Abstract
Background
Although the effects of P deficiency on tea (Camellia sinensis (L.) O. Kuntze) growth, P uptake and utilization as well as leaf gas exchange and Chl a fluorescence have been investigated, very little is known about the effects of P deficiency on photosynthetic electron transport, photosynthetic enzymes and carbohydrates of tea leaves. In this study, own-rooted 10-month-old tea trees were supplied three times weekly for 17 weeks with 500 mL of nutrient solution at a P concentration of 0, 40, 80, 160, 400 or 1000 μM. This objective of this study was to determine how P deficiency affects CO2 assimilation, Rubisco, carbohydrates and photosynthetic electron transport in tea leaves to understand the mechanism by which P deficiency leads to a decrease in CO2 assimilation.
Results
Both root and shoot dry weight increased as P supply increased from 0 to 160 μM, then remained unchanged. P-deficient leaves from 0 to 80 μM P-treated trees showed decreased CO2 assimilation and stomatal conductance, but increased intercellular CO2 concentration. Both initial and total Rubisco activity, contents of Chl and total soluble protein in P-deficient leaves decreased to a lesser extent than CO2 assimilation. Contents of sucrose and starch were decreased in P-deficient leaves, whereas contents of glucose and fructose did not change significantly except for a significant increase in the lowest P leaves. OJIP transients from P-deficient leaves displayed a rise at the O-step and a depression at the P-step, accompanied by two new steps at about 150 μs (L-step) and at about 300 μs (K-step). RC/CSo, TRo/ABS (or Fv/Fm), ETo/ABS, REo/ABS, maximum amplitude of IP phase, PIabs and PItot, abs were decreased in P-deficient leaves, while VJ, VI and dissipated energy were increased.
Conclusion
P deficiency decreased photosynthetic electron transport capacity by impairing the whole electron transport chain from the PSII donor side up to the PSI, thus decreasing ATP content which limits RuBP regeneration, and hence, the rate of CO2 assimilation. Energy dissipation is enhanced to protect P-deficient leaves from photo-oxidative damage in high light.
Background
Phosphorus (P) is one of essential macronutrients required for the normal growth and development of higher plants. Plant roots acquire P as phosphate (Pi), primarily in the form of H2PO4-, from the soil solution [1]. Although total Pi is abundant in many soils, the available Pi in the soil solution is commonly 1 – 2 μM due to its binding to soil mineral surfaces and fixation into organic forms [2]. Hence, P is one of the unavailable and inaccessible macronutrients in the soil [1] and is often the most limiting mineral nutrient in almost all soils [2]. Among the fertility constraints to crop production in China, low Pi availability is the primary limiting factor [3]. Pi availability is particularly limiting on the highly weathered acid soils of the tropics and subtropics, in which free iron and aluminum oxides bind native and applied Pi into forms unavailable to plants [2,3]. Therefore, Pi availability is often a major limiting factor for crop production in acid soils [2].
P deficiency affects photosynthesis in many plant species, including tea (Camellia sinensis (L.) O. Kuntze) [4], satsuma mandarin (Citrus unshiu Marc.) [5,6], pigeon pea (Cajanus cajan L. Millsp.) [7], soybean (Glycine max (L.) Merr.) [8], white clover (Trifolium repens L.) [9], sugar beet (Beta vulgaris L.) [10], tomato (Lycopersicon esculentum Mill.) [11], bean (Phaseolus vulgaris L.) [12], maize (Zea mays L.), sunflower (Helianthus annuus L.) [13]. In pigeon pea (cv. UPAS 120) [7] and tea [4], stomatal closure was at least partly responsible for the decreased photosynthetic rate under P deficiency, because the intercellular CO2 concentration was decreased. However, the lower CO2 assimilation in P-deficient leaves of soybean [14] and bean [12] was primarily caused by non-stomatal factors as the lower assimilation rate coincided with an increase of the intercellular CO2 concentration and the internal to ambient CO2 concentration ratio, respectively. Decreases in the activity and amount of Rubisco due to P deficiency have been reported for spinach (Spinacia oleracea L.) [15,16], sunflower [13], maize [17] and soybean [14,18]. However, experiments with sugar beet [10,19] and maize [13] showed that the effects of P deficiency on photosynthetic rate acted through RuBP regeneration rather than Rubisco activity. Jacob and Lawlor [20] concluded that the decreased CO2 assimilation in P-deficient sunflower and maize leaves was a consequence of a smaller ATP content and lower energy charge which limited the production of RuBP. A feedback inhibition of photosynthesis has been suggested as a cause of decreased CO2 assimilation at low P supply [21,22]. However, for tomato plants a decrease in starch accumulation and an increase in oxygen sensitivity of CO2 fixation with decreasing P supply suggest that feedback limitation is decreased under P deficiency [11,23]. P deficiency may also limit photosynthetic rate by altering leaf Chl and protein contents [24,25]. However, the decreased photosynthetic rate under P deficiency was not accompanied by decreased contents of Chl and protein per unit leaf area [10,15].
