Abstract
Apelin is a potent inodilator with recently described antiatherogenic properties. We hypothesized that apelin might also attenuate abdominal aortic aneurysm (AAA) formation by limiting disease-related vascular wall inflammation. C57BL/6 mice implanted with osmotic pumps filled with apelin or saline were treated with pancreatic elastase to create infrarenal AAAs. Mice were euthanized for aortic PCR analysis or followed ultrasonographically and then euthanized for histological analysis. The cellular expression of inflammatory cytokines and chemokines in response to apelin was also assessed in cultured macrophages, smooth muscle cells, and fibroblasts. Apelin treatment resulted in diminished AAA formation, with a 47% reduction in maximal cross-sectional area (0.74 vs. 1.39 mm2, P < 0.03) and a 57% reduction in macrophage infiltrate (113 vs. 261.3 cells/high-power field, P < 0.0001) relative to the saline-treated group. Apelin infusion was also associated with significantly reduced aortic macrophage colony-stimulating factor expression and decreased monocyte chemattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, interleukin (IL)-6, and tumor necrosis factor (TNF)-α mean mRNA levels. Apelin stimulation of cultured macrophages significantly reduced MCP-1 and TNF-α mRNA levels relative to baseline (2.03- and 1.89-fold reduction, P < 0.03, respectively) but did not affect intimal adhesion molecule expression or medial or adventitial cell cytokine production. Apelin significantly reduces aneurysm formation in the elastase model of human AAA disease. The mechanism appears to be decreased macrophage burden, perhaps related to an apelin-mediated decrease in proinflammatory cytokine and chemokine activation.
Keywords: abdominal aortic aneurysms, vascular disease, chemokine, inflammatory cytokine
abdominal aortic aneurysms (AAA) continue to provide an intractable clinical problem. As a disease that affects nearly 10% of the elderly population and claims over 15,000 lives/yr, it is remarkable that treatment of a ruptured AAA is still associated with mortality rates in excess of 80% (8, 24, 28). Many would argue that physicians remain largely incapable of altering the natural history of this disease, despite our growing understanding of the pathophysiology of the vascular lesion. Several promising therapies, including statins, β-blockers, and antibiotics, have all failed to conclusively improve outcomes in large clinical trials, and no medicine is currently approved to treat AAA formation (3, 11).
Aneurysms are complex entities that differ physiologically from stenotic and atherosclerotic vascular lesions. Although atheromas are dominated by neointimal proliferation and foam cell generation, the AAA is defined by the progressive loss of extracellular matrix and medial degeneration (9, 30). Macrophages are recruited to the involved vessel in both conditions, but have differing roles in each case. Unlike the subendothelial lipid-laden cells of the fatty streak, macrophages of the abdominal aneurysm accumulate in the medial layer where they present antigens to other leukocytes, secrete collagenases, and elaborate proinflammatory cytokines and chemoattractants (5, 23). Ultimately, they play a role in progressive aneurysmal dilation and clinical presentation. Novel therapies that can reverse this pathological course are eagerly sought.
There is emerging evidence that the endogenous hormone apelin may have beneficial effects in vascular physiology and disease. While initially appreciated as a potential inotropic and neurohormonal heart failure therapeutic, recent work has suggested that apelin may also have an important role in endothelial and vascular smooth muscle cell health (1, 4, 14). Apelin, which is widely expressed in the vascular endothelium, induces the phosphorylation of endothelial nitric oxide synthase and potentiates nitric oxide-dependent vasodilation (15, 17). APJ, the seven-transmembrane G protein-coupled apelin receptor, is also expressed by endothelial cells but may be expressed by certain smooth muscle cells, as well (18). There are data with smooth muscle cells in vivo and in culture suggesting that the vascular smooth muscle of both resistance and conduit vessels can express APJ (13, 17, 18). The activation of APJ has been shown to modulate the growth and contraction of smooth muscle cells ex vivo (13, 18, 19).
