Abstract
Doxorubicin (DOX) is a potent available antitumor agent; however, its clinical use is limited because of its cardiotoxicity. Cell death is a key component in DOX-induced cardiotoxicity, but its mechanisms are elusive. Here, we explore the role of superoxide, nitric oxide (NO), and peroxynitrite in DOX-induced cell death using both in vivo and in vitro models of cardiotoxicity. Western blot analysis, real-time PCR, immunohistochemistry, flow cytometry, fluorescent microscopy, and biochemical assays were used to determine the markers of apoptosis/necrosis and sources of NO and superoxide and their production. Left ventricular function was measured by a pressure-volume system. We demonstrated increases in myocardial apoptosis (caspase-3 cleavage/activity, cytochrome c release, and TUNEL), inducible NO synthase (iNOS) expression, mitochondrial superoxide generation, 3-nitrotyrosine (NT) formation, matrix metalloproteinase (MMP)-2/MMP-9 gene expression, poly(ADP-ribose) polymerase activation [without major changes in NAD(P)H oxidase isoform 1, NAD(P)H oxidase isoform 2, p22phox, p40phox, p47phox, p67phox, xanthine oxidase, endothelial NOS, and neuronal NOS expression] and decreases in myocardial contractility, catalase, and glutathione peroxidase activities 5 days after DOX treatment to mice. All these effects of DOX were markedly attenuated by peroxynitrite scavengers. Doxorubicin dose dependently increased mitochondrial superoxide and NT generation and apoptosis/necrosis in cardiac-derived H9c2 cells. DOX- or peroxynitrite-induced apoptosis/necrosis positively correlated with intracellular NT formation and could be abolished by peroxynitrite scavengers. DOX-induced cell death and NT formation were also attenuated by selective iNOS inhibitors or in iNOS knockout mice. Various NO donors when coadministered with DOX but not alone dramatically enhanced DOX-induced cell death with concomitant increased NT formation. DOX-induced cell death was also attenuated by cell-permeable SOD but not by cell-permeable catalase, the xanthine oxidase inhibitor allopurinol, or the NADPH oxidase inhibitors apocynine or diphenylene iodonium. Thus, peroxynitrite is a major trigger of DOX-induced cell death both in vivo and in vivo, and the modulation of the pathways leading to its generation or its effective neutralization can be of significant therapeutic benefit.
Keywords: heart failure, apoptosis, inducible nitric oxide synthase, hemodynamics
because of its unmatched efficacy and broad-spectrum effect, doxorubicin (DOX; Adriamycin), an anthracycline antibiotic, continues to be a widely used chemotherapeutic agent to treat a variety of cancers (55) despite its potential to elicit serious dose-dependent cardiotoxicity often leading to degenerative cardiomyopathy/heart failure (55).1 The therapeutic options for DOX-induced cardiomyopathy are very limited, mostly involving supportive treatment or cardiac transplantation. The proposed mechanism of DOX-induced cardiotoxicity is complex and involves increased oxidative/nitrosative stress and downstream effector pathways (2, 3, 11, 28, 43–45, 60, 65). Apoptotic cell death is a key component in DOX-induced cardiotoxicity (7, 20, 23, 31, 62); however, the exact triggers/mechanisms have not been fully established, and optimal therapeutic approaches for cardioprotection remain undefined (56).
Peroxynitrite is a reactive oxidant that is produced from the diffusion-controlled reaction between nitric oxide (NO) and another free radical, the superoxide anion (41). In addition to its diffusion-controlled reactions, NO under certain conditions may also react with glutathione to generate S-nitrosoglutathione to be stored in this complexed form (15). In vivo peroxynitrite generation and/or protein nitration have recently been demonstrated in various rodent models of myocardial ischemia-reperfusion and heart failure and in humans with these pathologies (14, 41, 49, 59). Previous studies (28, 43, 65) have also demonstrated increased nitrotyrosine (NT; a footprint of peroxynitrite generation) formation in the myocardium of DOX-treated rodents and hypothesized that the peroxynitrite-induced increased nitration of key myocardial proteins may contribute to the DOX-induced depressed cardiac function. However, these studies only partially addressed the potential sources of superoxide and NO leading to peroxynitrite generation and have not addressed the possible interplays of these free radicals, activities of antioxidant enzymes, and their roles in DOX-induced cell death (both apoptotic and necrotic), which is a major determining factor of its cardiotoxicity.
The goal of the present study was to investigate the role and sources of superoxide, NO, and peroxynitrite in DOX-induced cell death both in vivo and in vitro. To achieve this goal, we used pharmacological tools, inducible NO synthase (iNOS) knockout mice, flow cytometry, fluorescent and confocal microscopy, biochemistry, and molecular biology techniques. We also used a novel flow cytometric method (32) allowing the simultaneous quantitative detection of mitochondrial superoxide generation with apoptosis markers in live cells to gain insight into the role of mitochondrial superoxide generation in DOX-induced cell death. Herein, by using these multiple tools, we provide unequivocal evidence that peroxynitrite is the major trigger of DOX-induced cell death in cardiomyocytes both in vivo and in vitro.
MATERIALS AND METHODS
Animals.
All protocols were approved by the Institutional Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male C57BL/6J, iNOS+/+, and iNOS−/− mice weighing ∼30 g were administered a single dose of DOX HCl (Sigma, St. Louis, MO) at 20 mg/kg ip and used for functional and biochemistry measurements 5 days later, when severe cardiac dysfunction was well established (10, 31, 37, 43, 45, 65). This time point was chosen because it represents >5 final half-lives of elimination of DOX from both plasma and cardiac tissue in mice (63). Treatment with the peroxynitrite decomposition catalyst iron α,β,γ,δ-tetrakis(4-N-methylpyridyl)porphine (FeTMPyP; 10 mg/kg ip) or the peroxynitrite decomposition catalyst/SOD mimetic mangenese α,β,γ,δ-tetrakis(4-N-methylpyridyl)porphine (MnTMTPyP; 10 mg/kg ip) [also termed peroxynitrite “scavengers” (PSs)] started 1.5 h before the DOX injection and was continued (10 mg·kg−1·day−1 in drinking water) until the hemodynamic measurements were made and animals were killed for the isolation of hearts. In a separate set of experiments, mice were killed 6 h, 1 day, 2 days, 3 days, and 5 days after DOX administration with or without FeTMPyP/MnTMPyP (same doses as mentioned above).
Reagents and cell culture.
DOX, sodium nitroprusside (SNP), hydrogen peroxide, SOD-polyethylene glycol (PEG), catalase-PEG, diphenylene iodonium (DPI), allopurinol, and apocynin were purchased from Sigma; JC-1, diethylenetriamine DETANONoate, peroxynitrite, peroxynitrite decomposition catalysts (FeTMPyP and MnTMPyP), nitrotyrosine monoclonal antibody, and NT affinity sorbent were from Cayman (Ann Arbor, Michigan); and iNOS inhibitors {S,S′-[1,3-phenylene-bis(1,2-ethanediyl)]bis-isothiourea (1,3-PB-ITU) and l-N6-(1-iminorthyl)-lysine (l-NIL)} were from AXXORA (San Diego, CA). Antibodies were as follows: actin monoclonal antibody and polyclonal NT antibody were from Chemicon (Temecula, CA); cleaved caspase-3 and cytochrome c were from Cell Signaling (Danvers, MA); iNOS was from BD Pharmingen (San Diego, CA); endothelial NOS (eNOS) and and neuronal NOS (nNOS) were from Santa Cruz Biotechnology (Santa Cruz, CA); and xanthine oxidase was from Abcam (Cambridge, MA). The microscopy and flow cytometry reagents tetramethylrhodamine ethyl ester (TMRE), Mitotracker green, MitoSOX red, 4-amino-5-methylamino-2′,7′-dihydroethidium (DHE), Hoechst 33342, rhodamine 11, bis(l-aspartic acid amide), Sytox green, and annexinV-APC were from Molecular Probes (Invitrogen, Carlsbad, CA).
Hemodynamic measurements using the pressure-volume conductance system in mice.
