increasing doses of simvastatin to assess its effect on the release of MCP-1. Released MCP-1 was assessed by enzyme-linked immunosorbent assay and normalized by cell number. Data are means ± SE of secreted MCP-1 in ng/million cells. Cells showed a significant decreases in their release of MCP-1 per repeated-measures ANOVA, n = 4 with simvastatin treatment. Post hoc analysis showed that 1 μM simvastatin markedly reduced MCP-1 release (**P < 0.01 vs. control).
Effect of simvastatin on EPC paracrine activity.
To assess whether anti-inflammatory treatment of EPCs could mitigate the proinflammatory paracrine activity, we chose to treat EPCs with simvastatin, a commonly used anti-hyperlipidemic agent with known anti-inflammatory effects. Adult late EPCs were isolated from mononuclear cells as described above. Cells were switched to EBM for 24 h. To determine whether simvastatin could modulate the paracrine activity of EPCs, additional cell samples were prepared in which the EBM was supplemented with simvastatin (Sigma Chemicals) or the control vehicle dimethyl sulfoxide (Sigma Chemicals) during the 24-h period. The levels of the pro-inflammatory chemokine MCP-1 were determined by ELISA (R&D Systems, Minneapolis, MN). The number of adherent cells was quantified, and the data are expressed as secreted nanograms per 106 over a 24-h period. Statistical analysis of the dose-dependent simvastatin effect was performed by a repeated-measures ANOVA using the GraphPad Prism software. Following the ANOVA, a Tukey's post hoc test was performed to compare the effect of the varying simvastatin doses on MCP-1 release.
RESULTS
Distinct subtypes of EPC.
EPCs obtained by the long-term culture of mononuclear cells were able to form proliferative colonies, which stained positive for the endothelial surface membrane stain Ulex europaeus lectin as well as uptake of acetylated LDL (see Fig. 1A). As previously shown, short-term culture of mononuclear cells yields a myeloid cell population (CACs or early EPCs) that also stains positive for Ulex europaeus lectin and has uptake of acetylated LDL (25). The key distinguishing feature of late EPCs and early EPCs was their surface expression of marker proteins. All EPC types appeared to express the endothelial surface protein CD31 (PECAM), although its expression level was much lower for early EPCs (mean intensity of fluorescence 13.2 ± 1.2) than for late adult EPCs (mean intensity of fluorescence 186.0 ± 36.0) and late cord blood EPCs (mean intensity of fluorescence 185.7 ± 56.9). However, only early EPCs expressed the myeloid marker CD45 (mean intensity of fluorescence 33.4 ± 4.2), thus highlighting their hematopoietic origin (see Fig. 1B).
Release of chemokines by EPCs.
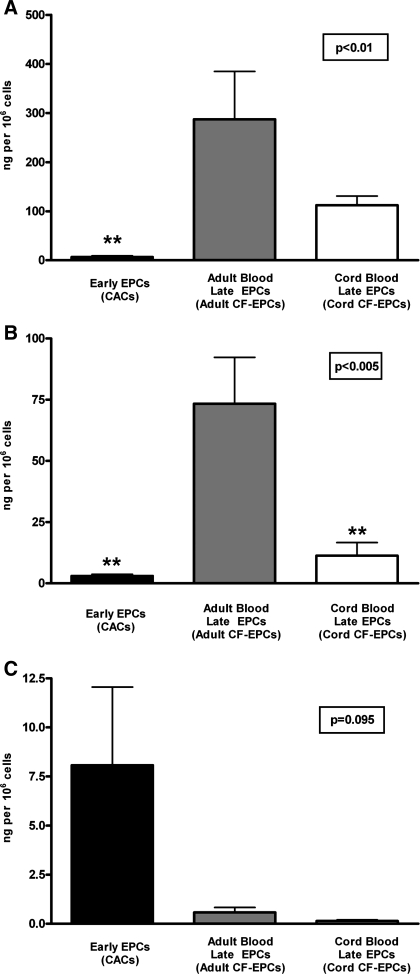
To investigate whether EPCs released chemokines that are either known to promote inflammatory or atherogenic processes (6), we examined the secretion of the chemokines MCP-1, IL-8, and RANTES by early EPCs, adult blood late EPCs, and cord-blood late EPCs (see Fig. 2). Interestingly, adult late EPCs had the highest release of MCP-1 (early EPCs: 6.5 ± 2.5 ng/million cells; adult late EPCs: 287.0 ± 98 ng/million cells; cord late EPC: 112 ± 18.8 ng/million cells; adult late EPCs vs. early EPCs P < 0.01) and IL-8 (early EPC: 3.0 ± 0.7 ng/million cells; adult late EPCs: 73.4 ± 19 ng/million cells; cord blood late EPC: 11.3 ± 5.3 ng/million cells; P < 0.005). Early EPCs had a trend toward the highest release of the chemokine RANTES (early EPCs: 8.1 ± 4.0 ng/million cells; adult late EPCs: 0.6 ± 0.3 ng/million cells; cord blood late EPCs: 0.1 ± 0.1 ng/million cells; P = 0.095).