All oxygenic photosynthetic materials investigated so far using direct, time-resolved fluorescence measurement show the polyphasic rise with the basic steps of O-J-I-P [26-28]. The OJIP transient has been found to be a sensitive indicator of photosynthetic electron transport processes [29]. The kinetics of the OJIP are considered to be determined by changes in the redox state of QA [28,30], but at the same time, the OJIP transient reflects the reduction of the photosynthetic electron transport chain [31]. The OJ phase represents the reduction of the acceptor side of PSII [29,31]. The JI phase parallels the reduction of the PQ-pool [29,32] and the IP phase represents the fractional reduction of the acceptor side of PSI or the last step in the reduction of the acceptor side of PSII and the amplitude of the IP phase may be a rough indicator of PSI content [31,33]. Reports concerning the effects of P deficiency on photosynthetic electron transport activity are some conflicting. Abadia et al. [34] reported that low P had no major effect on the structure and function of the photosynthetic electron transport system or on photosynthetic quantum yield of sugar beet leaves. Jacob and Lawor [20] concluded that in vivo photosynthetic electron transport did not limit photosynthetic capacity in P-deficient sunflower and maize leaves. However, P-deficient citrus exhibited a 6% decrease in Fv/Fm and a 49.5% decrease in electron transport rate [5]. Recently, Ripley et al. [35] reported that P deficiency decreased TRo/ABS (Fv/Fm), ETo/ABS of sorghum (Sorghum bicolor (L.) Moench) leaves, but had no significant effect on electron transport flux per RC (ETo/RC). Thus, it is not well known how P deficiency affects photosynthetic electron transport in plants.
Tea is an evergreen shrub native to China and is cultivated in humid and sub-humid of tropical, sub-tropical, and temperate regions of the world mainly on acid soils [4]. P deficiency is frequently observed in tea plantations [36,37]. For this reason, P fertilizers are being used annually in tea plantations in order to raise tea productivity and improve tea quality [4]. Although Salehi and Hajiboland [4] investigated the effects of P deficiency on tea growth, P uptake and utilization as well as leaf gas exchange and Chl a fluorescence, very little is known about the effects of P deficiency on photosynthetic electron transport, photosynthetic enzymes and carbohydrates of tea leaves. The objective of this study was to determine how P deficiency affects CO2 assimilation, Rubisco, non-structural carbohydrates and photosynthetic electron transport in tea leaves to understand the mechanism by which P deficiency leads to a decrease in CO2 assimilation.
Results
Leaf P content and plant growth characteristics
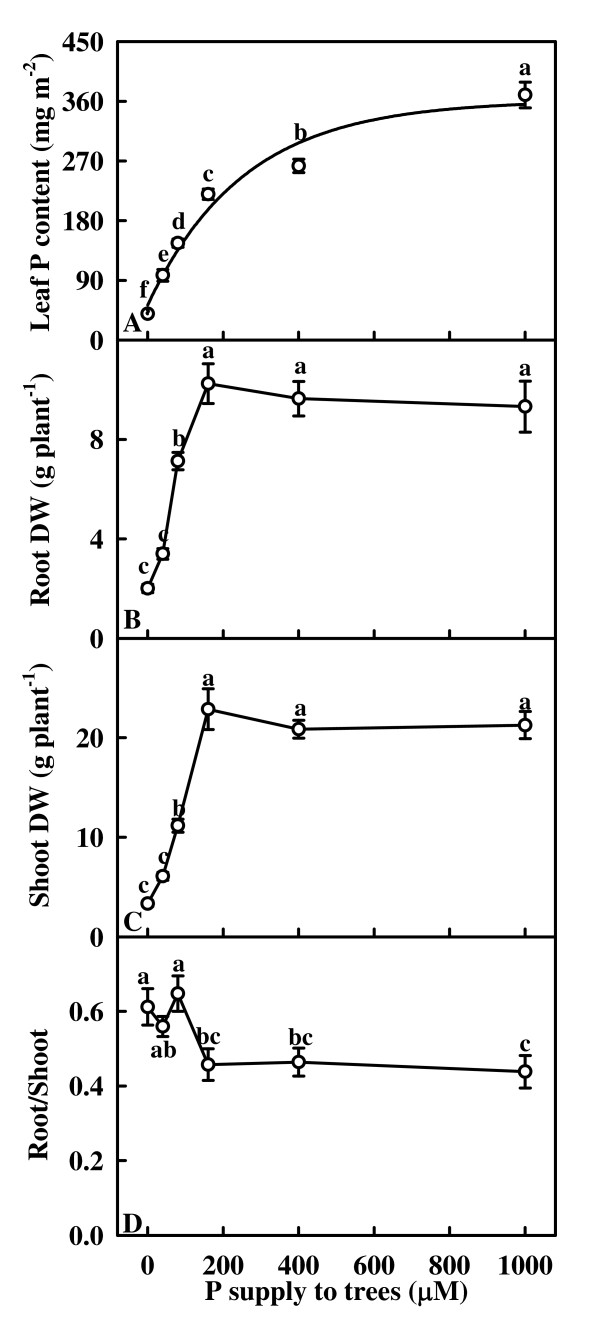
As P supply decreased, leaf P content decreased curvilinearly (Fig. 1A). Both root and shoot dry weight increased as P supply increased from 0 to 160 μM, then remained unchanged (Fig. 1B and 1C). The ratio of root/shoot dry weight in the 0 to 80 μM P-treated trees was higher than in the 160 μM to 1000 μM P-treated ones (Fig. 1D).