Left ventricular (LV) performance was analyzed in mice anesthetized with 2% isoflurane. Animals were placed on controlled heating pads, and their core temperature was measured via a rectal probe and maintained at 37°C. The trachea was cannulated, and animals were artificially ventilated using MiniVent respirator (Harvard Apparatus, Holliston, MA) at rates and tidal volumes adjusted to body weights. A 1-Fr microtip pressure-volume (P-V) catheter (PVR 1045, Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the LV as previously described (5, 31, 43, 45, 46). After stabilization for 20 min, signals were continuously recorded at a sampling rate of 1,000 s−1 using an ARIA P-V conductance system (Millar Instruments) coupled to a Powerlab/4SP analog-to-digital converter (AD Instruments, Mountain View, CA), stored, and displayed on a computer. All P-V loop data were analyzed using a cardiac P-V analysis program (PVAN3.6, Millar Instruments), and maximal LV systolic pressure (LVSP), maximal slope of the systolic pressure increment (+dP/dt), ejection fraction (EF), cardiac output (CO), and stroke work (SW) were computed. All hemodynamic parameters were calculated and corrected according to in vitro and in vivo volume calibrations (5, 31, 43, 45, 46). These parameters were also determined under conditions of changing preload, which was elicited by transiently compressing the inferior vena cava in ventilated anesthetized animals after the thoracotomy. Since +dP/dt may be preload dependent (17), in these animals, P-V loops recorded at different preloads were used to derive other useful systolic function indexes that may be less influenced by loading conditions and cardiac mass. These measures include the dP/dt-end-diastolic volume (EDV) relation (17), preload-recruitable SW (PRSW; which represents the slope of the relation between SW and EDV and is independent of chamber size and mass) (17), the end-systolic P-V relation, and maximal elastance (Emax) (35). After the hemodynamic measurements had been completed, animals were euthanized and tissue samples were collected.
Real-time PCR analyses.
Total RNA was isolated from tissue (heart) homogenates or from H9c2 cells using TRIzol LS reagents (Invitrogen) according to the manufacturer's instructions. Isolated RNA was treated with RNase-free DNase (Ambion, Austin, TX) to remove traces of genomic DNA contamination. Total RNA (1 μg) was reverse transcribed to cDNA using Super-Script II (Invitrogen). The target gene expression was quantified with gene-specific primers and iTaq Syber Green Mix (Bio-Rad, Hercules, CA) using the Bio-Rad Chromo 4/Opticon system. Each amplified sample was analyzed for homogeneity using melting curve analysis. Relative quantification was performed using the comparative threshold cycle method. The primers used are shown in Table 1.
Table 1.
Primers used in RT-PCR analysis
| Forward Primer | Reverse Primer | |
|---|---|---|
| iNOS | 5′-ATTCACAGCTCATCCGGTACG-3′ | 5′-GGATCTTGACCATCAGCTTGC-3′ |
| eNOS | 5′-CATTTTCGGACTCACATTGCG-3′ | 5′-TTGGTCAACCGAACGAAGTG |
| nNOS | 5′-CAGGCAAATCCCAAGCCTATG-3′ | 5′-CAAAGCACAGCCGAATTTCTC-3′ |
| NOX1 | 5′-TCGAACGCTACAGAAGAAGCC-3′ | 5′-TGGCAATCACTCCAGTAAGGC-3′ |
| NOX2 | 5′-GACCATTGCAAGTGAACACCC-3′ | 5′-AAATGAAGTGGACTCCACGCG-3′ |
| p22phox | 5′-ATGGAGCGATGTGGACAGAAG-3′ | 5′-TAGATCACACTGGCAATGGCC-3′ |
| p40phox | 5′-TTCAAAGACCTGCTAGCGCT-3′ | 5′-TCCTTCTGTGTGACATGCAGC-3′ |
| p47phox | 5′-TTCCATCCCCAAATGCAAAG-3′ | 5′-TCAGATGCCCTAAAACCGGAG-3′ |
| p67phox | 5′-GCCTTCACCAAAAGCATCAAC-3′ | 5′-ACCTCACAGGCAAACAGCTTG-3′ |
| Xanthine oxidase | 5′-AAAGGACCAGACGATTGCTCC-3′ | 5′-TCACACGTTCCCCTTCAAAAC-3′ |
| MMP-2 | 5′-CAAGGACCGGTTTATTTGGC-3′ | 5′-ATTCCCTGCGAAGAACACAGC-3′ |
| MMP-9 | 5′-TCTTCTGGCGTGTGAGTTTCC-3′ | 5′-CGGTTGAAGCAAAGAAGGAGC-3′ |
| Caspase-3 | 5′-GGACTGTGGCATTGAGACAG-3′ | 5′-CGACCCGTCCTTTGAATTTC-3′ |
| Caspase-9 | 5′-GGATGCTGTGTCAAGTTTGCC-3′ | 5′-CTTTCGCAGAAACAGCATTGG-3′ |
| Actin | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-GGATGCAGGGATGATGTTC-3′ |
iNOS, inducible nitric oxide synthase (NOS); eNOS, endothelial NOS; nNOS, neuronal NOS; NOX, NAD(P)H oxidase; MMP, matrix metalloproteinase.
Immunoprecipitation for NT detection.
Equal amounts of either 200 μg tissue homogenate or 400 μg cell lysate from each sample were incubated with 20 μg of NT affinity sorbent (NT antibody cross-linked to a protein A-agarose matrix) overnight at 4°C in a rotating wheel. The NT affinity sorbent is designed for immunoprecipitation of nitrated proteins from biological samples. Immunoprecipitates were washed five times with PBS containing 0.1% Triton X-100. Pellets were suspended in 1× Laemmli buffer with DTT. SDS-PAGE analysis and immunoblots were performed as described below followed by silver staining of protein gels. Silver staining (detection limit: 1 ng protein) was carried out as previously described (13, 34).
Immunoblot analyses.
Protein was extracted from tissue homogenates using RIPA lysis buffer containing protease inhibitor cocktail set III and phosphatase inhibitor cocktail set I (Calbiochem, EMD Biosciences, San Diego, CA). Equal amounts (40 μg/lane) were fractionated on NuPAGE 4–12% bis-Tris gel and transferred onto nitrocellulose membranes (Invitrogen) using a semidry transfer apparatus (Bio-Rad). Blocking was carried out for 2 h in 5% nonfat dry milk in PBS. Primary antibodies were added as per the manufacturer's recommended dilution in blocking buffer containing 0.1% Tween 20 at 4°C overnight. After three washes in PBS containing 0.1% Tween 20, secondary horseradish peroxidase conjugate (Pierce Biotechnology, Rockford, IL) was added followed by three washes with PBS containing 0.1% Tween 20. Blots were detected with Supersignal West Pico chemiluminescent substrate (Pierce Biotechnology) and developed using Kodak Biomax film (Perkin-Elmer, Wellesley, MA). All blots were normalized to the loading control (β-actin).
Mitochondrial membrane potential measurements by confocal microscopy.
For the determination of mitochondrial membrane potential, cells were loaded with 50 nM of the fluorescent potential-dependent indicator TMRE and the mitochondrial marker Mitotracker green for 30 min at 37°C. In an additional set of experiments, we also used ready-to-use JC-1 dye (as recommended by the supplier) for loading for 20 min. Digital images were taken by a LSM Pascal confocal microscope (Carl Zeiss) at a resolution of 2,048 × 2,048 pixels. Images were captured using either ×40 or ×100 objectives, and the optical section was <1 μm.
Flow cytometry.
Early apoptosis and cytotoxity were determined by flow cytometry using propidium iodide or Sytox green and annexin V staining (Molecular Probes) according to the manufacturer's recommendations. Flow cytometry analyses included 5,000 or 10,000 events using a FAScalibur (Becton Dickinson). For the quantitative determination of mitochondrial membrane potential, cells were loaded with 50 nM of the fluorescent potential-dependent indicator TMRE for 30 min at 37°C and collected after trypsinization. The fluorescence intensity was monitored at the FL-2 channel by FAScalibur, and 5,000 events were collected per sample. A mixture of the mitochondrial uncouplers FCCP (5 μM) and oligomycin A (10 μg/ml) was added to disrupt mitochondrial membrane potential as a negative control in flow cytometry experiments as previously described (42) for confocal microscopy. For the determination of mitochondrial superoxide by flow cytometry, cells were incubated with MitoSOX red as previously described (32, 33). All data were acquired and analyzed using Cell quest or Flow Jo (version 8.5) software.