Fig. 2.
Release of inflammatory chemokines by EPCs. Release of the chemokines monocyte chemoattractant protein-1 (MCP-1; A), interleukin-8 (IL-8; B), and regulated upon activation, normal T cell expressed and secreted (RANTES; C) by early EPCs (n = 4), adult blood late EPCs (n = 3), and cord blood late EPCs (n = 4) was measured over a 24-h period. The data for each chemokine are presented as means ± SE of secreted ng/million adherent cells. In A, the overall P value for comparative MCP-1 release by ANOVA was P < 0.01, but the only significant difference in the post hoc analysis was P < 0.01 (**) between early EPCs and adult late EPCs. In B, the overall ANOVA of the IL-8 release was highly significant (P < 0.005), and this was driven by the difference in IL-8 release between early EPCs and adult late EPCs (**P < 0.01) and by the difference between the cord blood late EPC group and adult late EPCs (**P < 0.01). In C, the overall ANOVA of the RANTES release was not significant (P = 0.095), so no post hoc analysis could be performed, although there was a trend toward higher RANTES release by early EPCs compared with either one of the late EPC groups.
Secretion of thromboinflammatory factors by EPCs.
Because thromboinflammatory factors like myeloperoxidase, tissue factor, and PAI-1 can serve as biomarkers and mediators of the thromboinflammatory cascade during plaque formation and plaque rupture (20), we measured their release by EPCs (see Fig. 3). Myeloperoxidase and tissue factor were primarily released by early EPCs. The myeloperoxidase release was 81.5 ± 19.9 ng/million cells in early EPCs (P < 0.01 vs. either adult or cord blood late EPCs), and the tissue factor release in early EPCs was 0.5 ± 0.1 ng/million cells (P < 0.05 vs. either adult or cord blood late EPCs), and their release by late EPCs was minimal. However, PAI-1 on the other hand was primarily released by adult blood late EPCs (23,933 ± 12,011 ng/million cells, P < 0.05 vs. early EPCs) and to a lesser degree by cord blood late EPCs (6,017 ± 1,510 ng/million cells).
Fig. 3.
Release of thromboinflammatory factors by EPCs. The release of the thromboinflammatory factors myeloperoxidase (MPO, A), tissue factor (B), and plasminogen activator inhibitor-1 (PAI-1, C) by early EPCs (n = 4), adult blood late EPCs (n = 3), and cord blood late EPCs (n = 4) is shown over a 24-h period. The data for each factor are presented as means ± SE of secreted ng/million adherent cells. In A, the overall P value for comparative MPO release by ANOVA was *P < 0.005, and post hoc analysis revealed that early EPCs had markedly higher MPO release (**P < 0.01) compared with either adult or cord blood late EPCs, both of which showed only minimal MPO release. The release of tissue factor (B) was also markedly higher in early EPCs (*P < 0.05 vs. either of the two late EPC groups), whereas there was no significant difference in tissue factor release between the two late EPC groups. In C, the release of PAI-1 is shown, and statistical analysis demonstrated that adult late EPCs had significantly higher PAI-1 release (P < 0.05) than early EPCs and a trend toward higher PAI-1 release compared with cord blood late EPCs.
Changes in EPC paracrine activity induced by TNF-α.
