Abstract
The present study tested the hypotheses that male and female rats respond differently to subcutaneous infusions of aldosterone (Aldo; 1.8 μg·kg−1·h−1, 1% NaCl to drink; 28 days) and that central estrogen plays a protective role against the development of hypertension. In rats with blood pressure (BP) and heart rate (HR) measured by Data Sciences International telemetry, chronic Aldo/NaCl treatment induced a greater increase in BP in males (Δ25.4 ± 2.4 mmHg) than in females (Δ7.1 ± 2.2 mmHg). Gonadectomy augmented Aldo/NaCl-induced hypertension in females (Δ18.2 ± 2.0 mmHg) but had no effect in males (Δ23.1 ± 2.9 mmHg). Immunohistochemistry for Fra-like activity was higher in the paraventricular nucleus of intact males, castrated males, and ovariectomized (OVX) females compared with intact females after 28 days of Aldo/NaCl treatment. In intact males, central 17β-estradiol (E2) inhibited the Aldo/NaCl increase in BP (Δ10.5 ± 0.8) compared with that in central vehicle plus systemic Aldo/NaCl (Δ26.1 ± 2.5 mmHg) rats. Combined administration of E2 and estrogen receptor antagonist ICI182780 (ICI) blocked the protective effect of E2 (Δ23.2 ± 2.4 mmHg). In intact females central, but not peripheral, infusions of ICI augmented the Aldo/NaCl (Δ20.4 ± 1.8 mmHg) BP increase. Finally, ganglionic blockade after Aldo infusions resulted in a smaller reduction in BP in intact females (−23.9 ± 2.5 mmHg) and in central estrogen-treated males (−30.2 ± 1.0 mmHg) compared with other groups (intact males, −39.3 ± 3.4; castrated males, −41.8 ± 1.9; intact males with central E2 + ICI, −42.3 ± 2.1; OVX females, −40.3 ± 3.3; and intact females with central ICI, −39.1 ± 1.3 mmHg). Chronic Aldo infusion produced increases in NaCl intake and decreases in HR that were both similar in all groups. Taken together, the results indicate that central estrogen plays a protective role in the development of Aldo/NaCl-induced hypertension and that this may result from reduced sympathetic outflow.
Keywords: estrogen receptor, blood pressure, heart rate, sympathetic nervous system
hypertension is one of the most important risk factors for the development of cardiovascular disease. Sex differences in the regulation of blood pressure (BP) have been found in both humans and in animal models (11). For example, in patients with familial hyperaldosteronism type I, females compared with males appear to be relatively protected against the development of early onset or severe hypertension and its complications (36). In rats with deoxycorticosterone pivalate (DOC)/NaCl-induced hypertension, BP rises more rapidly and reaches a higher level in male than in female rats, and the development of the hypertension is attenuated by gonadectomy in male rats and exacerbated by gonadectomy in female rats (9, 30). Recent evidence from clinical and experimental studies has suggested that direct cardiovascular effects of aldosterone (Aldo) contribute to the development of cardiovascular injury (7, 8, 46). However, there are few animal studies evaluating sex differences and related mechanisms in mineralocorticoid/NaCl-induced hypertension (9).
An observation from the Framingham Heart Study that plasma Aldo levels correlate positively with cardiac wall thickness in women but not in men suggests an important interaction between estrogens and mineralocorticoids (34, 39). In DOC or Aldo/NaCl-treated ovariectomized (OVX) female rats, 17β-estradiol (E2) attenuates the course of hypertension (9) and mineralocorticoid receptor (MR)-mediated cardiovascular injury (2, 3, 21). However, such studies do not provide insight regarding the sites where interactions between mineralocorticoids and estrogen may occur.
The central nervous system (CNS) appears to play a key role in the pressor effects caused by mineralocorticoid excess. Continuous intracerebroventricular infusions of Aldo at low rates in rats cause a significant increase in BP, whereas the same dose is ineffective when given systemically (15). Chronic intracerebroventricular infusion of an MR antagonist blocks intracerebroventricular Aldo-induced hypertension as well as hypertension resulting from subcutaneous Aldo infusion (16). Central Aldo antagonism also normalizes mineralocorticoid-altered baroreflex activity and reduces sympathetic tone (19, 20, 40). Recent studies from our laboratory have shown that central E2 and activation of estrogen receptors (ER) attenuate angiotensin II (ANG II)-induced hypertension in intact male and OVX female mice through a reduction of reactive oxygen species production in the subfornical organ (44, 45). Both MR and ER are present in brain areas of the hypothalamus and in circumventricular organs (1, 17, 29, 35) implicated in the neural control of BP and body fluids balance (6, 22, 33). These findings raise the possibility that there are central interactions between estrogen and Aldo and that central estrogen actions may protect against Aldo-induced cardiovascular pathology.
The present study tested the hypotheses that 1) there are sex differences in the development of Aldo/NaCl-induced hypertension and 2) central E2 and its receptors are involved in the protective effects of estrogen against Aldo/NaCl-induced hypertension.