Study of the apelin-APJ pathway has also been extended to vascular pathophysiological states. In early studies, apelin was shown to have salutary effects in various vascular disease models, e.g., reversing endothelial dysfunction in diabetic (db/db) mice (31). Studies from this laboratory employing an apolipoprotein E (apoE) null model have shown that apelin also antagonizes the actions of ANG II in vivo, with apelin infusion blocking both the ANG II-mediated increase in atherosclerotic disease burden and adverse vascular remodeling and aneurysm formation in the hyperlipidemic state (7). This ANG II blocking effect appeared to be independent of blood pressure and related to direct cross talk between the two signaling pathways. Breeding of apelin and APJ knockouts on the apoE null model by different groups has led to opposing results, however, most likely because of differences in dietary exposure and genetic background of the mice evaluated, leading to some confusion over apelin's true vascular disease-related effects (7, 12).
Studies reported here were therefore conducted to evaluate the actions of apelin in a AAA model relevant to human disease, with the aim of clarifying its in vivo pathophysiological properties. Despite the prevailing belief that macrophages do not express the APJ receptor, we administered apelin in the elastase-induced AAA and vascular inflammation model (6, 12). Apelin significantly inhibited the development of aneurysmal disease and dramatically reduced the macrophage burden at the site of injury. There was also a significant apelin-mediated decrease in the important differentiation factor macrophage colony-stimulating factor (M-CSF) in the vessel wall in vivo and evidence for a direct anti-inflammatory effect on macrophages in culture.
MATERIALS AND METHODS
Surgical procedures.
All protocols were reviewed and approved by the Administrative Panel on Laboratory Animal Care at Stanford University (http://labanimals.stanford.edu/). Osmotic minipumps were implanted subcutaneously according to the manufacturer's protocol (Osmotic pump model 1002; Alzet, Cupertino, CA) to deliver a dose of 2 mg·kg−1·day−1 pyroglutamated apelin-13 peptide ([pGlu]-apelin-13), which has been shown to have physiologic effects but not to lower blood pressure in normal mice (American Peptides, Sunnyvale, CA), or sterile saline (1, 7). Employing the most widely used animal model of AAA disease (28), infrarenal AAAs were created the day following pump implant via elastase infusion, as previously described (26). Briefly, C57BL/6 male mice, n = 7–8/group, 12–14 wk of age, weighing 25–30 g, were anesthetized with inhaled isoflurane for the surgical procedure. The abdominal aortas were then isolated from the level of the left renal vein to the aortic bifurcation. After temporary ligature aortic control, a 30-gauge needle was advanced in the aortic bifurcation and withdrawn. Heat-tapered PE-10 tubing was advanced through the aortotomy and used to infuse 0.05 ml saline solution containing 15 U/ml type 1 porcine pancreatic elastase (E-1250; Sigma) for 5 min. After infusion, the PE-10 tubing was withdrawn, the aortotomy was closed with 10–0 suture, and aortic flow was restored. Mice were recovered from surgery in separate cages with free access to food and water.
Aortic monitoring via ultra-high frequency ultrasound.
Transabdominal 40-MHz B-mode ultrasound (US) imaging was used to measure aortic diameter in vivo. Images were obtained on the Vevo 770 Imaging system using an RMV 704 microvisualization scan head (Visualsonics, Ontario, CA). Imaging was performed preaneurysm creation and at 7 days following elastase perfusion. Baseline suprarenal aortic diameter ranged from 0.5 to 0.65 mm. Imaging was performed under inhaled isoflurane anesthesia on a constant-temperature table using warm acoustic coupling gel. Aortic diameters were measured in the longitudinal scan plane. Pulse-wave Doppler images were acquired to confirm aortic velocity waveforms and assist with image interpretation. Validation of US-determined aortic measurements using similar imaging protocols has been described previously (2, 20).
Pathology and immunohistochemistry.
Immediately after the mice were killed, the aortas underwent pressure perfusion fixation at 100 mmHg with 4% paraformaldehyde and were embedded in paraffin. Aortic tissues were processed and embedded in paraffin blocks and sectioned at 4 μm for hematoxylin and eosin, Verhoeff elastic-Masson's trichrome, and immunohistochemical staining. For immunohistochemical staining, endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol, and nonspecific binding sites were blocked with 10% normal serum (Dako, Carpinteria, CA). Sections were incubated for 1 h at room temperature in a primary rat anti-mouse macrophage galactose-specific lectin-2 monoclonal antibody (0.5 μg/ml; Cedarlane, Burlington, NC), goat anti-mouse VE-cadherin antibody (0.5 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), rat anti-mouse vascular cell adhesion molecule (VCAM)-1 antibody (4 μg/ml; BD Pharmingen, San Jose, CA), chicken anti-mouse E-selectin antibody (10 μg/ml; R&D Systems, Minneapolis, MN), and goat anti-mouse intercellular adhesion molecule (ICAM)-1 antibody (2 μg/ml; R&D Systems). After being stained with the primary antibody, samples were incubated with the appropriate biotinylated secondary antibodies (each at 1:200 dilution; Dako, Biocare Medical, Concord, CA) for 30 min at room temperature. Color development was performed with the diaminobenzidine color development system (Dako). All slides were evaluated in a blinded fashion. Each section was divided into four quadrants for cell counting under a ×20 objective, and two slides were studied per aorta, for a total of eight measurements per animal.