Figure 1.
Effects of phosphorus (P) supply on leaf P content (A), root dry weight (B), shoot dry weight (C) and root/shoot dry weight ratio (D) of tea trees. Each point is mean ± standard error (n = 5 or 6). Regression equations: (A) y = 361.3948 – 308.8565 e-0.0039x (r2 = 0.9690, P = 0.0055). Different letters above or below standard error bars indicate significant difference at P < 0.05.
Specific leaf weight, Chl, Car, total soluble protein and N
Specific leaf weight did not change significantly as leaf P content decreased from 369.3 mg m-2 to 97.5 mg m-2, then dropped significantly in the lowest P leaves (Fig. 2A). Leaf Chl (Fig. 2B), Car (Fig. 2C) and total soluble protein (Fig. 2D) contents did not change significantly as leaf P decreased from 369.3 mg m-2 to 146.0 mg m-2, then decreased with further decreasing leaf P content. Leaf N content remained little changed with decreasing leaf P content, except for a decrease in the lowest P leaves (Fig. 2D). The ratio of Chl a/b remained unchanged over the range of leaf P content examined (Fig. 2B). The ratio of Car/Chl remained relatively constant as leaf P content decreased, except for an increase in the lowest P leaves (Fig. 2C).
Figure 2.
Specific leaf weight (A), Chl content and Chl a/b ratio (B), carotenoid (Car) content and Car/Chl ratio (C), total soluble protein and N contents (D) in relation to P content in tea leaves. Each point is mean ± standard error for the leaf P content (horizontal, n = 6) and the dependent variable (vertical, n = 5 or 6). Different letters above or below standard error bars indicate significant difference at P < 0.05.
Leaf gas exchange and Rubisco
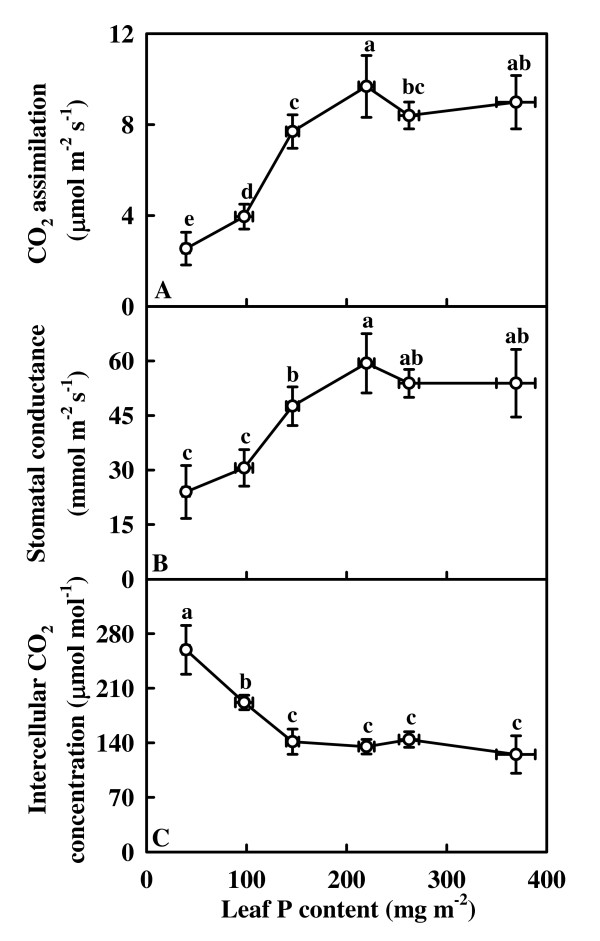
Both CO2 assimilation (Fig. 3A) and stomatal conductance (Fig. 3B) increased as leaf P content increased from 39.4 mg m-2 to 219.9 mg m-2, then remained relatively stable with further increasing leaf P content, whereas intercellular CO2 concentration decreased as leaf P content increased from 39.4 mg m-2 to 146.0 mg m-2, then did not change significantly with further increasing leaf P content (Fig. 3C).