METHODS
Animals
Sprague-Dawley rats (10–12 wk old) were obtained from Harlan Sprague-Dawley (Indianapolis, IN). They were housed in temperature- and light-controlled animal quarters and were provided with rat chow (7013 NIH-31 modified rat diet, 0.25% NaCl) ad libitum. The rats were divided into nine groups: intact males, castrated males, intact females, OVX females, intact females with peripheral ER antagonist ICI182780 (ICI) infusion, intact females with central ICI infusion, intact males with central vehicle infusion, intact males with central E2 infusion, and intact males with central E2 + ICI infusion. All of the groups were infused subcutaneously with Aldo (1.8 μg·kg−1·h−1) combined with 1% NaCl as the sole drinking fluid. Body weights and 1% NaCl intakes were measured daily. Gain of body weight and average 1% NaCl intake were calculated after 28 days of Aldo treatment. All experiments were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the University of Iowa Animal Care and Use Committee.
Surgical Procedures
Gonadectomy.
Ten days before implantation of Data Sciences International (DSI) telemetry probes, bilateral gonadectomies were performed in female and male rats anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). In the females, a single 2- to 3-cm dorsal midline incision was made in the skin and underlying muscles. The ovaries were isolated, tied off with sterile suture, and removed, and the incisions were then closed. In the males, a single incision was made in the skin covering the scrotum. The testicles were exteriorized, tied off, and removed, and the skin was then sutured.
Telemetry probe implantation.
Implantable rat BP transmitters (TA11PA-C40; DSI, St. Paul, MN) were used to directly measure arterial pressure in individual animals. The rats were anesthetized with a ketamine-xylazine mixture. The femoral artery of the rat was accessed with a ventral incision. The right femoral artery was isolated, and the catheter of a telemetry probe was inserted into the vessel. Through the same ventral incision a pocket along the right flank was formed. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture.
Chronic intracerebroventricular cannula and osmotic pump implantation.
After baseline BP and heart rate (HR) recordings were obtained, rats were again anesthetized with a ketamine-xylazine mixture, and an intracerebroventricular cannula with an osmotic pump (Model 2004; 28 days; Alzet, Cupertino, CA) was implanted into the right lateral ventricle (coordinates: 0.9 mm caudal, 1.5 mm lateral to bregma, and 5.0 mm below the skull surface) for chronic infusions of E2 (20 μg·kg−1·day−1; Sigma, St. Louis, MO), ICI (1.5 μg·kg−1·day−1; Tocris Bioscience, Ellisville, MO), or E2 + ICI. The dosages of E2 and ICI were chosen based on published in vivo studies (18, 44). At the same time, osmotic pumps (model 2004; Alzet) containing Aldo (1.8 μg·kg−1·h−1; Sigma) were implanted subcutaneously in the back and tap water was changed to 1% NaCl. At the end of the experiment, the animals were euthanized and perfused transcardially with saline followed by fixative. The location of the lateral ventricle cannula implantation was verified histologically.
Fluorescent immunohistochemistry.
Immunohistochemical studies were performed to assess neuronal activation in the paraventricular nucleus (PVN). Expression of Fra-like (fos family gene) activity was used as an indicator of chronic neuronal activation. Brain sections were incubated with a rabbit polyclonal anti-Fos antibody (Santa Cruz Biotechnology, Santa Cruz, CA; K-25, sc-253, 1:1,000) and a mouse monoclonal anti-MR antibody (generated in the laboratory of Dr. C. E. Gomez-Sanchez) in 5% donkey normal serum with 0.2% Triton X-100 for 72 h at 4°C. After being thoroughly washed with PBS, sections were incubated with Rhodamine Red-X-conjugated AffiniPure donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA) and Cy2-conjugated AffiniPure donkey anti-mouse IgG (1:100; Jackson) in PBS for 24 h at 4°C. Fluorescence was then identified using confocal microscopy. In each animal, Fra-like positive and double-labeled PVN neurons were counted manually, and the counts of two representative 40-μm transverse sections at a level ∼1.80 mm posterior to bregma were averaged.
Experimental Protocol
Measurement of BP and HR.
All rats were allowed 7 days to recover from transmitter implantation surgery before any measurements were made. Thereafter, BP and HR were telemetrically recorded and stored with the Dataquest ART data acquisition system (DSI).
To determine the effects of E2, ICI, or E2 + ICI on Aldo-induced hypertension in male and female rats, intracerebroventricular cannulas with osmotic pumps were implanted into the right lateral ventricle for chronic infusions of vehicle, E2, ICI, or E2 + ICI for 28 days. At the same time, the rats were infused subcutaneously with Aldo combined with 1% NaCl as the sole drinking fluid.
Evaluation of BP responses to autonomic blockade.
BP was also measured in the presence of the ganglionic blocker hexamethonium (30 mg/kg ip). Ganglionic blockade was repeated two times in each animal, during baseline and after 28 days of Aldo infusion. On the day of ganglionic blockade experiments, rats were allowed to stabilize for at least 60 min, after which time BP was recorded for 20 min before and after hexamethonium injection.