Cell culture.
Murine J774A.1 and RAW 264.7 macrophages, murine WEHI 78/24 monocytes, murine NIH 3T3 fibroblasts, and rat A7r5 aortic smooth muscle cells were cultured in DMEM with 10% FBS, 10 U/ml penicillin, and 100 μg/ml streptomycin. Activated macrophages were harvested from the peritoneum of male C57BL/6 mice 3 days after injection with 3 ml thioglycolate, as previously described (16). With the exception of the thioglycollate-stimulated macrophages, which were studied within 12 h of harvesting and plating, all cell types were serum starved for 12–18 h and then exposed to 10 nM [pGlu]-apelin-13 for 3–6 h for macrophage studies and 3–18 h for smooth muscle experiments. Immediately after stimulation, the cells were washed with sterile PBS and collected for expression analysis as described below.
Real-time PCR.
The snap-frozen aortic samples and fresh cell tissue samples were homogenized in RLT lysis buffer (Qiagen, Valencia, CA) followed by RNA isolation using RNeasy Micro and Mini Kits (Qiagen) as per the manufacturer's protocol. Purified RNA was reverse transcribed with SuperScript II (Invitrogen). RT-PCR was performed on a 7900 HT Sequence Detection System with TaqMan on Demand Gene Expression Probes [APJ Mm00442191_s1; monocyte chemoattractant protein (MCP)-1 Mm00441242_m1; IL-6 mm00446190_m1; tumor necrosis factor (TNF)-α mm00443259_g1; M-CSF Mm00432688_m1; macrophage inflammatory protein (MIP)-1α Mm00441258_m1; VCAM-1 Mm00449197_m1; matrix metalloproteinase (MMP) 2 Mm00439506_m1; MMP9 Mm00442991, systems and probes from Applied Biosystems, Foster City, CA]. Values were normalized to the relative amounts of 18S rRNA for each sample.
Cytokine enzyme-linked immunosobent assay measurement.
The concentrations of IL-6 and MCP-1 protein in cell culture supernatants and in the serum of the experimental animals were measured using mouse IL-6 and CCL2/MCP-1 enzyme-linked immunosobent assay (ELISA) kits (kits M6000B and MJE00, respectively; R&D Systems) according to the manufacturer's protocol.
Statistical analysis.
Data are presented as means ± SE. Data were subjected to the Kolmogorov-Smirnov test to determine distribution. Groups were compared using the two-tailed Students t-test for parametric data. Paired testing was performed to compare the aortic gene expression patterns between the suprarenal uninvolved portion and the infrarenal elastase-treated aneurysmal portion of the aorta of each animal. All other comparisons were made using unpaired t-tests between experimental groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Aneurysm formation.
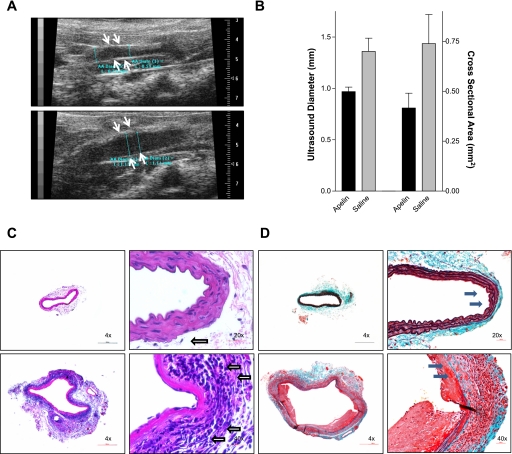
Seven days post-elastase infusion, a time point at which aortic dilation in known to have occurred but well before vessel rupture, the severity of the resulting aneurysms was assessed (n = 7 in each group) (27, 28). Mice treated with apelin had significantly smaller aortic diameters compared with mice treated with saline as measured by US, with maximal infrarenal aortic diameters of 0.97 and 1.36 mm, respectively (P < 0.01, Fig. 1, A and B). After these in vivo studies were completed, the animals were killed, and the aortas were embedded in paraffin for histological evaluation. Ex vivo analysis confirmed the observed reduction in aneurysm size, since apelin-treated animals had dramatically reduced (47% smaller) maximal cross-sectional area compared with saline-treated mice (0.74 vs. 1.39 mm2, respectively, P < 0.03) (Fig. 1B).