Mitochondrial superoxide measurement from isolated myocardial mitochondria.
Mitochondria were isolated from the myocardium of mice treated with vehicle, DOX, or DOX + FeTMPyP/MnTMPyP using a tissue mitochondrial isolation kit (Pierce Biotechnology). Isolated mitochondria were allowed to load for 30 min with 5 μM MitoSOX red, and measurements were taken at the FL-2 channel using FACScalibur. Fragmented mitochondria (mitochondrial debris) with low forward and side scatter were excluded from analyses. Mitochondrial debris was generated due to the procedure/method used and was not statistically significant in the different groups (vehicle: 25.6 ± 12.5, DOX: 27.8 ± 11.3, DOX + FeTMPYP: 29.2 ± 9.7, and DOX + FeTMPYP: 22.2 ± 17.2, n = 4 for each group).
Fluorescence microscopy for caspase activation.
Cells were grown in sterile glass-bottom dishes (MatTek), washed with PBS, stained with the DNA-binding dyes Hoechst 33342 and rhodamine 110 as well as bis-l-aspartic acid amide (final concentration: 1 μM) for 15 min, washed three times with PBS, and then observed under a Olympus IX81 at ×150 magnification for all samples using fluorescence microscopy (31).
Confocal microscopy of frozen heart sections for the determination of superoxide production.
Hearts were snap frozen in Tissue-Tek embedding medium (Sakura,Torrance, CA), and sections were made at 10 μm using microtome at −25°C. Sections were air dried and hydrated with PBS. Sections were then incubated with 5 μM DHE at 37°C for 30 min as previously described (21).
Detection of NT using confocal microscopy.
Cells were processed similarly to the procedure described under Fluorescence microscopy for caspase activation and stained with NT antibody after being fixed in 4% paraformaldehyde-PBS for 15 min, washed twice in PBS, and permeabilized for 5 min in 0.1% Triton X-100-PBS. Secondary anti-rabbit/mouse FITC or Texas red conjugates were used. For mitochondrial staining, cells were loaded with TMRE and Mitotracker green for 30 min followed by three washes with PBS buffer containing 1% BSA according to the manufacturer's recommendations. Digital images were taken by a LSM Pascal confocal microscope (Carl Zeiss) at a resolution of 2,048 × 2,048 pixels. Images were captured using either ×40 or ×60 objectives and the optical section was <1 μm.
Determination of NT by ELISA and flow cytometry.
NT was measured by the NT ELISA kit from Hycult Biotechnology (Cell Sciences, Canton, MA), and values are presented as fold changes compared with vehicle. Intracellular NT was also determined in H9c2 cardiomyocytes using flow cytometry. After treatment, cells were fixed and permeabilized. After being blocked, cells were incubated with NT monoclonal antibody at 4°C overnight and secondary anti-mouse FITC conjugate for an additional 4 h (27).
Myocardial caspase-3 and caspase-3/7 activity.
Caspase-3 activities in tissue extracts were determined using a commercially available kit (Chemicon). In brief, the assay is based on measuring the amount of the chromophore p-nitroaniline (pNA) liberated from the labeled substrate DEVD-pNA at 405 nM. Caspase-3 activities are expressed as fold increases over control (31). Caspase-3/7 activities were also measured using the Apo-one Homogeneous Caspase-3/7 assay kit (Promega) according to the manufacturer's instructions.
Myocardial catalase assay.
Myocardial catalase activity was measured according to the manufacturer's recommendations (Cayman). Briefly, the catalase assay kit uses the peroxidatic function of catalase for the determination of enzyme activity. The method is based on the reaction of the enzyme with methanol in the presence of an optimal concentration of hydrogen peroxide. The formaldehyde produced is measured spectrophotometrically with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald) as the chromogen. Purpald specifically forms a bicyclic heterocycle with aldehydes, which, upon oxidation, changes from colorless to a purple color (61).
Myocardial SOD assays.
Myocardial SOD activity was measured according to the manufacturer's instructions (Trevigen, Gaithersburg, MD). In this colorimetry-based assay, superoxide ions are generated from the conversion of xanthine and oxygen to uric acid and hydrogen peroxide by xanthine oxidase. The superoxide anion then converts WST-1 to WST-1 formazan, a colored product that absorbs light at 450 nm. SOD reduces the superoxide ion concentration and thereby lowers the rate of WST-1-formazan formation. A reduction in the appearance of WST-1-formazan is a measure of SOD activity present in the experimental sample (18).
Myocardial glutathione peroxidase assay.
Glutathione peroxide enzyme activity were assayed using a SpectraMax spectophotometer according to the manufacturer's instructions (Trevigen). One unit of glutathione peroxidase is defined as the amount of the enzyme that will cause the oxidation of 1 nmol of NADPH to NADP+ per minute at 25°C (40).
Myocardial PARP activity.
PARP activity was assayed by colorimetry according to the manufacturer's instructions (Trevigen). The PARP Universal Colorimetric Assay Kit measures the incorporation of biotinylated poly(ADP-ribose) onto histone proteins in a 96-well plate and has sensitivity down to 0.01 units of PARP per well (1).
Immunohistological analysis of heart tissue.
Heart tissue was fixed in 4% buffered formalin. After tissue had been embedded and cut into 5-μm slices, all sections were stained with hematoxylin and eosin. Myeloperoxidase (MPO) staining of neutrophils was done using anti-MPO antibody (1:100 dilution, DAKO, Carpinteria, CA) (4, 52) according to the manufacturer's protocol, and samples were counterstained with hematoxylin. In other staining procedures, NT (1:100 dilution, Cayman) and iNOS (1:100 dilution, BD Bioscience) were stained according to the manufacturer's instructions and counterstained with nuclear fast red and hematoxylin, respectively. Histological evaluation was performed in a blinded manner.
Statistical analysis.
Results are reported as means ± SE. Statistical significance among groups was determined by one-way ANOVA followed by post hoc Newman-Keuls analysis using GraphPad Prism 4.3 software (San Diego, CA). P values of <0.05 were considered significant. Statistical analyses between two measurements were determined by the two-tailed unpaired Student's t-test. Correlations were determined by GraphPad Prism 4.3 software.
RESULTS
DOX induces increased myocardial iNOS, but not eNOS and nNOS, expression and enhanced myocardial NT generation: effects of PSs.
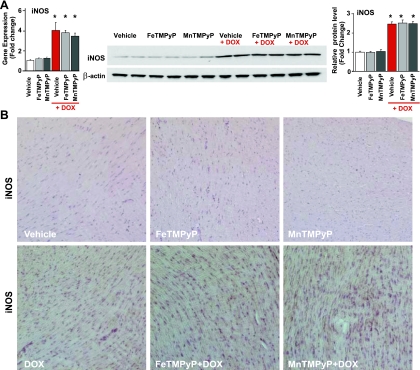
DOX induced ∼4- and ∼2.5-fold increases in myocardial iNOS (but not eNOS and nNOS) protein and mRNA expression (Fig. 1 A and Supplemental Fig. 3) at 5 days after exposure, which was not affected by PSs. DOX also increased iNOS expression in cardiomyocytes as measured by immunostaining (Fig. 1B) at day 5. DOX-induced significant increases in myocardial iNOS protein expression and NT generation and increases in caspase-3/7 activities were evident 1 day after exposure to the drug and peaked at around days 4–5 (Supplemental Fig. 1). PSs had no significant effects on iNOS protein expression; however, they attenuated the course of DOX-induced NT formation and decreased DOX-induced myocardial apoptosis.
Fig. 1.