Data Analysis
Mean arterial pressure (MAP) and HR were collected for 5 baseline days and then for 28 consecutive days during E2, ICI, or E2 + ICI and Aldo pump implantation. MAP and HR are presented as mean daily values.
All data are expressed as means ± SE. Statistical analyses of the effects of central administration of E2, ICI, or E2 + ICI on BP during Aldo infusion were performed with two-way ANOVA for repeated measures (Sigma Stat version 2.06). Post hoc analysis was performed with Fisher's least significant difference multiple comparison tests where appropriate. A one-way ANOVA was used for comparing changes in BP and 1% NaCl intake. Statistical significance was set at P < 0.05.
RESULTS
Baseline BP and HR in Conscious Rats
Baseline values for MAP were comparable in all groups including intact males (n = 7; 101.8 ± 1.8 mmHg), castrated males (n = 6; 102.3 ± 2.5 mmHg), intact females (n = 7; 99.6 ± 0.8 mmHg), and OVX females (n = 6; 101.6 ± 1.2 mmHg). However, baseline HR was significantly higher in intact and OVX female rats (376.4 ± 10.8 and 378.6 ± 7.4 beats/min, respectively; P < 0.05) than that in intact males (307.4 ± 5.6 beats/min) and castrated males (346.6 ± 8.8 beats/min).
Effects of Aldo/NaCl Treatment on BP and HR in Intact and Gonadectomized Male and Female Rats
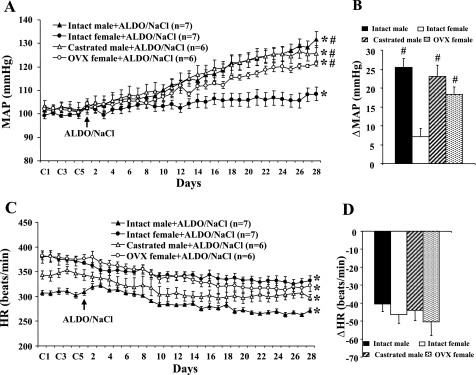
Over 28 days of Aldo + 1% NaCl treatment, male rats progressively developed hypertension (Δ25.4 ± 2.4 mmHg; P < 0.05), which was greater than that seen in intact females (Δ7.1 ± 2.2 mmHg; P < 0.05). Gonadectomy augmented Aldo + 1% NaCl-induced hypertension in females (Δ18.2 ± 2.0 mmHg; P < 0.05) but had no effect in males (Δ23.1 ± 2.9 mmHg; Fig. 1, A and B). Chronic Aldo infusion produced a significant decrease in HR in all groups (P < 0.05; Fig. 1C). However, the fall in HR during Aldo/NaCl treatment was similar in all groups (intact male, −40.5 ± 4.2; castrated male, −43.9 ± 5.7; intact female, −46.4 ± 4.9; and OVX female, −50.4 ± 7.6 beats/min; P > 0.05; Fig. 1D).
Fig. 1.
Sex difference in aldosterone (Aldo)-induced hypertension. Daily mean arterial pressures (MAP; A) and heart rate (HR; C) before and during systemic infusion of Aldo and 1% NaCl access in intact, castrated male rats and intact, ovariectomized (OVX) females are shown. B and D: average changes in MAP and HR induced by Aldo/NaCl treatment in all groups. Control (C) days (C1, C3, and C5) are followed by 28 days of systemic Aldo infusion. *P < 0.05 compared with baseline; #P < 0.05 compared with intact females.
Effects of Aldo/NaCl Treatment on Neuronal Excitation in the PVN
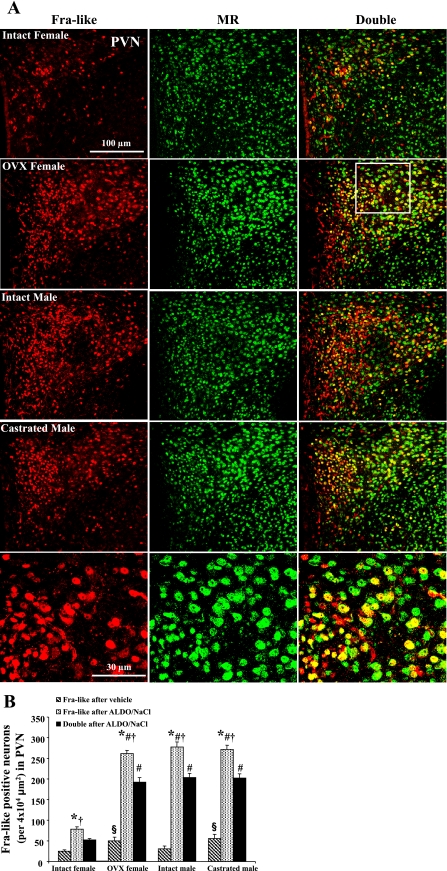
Although Fra-like activity in the PVN of gonadectomized male and female rats was higher (P < 0.05) than that in intact rats after vehicle infusion, Aldo/NaCl treatment induced a significant increase (P < 0.05) in Fra-like activity in PVN neurons in all groups (Fig. 2A). Moreover, the increases in Fra-like activity were greater in the PVN of the intact male, castrated male, and OVX female rats compared with intact female rats after 28 days of Aldo/NaCl treatment. Approximately 70% of Fra-like positive neurons showed MR-like immunocytochemical staining, and there were no significant differences in the percentage of Fra-like positive neurons with MR-like immunoreactivity in all groups (Fig. 2B).