Figure 3.
CO2 assimilation (A), stomatal conductance (B), and intercellular CO2 concentration (C) in relation to P content in tea leaves. Each point is mean ± standard error for the leaf P content (horizontal, n = 6) and the dependent variable (vertical, n = 5). Different letters above standard error bars indicate significant difference at P < 0.05.
On an area basis, both initial and total Rubisco activity kept relatively constant as leaf P content decreased from 369.3 mg m-2 to 219.9 mg m-2, then decreased with further decreasing leaf P content, whereas both initial and total activity expressed on a protein basis did not change significantly over the range of leaf P content examined, except for a slight decrease in initial activity in the lowest P leaves (Fig. 4A and 4B). Rubisco activation state remained unchanged as leaf P content decreased from 369.3 mg m-2 to 97.5 mg m-2, and then dropped in the lowest P leaves (Fig. 4C).
Figure 4.
Initial ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity (A), total Rubisco activity (B), and Rubisco activation state (C) in relation to P content in tea leaves. Each point is mean ± standard error for the leaf P content (horizontal, n = 6) and the dependent variable (vertical, n = 5). Different letters above or below standard error bars indicate significant difference at P < 0.05.
Leaf nonstructural carbohydrates
On an area basis, contents of glucose and fructose did not change significantly over the range of leaf P content examined except for a significant increase in the lowest P leaves (Fig. 5A and 5B). Contents of sucrose and starch remained little changed as leaf P content decreased from 369.3 mg m-2 to 219.9 mg m-2, then decreased with further decreasing leaf P content (Fig. 5C and 5D). When expressed on a dry weight basis, sucrose content did not change significantly as leaf P content decreased from 369.3 mg m-2 to 146.0 mg m-2 except for a decrease in the 39.4 mg m-2 and 97.5 mg m-2 P leaves (Fig. 5G), whereas the other results expressed on a dry weight basis were similar to those expressed on an area basis (Fig. 5E, 5F and 5H).
Figure 5.
Glucose (Glu, A and E), fructose (Fru, B and F), sucrose (Suc, C and G), and starch (D and H) contents expressed on an area (A-E) or DW (F-J) basis in relation to P content in tea leaves. Each point is mean ± standard error for the leaf P content (horizontal, n = 6) and the dependent variable (vertical, n = 6). Different letters above standard error bars indicate significant difference at P < 0.05.
Leaf OJIP transients and related parameters
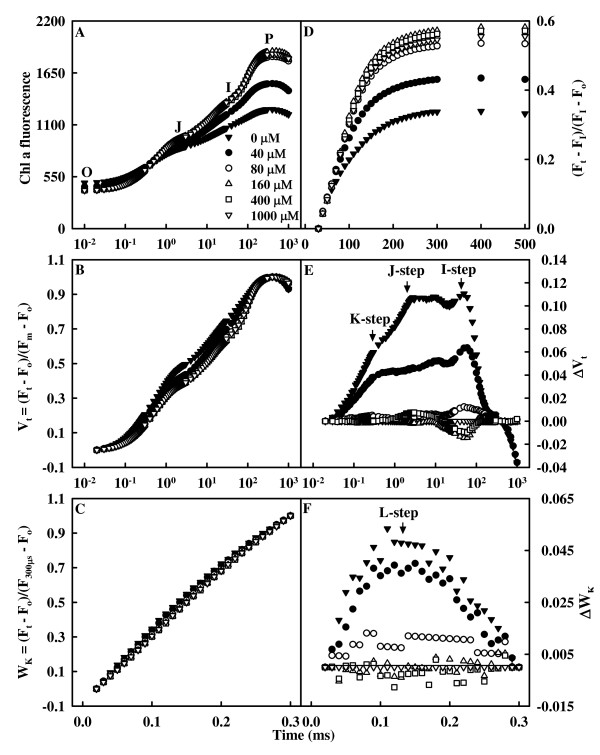
All OJIP transients showed a typical polyphasic rise with the basic steps of O-J-I-P. OJIP transients of leaves from 0 and 40 μM P-treated trees showed a rise at the O-step and a large depression at the P-step (Fig. 6A).
Figure 6.