Fig. 1.
Apelin inhibits abdominal aortic aneurysm (AAA) formation. A: aortic ultrasound 7 days post-elastase infusion revealed reduced in vivo maximal aneurysm diameter with apelin infusion (top) compared with saline infusion (bottom). B: apelin reduced the ultrasonographic aortic diameter by 29% (P < 0.01) and cross-sectional aortic area by 47% (P < 0.03) relative to saline. Representative hematoxylin and eosin (C)- and Verhoeff elastic-Masson's trichrome (D)-stained aortic samples reveal that apelin treatment (top) prevented aneurysmal dilation and inflammatory cell infiltration 1 wk after elastase infusion relative to saline treatment (bottom). Note the reduced leukocyte burden (open arrows) and preserved elastic and collagenous support structure (black arrows) in the apelin group.
Histology and immunohistochemistry.
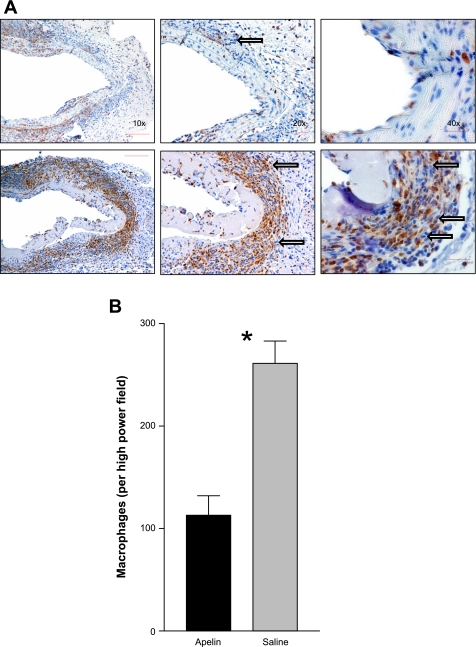
In addition to the histomorphometric differences in cross-sectional area already described, Verhoeff elastic-Masson's trichrome staining revealed that apelin treatment also resulted in dramatic preservation of the scaffolding and architectural support structures of the medial layer of the vessels. Unlike the saline-treated samples, which had clear disruption of their elastic and collagenous fibers (Fig. 1, C and D), the apelin-infused aortas had intact undigested connective tissue throughout. Quantitative immunohistochemistry analysis revealed a dramatic 57% reduction in macrophage number, with 113.0 ± 19.0 cells/field visualized in the apelin-treated aortas vs. 261.3 ± 21.8 cells/field in the saline-treated vessels (P < 0.0001, Fig. 2). However, the reduced inflammatory cell infiltrate appeared not to be associated with changes in endothelial expression of VCAM-1, ICAM-1, E-selectin, or VE-cadherin, since no qualitative difference in immunohistochemical staining was observed for these adhesion molecules (data not shown).
Fig. 2.
Apelin limits macrophage burden in diseased vessel wall. A: macrophage galactose-specific lectin-2 staining revealed that apelin-treated mice (top) had decreased macrophage burden relative to saline-treated mice (bottom). Note the dramatically reduced number of medial macrophages (arrows) in the apelin group. B: macrophage no. (macrophages/high-power field) showed a 57% reduction in the apelin-treated vessels (*P < 0.0001).
Aortic inflammatory cytokine expression.