Fig. 2.
Colocalization of Fra-like and mineralocorticoid receptor (MR) in the paraventricular nucleus (PVN). A: representative sections from each group showing Fra-like immunoreactivity (red), MR (green), and double-labeling (yellow) in PVN neurons after 28 days Aldo + 1% NaCl treatment. Top 4 rows have the same magnification. Bottom row is higher magnification of the sector indicated in second row. B: quantification of Fra-like-positive and double-labeled neurons in the PVN of each group. *P < 0.05 compared with vehicle treatment; §P < 0.05 compared with intact females with vehicle treatment; #P < 0.05 compared with intact female with Aldo; †P < 0.05 compared with double-labeling.
Effects of Intracerebroventricular Infusion of E2 or E2 + ICI on Aldo-induced Hypertension in Male Rats
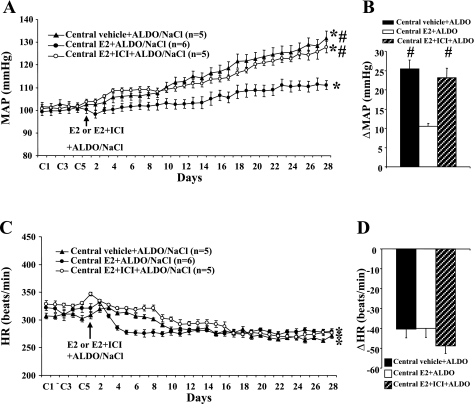
Central E2 (Fig. 3, A and B) inhibited the increase in MAP induced by Aldo/NaCl (Δ10.5 ± 0.8; n = 5; P < 0.05) compared with that seen in rats with central vehicle plus systemic Aldo (Δ26.1 ± 2.5 mmHg; n = 6). Concurrent administration of ICI prevented the protective effect of E2 (Δ23.2 ± 2.4 mmHg; n = 5). Systemic Aldo infusion produced significant, comparable decreases in HR in all groups (P < 0.05; Fig. 3C; central vehicle, −41.5 ± 5.2, central E2, −40.0 ± 4.6, and central E2 + ICI, −48.8 ± 3.7; P > 0.05; Fig. 3D).
Fig. 3.
The effect of chronic intracerebroventricular infusion of estrogen and estrogen receptor antagonist on Aldo/NaCl-induced hypertension in male rats. Daily MAP (A) and HR (C) before and during systemic infusion of Aldo in intracerebroventricular vehicle 17β-estradiol (E2) or E2 + ICI182780 (ICI)-treated male rats are shown. B and D: average changes in MAP and HR across days induced by Aldo infusion in all groups. Control (C) days (C1, C3, and C5) are followed by 28 days of central vehicle, E2, or E2 + ICI and systemic Aldo infusion. *P < 0.05 compared with baseline; #P < 0.05 compared with males given central E2.
Effects of Central and Peripheral Infusion of ICI on Aldo/NaCl-induced Hypertension in Female Rats
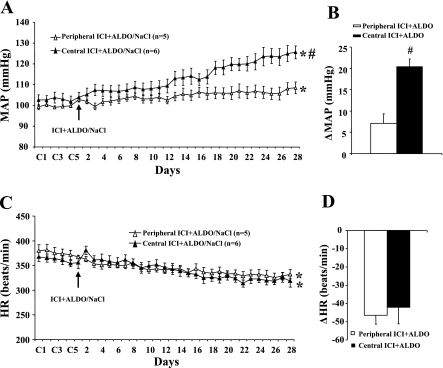
Twenty-eight days of Aldo infusion resulted in a 20.4 ± 1.8 mmHg (n = 6; P < 0.05) increase in MAP in female rats treated with central infusions of ICI versus a 7.1 ± 3.9 mmHg increase in female rats treated with the peripherally administrated antagonist (Fig. 4, A and B). As shown in Fig. 4C, Aldo infusion produced a significant decrease in HR in both groups (P < 0.05), but there was no difference in HR between groups treated with peripheral (−45.9 ± 4.9 beats/min) and central (−42.0 ± 9.0 beats/min) ICI (Fig. 4D).
Fig. 4.