Effects of P supply on the average Chl a fluorescence (OJIP) transients (average of 7 – 15 samples, A) and the different expressions of relative variable fluorescence: (B) between Fo and Fm: Vt = (Ft - Fo)/(Fm - Fo) and (E) the differences of the six samples to the reference sample treated with 1000 μM P (ΔVt), (C) between Fo and F300 μs: WK = (Ft - Fo)/(F300 μs - Fo) and (F) the differences of the six samples to the reference sample (ΔWK), (D) IP phase: (Ft - Fo)/(FI - Fo) - 1 = (Ft - FI)/(FI - Fo) [71] in dark-adapted tea leaves.
Fig. 6B and 6E shows the kinetics of relative variable fluorescence at any time Vt = (Ft - Fo)/(Fm - Fo) and the differences of normalized P-treated transients minus 1000 μM P-treated transient (ΔVt). The differences revealed three obvious bands: increase in the K-step (300 μs), in the 2 to 4 ms range J-step and in the 30 to 100 ms range I-step. The positive K-, J- and I-steps were very pronounced in the leaves from 0 and 40 μM P-treated trees. Fig. 6C and 6F depicts the relative variable fluorescence between Fo and F300 μs (WK) and the differences of normalized P-treated transients minus 1000 μM P-treated transient (ΔWK). The differences showed a clear L-step. OJIP transients from 0 to 80 μM P-treated trees had decreased maximum amplitude of IP phase and rise time, and the end-levels were lowered by P deficiency (Fig. 6D).
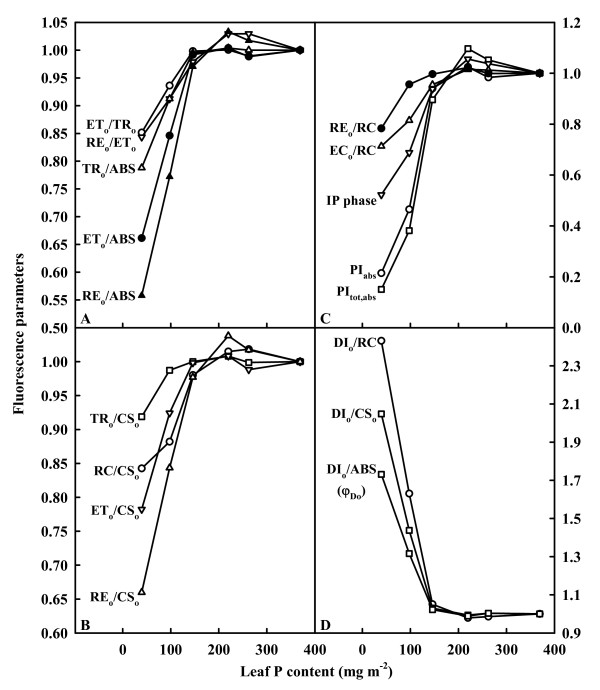
Fig. 7 depicts the behavior patterns of 17 fluorescence parameters. For each parameter the values were normalized on that of the sample treated with 1000 μM P. Generally speaking, leaves from 0 to 80 μM P-treated plants had decreased ETo/TRo, REo/ETo, TRo/ABS, ETo/ABS, REo/ABS (Fig. 7A), TRo/CSo, RC/CSo, ETo/CSo, REo/CSo (Fig. 7B), REo/RC, ECo/RC, maximum amplitude of IP phase, PIabs and PItot, abs (Fig. 7C), but increased DIo/RC, DIo/CSo and DIo/ABS (φDo) (Fig. 7D).
Figure 7.
Seventeen fluorescence parameters derived by the JIP-test from the average OJIP transients of Fig. 6A in relation to P content in tea leaves. All the values were expressed relative to the sample treated with 1000 μM P set as 1. Maximum amplitude of IP phase = (Fm - Fo)/(FI - Fo) - 1 [71].
