Abstract
ANG II type 1 receptors (AT1R) mediate most of the central effects of ANG II on cardiovascular function, fluid homeostasis, and sympathetic drive. The mechanisms regulating AT1R expression in the brain are unknown. In some tissues, the AT1R can be upregulated by prolonged exposure to ANG II. We examined the hypothesis that ANG II upregulates the AT1R in the brain by stimulating the intracellular mitogen-activated protein kinase (MAPK) signaling pathway. Using molecular and immunochemical approaches, we examined expression of the AT1R and phosphorylated MAPK in the paraventricular nucleus of the hypothalamus (PVN) and the subfornical organ (SFO) of rats receiving a chronic (4-wk) subcutaneous infusion of ANG II (0.6 μg/h) or saline (vehicle control), with or without concomitant (4-wk) intracerebroventricular (ICV) infusions of MAPK inhibitors or the AT1R blocker losartan. Subcutaneous infusion of ANG II markedly increased phosphorylation of MAPK and expression of AT1R mRNA and protein and AT1R-like immunoreactivity in the PVN and SFO. ANG II-induced AT1R expression was blocked by ICV infusion of the p44/42 MAPK inhibitor PD-98059 (0.025 μg/h) and the JNK inhibitor SP-600125 (0.125 μg/h), but not by the p38 MAPK inhibitor SB-203580 (0.125 μg/h). Upregulation of the AT1R in the PVN and SFO by peripheral ANG II was abolished by ICV losartan (10 μg/h). The data indicate that blood-borne ANG II upregulates brain AT1R by activating intracellular p44/42 MAPK and JNK signaling pathways.
Keywords: paraventricular nucleus, subfornical organ, angiotensin II type 1 receptor, autonomic regulation
high densities of the ANG II type 1 receptor (AT1R) are found in brain regions critical for the regulation of blood pressure, sodium-water balance, neurohumoral release, and sympathetic drive (33), including the organum vasculosum of the laminae terminalis, median preoptic nucleus, subfornical organ (SFO), paraventricular nucleus of the hypothalamus (PVN), supraoptic nuclei (SON), lateral parabrachial nucleus, rostral ventrolateral medulla, and nucleus of the solitary tract (26). The level of AT1R expression in these regions may decisively influence the activity and responsiveness of the brain renin-angiotensin system (RAS).
Many in vivo and in vitro studies have shown that AT1R gene expression, similar to expression of several other G protein-coupled receptors (18), is affected by its own agonist. Interventions that increase plasma ANG II level, such as water deprivation (6, 42), sodium depletion (12), and treatment with furosemide (11), cause significant increases in AT1R gene expression in cardiovascular regions of the brain. AT1R expression in specific brain regions is also increased in pathophysiological states characterized by activation of the systemic RAS, such as hypertension (41) and heart failure (46, 48). However, the molecular mechanisms regulating AT1R expression in brain tissues have not been elucidated.
In a recent study (48), mitogen-activated protein kinase (MAPK) signaling pathways were implicated in upregulation of the AT1R in the hypothalamus of rats with heart failure. However, the neurochemical milieu in heart failure is rich in factors that affect MAPK signaling. The present study utilizes a simpler model to test the hypothesis that ANG II-induced upregulation of AT1R expression in the brain is dependent on MAPK signaling. Normal rats received a chronic systemic infusion of ANG II, with or without concomitant central administration of MAPK inhibitors. AT1R expression was examined in two representative forebrain regions: the SFO, which is directly exposed to circulating ANG II via a deficient blood-brain barrier, and the PVN, which lies within the blood-brain barrier. In both regions, the AT1R level increases in response to experimental manipulations that increase circulating ANG II (6, 11, 12, 42).
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (275–300 g body wt; Harlan Sprague Dawley, Indianapolis, IN) were housed in temperature-controlled (23 ± 2°C) rooms in the University of Iowa Animal Care Facility, where they were exposed to a normal 12:12-h light-dark cycle, and rat chow was provided ad libitum. These studies were performed in accordance with the American Physiological Society's guidelines for research involving animals and human beings (3). The experimental procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Surgical preparation.
Surgery was performed under sterile conditions. Rats were anesthetized with ketamine (87.5 mg/kg ip) + xylazine (12.5 mg/kg ip) and fixed in a stereotaxic instrument. A 26.5-gauge 15-mm-long stainless steel cannula was positioned in the brain, with the tip placed in the left lateral cerebral ventricle (−1.0 mm anteroposterior, −4.5 mm dorsoventral, and −1.5 mm mediolateral, with bregma as a reference) (38). Polymerizing dental orthodontic resin (Dentsply International, Milford, DE) was applied to the surface of the skull, and three protective screws were placed around the cannula. The cannula was bent at a 90° angle and fixed again with dental orthodontic resin. An osmotic minipump (model 2004, Alzet; 0.25 μl/h) containing dissolved drugs was positioned subcutaneously at the back of the neck and connected with sterilized tubing to the free end of the cannula. A second osmotic minipump (model 2002, Alzet; 0.5 μl/h) was subcutaneously implanted at the back of the neck for infusion of ANG II or vehicle (VEH, saline). The skin incisions were sutured. Proper location of the intracerebroventricular (ICV) cannula was verified at the end of the experiments by injection of pontamine sky blue (2 μl). Data from animals with incorrectly placed cannulas were excluded from the analyses.