The effect of chronic intracerebroventricular infusions of an estrogen receptor antagonist on Aldo/NaCl-induced hypertension in female rats. Daily MAP (A) and HR (C) before and during systemic infusions of Aldo and access to 1% NaCl in peripheral ICI or intracerebroventricular ICI-treated female rats are shown. B and D: average changes in MAP and HR induced by Aldo infusion in all groups. Control (C) days (C1, C3, and C5) are followed by 28 days of peripheral or central ICI and systemic Aldo infusion. *P < 0.05 compared with baseline; #P < 0.05 compared with females given peripheral ICI.
Effects of Autonomic Blockade on BP
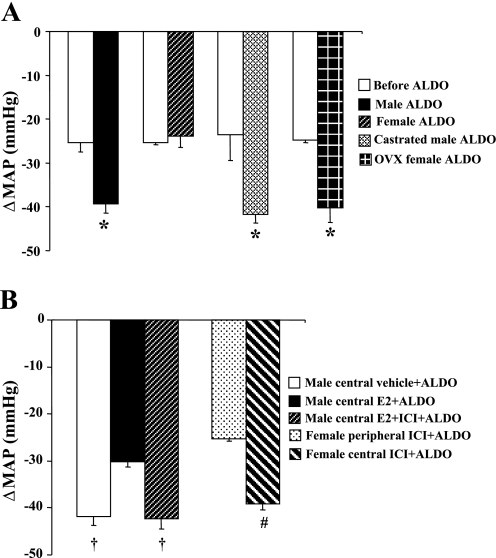
Figure 5 shows decreases in BP with acute ganglionic blockade in all groups. The average reduction in the BP response to hexamethonium injection before infusion of Aldo was −24.7 ± 3.5 mmHg, and there was no difference between males and females (Fig. 5A). In males, following 28 days of Aldo infusion, acute hexamethonium injection resulted in a greater reduction in BP in intact (−39.3 ± 3.4 mmHg; Fig. 5A), castrated (−41.8 ± 1.9 mmHg; Fig. 5A), central vehicle (−42.1 ± 3.4 mmHg; Fig. 5B), or E2 + ICI-treated (−42.3 ± 2.1 mmHg; Fig. 5B) rats compared with central E2-treated rats (−30.2 ± 1.0; P < 0.05). In females, the BP reduction was greater in response to hexamethonium injection in OVX (−40.3 ± 3.3 mmHg; Fig. 5A) or central ICI-treated (−39.1 ± 1.3 mmHg; Fig. 5B) rats than that seen in intact (−23.9 ± 2.5 mmHg; P < 0.05) or peripheral ICI-treated (−25.4 ± 0.5 mmHg; P < 0.05) rats.
Fig. 5.
MAP in response to ganglionic blockade with hexamethonium before beginning Aldo infusions and 1% NaCl access (control) and on day 28 of Aldo infusion in all groups (A and B). *P < 0.05 compared with control or Aldo-treated intact female rats; †P < 0.05 compared with males with central E2; #P < 0.05 compared with females with peripheral ICI.
Effects of Aldo Infusion on 1% NaCl Intake
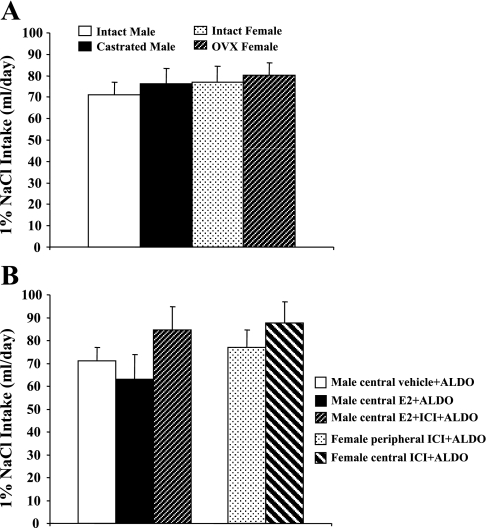
There was no difference in 1% NaCl intake between male (37.8 ± 8.0 ml/day) and female (33.8 ± 6.0 ml/day) rats when given Aldo vehicle alone (not shown in Fig. 6). Systemic infusion of Aldo significantly increased 1% NaCl intake in all groups of rats with or without central treatment. There was also no difference between males and females (Fig. 6, A and B).
Fig. 6.
Mean daily 1% NaCl intake during chronic Aldo infusions in intact and gonadectomized male and female rats (A). Mean daily 1% NaCl intake during Aldo infusions in male rats treated with central vehicle, E2, or E2 + ICI and in female rats treated with peripheral or central ICI (B).
Effects of Aldo/NaCl on Body Weight
Gonadectomy increased the body weight in female (45.2 ± 5.7 g; P < 0.05) compared with male (12.0 ± 4.2 g) rats. Twenty-eight days of Aldo/NaCl treatment resulted in a further increase in body weight in OVX females (42.1 ± 4.2 g), but this was not statistically different from the gains of body weight in intact males (39.1 ± 7.5 g), intact females (38.4 ± 3.9 g), castrated males (36.7 ± 5.7 g), central E2 + ICI-treated males (38.0 ± 5.1 g), central ICI-treated females (40.5 ± 4.7 g), and peripheral ICI-treated females (42.5 ± 5.2 g). However, in intact male rats central E2 infusion significantly reduced the body weight during Aldo/NaCl treatment (−25.0 ± 4.7 g; P < 0.05).