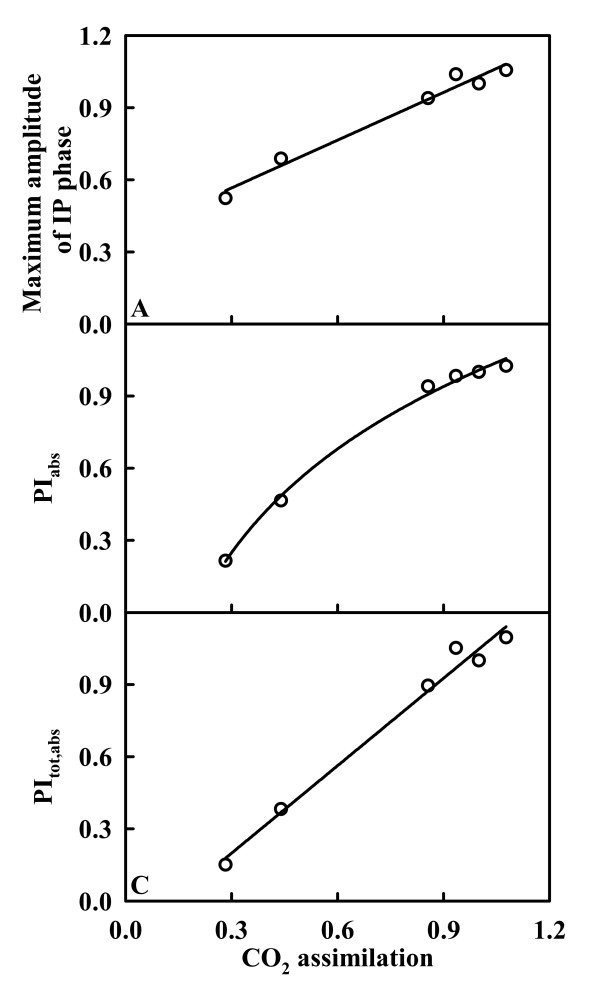
Leaf maximum amplitude of IP phase, PIabs and PItot, abs in relation to CO2 assimilation
Leaf CO2 increased linearly or curvilinearly with increasing maximum amplitude of IP phase (Fig. 8A), PIabs (Fig. 8B) and PItot, abs (Fig. 8C), respectively.
Figure 8.
Maximum amplitude of IP phase (A), PIabs (B) and PItot, abs (C) in relation to CO2 assimilation in tea leaves. All the values were expressed relative to the sample treated with 1000 μM P set as 1. Regression equations: (A) y = 0.5070 + 0.5208 × (r2 = 0.9556, P = 0.0007); (B) y = -11.9070 + 12.9149 x0.0503 (y2 = 0.9951, P = 0.0003); (C) y = -0.1650 + 1.2127 × (y2 = 0.9839, P < 0.0001).
Discussion
Our results showed that 0, 40 and 80 μM P treatments decreased root and shoot dry weight (Fig. 1B and 1C), and foliar P content for the three treatments was lower than the sufficiency range of 1.9 to 2.5 mg g-1 DW [38]. In addition, nearly all physiological and biochemical activities reached their maximum in the leaves of about 220 mg m-2 from 160 μM P-treated trees (Figs. 2, 3, 4, 5, 6, 7). Based on these results, trees treated with 0, 40 or 80 μM P are considered P deficient. P deficiency resulted in an increase in the ratio of root/shoot dry weight (Fig. 1D), as previously observed in different plant species growing under different growth conditions [10,39-42]. The increase of the root/shoot dry weight ratio in response to P deficiency may be associated with stronger sink competition of the roots for P and photosynthates [7,40,43-45].
Despite decreased CO2 assimilation, P deficiency causes increased starch content and decreased sucrose content in leaves of several plant species including soybean [44,46], tobacco (Nicotiana tabacum L.) [22], spinach, barley (Hordeum vulgare L.) [47] and Brachiaria hybrid [48]. Increased partitioning of photosynthetically fixed carbon into the starch at the expense of sucrose synthesis in leaves [22,44] and decreased demand from growth [22,46,49] have been shown to contribute to increased starch accumulation in P-deficient leaves. However, a simultaneous increase in starch and sucrose contents in the leaves of P-deficient soya (G. max (L.) Merr.) [47], bean [50] and sugar beet [51] plants has been observed while chloroplastic and leaf levels of sugar phosphates decreased markedly [19]. In our study, P-deficient leaves had decreased sucrose (Fig. 5C and 5G) and starch (Fig. 5D and 5H) contents, as previously found for trifoliate orange (Poncirus trifoliata (L.) Raf.), Swingle citrumelo (C. paradisi Macf. × P. trifoliata), Carrizo citrange (C. sinensis (L.) Osb. × P. trifoliata) [52] and rice (Oryza sativa L.) [48]. There appears to be considerable variation in the responses of leaf carbohydrate metabolism during P deficiency. Some of the variation may result from different degree of P deficiency, time of exposure to P deficiency, plant species, light intensities used in different studies [8,22,23,47,52]. It is noteworthy that specific leaf weight decreased in the lowest P leaves (Fig. 2A). This contrasts with previous data obtained for soybean [44] and sugar beet [10], whose leaves accumulated starch under P deficiency [10,44]. Regressive analysis showed that specific leaf weight decreased linearly with decreasing leaf starch content expressed on a leaf area basis (P = 0.0053, data not shown). Therefore, the decrease in specific leaf weight under P deficiency may be explained, at least in part, by the decrease in starch content.