Drugs infused.
ANG II was obtained from Sigma (St. Louis, MO). The AT1R antagonist losartan was a gift from DuPont. ANG II was dissolved in saline and losartan in artificial cerebrospinal fluid (aCSF). The selective p44/42 MAPK inhibitor PD-98059, the JNK inhibitor SP-600125, and the p38 MAPK inhibitor SB-203580 were obtained from Tocris (Ellisville, MO). These inhibitors were dissolved in DMSO and then diluted in aCSF to make a 0.1–0.5% final DMSO concentration. The vehicle control (VEH) for ICV infusions was aCSF containing 0.5% DMSO.
To avoid confounding secondary effects, we infused ANG II subcutaneously at a dose that does not increase arterial pressure or circulating aldosterone in rats fed a normal-salt diet (19). The MAPK inhibitors were infused ICV at doses we showed previously to inhibit AT1R expression in the brain in rats with heart failure (48); in our previous study, the specificity of the MAPK inhibitors was confirmed by demonstration of their ability to reduce phosphorylated expression of their own MAPK in the brain by immunohistochemistry (48). Their specificity has previously been demonstrated by others in a variety of experimental protocols (21, 30, 50).
Experimental protocols.
ANG II (0.6 μg/h) or saline (0.5 μl/h) was infused subcutaneously (SC) over a 4-wk interval concomitantly with an ICV infusion (infusion rate 0.25 μl/h) of the AT1R antagonist losartan (10 μg/μl) or one of the following MAPK inhibitors: PD-98059 (0.1 μg/μl), SP-600125 (0.5 μg/μl), or SB-203580 (0.5 μg/μl). The six experimental groups were as follows: 1) saline + ICV VEH (n = 24), 2) SC ANG II + ICV VEH (n = 24), 3) SC ANG II + ICV losartan (n = 24), 4) SC ANG II + ICV PD-98059 (n = 24), 5) SC ANG II + ICV SP-600125 (n = 24), and 6) SC ANG II + ICV SB-203580 (n = 24). Animals from each group were assigned to one of three study protocols: RT-PCR (n = 6), Western blot (n = 12), and immunocytochemistry (n = 6).
At the conclusion of the treatment protocols, some (n = 8) saline-infused control rats and some (n = 8) ANG II-infused rats were anesthetized with ketamine (90 mg/kg ip) + xylazine (10 mg/kg ip) for implantation of a catheter in the left femoral artery. Blood pressure and heart rate were recorded from these rats in the conscious state on the following day.
Tissue preparation.
The rats were euthanized with urethane, and brain tissue was collected for immunohistochemical or molecular studies. For Western blot and real-time PCR, the brains were immediately removed, frozen in liquid nitrogen, and stored at −80°C for subsequent use. The frozen brain was cut into 300-μm coronal sections, and the PVN and SFO were punched using a 15-gauge needle (1.5 mm ID) centered over the PVN and SFO. PVN tissues were collected from both sides in two sections from each rat. SFO tissues were collected from two or three sections from each rat, depending on whether the third section contained SFO. Some immediately surrounding tissue was usually included. The punched tissues were homogenized in cell lysis buffer (Cell Signaling Technology, Beverly, MA) to extract protein for Western assay or in TriReagent (Molecular Research Center, Cincinnati, OH) to extract RNA for real-time PCR. To obtain a sufficient amount of tissue to detect AT1R protein by Western blot in each region, we combined the samples from two different rats. To collect tissues for immunostaining, we transcardially perfused the rats with 4% paraformaldehyde. Brains were then embedded with OCT compound and rapidly frozen in alcohol-chilled dry ice. Coronal forebrain sections (12 μm) of target tissues were made using a cryostat and then stored at −80°C.
Western blot.
AT1R protein level in the PVN and SFO was assessed by Western blotting. Briefly, protein samples (30 μg) were separated by 10% SDS-polyacrylamide gel and then transferred to a PVDF membrane. Nonspecific binding was blocked by incubation with 5% nonfat dry milk for 1 h at room temperature. Membranes were then incubated overnight at 4°C with rabbit anti-rat AT1R polyclonal antibody (1:500 dilution; catalog no. sc-1173, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-rat β-actin monoclonal antibody (1:2,000 dilution; catalog no. 4970, Cell Signaling Technology), respectively, and then with goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; catalog no. sc-2004, Santa Cruz Biotechnology) for 1 h at room temperature. Immunoblots were visualized with an enhanced chemiluminescence reagent. Band intensities were quantified with NIH Image J software. AT1R protein was normalized by the total content of β-actin.
Real-time PCR.