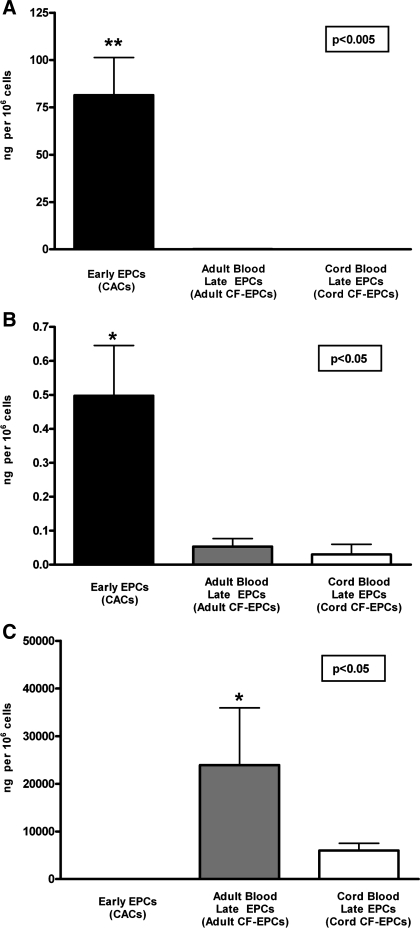
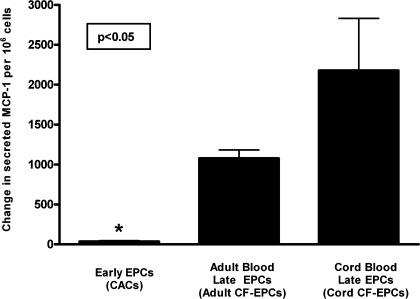
Following a therapeutic delivery of EPCs in the cardiovascular system of an atherosclerosis patient, cells could potentially be exposed to proinflammatory stimulation since cytokines are commonly upregulated within the milieu of the vasculature and myocardium (20). To determine if inflammatory stimulation of EPCs could upregulate the release of proinflammatory and proatherogenic factors, we exposed all three EPC types to the cytokine TNF-α and measured the change in paracrine activity of the MCP-1, which plays a key role in the development of atherosclerosis (6, 8). As shown in Fig. 4, stimulation with TNF-α augmented the paracrine release of MCP-1 in an EPC subtype-specific manner. Early EPCs not only had the lowest baseline release of MCP-1 (Fig. 2) but also showed the lowest induction by TNF-α (35.5 ± 9.7 ng/million cells), whereas cord blood late EPCs demonstrated the greatest induction of MCP-1 release with inflammatory stimulation (2,180 ± 651 ng/million cells, P < 0.05 vs. early EPCs).
Fig. 4.
Changes in EPC paracrine activity induced by tumor necrosis factor (TNF)-α. To assess whether inflammatory stimulation of EPCs could modulate their paracrine activity, EPCs were exposed to 10 ng/ml of the proinflammatory cytokine TNF-α during a 24-h period. The concentration of the proinflammatory chemokine MCP-1 was determined, and the change in paracrine activity was expressed as the difference in ng MCP-1 released/million cells between baseline and after TNF-α stimulation. Data are presented as means ± SE of secreted ng/million adherent cells. Statistical analysis is performed as a between-group ANOVA on the degree of change in MCP-1 release, and post hoc analysis revealed that early EPCs (n = 4) had minimal induction of MCP-1 release by TNF-α (*P < 0.05) compared with cord blood late EPCs (n = 4), whereas adult late EPCs (n = 3) had an intermediate increase in MCP-1 release.
Comparison of inflammatory vulnerability to TNF-α between mature endothelial cells and EPCs.