DISCUSSION
The main findings of this study are 1) Aldo/NaCl treatment induces greater hypertension in males than in females, 2) gonadectomy augments hypertension in females, 3) central E2 attenuates Aldo/NaCl-induced hypertension in males, 4) central ER antagonist augments this form of hypertension in females, and 5) the attenuated BP effect on Aldo/NaCl-induced hypertension in intact females and males produced by central E2 administration involves decreased sympathetic outflow. Taken together, these results suggest that female rats are protected against the development of Aldo/NaCl-induced hypertension and that central estrogen plays an important modulatory role in the pathogenesis of Aldo/NaCl-induced hypertension.
Aldo has been identified as an independent risk factor for cardiovascular disease (46). Increased serum Aldo levels in patients with heart failure have been shown to correlate directly with the risk of cardiovascular events (37). In several species including mouse, rat, and dog, administration of Aldo, DOC, or the water soluble form of DOC [i.e., deoxycorticosterone acetate (DOCA)] leads to sustained hypertension (9, 23, 27). Male animals with sufficiently elevated Aldo became hypertensive after ∼10 days of hormone administration in combination with moderately increased sodium intake (24).
Crofton and Share (9) were the first to report sex differences in the development of mineralocorticoid-induced hypertension by demonstrating the relative protection of females to DOC/NaCl-induced hypertension and that OVX exacerbated the rise in BP in female rats (9). The present investigations are consistent with the previous study by demonstrating that infusion of the primary mineralocorticoid in rat, Aldo, combined with 1% NaCl as the sole drinking fluid increased BP significantly in males but not in females and that OVX augmented the pressor effect in the mineralocorticoid/NaCl-treated females.
It has been shown that activation of central MR is essential for the pressor effects caused by systemic Aldo excess (14). In the brain, like the periphery, Aldo exerts its actions through binding to MR. MR in the PVN and the two forebrain circumventricular organs, the subfornical organ and the organum vasculosum of the lamina terminalis, are heavily implicated in Aldo/NaCl-induced hypertension (10, 14, 47, 48). It has been shown that central Aldo excess produces hypertension through an increase in the release of arginine vasopressin and elevated central sympathetic drive to the kidneys, heart, and vascular smooth muscle (14). Central infusion of an MR antagonist reduces sympathetic tone (19, 20, 40). However, the neuroanatomical sites where Aldo acts to increase sympathetic activity remain uncertain. Neurons of the PVN are known to be involved in the regulation of autonomic and neuroendocrine activity. Efferent projections from the PVN to the rostral ventrolateral medulla and to the spinal cord modulate the excitability of sympathetic preganglionic neurons (25, 33). This combined with the role of the PVN in the control of vasopressin synthesis and release (6) makes the PVN a prime forebrain candidate as a central site responsible for mediating sympathetic outflow and vasopressin release during Aldo excess. Recent immunohistochemical and electrophysiological studies by Zhang and colleagues (48) support this idea. In their studies, intracerebroventricular infusion of Aldo increased Fra-like activity (indicating neuronal activation) in the neurons of the PVN and enhanced sympathetic nerve activity by upregulating the brain renin-angiotensin system activity and superoxide production in the PVN (48). The present study extends this finding by showing that most of the Aldo-activated neurons in the PVN also possess MR. Moreover, after Aldo/NaCl treatment, the number of activated neurons in the PVN was low in intact females, and OVX increased the activated neurons in the PVN to an extent similar to that seen in intact males. These observations suggest that in females estrogen may counteract the activating effect of Aldo to influence the activity of PVN neurons. The sympathetic nervous system is further implicated by our functional study in which the reduction of BP in response to ganglionic blockade was less in both intact males treated with central E2 and intact females compared with either intact or castrated males or OVX females. These results may account for the observed sex differences in the central effects of Aldo on Aldo/NaCl-induced hypertension and on sympathetic drive.
Crofton and Share (9) demonstrated that gonadectomy attenuates the development of DOC/NaCl-induced hypertension in males. In the present study, we did not find that castration affected Aldo/NaCl-induced hypertension. Crofton and Share (9) studied uninephrectomized rats treated with DOC and measured BP by tail-cuff in lightly anesthetized, slightly heated animals. The present study employed rats with intact kidneys, treated with the primary rodent mineralocorticoid Aldo, and chronically monitored arterial BP and HR by telemetry. The alternative methods used in these two studies may account for apparent differences on the effects of castration on mineralocorticoid-induced hypertension.