Effects of doxorubicin (DOX) with or without peroxynitrite scavengers (PSs) on myocardial inducible nitric oxide (NO) synthase (iNOS) expression in vivo. A: DOX-induced increased myocardial iNOS mRNA (left) and protein (right) levels, which were not affected by PSs. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group for protein samples and n = 9 per group for mRNA samples. B: immunohistochemistry demonstrated widespread DOX-induced increased myocardial iNOS expression. FeTMPyP, iron α,β,γ,δ-tetrakis(4-N-methylpyridyl)porphine; MnTMPyP, mangenese α,β,γ,δ-tetrakis(4-N-methylpyridyl)porphine.
DOX increases myocardial ROS generation: effects of PSs.
DOX induced an approximately sixfold increase in superoxide/ROS generation as measured by DHE fluorescence using confocal microscopy from frozen sections, which was not significantly decreased by PSs (Fig. 2A). There were no statistically significant increases in the gene expression (expressed as fold changes compared with vehicle) of NAD(P)H oxidase isoform 1 (NOX1; 1.0 ± 0.17, 0.92 ± 0.18, 0.73 ± 0.14, and 0.91 ± 0.19), NAD(P)H oxidase isoform 2 (NOX2; 1.0 ± 0.09, 1.18 ± 0.23, 1.21 ± 0.26, and 1.1 ± 0.27), p22phox (1.0 ± 0.09, 0.78 ± 0.12, 0.91 ± 0.16, and 0.83 ± 0.18), p40phox (1.0 ± 0.19, 0.79 ± 0.14, 0.84 ± 0.21, and 0.81 ± 0.18), p47phox (1.0 ± 0.12, 1.1 ± 0.18, 0.92 ± 0.19, and 1.05 ± 0.17), and p67phox (1.0 ± 0.15, 1.31 ± 0.19, 1.26 ± 0.13, and 1.15 ± 0.27) in the vehicle-, DOX-, DOX + FeTMPyP-, and DOX + MnTMPyP-treated groups, respectively (n = 6 per group). Xanthine oxidase mRNA but not protein levels were increased to ∼1.8 ± 0.2-fold by DOX (Fig. 2B).
Fig. 2.
Effects of DOX with or without PSs on myocardial superoxide/ROS formation, its sources, and antioxidant enzyme activities in vivo. A: effects of PSs on DOX-induced superoxide/ROS formation from frozen heart sections using dihydroethidium (DHE). *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. B: effects of PSs on DOX-induced myocardial xanthine oxidase mRNA (left) and protein (right) expression. A representative blot from 3 sets of experiments is shown. The blot was also probed for β-actin as a loading control. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. C: effects of DOX with or without PSs on mitochondrial superoxide/ROS generation in isolated mitochondria. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. D: effects of DOX with or without PSs on myocardial SOD activity. n = 6 per group. E and F: effects of DOX with or without PSs on myocardial catalase (E) and glutathione peroxidase (F) activities. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group.
DOX treatment of mice markedly increased mitochondrial superoxide generation as measured from isolated cardiac mitochondria loaded with MitoSOX, which was not significantly attenuated by PSs (Fig. 2C).
To evaluate the possible role of SOD, catalase and glutathione peroxidase activities were measured (Fig. 2, D–F). SOD activity was not significantly different among all groups studied (Fig. 2D), but DOX decreased catalase and glutathione peroxidase activities, and these decreases were attenuated by PSs (Fig. 2, E and F).
DOX increases myocardial NT formation, matrix metalloproteinase-2/9 gene expression, and PARP activity, which are attenuated by PSs.
DOX induced a marked increase of myocardial NT formation (as measured using immunoprecipitaion of NT-modified protein) 5 days after DOX administration to mice, which was largely prevented by PSs (Fig. 3A; see also Supplemental Fig. 1 for ELISA data). Since matrix metalloproteinases (MMPs) and PARP are well-known downstream effectors of peroxynitrite/ROS-induced myocardial injury (41, 54), we also measured MMP-2 and MMP-9 gene expression and PARP activity from the myocardium of mice, which are known to be increased by DOX (3). DOX increased myocardial MMP-2 and MMP-9 mRNA expression and PARP activity by approximately two- to threefold, which were attenuated by FeTMPyP/MnTMPyP (Fig. 3, B and C).
Fig. 3.
Effects of DOX with or without PSs on myocardial nitrotyrosine (NT) formation, matrix metalloproteinase (MMP)-2 and MMP-9 gene expression, and poly(ADP-ribose) polymerase (PARP) and myeloperoxidase (MPO) activities in vivo. A: effects of PSs on DOX-induced NT formation from heart tissue homogenates. B: effects of PSs on DOX-induced myocardial MMP-2 and MMP-9 mRNA expression. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 9 per group. C: effects of PSs on DOX-induced myocardial PARP activity. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. D: effects of DOX with or without PSs on myocardial MPO staining. A representative sample from liver ischemia-reperfusion (I/R) injury with marked neutrophil infiltration as previously described (4, 52) and stained under the same conditions was used as a positive control.
DOX triggers myocardial apoptosis, which is markedly attenuated by PSs.
DOX treatment markedly increased (by 3- to 5-fold) cytochrome c release into the cytoplasm, myocardial caspase-3 cleavage, caspase-3 activity, caspase-3 and -9 gene expression, and DNA fragmentation as measured by quantitative TUNEL assay, which were markedly attenuated by PSs (Fig. 4, A–E).
Fig. 4.
Effects of DOX with or without PSs on myocardial apoptosis in vivo. A: effects of PSs on DOX-induced myocardial cytochrome c (Cyt-C) release from mitochondria. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. B: effects of PSs on DOX-induced myocardial caspase-3 activation. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. C: effects of PSs on DOX-induced caspase-3 activity. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. D: effects of PSs on DOX-induced myocardial DNA fragmentation (TUNEL assay). Eu, europium; AU, arbitrary units. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. E: effects of PSs on DOX-induced caspase-3 and caspase-9 gene expression. Data were analyzed using two housekeeping genes, and the data presented here were normalized to β-actin. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 9 per group.
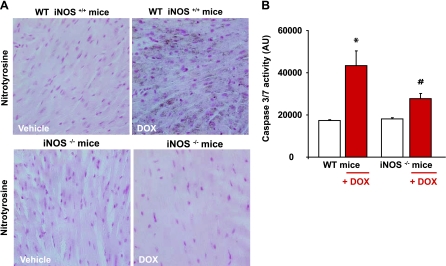
Attenuated DOX-induced myocardial NT formation and cell death in iNOS knockout mice compared with their wild-type littermates.
DOX induced a marked increase of myocardial NT formation and caspase-3/7 activity in wild-type mice, whereas these effects were significantly attenuated in iNOS knockout mice (Fig. 5, A and B).
Fig. 5.
Effects of DOX on myocardial NT generation and caspase-3/7 activity in iNOS knockout mice. A and B: decreased DOX-induced NT formation (A) and apoptosis (B) as measured by myocardial caspase-3/7 activity in iNOS−/− mice compared with wild-type (WT) mice. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 9 per group.
DOX-induced cardiac dysfunction is attenuated by PSs.
DOX-induced cardiac dysfunction fully developed after 3 days after the administration of the drug (Supplemental Fig. 2), consistently with other reports (31, 43–45, 53, 65), and was similar to that previously described in more chronic models of this cardiomyopathy (10, 43). PSs markedly improved the DOX-induced decline in various load-dependent indexes (+dP/dt, LVSP, EF, SW, and CO) and load-independent indexes (dP/dt-EDV, PRSW, and Emax) of myocardial contractility (Fig. 6, A and B).
Fig. 6.
Effects of DOX with or without PSs on myocardial function in vivo. A: effect of PSs on the DOX-induced depression of left ventricular systolic pressure (LVSP), maximal slope of the systolic pressure increment (+dP/dt), ejection fraction, stroke work (SW), and cardiac output in mice. B: effects of PSs on the DOX-induced depression of load-independent indexes of cardiac contractility [dP/dt-end-diastolic volume (EDV) relation, preload-recruitable SW (PRSW), and maximal elastance (Emax)]. Hemodynamic parameters were measured 5 days after DOX administration. Results are means ± SE of 6–12 experiments in each group. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX.
Effects of PSs on DOX-induced increased mitochondrial superoxide formation in vitro.