The higher intercellular CO2 concentration in P-deficient leaves indicates that the low CO2 assimilation under P deficiency (Fig. 3A and 3C) is primarily caused by non-stomatal factors, as earlier reported for soybean [14] and bean [12]. However, Salehi and Hajiboland [4] proposed that lower stomatal conductance was the main cause for the decreased CO2 assimilation rate in P-deficient tea leaves as the decrease in assimilation rate was accompanied by a decrease in the intercellular CO2 concentration. Similar result has been obtained for pigeon pea (cv. UPAS 120) [7].
It has been suggested that low sink demand limits photosynthesis under P deficiency [21,22]. In our study, however, the decrease of assimilation CO2 rate under P deficiency was accompanied by a decrease in the starch accumulation (Fig. 3A, 5D and 5H), as previously reported for tomato grown in high light [23]. This indicates that the production, rather that the utilization of photosynthates, is limiting. Evidence shows that soluble sugars, specifically hexoses, may repress photosynthetic gene expression, particularly of the nuclear-encoded small sub-unit of Rubisco, thus decreasing Rubisco content and CO2 assimilation [53]. The lack of accumulation of sucrose and hexoses in the leaves from 40 and 80 μM P-treated trees (Fig. 5A–C and 5E–G) means that the feedback repression mechanism via accumulation of soluble sugars does not play a major role in determining the activity of Rubisco and the rate of CO2 assimilation in these leaves. However, this is not to deny that the decrease in CO2 assimilation in the lowest P leaves can be due to the accumulation of hexoses, because the levels of glucose + fructose observed was higher than the reported threshold level (4.5 mmol m-2) for hexose regulation of gene expression in tobacco [54]. The decrease in initial and total Rubisco activity expressed on an area basis in response to P deficiency was probably not the primary factor limiting CO2 assimilation, because there was a greater decrease in CO2 assimilation than in Rubisco activity (Fig. 3A, 4A and 4B). In our study, the observed lower initial and total Rubisco activity expressed on an area basis in P-deficient leaves could be associated with decreased total soluble protein content (Fig. 2D), because both initial and total activity expressed on a protein basis did not change significantly over the range of leaf P content examined, except for a slight decrease in the initial activity in the lowest P leaves (Fig. 4A and 4B). The decrease in CO2 assimilation in P-deficient leaves cannot be attributed to a decrease in Chl and protein contents, because the decrease in leaf Chl (Fig. 2B) and total soluble protein (Fig. 2D) contents was much less than CO2 assimilation (Fig. 3A). Similar results have been reported for spinach [15], sugar beet [10], and bean [12].
The presence of a positive L-step at ca. 150 μs in P-deficient leaves (Fig. 6F) means that the OJIP transients from P-deficient leaves are less sigmoidal than from P-sufficient ones and that the PSII units are less grouped or less energy is being exchanged between the independent PS II units. Because the grouped conformation is more stable than the ungrouped one, the decreased grouping implies that the PSII units of P-deficient leaves have lost stability and become more fragile. Similar results have been reported for N-deficient cowpea (Vigna unguiculata L.) [28] and Al-treated Citrus grandis (L.) Osbeck [55].
The decrease of Fv/Fm in P-deficient leaves was caused by both a decrease in Fm and an increase in Fo (Fig. 6A and 7A), as previously found for tea [4], satsuma mandarin [5] and sorghum [35]. The decrease in Fv/Fm under stress is considered to reflect the photoinhibitory damage to PSII complexes [56,57]. The higher Fo may be caused by both the damage of OEC and the inactivation of some of the PSII RCs [58,59], because P-deficient leaves had decreased RC/CSo (Fig. 7B) and increased damage to OEC, or it may be related to the accumulation of reduced QA [60], because the physiological fractional reduction of QA to QA -, as indicated by the increase in Mo (Fig. 6B and 6E), increased in P-deficient leaves. Quenching of Fm in P-deficient leaves may arise from the photoinhibitory quenching (qI), because an increase in Fo with a quenched Fm was observed in P-deficient leaves (Fig. 6A) [61] and from the xanthophyll cycle-dependent thermal energy dissipation, which was significantly higher in P-deficient satsuma mandarin leaves than in P-sufficient ones [6].
The J-step, I-step and IP phase of OJIP transients are correlated with the redox state of QA, the redox state of plastoquinone, and the redox state of end acceptors at PSI electron acceptor side, respectively [27,28,30,32]. The finding that P-deficient leaves had increased VJ and VI (Fig. 6B and 6E), but decreased maximum amplitude of IP phase (Fig. 6D) suggests that acceptor side of PSII became more reduced under P deficiency, but the acceptor side of PSI become more oxidized. P deficiency-induced photoinhibitory damage at PSII acceptor is also supported by the fact that Fv (Fv = Fm - Fo) was decreased in P-deficient leaves along with an increase in Fo (Fig. 6A), which is the characteristic of photoinhibitory damage at PSII acceptor side [62]. A positive K-step appeared at ca. 300 μs in the OJIP transients in P-deficient leaves. This means that the oxygen evolving complex (OEC) is damaged [63,64]. A positive K-step has also been found in N-deficient cowpea leaves [28].