AT1R mRNA levels in the PVN and SFO were measured with real-time PCR following reverse transcription of total RNA, as described previously (13, 51). The sequences for primers and probe were as follows: 5′-GTA-GCC-AAA-GTC-ACC-TGC-ATC A-3′ (sense) and 5′-GGT-AGA-TGA-CGG-CTG-GCA-AA-3′ (antisense) for primer and 5′-CAT-CTG-GCT-AAT-GGC-TGG-CTT-GGC-3′ for probe. TaqMan primer and probe for rat GAPDH were purchased from Applied Biosystems (Foster City, CA). Real-time PCR was performed using the Prism 7700 Sequence Detection System (Applied Biosystems). Rat GAPDH mRNA was quantified as an internal control for each sample, and the final results of real-time PCR were expressed as the ratio of the mRNA of interest to GAPDH mRNA.
Immunohistochemistry.
Immunohistochemical visualization of phosphorylated MAPK expression in the PVN and SFO was performed on frozen sections using antibodies and avidin-biotin-peroxidase methods, as previously described (32). The primary antibodies for detection of immunoreactivity of phosphorylated MAPK were rabbit monoclonal antibody (Cell Signaling) to phosphorylated p44/42 MAPK (1:250 dilution; catalog no. 4376), phosphorylated p38 MAPK (1:250 dilution; catalog no. 4631), and phosphorylated SAPK/JNK (1:250 dilution; catalog no. 9251). For immunohistochemical analysis, the numbers of phosphorylated MAPK-positive cells in a 100 × 100 μm window positioned over the dorsal parvocellular (PVN-dp), the ventrolateral parvocellular (PVN-vlp), or the posterior magnocellular (PVN-pm) subdivision of the PVN (44) and a 200 × 200 μm window positioned over the central SFO were counted manually. The subdivisions of PVN were defined as described in previous studies (43–45). In each rat, the number of positive cells was counted in three sections from each subdivision of the PVN and three sections from the SFO and averaged to obtain a single value for statistical analysis. Data were represented as positive cells per 104 μm2.
Immunofluorescence.
Immunofluorescent staining was used to localize AT1R expression. The primary antibody for detection of AT1R-like immunoreactivity was a rabbit polyclonal antibody to the AT1R (1:100 dilution; catalog no. sc-1173, Santa Cruz Biotechnology). The sections were then incubated with a secondary antibody, Alexa Fluor 488 goat anti-rabbit IgG (1:200 dilution; catalog no. A-11070, Invitrogen), and further incubated with To-Pro-3 (1:2,000 dilution; Invitrogen) to counterstain cell nuclei. Immunostaining was visualized with a confocal laser-scanning microscope (model LSM 510, Carl Zeiss). Immunofluorescent intensity [arbitrary units (AU)] was quantified with NIH Image J software, as described previously (9). The fluorescent intensity of AT1R-like immunoreactivity in the PVN and SFO, over the same areas used for immunohistochemistry, was measured from three representative transverse sections in each animal and averaged to obtain a value for statistical analysis.
Statistics.
Values are means ± SE. The significance of differences among groups was analyzed by two-way repeated-measures ANOVA followed by post hoc Fisher's least significant difference test. Differences were considered significant at P < 0.05.
RESULTS
Hemodynamic effects of the ANG II infusion.
The 4-wk subcutaneus infusion of ANG II had no significant effect on blood pressure (107.9 ± 4.5 vs. 101.2 ± 4.3 mmHg, n = 8, P > 0.05) or heart rate (403.1 ± 13.3 vs. 389.4 ± 12.1 beats/min, n = 8, P > 0.05) compared with saline-infused control rats.
Quantitative assessment of AT1R mRNA and protein expression in the PVN and SFO.
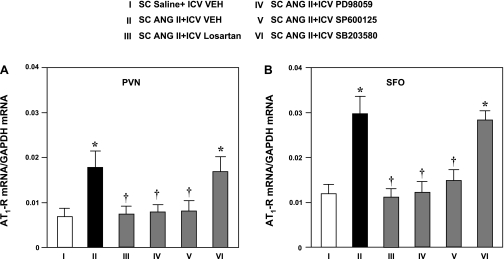
ANG II-infused rats exhibited significant increases in AT1R mRNA expression compared with the saline-infused control rats (Fig. 1) that were similar in magnitude in the SFO and PVN. Concomitant ICV administration of losartan, the p44/42 MAPK inhibitor PD-98059, and the JNK inhibitor SP-600125 prevented the ANG II-induced increase in AT1R mRNA in the PVN and SFO. AT1R mRNA in the PVN and SFO in ANG II-infused rats treated with ICV losartan, PD-98059, and SP-600125 was not significantly different from that in saline-infused control rats. ICV infusion of the p38 MAPK inhibitor SB-203580 had no effect on ANG II-induced AT1R mRNA in the PVN or SFO (Fig. 1).
Fig. 1.
ANG II type 1 receptor (AT1R) mRNA in the paraventricular nucleus of the hypothalamus (PVN, A) and subfornical organ (SFO, B) of rats treated concomitantly with a 4-wk subcutaneous (SC) infusion of saline (VEH) or ANG II and an ICV infusion of VEH, the AT1R antagonist losartan, the p44/42 MAPK inhibitor PD-98059, the JNK inhibitor SP-600125, or the p38 MAPK inhibitor SB-203580. Data were acquired by real-time PCR and normalized to GAPDH. Values are means ± SE (n = 6 for each group). *P < 0.05 vs. SC saline + ICV VEH. †P < 0.05 vs. SC ANG II + ICV VEH.