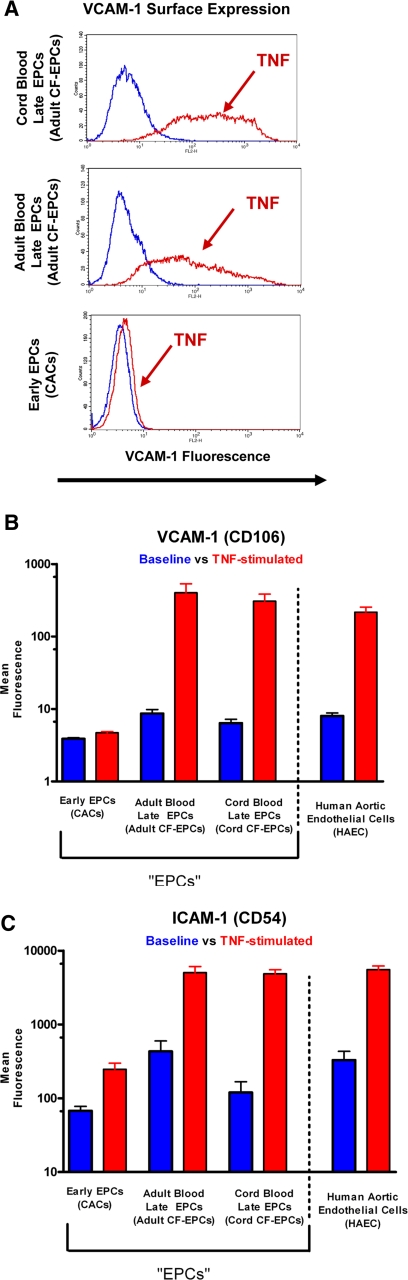
Because a significant component of the atherogenic process involves the upregulation of inflammatory adhesion molecules, we investigated whether EPC subtypes would differ in their inflammatory response, compared with mature aortic endothelial cells. Early EPCs did not express the adhesion molecule VCAM-1 at baseline (mean fluorescence: 4 ± 0.1) or with stimulation (mean fluorescence: 5 ± 0.2). Adult and cord blood late EPCs demonstrated a significant degree of VCAM-1 upregulation with TNF-α stimulation: 1) adult baseline: 9 ± 1.2 and adult stimulation: 402 ± 1.2, 2) cord baseline: 6 ± 0.8 and cord stimulation: 307 ± 77 (representative flow cytometry histograms in Fig. 5A). The late EPC response was comparable to that of mature endothelial cells (mean fluorescence at baseline 8 ± 0.8 and with stimulation 216 ± 38). The surface adhesion molecule ICAM-1 was expressed on all studied cells at baseline but upregulated to a much greater extent on late EPCs or mature endothelial cells than on early EPCs (Fig. 5).
Fig. 5.
Cell surface expression of proinflammatory cell adhesion molecules [vascular cell adhesion molecule (VCAM)-1 (CD106) and intercellular adhesion molecule (ICAM)-1 (CD54)] on EPCs. A: representative flow cytometry histograms of VCAM-1 expression on early EPCs, adult blood-derived late EPCs, and cord blood-derived late EPCs at baseline and with TNF-α stimulation (20 h, 10 ng/ml). All three EPC subtypes express minimal VCAM-1 at baseline; however, only late EPCs show a marked upregulation of VCAM-1, a surface marker characteristic of endothelial inflammation. The quantitative assessment of proinflammatory surface adhesion molecule expression demonstrates that early EPCs upregulate ICAM-1 (C) but not VCAM-1 (B) upon stimulation. Because of the massive response to the stimulation, the mean fluorescence of ICAM-1 and VCAM-1 is shown on the y-axis on a log scale. Late EPCs (both adult and cord blood derived) have a similar response to inflammatory stimulation as mature human aortic endothelial cells (HAECs), thus highlighting their physiological similarity.
Modulation of proinflammatory paracrine activity with simvastatin.
Adult late EPCs were cultured, and their release of the proinflammatory chemokine MCP-1 was measured with or without the presence of simvastatin. Because statins do not just control lipids but also have anti-inflammatory effects on the mature endothelium (22), we evaluated whether addition of simvastatin to the cultured EPCs was able to reduce the release of MCP-1 by EPCs. As shown in Fig. 6, when EPCs were treated with simvastatin, they showed a marked reduction of MCP-1 release of up to 70% with addition of 1 μM simvastatin (P < 0.01 vs. control).
DISCUSSION
The paracrine activity of EPCs.
The release of paracrine factors by stem and progenitor cells in the setting of cardiovascular cell therapy appears to be a major underlying component of the therapeutic effect (7, 14, 25, 26, 30). Furthermore, EPCs are thought to release multiple synergistic, therapeutic angiogenesis factors(7, 25), which may explain the potent neovascularization observed in animal models after EPC transplantation. However, it was recently shown in a preclinical study that EPC transplantation can also promote the growth of atherosclerotic plaques(5, 10), and concerns have been raised about potential risks of cardiovascular stem and progenitor cell therapy (5, 10). Little is known about the release of proinflammatory factors by EPCs and whether the various EPC subtypes that are frequently used in animal models and patients differ in their ability to release proinflammatory cytokines.