Because maximal aortic inflammation is known to occur 3 days after elastase infusion, a second cohort of animals was killed at this time point to observe apelin's effects at the height of vascular injury (n = 7–8 animals/group) (21, 28). The gene expression profile of the homogenized aneurysmal portion of the infrarenal aortas was compared with suprarenal aorta (nonaneurysmal and nonelastase treated) of each experimental animal. Using paired t-testing, the relative amount by which the cytokines were upregulated in the AAA was thus determined, normalized to the expression in that animal's uninvolved aorta. Additionally, a set of littermate control animals was studied to establish the baseline aortic expression signature for each gene studied, since elastase is known to dramatically increase the expression of several of these mediators in this animal model (28).
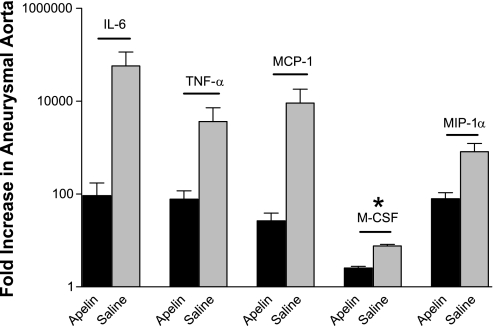
With the use of the uninvolved nonaneurysmal suprarenal aorta of each animal as an internal control, exogenous apelin administration was associated with a much smaller increase in the aneurysmal expression of several pathological genes post-elastase infusion than was observed in the saline group. For example, a protective effect was seen for pathological macrophage-specific chemokines, with apelin significantly reducing the increase in M-CSF expression (2.56 ± 0.23 vs. 7.6 ± 0.66, P < 0.01), and also a trend towards less MIP-1α (79.1 ± 27.9 vs. 818.7 ± 412, P = 0.09) and MCP-1 (71.1 ± 27.9 vs. 17,290 ± 17,210, P = 0.26) compared with saline (Fig. 3). Also, the elastase-related increase in cytokines IL-6 and TNF-α was much lower in apelin-treated mice than in saline-treated animals (92.9 ± 81.3 vs. 57,440 ± 57,240; P = 0.29; and 77.5 ± 39.9 vs. 3,644± 3,558; P = 0.26, respectively). These dramatic differences in mean expression change did not reach statistical significance because of large interanimal expression variation, despite their being normally distributed. Apelin had no effect on VCAM-1 expression (6.13 ± 2.13 vs. 7.12 ± 1.39, P = 0.76), confirming the qualitative histological data. Interestingly, elastase infusion was associated with downregulated vascular APJ expression in the saline group, but concomitant apelin infusion reversed this trend and was associated with a nonsignificant increase in aneurysmal receptor expression (−1.49 ± 0.32 vs. 2.35 ± 0.78, respectively, P = 0.20).
Fig. 3.
Apelin reduces aortic cytokine and chemokine expression. Relative to saline, apelin significantly limited the mean increase in aneurysmal expression of macrophage colony-stimulating factor (M-CSF) 3 days postinfusion (*P < 0.01). Mean interleukin (IL)-6, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, M-CSF, and macrophage inflammatory protein (MIP)-1α expression levels were also decreased by apelin, although these differences did not reach statistical significance.
Cell-based experiments.