Several studies have shown that intracerebroventricular Aldo increased HR and impaired arterial baroreflex control of HR (19, 20, 40). With regard to basal HR in the present study, the opposite was observed in that Aldo/NaCl treatment produced gradual reductions over the course of the experiment that were similar in all groups, regardless of sex or treatment. The explanation for the discrepancy between the previous studies and the present study may also be due to differences in the methods used to collect BP and HR measures. Previous work used either direct measurements of BP and HR made shortly after implanting indwelling arterial catheters or the tail-cuff method. These methods are likely to have marked nonspecific stress components. In contrast, the present studies used telemetry to allow collection of cardiovascular data with minimal stress and maximal time for recovery after implantation of vascular lines.
There is a growing consensus from a body of cardiovascular research that ER activation is beneficial. Endogenous estrogen as well as ER activation by selective or nonselective agonists lowers elevated BP and significantly improves several key features of cardiovascular injury in DOC/NaCl- or Aldo/NaCl-treated rats (2, 9) and in OVX spontaneously hypertensive rats (21). Several clinical studies have also demonstrated important interactions between mineralocorticoids and ER function. A new hormone therapy combining E2 with drospirenone, which is a progestin with anti-aldosterone activity, has been shown to have a BP lowering effect in postmenopausal women and to effectively relieve symptoms of menopause (41, 42).
A growing number of recent studies have demonstrated the protective effects of estrogen in the CNS. In the in vitro experiments, E2 facilitates area postrema calcium-activated K+ currents and inhibits area postrema neuronal activity (26). E2 also attenuates the increase in intracellular Ca2+ concentration induced by ANG II in cultured area postrema neurons (31). In in vivo experiments, central infusion of E2 prevents the increase in BP induced by ANG II in male mice and in OVX female mice (44, 45). Central infusion of an ER antagonist augments ANG II-induced hypertension in female mice (44).
Moreover, sex differences in MR mRNA levels have been reported in the rat brain (38). Chronic E2 treatment decreases MR mRNA expression and MR binding in the hippocampus (4, 5). Given the importance of the central activation of MR in the development of Aldo/NaCl-induced hypertension and in light of the evidence that estrogen protects centrally against various components of the renin-angiotensin-Aldo system (12), one could hypothesize that central estrogen and activation of its receptors contribute to the attenuated BP and the reduced BP response to automatic blockade in Aldo-treated females. In the present study, central E2 inhibited the increase in BP induced by Aldo/NaCl treatment in male rats. In intact females, central (but not peripheral) infusions of the ER antagonist augmented the pressor effects of Aldo. Ganglionic blockade resulted in a smaller reduction in BP in central E2-treated males compared with females with central ER antagonist treatment. This is consistent with the aforementioned hypothesis that central estrogen and ER activation reduces sympathetic outflow and Aldo/NaCl-induced increases in BP.
The effects of estrogen on the cardiovascular system are mediated by two different ER subtypes: ER-α and ER-β. The present study used a nonselective ER agonist and a nonselective ER antagonist. In a recent study on the effects of estrogen in the PVN on BP, microinjections of E2 into the PVN dose-dependently attenuated the pressor response to glutamate (13). These effects of E2 appear to be mediated by ER-β (13). Future studies could focus on determining whether ER-α and/or ER-β are responsible for the protective effects of E2 in Aldo/NaCl-induced hypertension (43).
It has been shown that intracerebroventricular Aldo produced sustained dose-responsive increases in BP, which appear to be independent of alterations in sodium ingestion (32). Intracerebroventricular administration of the MR antagonist markedly attenuates hypertension in the DOCA/NaCl model but has no effect on saline intake (20). These results suggest that the pathways mediating the BP responses to Aldo/NaCl treatment may be separate from those controlling thirst and salt appetite (28). Although OVX, central E2, or ICI changed the BP responses to Aldo infusion in the present studies, saline intakes in all groups were similar regardless of sex or treatment condition. Thus the sex differences in Aldo-induced hypertension and the effects of OVX, central E2, or ICI on Aldo-induced hypertension in the present study are unlikely to be due to saline intake.
Body weights were reduced in intact male rats chronically treated with E2, whereas in the other group of rats they were increased after Aldo/NaCl treatment. Although hypertension was attenuated in the E2-treated males, we are unaware of data that support an influence of growth rate or body mass on the development of Aldo-induced hypertension. Indeed, the gain of body weight in intact females with Aldo/NaCl treatment was similar to that in male or gonadectomized rats, but the development and severity of Aldo-induced hypertension was much less than that seen in intact male or gonadectomized male and female rats.
In summary, female gender and the central actions of estrogen on ER play an important protective role in the development of Aldo/NaCl-induced hypertension. This protective effect at least in part seems to involve actions to decrease sympathetic outflow, which may arise from influences mediated through the hypothalamic PVN.
GRANTS
This work was supported by NIH Grants HL-62261, HL-59676, HL-14388, and DK-66086.