We therefore studied the chemokines MCP-1, IL-8, and RANTES, which have all been shown to be involved in the growth and formation of the atherosclerotic plaque by recruiting circulating inflammatory cells (6, 8). Because thrombus formation and plaque rupture is another major aspect of cardiovascular disease, we also studied thromboinflammatory factors like myeloperoxidase, tissue factor, and PAI-1, which are biomarkers and mediators of the thromboinflammatory cascade during plaque formation and plaque rupture (20). Our study focused on identifying potential inflammatory factors that could mediate such proatherogenic effects of EPCs. The key findings of our study are that the studied types of EPCs have a distinct pattern of chemokine and thromboinflammatory factor release. Additionally, we were able to show that the paracrine release is significantly augmented when EPCs are exposed to the proinflammatory cytokine TNF-α and that TNF-α can induce a similar degree of surface adhesion molecule upregulation in EPCs as in mature endothelium. Patients requiring EPC therapy due to endothelial dysfunction or chronic coronary artery disease are likely to contain proinflammatory cytokines such as TNF-α in their vasculature (20). Based on our results, it appears that transplanted EPCs would be vulnerable to an inflammatory environment. While it is important for transplanted cells to maintain their physiological ability to respond to stimuli, our findings highlight that it may be necessary to monitor the inflammatory activity following EPC transplantation and assess if transplanted cells are participating in pathological inflammatory processes.
Finding the ideal stem or progenitor cell for cardiovascular cell therapy.
A major finding in our study was that all three studied cell types released proinflammatory factors and that the paracrine profile was distinct for early EPCs vs. late EPCs. A cell type-specific function and phenotype profile may need to be established for each cell type and matched with a specific therapeutic goal such as postinfarct repair vs. collateral formation. Recent arteriogenesis studies are raising the importance of individualizing therapeutic approaches based on the patient's need and microenvironment (27). Monocyte/macrophages play key roles in the postinfarct repair by limiting infarct extension (19) and in collateral formation (8). It may be the proinflammatory monocytic-myeloid character of early EPCs that is responsible for their therapeutic effects in the setting of postinfarct repair or collateral formation (4, 23) and may, for example, make them ideal cell therapies in the postinfarct setting. However, because of their proatherogenic paracrine activity, they may not be the optimal choice to restore endothelial function and reduce atherosclerosis progression.
“Janus” phenomenon in cardiovascular cell therapy: safety vs. efficacy.
Preclinical studies using angiogenesis gene therapy have been highly successful in animal models but have had more modest benefits in the clinical setting. One reason for the limited success in translating angiogenesis therapies to the clinical setting could be the “Janus” phenomenon (8). It is a term used to describe the intricacy in approaching therapeutic angiogenesis, as many of the factors involved in desired collateral growth are also involved in the growth of the atherosclerotic plaque (8). One such example is the chemokine MCP-1, which is an extremely potent arteriogenic factor but is also thought to promote vascular inflammation and atherosclerosis (8). EPCs release a synergistic cocktail of angiogenic factors that (25) may account for their considerable therapeutic effects, but our work now demonstrates that they also release a combination of detrimental factors that could result in a negative synergy, affecting plaque growth and rupture, or promote a proinflammatory environment in the vasculature, and possibly also damaging the myocardium.
Role of pharmacotherapy in modulating EPC paracrine activity.
We examined the release of MCP-1, which appears to be a key mediator in atherosclerosis progression and plaque rupture (6), from late EPCs in the presence of simvastatin. We chose to study late EPCs, since a recent study confirmed that the late EPCs have long-term growth potential and can form actual vascular tubes, thus making them candidates for future therapies targeting vascular tissue engineering (29). We found that the release of the proatherogenic chemokine MCP-1 could be markedly reduced by coculturing the cells with simvastatin, a known anti-inflammatory agent in endothelial cells (22). This finding points to an important novel therapeutic option in cardiovascular cell therapy: The use of anti-inflammatory agents to treat cells before cell transplantation and thus improve their safety. Our study only investigated simvastatin, but future studies could evaluate other statins as well as other anti-inflammatory agents during the cell culture period, with the specific goal to reduce the proinflammatory potential of cells. Clinical studies using cell therapies could also consider modifying the therapeutic regimen in patients in the peritransplantation time period to improve the safety of cardiovascular cell transplantation.
Limitations.
All of our assessments of cell activity were performed on cultured EPCs because that is how they are currently defined and isolated (11, 29, 32). The culture process and differences in culture protocols for early and late EPCs also make it difficult to make direct inferences regarding possible native inflammatory function of the circulating cell populations that give rise to the cultured early and late EPCs. Furthermore, adult late EPCs may also contain some mature endothelial cells, and the culture process can result in maturation of EPCs to more mature endothelial cells.