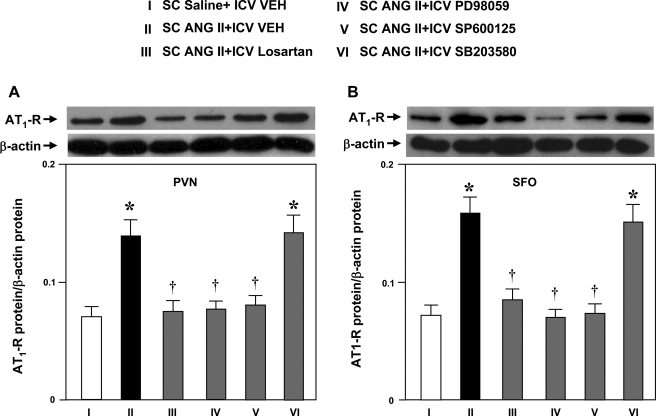
AT1R protein in the PVN and SFO was higher in ANG II-infused rats than in saline-infused control rats (Fig. 2). The ANG II-induced increase in AT1R protein expression in the PVN and SFO was prevented by concomitant ICV infusion of losartan (Fig. 2), the p44/42 MAPK inhibitor PD-98059, or the JNK inhibitor SP-600125. The level of AT1R protein in ANG II-infused rats treated with ICV losartan, PD-98059, and SP-600125 was not significantly different from that in saline-infused control rats. The p38 MAPK inhibitor SB-203580 had no effect on ANG II-induced AT1R protein expression in the PVN or SFO (Fig. 2).
Fig. 2.
AT1R protein levels by Western blot from PVN (A) and SFO (B) tissues in rats treated concomitantly with a 4-wk SC infusion of saline or ANG II and an ICV infusion of VEH, losartan, PD-98059, SP-600125, or SB-203580. Representative Western bands of AT1R and β-actin are shown above each bar. Values are means ± SE [n = 6 (each sample includes tissue from 2 animals) for each group]. *P < 0.05 vs. SC saline + ICV VEH. †P < 0.05 vs. SC ANG II + ICV VEH.
Immunohistochemical assessment of MAPK expression in the PVN and SFO.
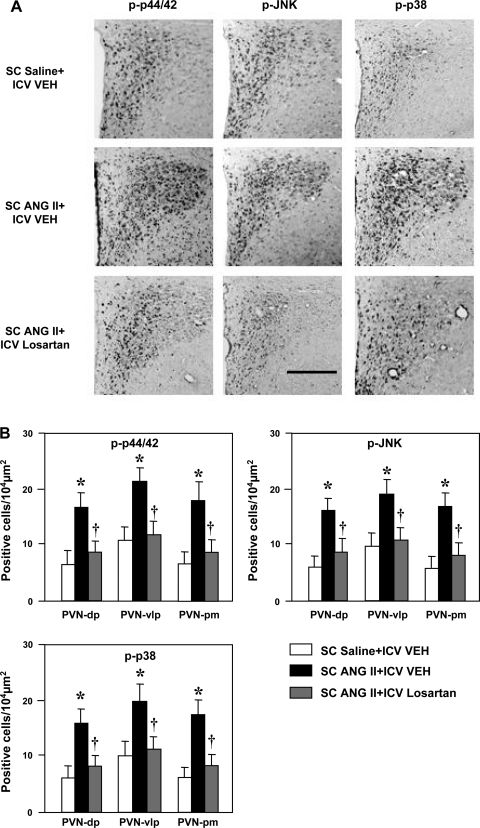
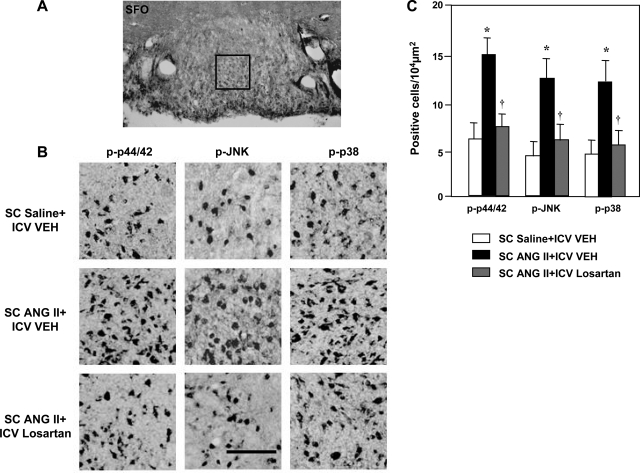
More cells in the three PVN regions (PVN-dp, PVN-vlp, and PVN-pm; Fig. 3) and in the SFO (Fig. 4) expressed phosphorylated p44/42 MAPK, p38 MAPK, and JNK in ANG II-infused rats than in saline-infused control rats. The numbers of cells expressing MAPK was greatly attenuated in the PVN (Fig. 3) and SFO (Fig. 4) by concomitant ICV infusion of losartan.