REFERENCES
- 1.Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol 313: 522–538, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, Konig S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 50: 432–438, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Arias-Loza PA, Hu K, Schafer A, Bauersachs J, Quaschning T, Galle J, Jazbutyte V, Neyses L, Ertl G, Fritzemeier KH, Hegele-Hartung C, Pelzer T. Medroxyprogesterone acetate but not drospirenone ablates the protective function of 17 beta-estradiol in aldosterone salt-treated rats. Hypertension 48: 994–1001, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralocorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Mol Cell Neurosci 4: 191–198, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Castren M, Patchev VK, Almeida OF, Holsboer F, Trapp T, Castren E. Regulation of rat mineralocorticoid receptor expression in neurons by progesterone. Endocrinology 136: 3800–3806, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Ciriello J Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol 275: R1745–R1754, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Conlin PR Interactions of high salt intake and the response of the cardiovascular system to aldosterone. Cardiol Rev 13: 118–124, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Connell JM, Davis E. The new biology of aldosterone. J Endocrinol 186: 1–20, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 29: 494–499, 1997. [DOI] [PubMed] [Google Scholar]
- 10.De Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int 57: 1329–1336, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res 53: 688–708, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Gingerich S, Krukoff TL. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 48: 1130–1136, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez EP Central hypertensive effects of aldosterone. Front Neuroendocrinol 18: 440–462, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez EP Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology 118: 819–823, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Sanchez EP, Fort CM, Gomez-Sanchez CE. Intracerebroventricular infusion of RU28318 blocks aldosterone-salt hypertension. Am J Physiol Endocrinol Metab 258: E482–E484, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res 51: 371–381, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hegele-Hartung C, Siebel P, Peters O, Kosemund D, Müller G, Hillisch A, Walter A, Kraetzschmar J, Fritzemeier KH. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci USA 101: 5129–5134, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang BS, Wang H, Leenen FH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol 288: H517–H524, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Janiak PC, Lewis SJ, Brody MJ. Role of central mineralocorticoid binding sites in development of hypertension. Am J Physiol Regul Integr Comp Physiol 259: R1025–R1034, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res 77: 774–781, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678–686, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension 11: 750–753, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Kanagy NL, Pawloski CM, Fink GD. Role of aldosterone in angiotensin II-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 259: R102–R109, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Hay M. 17-Beta-estradiol modulation of area postrema potassium currents. J Neurophysiol 84: 1385–1391, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Obst M, Gross V, Luft FC. Systemic hemodynamics in non-anesthetized l-NAME and DOCA-salt-treated mice. J Hypertens 22: 1889–1894, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Osborn JW, Jacob F, Hendel M, Collister JP, Clark L, Guzman PA. Effect of subfornical organ lesion on the development of mineralocorticoid-salt hypertension. Brain Res 1109: 74–82, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res 54: 175–180, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi Y, Share L, Crofton JT, Iitake K, Brooks DP. Sex difference in pressor responsiveness to vasopressin and baroreflex function in DOC-salt hypertensive rats. J Hypertens 6: 381–387, 1988. [PubMed] [Google Scholar]
- 31.Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol 285: H1515–H1520, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Peysner K, Henry CA, Malvin RL. Central infusion of aldosterone increases blood pressure by mechanisms independent of Na retention. Clin Exp Hypertens A 12: 399–414, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Schunkert H, Hense HW, Muscholl M, Luchner A, Kurzinger S, Danser AH, Riegger GA. Associations between circulating components of the renin-angiotensin-aldosterone system and left ventricular mass. Heart 77: 24–31, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 436: 64–81, 2001. [PubMed] [Google Scholar]
- 36.Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Severity of hypertension in familial hyperaldosteronism type I: relationship to gender and degree of biochemical disturbance. J Clin Endocrinol Metab 85: 2160–2166, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Struthers AD The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail 6: 539–545, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Turner BB Influence of gonadal steroids on brain corticosteroid receptors: a minireview. Neurochem Res 22: 1375–1385, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension 43: 957–962, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Huang BS, Leenen FH. Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol 285: H2516–H2523, 2003. [DOI] [PubMed] [Google Scholar]
- 41.White WB Drospirenone with 17β-estradiol in the postmenopausal woman with hypertension. Climacteric 10 Suppl 1: 25–31, 2007. [DOI] [PubMed] [Google Scholar]
- 42.White WB, Hanes V, Chauhan V, Pitt B. Effects of a new hormone therapy, drospirenone and 17-beta-estradiol, in postmenopausal women with hypertension. Hypertension 48: 246–253, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Xue B, Badaue-Passos D Jr, Beltz TG, Johnson RF, Johnson AK, Hay M. Central injections of adeno-associated virus-siRNA gene to silence estrogen receptor beta (ERβ) augment aldosterone-induced hypertension in female rats (Abstract). FASEB J 22: 737.35, 2008. [Google Scholar]
- 44.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-α mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 292: H1770–H1776, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008. [DOI] [PMC free article] [PubMed]
- 46.Yoshimoto T, Hirata Y. Aldosterone as a cardiovascular risk hormone. Endocr J 54: 359–370, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZH, Kang YM, Yu Y, Wei SG, Schmidt TJ, Johnson AK, Felder RB. 11β-Hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation. Hypertension 48: 127–133, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008. [DOI] [PubMed] [Google Scholar]