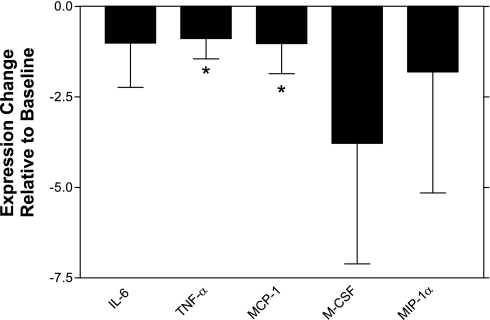
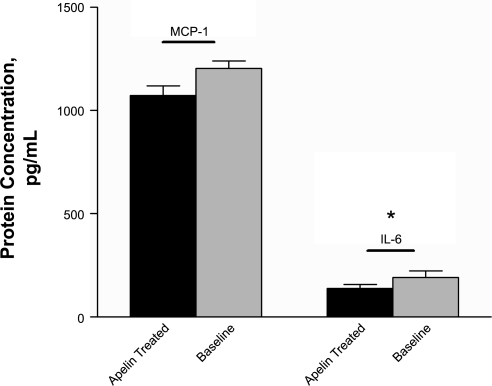
Because endothelial adhesion molecule expression did not appear to be modulated in the presence of apelin, we further investigated the mechanism by which medial macrophage number was decreased in those animals with infusion of apelin. PCR-based expression studies with cultured aortic vascular smooth muscle cells and representative mouse fibroblast cells (both of which were confirmed to express APJ by RT-PCR) failed to provide evidence for modulation of cytokines or chemokines in these cell types when treated with apelin, arguing that apelin's anti-inflammatory effects were not occurring because of a direct modulation of resident cells in the tunica media or adventitia (P = not significant for all cytokines and chemokines evaluated). Thus we investigated the effect of apelin on macrophages themselves. We studied three different types of murine macrophages and one murine monocytic line to thoroughly investigate apelin's effects on this cell type. Interestingly, all four lines possessed the APJ receptor. Although RAW 264.7 cells expressed a relatively small amount of APJ [cycle threshhold (Ct) ∼28–31], the J774A.1 cells and the activated thioglycollate-induced intraperitoneal macrophages and cultured monocytes expressed much higher amounts of the receptor (Ct ∼24–27, with expression confirmed by Western Blot- data not shown). Unlike the smooth muscle cells, which did not show decreased inflammatory gene expression with apelin treatment, the macrophage cells did in fact produce less proinflammatory cytokines when cultured in the presence of 10 nM [pGlu]-apelin-13 than they expressed after serum starvation alone. Compared with baseline, the J774 macrophages expressed less MCP-1 and TNF-α (2.03- and 1.89-fold reduction, P < 0.03 and <0.01, respectively) and produced lower mean IL-6, MIP-1α, and M-CSF when treated with apelin (2.01-, 2.81-, and 4.78-fold reduction, respectively), although these latter differences did not reach statistical significance (Fig. 4). Although apelin did not affect the levels of the representative cytokines IL-6 or MCP-1 in the serum of the experimental mice by ELISA (P = 0.44 and 0.49, respectively), it did reduce the elaboration of these proteins in the media of cultured J774 macrophages (Fig. 5), additionally supporting the possibility that apelin acts locally on the cells at the site of injury independent of its systemic properties (IL-6: 139.4 vs. 192.1 pg/ml, P = 0.04; MCP-1: 1,073 vs. 1,205 pg/ml, P = 0.07). Apelin did not modulate cellular MMP-2 or MMP-9 gene expression, however, arguing that it was not directly reducing the production of these digestive enzymes (P = 0.59 and 0.70, respectively). Thus circulating monocytes and activated tissue macrophages are capable of responding to apelin, which has a significant anti-inflammatory effect on this cell lineage.
Fig. 4.
Apelin reduces the expression of inflammatory cytokines and chemokines by macrophages in culture. J774 macrophages cultured in the presence of 10 nM [pGlu]-apelin-13 had significant downregulation of MCP-1 and TNF-α expression (*P < 0.05). IL-6, M-CSF, and MIP-1α expression levels were also decreased by apelin, although these differences did not reach statistical significance.
Fig. 5.
Apelin reduces the elaboration of inflammatory proteins in culture. The concentrations of IL-6 and MCP-1 protein were reduced in the supernatant of cultured J774 macrophages when treated with apelin compared with baseline (*P < 0.05). No such difference was found in the serum of the elastase-treated animals, arguing that apelin's effects likely occur locally at the site of injury and are not the result of systemic actions.
DISCUSSION
Aneurysmal expansion precedes aortic rupture and AAA-related mortality (22). Despite recent advances in surgical and endovascular approaches, no therapies exist that can effectively retard the progressive inflammation and elastolysis that typifies this devastating disease. Here we report that apelin significantly inhibits the development of AAA disease and diminishes the production of several deleterious inflammatory mediators known to potentiate aneurysm expansion. Interestingly, apelin also dramatically reduced macrophage burden in the arterial wall, and thus had a direct anti-inflammatory effect in this model. In vitro mechanistic studies indicated that apelin inhibits production of chemokines by macrophage cell lines, without an observed effect on representative cells from the tunica intima, media, or adventitia. Taken together, these findings suggest that apelin may have potent anti-aneurysmal activity and represent a novel vascular therapeutic.