Fig. 3.
Immunohistochemical analysis of phosphorylated (p-) p44/42 MAPK, JNK, and p38 MAPK in the PVN of rats treated concomitantly with a 4-wk SC infusion of saline or ANG II and an ICV infusion of VEH or losartan. A: representative sections showing p-p44/42, p-JNK, and p-p38 in the PVN of saline-infused control rats (top), ANG II-infused rats treated with ICV VEH (middle), and ANG II-infused rats treated with losartan (bottom). Third ventricle is at left. Scale bar, 300 μm. B: grouped data showing numbers of p-p44/42-, p-JNK-, and p-p38-positive cells in dorsal parvocellular (PVN-dp), ventrolateral parvocellular (PVN-vlp), and posterior magnocellular (PVN-pm) subdivisions of the PVN. Values are means ± SE (n = 6 for each group). *P < 0.05 vs. SC saline + ICV VEH. †P < 0.05 vs. SC ANG II + ICV VEH.
Fig. 4.
Immunohistochemical analysis of p-p44/42 MAPK, p-JNK, and p-p38 MAPK in the SFO of rats treated concomitantly with a 4-wk SC infusion of saline or ANG II and an ICV infusion of VEH or losartan. A: low-power micrograph of SFO indicating location of a 200 × 200 μm2 window that was used to select higher-power images for quantitative analysis. B: representative images showing p-p44/42, p-JNK, and p-p38 in the SFO of saline-infused control rats (top), ANG II-infused rats treated with ICV VEH (middle), and ANG II-infused rats treated with ICV losartan (bottom). Scale bar, 100 μm. C: grouped data showing numbers of p-p44/42-, p-JNK-, and p-p38-positive cells counted in the SFO. Values are means ± SE (n = 6 for each group). *P < 0.05 vs. SC saline + ICV VEH. †P < 0.05 vs. SC ANG II + ICV VEH.
Immunofluorescent assessment of AT1R expression in the PVN and SFO.
Laser confocal images revealed increased fluorescent intensity of AT1R-like immunoreactivity in the PVN (Fig. 5) and SFO (Fig. 6) in ANG II-infused rats compared with the saline-infused control rats. AT1R-like immunoreactivity was increased in the three PVN regions (PVN-dp, PVN-vlp, and PVN-pm). There was strong expression of the AT1R-like immunoreactivity throughout the SFO in ANG II-infused rats compared with the saline-infused control rats.
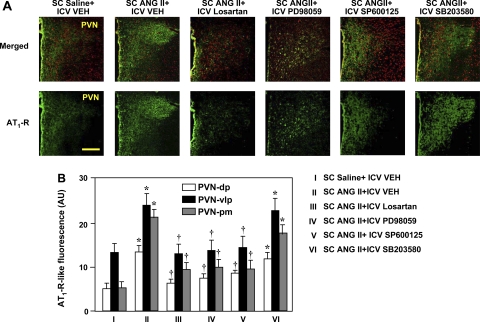
Fig. 5.
A: laser confocal views of AT1R-like immunoreactivity in the PVN of rats treated concomitantly with a 4-wk SC infusion of saline or ANG II and an ICV infusion of VEH, losartan, PD-98059, SP-600125, or SB-203580. Bright green, AT1R-like staining; red, nuclear staining. Top: merged images of AT1R-like and nuclear staining. Bottom: images of AT1R-like immunoreactivity. Third ventricle is at left. Scale bar, 200 μm. B: quantification of AT1R-like immunoreactivity in PVN-dp, PVN-vp, and PVN-mp. Values are means ± SE (n = 6 for each group). AU, arbitrary units. *P < 0.05 vs. SC saline + ICV vehicle. †P < 0.05 vs. SC ANG II + ICV VEH.
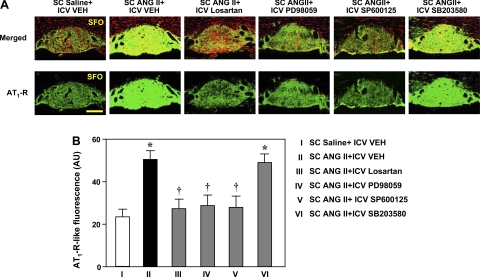
Fig. 6.
A: laser confocal immunofluorescent views of AT1R expression in the SFO of rats treated concomitantly with a 4-wk SC infusion of saline or ANG II and an ICV infusion of VEH, losartan, PD-98059, SP-600125, or SB-203580. Bright green, AT1R-like staining; red, nuclear staining. Top: merged images of AT1R-like and nuclear staining. Bottom: images of AT1R-like immunoreactivity. Scale bar, 300 μm. B: quantification of AT1R-like immunoreactivity in the SFO. Values are means ± SE (n = 6 for each group). *P < 0.05 vs. SC saline + ICV VEH. †P < 0.05 vs. SC ANG II + ICV VEH.