Therefore, our focus was not to understand the physiology of circulating EPCs but instead what potential risk cultured EPCs may harbor when transplanted into patients. Because the analysis was performed on a small group of patients, we could not extensively analyze subgroups and the effects of risk factors on the paracrine activity. However, by identifying some of the proinflammatory factors released by cultured EPCs, we are providing the tools for future large-scale trials to monitor serum levels in patients who are recipients of EPC transplantation. Since a recent study demonstrated in an animal model that factors released by transplanted EPCs can be measured in the serum (7), future clinical trials could monitor the appropriate serum factors in patients. The EPC subtype-specific chemokines, adhesion molecules, and thromboinflammatory markers identified in this study could serve as a starting point to monitor inflammatory activation in patients following EPC transplantation. This approach will ultimately allow for greater safety of patients receiving cell therapy. While we chose specific representatives of the class of chemokines and thromboinflammatory factors for our study and based our choice on some factors that have a well-established role in atherosclerosis, it is possible and likely that EPCs also release multiple additional factors that play a major role in atherosclerosis.
In conclusion, cardiovascular cell therapy with EPCs needs to be accompanied with the necessary caution and monitoring of possible inflammatory activation in the host vasculature to prevent the development of pathological vascular inflammation. Furthermore, there is an emerging recognition that angiogenic therapy needs to be individualized for each patient (24), and our findings demonstrate that paracrine profiling of cells may also allow for a more optimal choice of EPC subtype. If the release of chemokines by EPCs is of significant concern in a selected patient group, pharmacological treatment of cells (and possibly patients) during the peritransplantation period may modulate the release of proinflammatory chemokines. Careful matching of specific therapeutic goals with well-defined and appropriate cell therapies will allow for achieving an optimal balance between therapeutic effects and the risk of side effects such as atherogenesis and plaque rupture.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant K08-HL080082 (J. Rehman).
DISCLOSURES
D. Ingram owns stock in EndGenitor Technologies, Inc. (Indianapolis, IN). He has also acted as a consultant to and member or officer of the Board of EndGenitor Technologies within the last 3 years.
REFERENCES
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 167: 989–997, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 355: 1222–1232, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol 26: 758–764, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res 95: 858–866, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon Ys. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 204: 3257–3269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L, Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation 109: 2826–2831, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 45: 1449–1457, 2005. [DOI] [PubMed] [Google Scholar]
- 10.George J, Afek A, Abashidze A, Shmilovich H, Deutsch V, Kopolovich J, Miller H, Keren G. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 25: 2636–2641, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93: 1023–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol 48: 1579–1587, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002. [DOI] [PubMed] [Google Scholar]
- 14.He T, Peterson TE, Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol 289: H968–H972, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348: 593–600, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 97: 3422–3427, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambiase PD, Edwards RJ, Anthopoulos P, Rahman S, Meng YG, Bucknall CA, Redwood SR, Pearson JD, Marber MS. Circulating humoral factors and endothelial progenitor cells in patients with differing coronary collateral support. Circulation 109: 2986–2992, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Leor J, Marber M. Endothelial progenitors: a new Tower of Babel? J Am Coll Cardiol 48: 1588–1590, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A, Danon D. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation 114: I94–I100, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 111: 3481–3488, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113: 1287–1294, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 103: 2531–2534, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Rabelink TJ, de Boer HC, de Koning EJ, van Zonneveld AJ. Endothelial progenitor cells: more than an inflammatory response? Arterioscler Thromb Vasc Biol 24: 834–838, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Rehman J An inconvenient truth: recognizing individual differences in arteriogenesis. Circ Res 102: 1146–1147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells 25: 1746–1752, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Schirmer SH, Fledderus JO, Bot PT, Moerland PD, Hoefer IE, Baan J Jr, Henriques JP, van der Schaaf RJ, Vis MM, Horrevoets AJ, Piek JJ, van Royen N. Interferon-beta signaling is enhanced in patients with insufficient coronary collateral artery development and inhibits arteriogenesis in mice. Circ Res 102: 1286–1294, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 49: 741–752, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Sieveking DP, Buckle A, Celermajer DS, Ng MKC. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol 51: 660–668, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39: 733–742, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353: 999–1007, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-α via the p38 mitogen-activated protein kinase pathway. Faseb J. In Press. [DOI] [PMC free article] [PubMed]