Treatment of H9c2 cells with DOX at 1 μM for 16 h induced an ∼5.7-fold increase in mitochondrial superoxide production. Treatment of cells with MnTMPyP, but not FeTMPyP, attenuated the DOX-induced mitochondrial superoxide generation (Fig. 7A).
Fig. 7.
Effects of DOX with or without PSs and/or iNOS inhibitors on mitochondrial superoxide generation and cell death in H9c2 cardiomyocytes in vitro. A: effects of DOX with or without PSs on cell death and mitochondrial superoxide generation in vitro as measured by quantitative flow cytometry. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. B: effects of iNOS inhibitors {S,S′-[1,3-phenylene-bis(1,2-ethanediyl)]bis-isothiourea (1,3-PB-ITU) and l-N6-(1-iminorthyl)-lysine (l-NIL)} on DOX-induced cell death in vitro. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 9 per group.
Effects of selective iNOS inhibitors on DOX-induced cell death.
The selective iNOS inhibitors 1,3-PB-ITU and l-NIL significantly attenuated DOX-induced cell death (Fig. 7B).
Effect of PSs on DOX-induced mitochondrial dysfunction, cell death, and peroxynitrite-dependent NT formation in vitro.
DOX dose dependently decreased mitochondrial membrane potential [as measured by confocal microscopy (Supplemental Fig. 4) or flow cytometry (Fig. 8A) using TMRE or by confocal microscopy using JC-1 (Fig. 8B)] and increased apoptosis in H9c2 cardiomyocytes (Figs. 9, A–C); these effects were largely preventable by PSs. PSs also prevented DOX- or peroxynitrite-induced increased NT formation and cell death in vitro (Figs. 9 and 10). DOX-induced NT formation was localized in the mitochondria, as shown by the yellow color in Fig. 11 (middle left overlay image), and was also present in the cytosol, as shown by the red color in Fig. 11 (middle left overlay image).
Fig. 8.
Effects of PSs on DOX-induced mitochondrial dysfunction in vitro. A: effects of PSs (200 μM) on DOX-induced dissipation of mitochondrial membrane potential (as measured by TMRE) in H9c2 cells using flow cytometry. Various controls are also shown at the far right, including vehicle without TMRE, vehicle with TMRE, and dissipation of mitochondrial potential by FCCP + oligomycin A (Oligo A) for 2 and 30 min after exposure. B: effects of PSs (200 μM) on DOX-induced dissipation of mitochondrial membrane potential measured in H9c2 cells loaded with JC-1 using confocal microscopy. Magnification: ×600.
Fig. 9.
Effects of PSs on DOX-induced apoptosis/necrosis in vitro. A: effects of PSs (200 μM) on DOX-induced apoptosis/necrosis as measured by flow cytometry in H9c2 cardiomyocytes. Representative data from 6 separate experiments were analyzed. PI, propidium iodide. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. B: effects of PSs on DOX-induced Cyt-C release as analyzed by Western blot from H9c2 cells. Shown is a representative blot from 3 separate experiments. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 6 per group. C: effects of PSs on DOX-induced active caspase expression (green) and the nuclear staining pattern by Hoechst 33342 dye (blue). Representative data from 10 experiments were analyzed. Magnification: ×150.
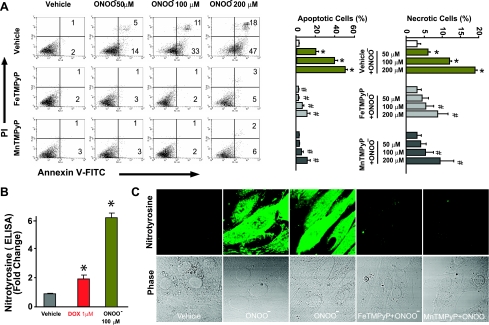
Fig. 10.
Effects of PSs on peroxynitrite-induced apoptosis/necrosis and NT generation in vitro. A: effects of PSs (200 μM) on peroxynitrite-induced apoptosis/necrosis as measured by flow cytometry in H9c2 cardiomyocytes. The treatment of peroxynitrite was described previously (Ref. 22). Representative data from 4 experiments were analyzed. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 4 per group. B: effects of DOX and peroxynitrite on NT formation in H9c2 cells as measured by quantitative ELISA. *P < 0.05 vs. vehicle; n = 4 per group. C: effects of PSs on peroxynitrite-induced NT formation (green). Representative data from 8 experiments are shown. Magnification: ×600.
Fig. 11.
Effects of DOX with or without PSs on intracellular NT formation. Shown are the effects of PSs on the intracellular localization of DOX-induced NT formation (red) and the mitochondrial dye Mitotracker green by confocal microscopy. Magnification: ×800.
NO donors enhance DOX-induced cell death and NT formation without major effects without DOX.
NO donors [DETA-NONOate (Figs. 12 and 13A) and SNP and SIN-1 (Supplemental Fig. 7)] enhanced DOX-induced cell death and NT formation (Fig. 13 and Supplemental Fig. 8) without major effects without DOX. DOX-induced increased NT formation positively correlated with apoptotic/necrotic cell death in H9c2 cells (Fig. 13).
Fig. 12.
Effects of the NO donor diethylenetriamine (DETA) NONOate on DOX-induced apoptosis/necrosis in vitro. A: effects of the NO donor DETA NONOate (30 and 100 μM) on DOX-induced apoptosis/necrosis as measured by flow cytometry in H9c2 cardiomyocytes. Representative data from 4 experiments were analyzed. B: percentages of apoptotic (top) and necrotic (bottom) cells. *P < 0.05 vs. vehicle; #P < 0.05 vs. DOX. n = 4 per group.
Fig. 13.
Effect of DOX with or without the NO donor DETA NONOate on cell death and intracellular NT generation in vitro. A: DOX dose dependently increased apoptosis and necrosis in H9c2 cardiomyocytes (left), which were drastically enhanced by the addition of the NO donor DETA NONOate (right). DETA NONOate by itself only slightly increased apoptosis but not necrosis at high concentrations. Similar results were observed with 2 additional NO donors (see supplemental Fig. 7). B: DOX dose dependently increased intracellular NT formation in H9c2 cardiomyocytes (left), which was enhanced by the addition of the NO donor DETA NONOate (left and right). DETANONOate by itself did not increase intracellular NT formation in H9c2 cardiomyocytes. C: correlation between intracellular NT formation (as measured by ELISA) and apoptosis/necrosis in H9c2 cardiomyocytes. The correlation analysis included vehicle-, DOX-, and DOX + DETA NONOate-treated cells. Each point in the graph represents a flow cytometry experiment.
DETA NONOate attentuates DOX-induced superoxide generation with concomitant increased cell death and increased intracellular NT generation.
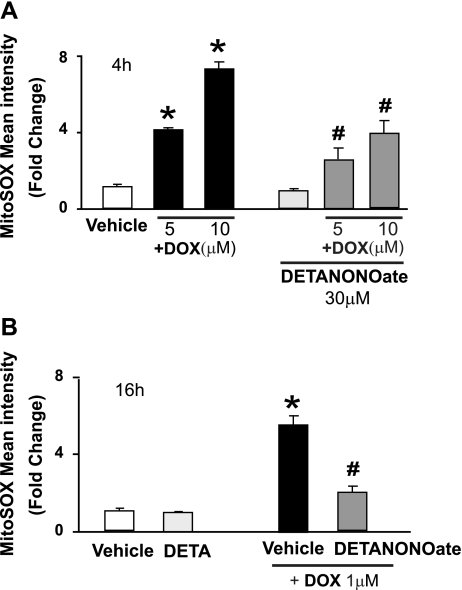
The NO donor DETA NONOate attenuated the DOX-induced superoxide generation (Fig. 14) with concomitant increased cell death and increased intracellular NT generation (see above and Figs. 12 and 13 and Supplemental Fig. 7).
Fig. 14.
Effects of NO donors on DOX-induced mitochondrial superoxide formation in vitro. Shown are the effects of NO donors in both short-term (4 h; A) and long-term (16 h; B) DOX-induced mitochondrial superoxide formation. *P < 0.001 vs. vehicle; #P < 0.001 vs. the corresponding DOX concentration. n = 6 per group.