Concomitant ICV administration of losartan, the p44/42 inhibitor PD-98059, and the JNK inhibitor SP-600125 prevented the increase in AT1R-like immunoreactivity in the PVN-vlp and significantly reduced the increases in AT1R-like immunoreactivity in the PVN-dp and PVN-pm (Fig. 5) and in the SFO (Fig. 6) of ANG II-infused rats. Concomitant ICV administration of the p38 MAPK inhibitor SB-203580 had no significant effect on the ANG II-induced increase in AT1R-like immunoreactivity in any region of the PVN (Fig. 5) or in the SFO (Fig. 6).
DISCUSSION
MAPK signaling pathways have been implicated in the upregulation of the AT1R in the rat brain in pathophysiological states characterized by high circulating levels of ANG II (28, 48). Under those conditions, multiple factors that may affect MAPK signaling are present. The present study examined the isolated influence of one of these factors, ANG II. The results demonstrate that ANG II upregulates the AT1R in two key cardiovascular regulatory centers of the brain, the SFO and the PVN, and that phosphorylation of p44/42 MAPK and JNK is a necessary step in that process. p38 MAPK does not appear to be involved.
Aldosterone is another factor that may activate MAPK signaling pathways in peripheral tissues (10, 31) and has been shown to increase AT1R expression in the brain of normal rats and rats with heart failure (51, 52). To avoid inducing release of aldosterone from the adrenal glands, we used a low dose of ANG II in the present study. A previous study demonstrated that this dose of ANG II does not significantly raise plasma aldosterone levels in rats that are not sodium restricted (19). Other neurohumoral factors that might activate MAPK, e.g., proinflammatory cytokines (5, 20) and corticotropin-releasing hormone (CRH) (39), are not likely to be affected by this low-dose ANG II infusion protocol. It is interesting, however, that even this low dose of ANG II, infused chronically over several weeks, increased AT1R mRNA and protein in the SFO and PVN to levels comparable to those observed in rats with heart failure (48). This observation is consistent with the findings of a previous study demonstrating that plasma renin activity peaked in the first 2 wk after induction of heart failure in rats and subsequently remained only minimally elevated (15).
An intriguing finding of the present study is that a systemic infusion of ANG II induced MAPK-dependent AT1R expression in brain tissues directly exposed to circulating ANG II in the SFO and in tissues inside the blood-brain barrier in the PVN. Similar ANG II-induced increases in AT1R mRNA and protein were observed in both regions, and the ANG II-induced increases in the AT1R in both regions were blocked to a similar extent by ICV losartan and by ICV p44/42 MAPK and JNK inhibitors. These observations imply that the same ANG II-driven MAPK-mediated mechanism is operative at both sites, despite the fact that circulating ANG II does not cross the blood-brain barrier. At the PVN, inside the blood-brain barrier, angiotensinergic neurons projecting from circumventricular organs to the hypothalamus (27, 37) may play a role. It has been reported that microinjection of ANG II into the SFO significantly raises ANG II release in the PVN (49).
The immunohistochemical and immunofluorescent images reveal that MAPK activity and AT1R-like immunofluorescence are increased diffusely throughout the SFO and PVN. This is particularly interesting in the PVN, in which previous work demonstrated a predominant localization of the AT1R to the parvocellular PVN and, particularly, to those neuroendocrine neurons containing CRH that are destined for the median eminence (36). Notably, that study was done in normal, unstressed rats. The observation that a chronic ANG II infusion increases MAPK and AT1R-like immunofluorescence in the PVN-dp and PVN-vlp, regions that are associated with sympathetic outflow, and in the PVN-pm, which is associated with vasopressin release, is consistent with the fact that ANG II activates a variety of neurohumoral systems, releasing ACTH (8) and AVP (22) as well as augmenting sympathetic drive (47, 53). A similar distribution of AT1R-like immunofluorescence has been observed in the PVN of rats with heart failure (48), in which the systemic RAS is activated (15) and circulating ANG II levels are increased (25). These observations suggest that increased ANG II may upregulate AT1R expression on neurons not expressing those receptors under resting conditions, resembling the de novo appearance of CRH receptors in the PVN under conditions of stress (29, 40).
This study has focused on a single, but apparently essential, aspect of the cell signaling cascade mediating ANG II-induced upregulation of the AT1R. Importantly, MAPK signaling is redox dependent, and recent work by others has emphasized the importance of NAD(P)H oxidase as another essential step in ANG II-induced upregulation of the AT1R (16). An important downstream component of this same process is activator protein 1 (AP-1), a nuclear transcription factor that has also been implicated in ANG II-induced upregulation of the AT1R (28). Thus, activation of p44/42 MAPK and JNK is one of several signaling mechanisms that are necessary for ANG II-induced increase in AT1R expression. Similarly, MAPK is integral to other signaling mechanisms, mediating, e.g., the effects of proinflammatory cytokines (20), aldosterone (31), and CRH (39), and activation of the several different MAPK pathways by different stimuli will likely result in different outcomes. For example, p38 MAPK had no apparent effect on ANG II-induced AT1R expression in this study, but it plays a key role in LPS- and cytokine-induced inflammatory responses (2, 17, 23).