The proteolysis of collagen and elastin within the arterial wall is a hallmark of advanced AAA. Furthermore, the activity of digestive enzymes such as elastase and the proinflammatory cytokines that promote their production have been shown to correlate with aneurysm diameter in humans (10). By protecting the scaffolding of the aorta in the face of an exogenous elastase infusion while simultaneously reducing the expression of IL-6 and TNF-α in the vessel wall, apelin appears to be directly involved in two of the most important pathophysiological processes of aneurysm generation. These anti-inflammatory and anti-aneurysmal properties complement our recent discovery that apelin also inhibits atherosclerogenesis and antagonizes ANG II signaling independent of a blood pressure effect, greatly extending the significance of APJ signaling in the biology of the vessel wall (7). Whereas that previous study also showed a reduction in AAA formation, the positive findings presented here in the elastase infusion model, which is considered a more representative model of human AAA disease, confirm and extend apelin's therapeutic potential in vascular disease (28).
An unexpected finding of this study was the inhibitory action of apelin on macrophage accumulation in the vessel wall. Prior studies have reported that macrophages do not possess the APJ receptor and are incapable of responding to apelin (6, 13). We therefore began by examining other vascular constituents to elucidate the mechanism by which apelin downregulated inflammatory cell recruitment. Because apelin is known to reverse endothelial dysfunction, its effect on the intima of the aneurysm was investigated first. Interestingly, apelin did not modulate the expression of VCAM-1, ICAM-1, VE-cadherin, or E-selectin on the vessel wall, arguing that the observed reduction in macrophage burden may be because of a modification of local chemotactic signals rather than a diminution of adhesion molecule expression. This hypothesis was subsequently confirmed when demonstrably less chemokines were found in the aortas of apelin-treated animals. Compared with saline-treated controls, apelin-infused aneurysms were associated with significantly decreased expression of the macrophage differentiation factor M-CSF and decreased expression of the macrophage trafficking chemokines MIP-1α and MCP-1, although the latter did not reach statistical significance in these experiments. The medial smooth muscle cell and adventitial fibroblast were considered as other possible sites of apelin action, since these cell types are known to express APJ in vivo and to have prominent roles in AAA-related leukocyte recruitment and aneurysm progression (23, 29). It was surprising to find, however, that apelin had no effect on chemokine production by either aortic smooth muscle cells or fibroblasts in culture, reinforcing the notion that apelin was likely acting through the infiltrating macrophages themselves rather than resident cells of the unperturbed aorta.
Despite prior studies showing that neural macrophages do not express the apelin receptor, our quantitative RT-PCR data confirmed that both murine monocytes and several lines of mouse macrophages express APJ. It is notable that thioglycollate-stimulated macrophages, which represent phagocytic cells in an activated state, expressed relatively high levels of APJ, suggesting that macrophages are responsive to apelin during periods of activation (23). Most importantly, apelin was found to have a potent direct anti-inflammatory effect on these macrophages, inducing a significant downregulation of TNF-α and MCP-1 expression and a trend toward less IL-6, MIP-1α, and M-CSF in cultured cells. Apelin's effect on the macrophage is specific, since these harmful mediators were reduced without a modulation of cellular migration or phagocytosis (data not shown). Thus apelin's capacity to reduce AAA formation may largely be explained by its anti-inflammatory effect on macrophages, and not effects on the endothelium or smooth muscle cell, as might be expected based on our prior understanding of apelin-APJ biology. When exposed to apelin, macrophages produce less of the deleterious cytokines that are known to promote the activation of extracellular matrix-digesting enzymes (25). Also, apelin downregulates the expression of several critical chemokines, thereby limiting the subsequent recruitment of circulating monocytes to the site of injury. And while the smaller absolute number of cells present in the aneurysmal wall is certainly important, it is also possible that the loss of autocrine and paracrine circuits on the existing macrophages is even more critical. By blocking the production of MCP-1, apelin would be expected to limit the elaboration of MMPs by cells already present in the media, thereby protecting the architectural support structure of the diseased aorta (30).