DOX-induced cell death is attenuated by SOD-PEG but not by allopurinol, apocynin, DPI, or catalase-PEG.
DOX-induced cell death was attenuated by cell-permeable SOD-PEG but not the xanthine oxidase inhibitor allopurinol, the NADPH oxidase inhibitors apocynin and DPI, or cell-permeable catalase-PEG (Supplemental Figs. 5 and 6). In contrast, catalase-PEG attenuated hydrogen peroxide-induced dose-dependent cell death (Supplemental Fig. 6), indicating that hydrogen peroxide is not the main mediator of DOX-induced cell death in vitro. Consistently with several recent reports (e.g., Ref. 26), DPI by itself induced marked cell death (Supplemental Fig. 5), suggesting a serious note of caution in using this compound as a NADPH oxidase inhibitor for in vitro studies.
DISCUSSION
Because of its superior efficacy and broad-spectrum action, DOX continues to be a commonly used chemotherapeutic agent despite its cardiotoxicity (55). Preclinical and clinical studies have suggested that increased oxidative/nitrosative stress associated with an impaired antioxidant defense status plays a critical role in DOX-induced subcellular remodeling, Ca2+-handling abnormalities, and alteration of cardiac energetics, eventually culminating into cellular dysfunction and death, with subsequent cardiomyopathy and heart failure (3, 11, 28, 43–45, 56, 57, 60, 65).
Our results indicated that the mitochondrion is a pivotal source of superoxide generation after DOX exposure both in vivo and in vitro, and peroxynitrite formed in cardiomyocytes and most likely endothelial cells through diffusion-limited reaction of iNOS-derived NO and superoxide is a major trigger/mediator of DOX-induced apoptotic cell death, which is a key component of DOX-induced cardiotoxicity. Treatment with two PSs prevented the DOX-induced marked increase in myocardial apoptotic cell death as measured by multiple markers, contractile dysfunction, and myocardial NT formation in a well-established mouse model of DOX-induced acute heart failure in vivo. The time point of 5 days after a single dose of DOX for functional and other assessments was chosen because by this time, DOX is eliminated and no longer present in the blood or cardiac tissues (63). This is very important from the clinical point of view, because the cardiotoxicity of DOX in most patients develops a long time after the initial exposure to the drug.
NT formation was initially considered as a specific marker of in vivo peroxynitrite generation, but now it is rather used as a collective index of reactive nitrogen species, because other pathways inducing tyrosine nitration have also been proposed [e.g., MPO in certain inflammatory conditions (41)]. However, our experiments demonstrated that MPO cannot contribute to NT generation in this model, since normal and DOX-treated mouse hearts were MPO negative (Fig. 3D); thus, the increased NT most likely originates from increased endogenous peroxynitrite formation in our model. This was also supported by decreased NT formation in iNOS knockout mice treated with DOX (Fig. 5). We observed a time-dependent increase in myocardial NT formation after DOX exposure (Supplemental Fig. 1), which peaked at day 5, when the myocardial dysfunction was fully developed in our in vivo model (31, 43–45, 65) (Supplemental Fig. 2). This is also consistent with the highly significant inverse relationship between LV fractional shortening and cardiac NT immunoprevalence observed in a previous study (65).
Using flow cytometry, fluorescent and confocal microscopy, biochemistry, and molecular biology techniques, we also demonstrated that DOX, similarly to exogenously applied peroxynitrite (22), induces marked dose-dependent increases in cellular NT formation, dissipation of mitochondrial membrane potential, cytochrome c release, and execution of the mitochondrial phase of caspase-3-dependent apoptosis in H9c2 cardiomyocytes. Likewise, as observed in an in vivo model, these proapoptotic effects of DOX (similar to the effects of exogenously applied peroxynitrite) could be prevented by two different PSs in vitro (Figs. 9 and 10). DOX-induced NT formation was strongly colocalized with mitochondria and was also present throughout the cytosol in cardiomyocytes. Mitochondria are particularly vulnerable targets of peroxynitrite toxicity, and peroxynitrite can lead to alterations of mitochondrial energy and Ca2+ homeostasis and promotes the opening of the permeability transition pore, cytochrome c release, and execution of the mitochondrial phase of apoptosis (41, 51). In addition, oxidative/nitrosative stress may also impair various key mitochondrial enzymes of the respiratory chain (these enzymes are particularly sensitive targets of peroxynitrite-induced damage), leading to sustained ROS generation in mitochondria after the initial insult/injury (12, 29, 51). Since peroxynitrite is produced from the diffusion-controlled reaction between NO and superoxide anion, it can easily be formed in large amounts when superoxide and NO are produced simultaneously in close proximity (41, 49, 59).
It has previously been demonstrated using isolated heart mitochondria that DOX forms a complex with cardiolipin within the mitochondrial inner membrane of cardiomyocytes, where it is reduced by NADH dehydrogenase from the respiratory chain to form a semiquinone radical. The semiquinone radical is then oxidized back to the parental compound by passing the electron received onto molecular oxygen, forming a superoxide radical (11, 41). This is consistent with our present observation showing markedly increased superoxide generation in isolated cardiac mitochondria of DOX-treated mice or in mitochondria of live H9C2 myocytes exposed to DOX (32). The pivotal role of mitochondrial superoxide generation in DOX-induced cardiotoxicity is also supported by the lack of major changes in NOX1, NOX2, p22phox, p40phox, p47phox, p67phox, and xanthine oxidase expression in hearts of DOX-treated mice and the inability of allopurinol, apocynin, and DPI to attenuate DOX-induced cell death in vitro. However, some minor contribution of NAD(P)H and xanthine oxidase to in vivo superoxide generation cannot be ruled out, and a recent study (36) using cardiospecific eNOS knockout mice has also proposed that under such conditions, eNOS is uncoupled and produces superoxide rather than NO (36).
We found increased iNOS (but not eNOS and nNOS) protein and mRNA expression in the myocardium of DOX-treated mice, suggesting that iNOS is the predominant source of DOX-induced increased NO formation to generate peroxynitrite in the mitochondria and cytosol [NO can easily diffuse through cellular compartments and membranes to form peroxynitrite when superoxide is readily available (41)]. The increase in myocardial iNOS protein expression after DOX exposure peaked around days 4–5, when myocardial dysfunction and NT formation were also evident (Supplemental Fig. 1). In agreement with our results, in the same mouse model of DOX-induced acute heart failure, increased iNOS expression has been previously reported by immunohistochemistry (65), which was recently confirmed in both acute and chronic rodent models by other groups (2, 24, 38). This is also consistent with the better preservation of cardiac function in DOX-exposed iNOS knockout mice (43) compared with their wild-type littermates and impoved cardiac function and decreased DOX-induced histological damage in aminoguanidine-treated mice or rats (8, 30, 43). In contrast to all of these studies, Cole et al. (9), using iNOS knockout mice on different background, suggested that the inhibition of iNOS may be deleterious.
To further investigate the role of NO, iNOS, superoxide, and peroxynitrite in DOX-induced cell death, we studied the effect of two selective iNOS inhibitors (1,3-PB-ITU and l-NIL) on DOX-induced apoptosis in H9c2 cells using quantitative flow cytometry. These experiments revealed that iNOS inhibitors attenuated DOX-induced apoptosis in vitro, consistently with attenuated apoptosis in the myocardium of DOX-treated iNOS knockout mice compared with their wild types. Our results also demonstrated that various NO donors (DETA NONOate, SNP, and SIN-1) dramatically enhanced DOX-induced cell death with concomitant increases in intracellular NT formation while having only a modest effect in the absence of DOX. Importantly, the cellular NT content positively correlated with cell execution. Consistently, the natural phenolic antioxidant oleuropein protects against DOX-induced histological damage in an acute model of rat heart failure by reducing myocardial iNOS expression and NT formation (2). Furthermore, recent studies have also demonstrated that the cardiac-targeted expression of soluble Fas (38) or fluvastatin (53) attenuated DOX-induced cardiotoxicity by decreasing either iNOS expression or NT formation or both.