Several technical limitations of this study deserve comment. First, the specificity of AT1R antibodies is recognized to be controversial. The antibody we used for Western blot quantification of AT1R protein and immunofluorescent localization of AT1R-like immunoreactivity was found in a prior study (48) to produce a single band at the appropriate molecular mass (43–45 kDa) for the AT1R, whereas another AT1R antibody produced multiple bands. This antibody identified immunoreactivity in an anatomic distribution consistent with the known functions of the AT1R in the brain, as previously reported (14, 24). For example, AT1R-like immunoreactivity was identified not only in the PVN and SFO, but also in the supraoptic nucleus (48), in which increased ANG II levels are associated with increased expression of the AT1R (34). However, the recent report that this same antibody detected a single band on Western blot at the same molecular weight in renal cortex tissue from AT1R-knockout mice (1) raises doubt about its specificity. Thus, until an antibody with undisputed specificity for the AT1R becomes available, the results of this study and others reporting changes in AT1R expression must be interpreted with caution.
Second, because we did not utilize markers for specific cell types, we cannot exclude the possibility that at least some of the changes we observed in MAPK and AT1R expression in the SFO and PVN occurred in nonneuronal elements. Finally, because we did not demonstrate colocalization of MAPK in the same cells in which AT1R-like immunoreactivity was expressed, we cannot exclude the possibility of a local paracrine interaction between different cell types. However, since ANG II-induced upregulation of AT1R expression was blocked by inhibition of the intracellular MAPK signaling pathways and ANG II-induced MAPK and AT1R-like immunoreactivity have the same general anatomic distribution, it seems likely that the increased MAPK activity is occurring in the same cells that are expressing increased AT1R activity. Further studies are required to more precisely delineate the effects of ANG II on the activation of MAPK pathways and upregulation of the AT1R in specific cell types.
Perspectives
By binding to its receptor, ANG II can induce the expression of a variety of AP-1 subunits (i.e., c-Fos, FosB, JunB, JunD, and c-Jun) in key cardiovascular-related brain areas, including the SFO, median preoptic nucleus, PVN, SON, and organum vasculosum of the laminae terminalis (7). It is well established that the MAPK family members p44/42 and JNK are predominantly responsible for the induction of c-Fos and c-Jun, the two major components of AP-1 (4). AP-1 DNA binding sites have been identified in the promoter region of the rat AT1R gene (35). Therefore, it has been predicted that AP-1 is a key transcription factor involved in the ANG II-induced upregulation of the AT1R in the central nervous system (28). The results of the present study in ANG II-infused normal rats, similar to results of a previous study of rats with heart failure (48), are consistent with the view that ANG II upregulates the AT1R by an AP-1-dependent mechanism.
GRANTS
This work was supported in part by the Department of Veterans Affairs; Veterans Health Administration; Office of Research and Development; Biomedical Laboratory Research and Development; National Heart, Lung, and Blood Institute Grant RO1-HL-073986 (to R. B. Felder); a Merit Review Award from the Department of Veterans Affairs; and institutional funds provided by the University of Iowa.
Acknowledgments
This work is part of S.-G. Wei's Ph.D. thesis. S.-G. Wei thanks his thesis committee members (Drs. Mark Chapleau, Donald Heistad, Alan K. Johnson, and Thomas Schmidt) for their contributions to this study.
REFERENCES
- 1.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 51: 199–208, 2005. [DOI] [PubMed] [Google Scholar]
- 3.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Barbin G, Roisin MP, Zalc B. Tumor necrosis factor-α activates the phosphorylation of ERK, SAPK/JNK, and p38 kinase in primary cultures of neurons. Neurochem Res 26: 107–112, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Barth SW, Gerstberger R. Differential regulation of angiotensinogen and AT1A receptor mRNA within the rat subfornical organ during dehydration. Brain Res 64: 151–164, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Blume A, Herdegen T, Unger T. Angiotensin peptides and inducible transcription factors. J Mol Med 77: 339–357, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Brooks VL Vasopressin and ANG II in the control of ACTH secretion and arterial and atrial pressures. Am J Physiol Regul Integr Comp Physiol 256: R339–R347, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Cai WJ, Koltai S, Kocsis E, Scholz D, Kostin S, Luo X, Schaper W, Schaper J. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol 284: H31–H40, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Callera GE, Touyz RM, Tostes RC, Yogi A, He Y, Malkinson S, Schiffrin EL. Aldosterone activates vascular p38 MAP kinase and NADPH oxidase via c-Src. Hypertension 45: 773–779, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Charron G, Laforest S, Gagnon C, Drolet G, Mouginot D. Acute sodium deficit triggers plasticity of the brain angiotensin type 1 receptors. FASEB J 16: 610–612, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, da Rocha MJ, Morris M. Osmotic regulation of angiotensin AT1 receptor subtypes in mouse brain. Brain Res 965: 35–44, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol 22: 611–616, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Davisson RL Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 285: R498–R511, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1734–R1745, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR. Interleukin-1β-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem 273: 28670–28676, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Imaki T, Naruse M, Harada S, Chikada N, Imaki J, Onodera H, Demura H, Vale W. Corticotropin-releasing factor up-regulates its own receptor mRNA in the paraventricular nucleus of the hypothalamus. Mol Brain Res 38: 166–170, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Kanagy NL, Pawloski CM, Fink GD. Role of aldosterone in angiotensin II-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 259: R102–R109, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Kida Y, Kobayashi M, Suzuki T, Takeshita A, Okamatsu Y, Hanazawa S, Yasui T, Hasegawa K. Interleukin-1 stimulates cytokines, prostaglandin E2 and matrix metalloproteinase-1 production via activation of MAPK/AP-1 and NF-κB in human gingival fibroblasts. Cytokine 29: 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19: 2026–2028, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Klingbeil CK, Keil LC, Chang D, Reid IA. Effects of CRF and ANG II on ACTH and vasopressin release in conscious dogs. Am J Physiol Endocrinol Metab 255: E46–E53, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 40: 175–183, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lazartigues E, Dunlay SM, Loihl AK, Sinnayah P, Lang JA, Espelund JJ, Sigmund CD, Davisson RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res 90: 617–624, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Leenen FH, Skarda V, Yuan B, White R. Changes in cardiac ANG II postmyocardial infarction in rats: effects of nephrectomy and ACE inhibitors. Am J Physiol Heart Circ Physiol 276: H317–H325, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18: 383–439, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Lind RW, Swanson LW, Ganten D. Angiotensin II immunoreactive pathways in the central nervous system of the rat: evidence for a projection from the subfornical organ to the paraventricular nucleus of the hypothalamus. Clin Exp Hypertens A 6: 1915–1920, 1984. [DOI] [PubMed] [Google Scholar]
- 28.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: activation of activator protein 1 and Jun N-terminal kinase. Circ Res 99: 1004–1011, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Kiss A, Makara G, Lolait SJ, Aguilera G. Stress-specific regulation of corticotropin releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Neuroendocrinol 6: 689–696, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Marra F, Delogu W, Petrai I, Pastacaldi S, Bonacchi A, Efsen E, Aleffi S, Bertolani C, Pinzani M, Gentilini P. Differential requirement of members of the MAPK family for CCL2 expression by hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287: G18–G26, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mazak I, Fiebeler A, Muller DN, Park JK, Shagdarsuren E, Lindschau C, Dechend R, Viedt C, Pilz B, Haller H, Luft FC. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation 109: 2792–2800, 2004. [DOI] [PubMed] [Google Scholar]
- 32.McInvale AC, Harlan RE, Garcia MM. Immunocytochemical detection of two nuclear proteins within the same neuron using light microscopy. Brain Res Protoc 5: 39–48, 2000. [DOI] [PubMed] [Google Scholar]
- 33.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun CJ, Unger T. Effect of repetitive icv injections of ANG II on c-Fos and AT1-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol 280: R1095–R1104, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Murasawa S, Matsubara H, Urakami M, Inada M. Regulatory elements that mediate expression of the gene for the angiotensin II type 1a receptor for the rat. J Biol Chem 268: 26996–27003, 1993. [PubMed] [Google Scholar]
- 36.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 1986.
- 39.Refojo D, Echenique C, Muller MB, Reul JM, Deussing JM, Wurst W, Sillaber I, Paez-Pereda M, Holsboer F, Arzt E. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc Natl Acad Sci USA 102: 6183–6188, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivest S, Laflamme N, Nappi RE. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci 15: 2680–2695, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saavedra JM, Correa FM, Kurihara M, Shigematsu K. Increased number of angiotensin II receptors in the subfornical organ of spontaneously hypertensive rats. J Hypertens Suppl 4: S27–S30, 1986. [PubMed] [Google Scholar]
- 42.Sanvitto GL, Johren O, Hauser W, Saavedra JM. Water deprivation upregulates ANG II AT1 binding and mRNA in rat subfornical organ and anterior pituitary. Am J Physiol Endocrinol Metab 273: E156–E163, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Serrats J, Sawchenko PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol 495: 236–254, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol 286: H1665–H1671, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Angiotensin II-triggered p44/42 mitogen-activated protein kinase mediates sympathetic excitation in heart failure rats. Hypertension 52: 342–350, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei SG, Yu Y, Zhang ZH, Weiss RM, Felder RB. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension 52: 679–686, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JW, Roberts KA, Stubley LA, Hanesworth JM, Harding JW. Hypothalamic angiotensin release in response to AII or glutamic acid stimulation of the SFO in rats. Brain Res Bull 31: 649–654, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Yoo K, Choi JW, Choi MS, Ryu MK, Park GH, Jeon MJ, Ko KH. Mitogen-activated protein kinases (MAPKs) mediate SIN-1/glucose deprivation-induced death in rat primary astrocytes. Arch Pharmacol Res (Seoul) 28: 942–947, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension 51: 727–733, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 282: H2039–H2045, 2002. [DOI] [PubMed] [Google Scholar]