The potential utility for apelin as a therapeutic for decompensated heart failure is increasingly recognized. The current findings, however, suggest that apelin may represent a viable treatment option for the patient with vascular disease as well. By blocking macrophage burden, as well as inflammatory chemokine and cytokine production in the aneurysmal aorta, apelin may have a beneficial therapeutic effect combating AAA development and vascular disease.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-077676 (T. Quertermous), HL-064338-07 (R. L. Dalman), KO8 HL-083914-01 (E. A. Ashley), PO1 1P50HL-083800 (P. S. Tsao), and F32 HL-090203-01 (N. J. Leeper) and by American Heart Association Western Affiliates Postdoctoral Fellowships 0625171Y (M. M. Tedesco) and 0725196Y (N. J. Leeper).
REFERENCES
- 1.Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R, Greve J, Robbins R, Patterson AJ, Bernstein D, Quertermous T. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 65: 73–82, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg 44: 372–376, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation 117: 1883–1889, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, Woo YJ. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 110: II187–II193, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cathcart MK Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24: 23–28, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Choe W, Albright A, Sulcove J, Jaffer S, Hesselgesser J, Lavi E, Crino P, Kolson DL. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J Neurovirol 6, Suppl 1: S61–S69, 2000. [PubMed] [Google Scholar]
- 7.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JA, Tsao P, Lenardo MJ, Ashley EA, Quertermous T. Apelin signaling antagonizes angiotensin II effects in experimental atherosclerosis. J Clin Invest 118: 3343–3354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creager MA, Halperin JL, Whittemore AD. Aneurysmal disease of the aorta and its branches. In: Vascular Medicine, edited by Loscalzo J, Creager MA, and Dzau VJ. New York: Little Brown, 1996, p. 901.
- 9.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 24: 429–434, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Diehm N, Dick F, Schaffner T, Schmidli J, Kalka C, Di Santo S, Voelzmann J, Baumgartner I. Novel insight into the pathobiology of abdominal aortic aneurysm and potential future treatment concepts. Prog Cardiovasc Dis 50: 209–217, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Golledge J, Powell JT. Medical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 34: 267–273, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Kihara M, Imai N, Yoshida S, Shimoyamada H, Yasuzaki H, Ishida J, Toya Y, Kiuchi Y, Hirawa N, Tamura K, Yazawa T, Kitamura H, Fukamizu A, Umemura S. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol 171: 1705–1712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, Kihara M, Ishida J, Imai T, Yoshida S, Toya Y, Fukamizu A, Kitamura H, Umemura S. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 26: 1267–1272, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami KI, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to AT1, in blood pressure in vivo. J Biol Chem 279: 26274–26279, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, Yu F, Duan XH, Tang CS, Qi YF. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides 28: 2023–2029, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 109: 2213–2220, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 118: 119–125, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept 126: 233–240, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Li L, Qin X, Pan W, Feng F, Chen F, Zhu B, Liao D, Tanowitz H, Albanese C, Chen L. Apelin-induced vascular smooth muscle cell proliferation: the regulation of cyclin D1. Front Biosci 13: 3786–3792, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Martin-McNulty B, Vincelette J, Vergona R, Sullivan ME, Wang YX. Noninvasive measurement of abdominal aortic aneurysms in intact mice by a high-frequency ultrasound imaging system. Ultrasound Med Biol 31: 745–749, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Nakahashi TK, Hoshina K, Tsao PS, Sho E, Sho M, Karwowski JK, Yeh C, Yang RB, Topper JN, Dalman RL. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol 22: 2017–2022, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Nevitt MP, Ballard DJ, Hallett JW Jr. Prognosis of abdominal aortic aneurysms. A population-based study. N Engl J Med 321: 1009–1014, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Shah PK Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation 96: 2115–2117, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26: 987–994, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL. Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol 76: 108–116, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol 24: 1916–1921, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Sinha I, Pearce CG, Cho BS, Hannawa KK, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR Jr. Differential regulation of the superoxide dismutase family in experimental aortic aneurysms and rat aortic explants. J Surg Res 138: 156–162, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann NY Acad Sci 1085: 59–73, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res 102: 1368–1377, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Webb NR, Moore KJ. Macrophage-derived foam cells in atherosclerosis: lessons from murine models and implications for therapy. Curr Drug Targets 8: 1249–1263, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Zhong JC, Huang Y, Yung LM, Lau CW, Leung FP, Wong WT, Lin SG, Yu XY. The novel peptide apelin regulates intrarenal artery tone in diabetic mice. Regul Pept 144: 109–114, 2007. [DOI] [PubMed] [Google Scholar]