Collectively, our results suggest that superoxide is not the primary mediator of the mitochondrial phase of DOX-induced apoptosis, since prevention of the DOX-induced increase in NO by iNOS inhibition or by using iNOS knockout mice attenuated cell death but not the increase in mitochondrial superoxide generation induced by DOX. Furthermore, various NO donors when coadministered with DOX but not alone dramatically enhanced DOX-induced cell death and NT generation (Figs. 12 and 13 and Supplemental Fig. 7) with concomitant attenuation of mitochondrial superoxide formation (Fig. 14; because the excess NO reacted with DOX-induced increased superoxide to form peroxynitrite). Furthermore, in vitro, the mitochondrial superoxide generation occurs within minutes after DOX administration to cardiomyocytes or human or murine endothelial cells (32, 33), whereas cell death is evident only ∼8–14 h later (present study) when significant amounts of intracellular peroxynitrite is generated, which significantly correlates with cell death (both apoptotic and necrotic; Fig. 13C). DOX-induced increased superoxide/ROS generation is also an early event in vivo, as demonstrated by numerous studies (e.g., Refs. 6, 16, and 43), whereas more significant histological damage, cell death, and cardiac dysfunction peak only later (when DOX is no longer present in the circulation/myocardium), concomitantly with the increased myocardial NT formation (43, 53, 65) (Supplemental Figs. 1 and 2).
NO is not likely to be the trigger of apoptosis either, since NO donors by themselves (without DOX-induced increased superoxide production) have only modest effects on cell death and NT formation, whereas cell death is dramatically enhanced in the presence of DOX. It also appears that hydrogen peroxide is not involved in DOX-induced cell death, at least not in our in vitro system, since cell-permeable catalase was not able to attenuate DOX-induced cell death, whereas it almost completely prevented the cell death induced by various concentrations of hydrogen peroxide (Supplemental Fig. 6).
The most likely trigger of DOX-induced apoptosis/necrosis is peroxynitrite, since PSs effectively prevented not only DOX-induced cell death and contractile dysfunction but also reduce NT formation in cardiomyocytes, which positively correlates with cell death. This is further supported by the findings that PSs also attenuate the activation of known effector pathways for myocardial injury triggered by peroxynitrite (e.g., the activation of the nuclear enzyme PARP-1 and MMPs) in our DOX-induced heart failure model. Pharmacological inhibition of PARP or genetic deletion of PARP-1 is known to be protective in mouse models of DOX-induced cardiomyopathy/heart failure (44, 45), whereas the precise role and consequences of MMP activation (3, 19, 58) deserve additional exploration.
We propose that DOX initially rapidly increases mitochondrial superoxide and other ROS generation in cardiomyocytes and/or endothelial cells by redox cycling [marked DOX-induced superoxide/ROS generation already occurs in exposed cardiomyocytes and endothelial cells within several minutes after exposure (39, 64)], leading to the activation of transcription factor NF-κB (and perhaps other transcription factors to be explored in the future studies), which is an important early event after DOX exposure both in vitro and in vivo (39, 64). This is followed by increased NF-κB (or other transcription factor dependent) iNOS expression and consequent NO generation, the mechanisms of which should be evaluated in future studies. A minor contribution of other NOS isoforms to overall DOX-induced increased NO formation, e.g., in endothelial cells, cannot be excluded.
However, irrespective of the source of its generation, excess NO reacts with superoxide to form peroxynitrite both in the cytosol and mitochondria, inducing cell damage via lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration (e.g., proteins involved in the contractile function and mitochondrial respiration), and also activation of stress signaling pathways (41) [e.g., MAPK (50), which is also involved in DOX-induced cardiotoxicity (25)], MMPs (3, 19, 54, 58), and PARP-1 (41, 47). In the mitochondria, peroxynitrite, in concert with other ROS/reactive nitrogen species, may impair various key mitochondrial enzymes leading to more sustained intracellular ROS generation (persistent even after DOX is already metabolized and no longer present in the circulation/myocardium), triggering the further activation of transcription factor(s) and iNOS expression, resulting in an amplification of oxidative/nitrosative stress. In the mitochondria, peroxynitrite also triggers the release of proapoptotic factors such as cytochrome c and apoptosis-inducing factor, which mediate caspase-dependent and -independent apoptotic death pathways (41), which are also pivotal in DOX-induced cardiotoxicity. Peroxynitrite, in concert with other oxidants, also causes strand breaks in DNA, activating the nuclear enzyme PARP-1. Once excessive oxidative and nitrosative stress-induced DNA damage occurs, overactivated PARP initiates an energy-consuming cycle by transferring ADP-ribose units from NAD+ to nuclear proteins, resulting in the rapid depletion of intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading to cellular dysfunction and death, mostly by necrosis (41). Overactivated PARP may also facilitate the expression of a variety of inflammatory genes leading to increased inflammation and associated oxidative stress (48, 49), thus facilitating the progression of cardiovascular dysfunction and heart failure (Fig. 15). At a later stage of severe cardiac dysfunction/heart failure, numerous secondary pathways may also be activated [e.g., neuropeptides/neurohormones (angiotensin II, norepinephrine, and endothelin) or proinflammatory cytokines (TNF-α and IL-6)] acting directly on the myocardium or indirectly via changes in hemodynamic loading conditions to cause additional oxidative/nitrosative stress, endothelial and myocardial dysfunction, cardiac and vascular remodeling with hypertrophy, fibrosis, cardiac dilation, and myocardial necrosis, leading eventually to heart failure (47).
Fig. 15.
Schematic diagram of DOX-induced cardiotoxicity: role of superoxide, NO, and peroxynitrite. DOX initially increases mitochondrial superoxide and, consequently, the generation of other ROS (e.g., H2O2) in cardiomyocytes and/or endothelial cells by redox cycling. Increased DOX-induced ROS generation in cardiomyocytes triggers the activation of the transcription factor NF-κB, leading to enhanced iNOS expression and NO generation. NO reacts with superoxide to form peroxynitrite both in the cytosol and mitochondria, which, in turn, induces cell damage via lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration, and activation of stress signaling pathways (e.g., MAPK), MMPs, and PARP-1, among others. In the mitochondria, peroxynitrite, in concert with other ROS/reactive nitrogen species, impairs various key mitochondrial enzymes, leading to more sustained intracellular ROS generation (persistent even after DOX already metabolized), triggering further activation of transcription factor(s) and iNOS expression, resulting in the amplification of oxidative/nitrosative stress. In the mitochondria, peroxynitrite also triggers the release of proapoptotic factors (e.g., Cyt-C and apoptosis-inducing factor) mediating caspase-dependent and -independent cell death pathways, which are also pivotal in DOX-induced cardiotoxicity. Peroxynitrite, in concert with other oxidants, also causes strand breaks in DNA, activating the nuclear enzyme PARP-1. Once excessive oxidative and nitrosative stress-induced DNA damage occurs, overactivated PARP initiates an energy-consuming cycle by transferring ADP-ribose units from NAD+ to nuclear proteins, resulting in the rapid depletion of intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, eventually leading to cellular dysfunction and death, mostly by necrosis. Overactivated PARP may also facilitate the expression of a variety of inflammatory genes leading to increased inflammation (PARP-1 is a known coactivator of NF-κB) and associated oxidative stress, thus facilitating the progression of cardiovascular dysfunction and heart failure. PARG, poly(ADP-ribose) glycohydrolase.
Collectively, our data suggest that endogenous peroxynitrite formation is the major trigger of DOX-induced mitochondrial and PARP-dependent cell death in cardiomyocytes, and the modulation of the pathways leading to its generation or its effective neutralization can be of significant therapeutic benefit (Fig. 15). These results are in line with previous reports (41, 47, 49, 59) suggesting that in vivo peroxynitrite generation and/or protein nitration represents a crucial pathogenic mechanism in various cardiovascular pathophysiologies. Our results also suggest that the administration of NO donors should be avoided during the course of acute DOX infusion therapy, because of the increased chance of myocardial peroxynitrite formation and consequent cardiotoxicity.
GRANTS
This work was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism/National Institutes of Health (NIH) (to P. Pacher), NIH Grant R01-GM-60915 (to C. Szabó), and Swiss National Fund PP00B-68882/1 (to L. Liaudet).
Supplementary Material
Acknowledgments
This report is dedicated to P. Pacher's mother, Iren Bolfert, who died from cardiovascular complications of chemotherapy.
Footnotes
Supplemental material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Altmann SM, Muryshev A, Fossale E, Maxwell MM, Norflus FN, Fox J, Hersch SM, Young AB, MacDonald ME, Abagyan R, Kazantsev AG. Discovery of bioactive small-molecule inhibitor of poly ADP-ribose polymerase: implications for energy-deficient cells. Chem Biol 13: 765–770, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andreadou I, Sigala F, Iliodromitis EK, Papaefthimiou M, Sigalas C, Aligiannis N, Savvari P, Gorgoulis V, Papalabros E, Kremastinos DT. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol 42: 549–558, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bai P, Mabley JG, Liaudet L, Virág L, Szabó C, Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep 11: 505–508, 2004. [PubMed] [Google Scholar]
- 4.Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21: 1788–1800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, Csiszár A, Ungvári Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293: H909–H918, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaiswing L, Cole MP, St Clair DK, Ittarat W, Szweda LI, Oberley TD. Oxidative damage precedes nitrative damage in adriamycin-induced cardiac mitochondrial injury. Toxicol Pathol 32: 536–547, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 62: 4592–4598, 2002. [PubMed] [Google Scholar]
- 8.Cigremis Y, Parlakpinar H, Polat A, Colak C, Ozturk F, Sahna E, Ermis N, Acet A. Beneficial role of aminoguanidine on acute cardiomyopathy related to doxorubicin-treatment. Mol Cell Biochem 285: 149–154, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cole MP, Chaiswing L, Oberley TD, Edelmann SE, Piascik MT, Lin SM, Kiningham KK, St Clair DK. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res 69: 186–197, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Delgado RM 3rd, Nawar MA, Zewail AM, Kar B, Vaughn WK, Wu KK, Aleksic N, Sivasubramanian N, McKay K, Mann DL, Willerson JT. Cyclooxygenase-2 inhibitor treatment improves left ventricular function and mortality in a murine model of doxorubicin-induced heart failure. Circulation 109: 1428–1433, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261: 3068–3074, 1986. [PubMed] [Google Scholar]
- 12.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Dunn MJ Protein Purification Applications: a Practical Approach, edited by Harris ELV, Angal S. Oxford: Oxford Univ. Press, 1989, p. 34–35.
- 14.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Brit J Pharmacol 138: 532–543, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett 382: 223–228, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Jungsuwadee P, Cole MP, Sultana R, Joshi G, Tangpong J, Butterfield DA, St Clair DK, Vore M. Increase in Mrp1 expression and 4-hydroxy-2-nonenal adduction in heart tissue of Adriamycin-treated C57BL/6 mice. Mol Cancer Ther 5: 2851–2860, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation 76: 1422–1436, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther 9: 173–181, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kizaki K, Ito R, Okada M, Yoshioka K, Uchide T, Temma K, Mutoh K, Uechi M, Hara Y. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharmacol Res 53: 341–346, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3: 135–145, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res 98: 1479–1489, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Levrand S, Vannay-Bouchiche C, Pesse B, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite is a major trigger of cardiomyocyte apoptosis in vitro and in vivo. Free Radic Biol Med 41: 886–895, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li K, Sung RY, Huang WZ, Yang M, Pong NH, Lee SM, Chan WY, Zhao H, To MY, Fok TF, Li CK, Wong YO, Ng PC. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation 113: 2211–2220, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Li H, Qu H, Sun B. Nitric oxide synthase expressions in ADR-induced cardiomyopathy in rats. J Biochem Mol Biol 39: 759–765, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Mao W, Ding B, Liang CS. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H1956–H1965, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longpre JM, Loo G. Paradoxical effect of diphenyleneiodonium in inducing DNA damage and apoptosis. Free Radic Res 42: 533–543, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Marfella R, D'Amico M, Di Filippo C, Baldi A, Siniscalchi M, Sasso FC, Portoghese M, Carbonara O, Crescenzi B, Sangiuolo P, Nicoletti GF, Rossiello R, Ferraraccio F, Cacciapuoti F, Verza M, Coppola L, Rossi F, Paolisso G. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. J Am Coll Cardiol 47: 2444–2455, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Brit J Pharmacol 135: 581–588, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology 135: 1344–1357, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mostafa AM, Nagi MN, Al Rikabi AC, Al-Shabanah OA, El-Kashef HA. Protective effect of aminoguanidine against cardiovascular toxicity of chronic doxorubicin treatment in rats. Res Commun Mol Pathol Pharmacol 106: 193–202, 1999. [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50: 528–536, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay P, Rajesh M, Haskó G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukhopadhyay P, Roy KB. Protein engineering of BamHI restriction endonuclease: replacement of Cys54 by Ala enhances catalytic activity. Protein Eng 11: 931–935, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Nakano K, Sugawara M, Ishihara K, Kanazawa S, Corin WJ, Denslow S, Biederman RW, Carabello BA. Myocardial stiffness derived from end-systolic wall stress and logarithm of reciprocal of wall thickness. Contractility index independent of ventricular size. Circulation 82: 1352–1361, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, Furutani E, Perez-Sanz TM, Graveline A, Janssens SP, Picard MH, Scherrer-Crosbie M, Bloch KD. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 116: 506–514, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J 27: 1868–1875, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Niu J, Azfer A, Wang K, Wang X, Kolattukudy PE. Cardiac-targeted expression of soluble Fas attenuates doxorubicin-induced cardiotoxicity in mice. J Pharmacol Exp Ther 328: 740–748, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nozaki N, Shishido T, Takeishi Y, Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110: 2869–2874, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Ozdemir G, Ozden M, Maral H, Kuskay S, Cetinalp P, Tarkun I. Malondialdehyde, glutathione, glutathione peroxidase and homocysteine levels in type 2 diabetic patients with and without microalbuminuria. Ann Clin Biochem 42: 99–104, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J 20: 4107–4121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabó E, Ungvári Z, Wolin MS, Groves JT, Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107: 896–904, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Pacher P, Liaudet L, Bai P, Virág L, Mabley JG, Haskó G, Szabó C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 300: 862–867, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Pacher P, Liaudet L, Mabley JG, Cziraki A, Haskó G, Szabó C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med 17: 369–375, 2006. [PMC free article] [PubMed] [Google Scholar]
- 46.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacher P, Schulz R, Liaudet L, Szabó C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci 26: 302–310, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev 25: 235–260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pacher P, Szabó C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol 173: 2–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pesse B, Levrand S, Feihl F, Waeber B, Gavillet B, Pacher P, Liaudet L. Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol 38: 765–775, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33: 1451–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol 82: 1382–1389, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U, Kroemer HK, Schultheiss HP, Tschope C. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res 69: 695–699, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Schulz R Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Ann Rev Pharmacol Toxicol 47: 211–242, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207: 77–86, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Siveski-Iliskovic N, Kaul N, Singal PK. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation 89: 2829–2835, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: the role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res 69: 736–745, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Szenczi O, Kemecsei P, Holthuijsen MF, van Riel NA, van der Vusse GJ, Pacher P, Szabó C, Kollai M, Ligeti L, Ivanics T. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol 69: 725–732, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol 289: H701–H707, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Ueno M, Kakinuma Y, Yuhki K, Murakoshi N, Iemitsu M, Miyauchi T, Yamaguchi I. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci 101: 151–158, 2006. [DOI] [PubMed] [Google Scholar]
- 63.van der Vijgh WJ, Maessen PA, Pinedo HM. Comparative metabolism and pharmacokinetics of doxorubicin and 4′-epidoxorubicin in plasma, heart and tumor of tumor-bearing mice. Cancer Chemother Pharmacol 26: 9–12, 1990. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J 367: 729–740, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther 294: 396–401, 2000. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.