Our result showed that P deficiency decreased the total electron carriers per RC (ECo/RC; Fig. 7C), the yields (TRo/ABS (Fv/Fm), ETo/TRo, REo/ETo, ETo/ABS, and REo/ABS; Fig. 7A), the fluxes (REo/RC and REo/CSo; Fig. 7B and 7C) and the fractional reduction of the PSI end electron acceptors, as indicated by the decreased maximum amplitude of IP phase (Fig. 6D), and damaged all of the photochemical and non-photochemical redox reactions, as indicated by the decreases in PIabs and PItot, abs (Fig. 7D). This means that leaves from P-deficient trees have a decreased capacity for electron transport, thus limiting ATP synthesis and RuBP regeneration. Lacking ATP has the consequence that Rubisco is not fully activated [65]. This might partly explain why P-deficient leaves had lower Rubisco activity and activation state (Fig. 4). Regressive analysis showed that CO2 assimilation decreased linearly or curvilinearly with decreasing maximum amplitude of IP phase (Fig. 8A), PIabs (Fig. 8B) and PItot, abs (Fig. 8C), respectively. Therefore, we conclude that the decreased photosynthetic electron transport capacity, in conjunction with the lack of ATP which limit RuBP regeneration are probably the main factors contributing to decreased CO2 assimilation under P deficiency.
Because P-deficient leaves only utilized a small fraction of the absorbed light energy in photosynthetic electron transport, as indicated by the decreases in ECo/RC, ETo/ABS and REo/ABS (Fig. 7A and 7C), compared with the P-sufficient ones, more excess excitation energy existed in P-deficient than in P-sufficient leaves in high light. Correspondingly, energy dissipation, as indicated by DIo/CSo, DIo/RC, and DIo/ABS (φDo), increased in P-deficient leaves (Fig. 7D). In addition to this, the excess absorbed light in turn can lead to the production of 1O2 and reduced active oxygen species, causing damage to photosynthetic apparatus and cell structure [35,66]. Indeed, photoinhibitory damage to both donor side and acceptor side has been demonstrated to increase the production of reactive oxygen species [61,67].
Conclusion
P deficiency decreased photosynthetic electron transport capacity by impairing the whole electron transport chain from the PSII donor side up to the PSI, thus decreasing ATP content which limits RuBP regeneration, and hence, the rate of CO2 assimilation. In addition to decrease light absorption by lowering Chl content, energy dissipation is enhanced to protect P-deficient leaves from photo-oxidative damage in high light.
Methods
Plant culture and P treatments
This study was conducted outdoors from March to November 2007 at Fujian Agriculture and Forestry University, Fuzhou. Own-rooted 10-mouth-old uniform tea (Camellia sinensis (L.) O. Kuntze cv. Huangguanyin) trees were transplanted into 6 L plastic pots containing sand. Each pot contained two trees, and was supplied twice weekly with 500 mL of 1/2 strength nutrient solution. Full-strength nutrient solution contained 1 mM (NH4)2SO4, 0.8 mM K2SO4, 1 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4H2PO4, 0.05 mM CaCl2, 0.6 mM MgSO4, 46 μM H3BO3, 9 μM MnSO4, 9 μM ZnSO4, 2 μM CuSO4, 2.6 μM Na2MoO4, and 30 μM Fe-EDTA. Six weeks after transplanting, the treatment was applied for 17 weeks: until the end of the experiment, each pot was supplied three times weekly with 500 mL of nutrient solution at a P concentration of 0, 40, 80, 160, 400 or 1000 μM from NH4H2PO4 at pH of 5.5. N concentration was maintained at a constant by the addition of (NH4)2SO4. At the end of the experiment, the fully-expanded (about seven weeks old) leaves from different replicates and treatments were used for all the measurements. Leaf discs (0.61 cm2 in size) were collected at noon under full sun and immediately frozen in liquid N2. Samples were stored at -80°C until they were used for the determination of Chl, carotenoids (Car), Rubisco, carbohydrates, and protein. Special care was taken to ensure that all samples were transferred directly from liquid N2 to freezer of -80°C, at no time were any samples exposed to room temperature.
Measurements of root and shoot dry weight, and specific leaf weight
At the end of the experiment, six trees per treatment from different pots were harvested. The trees were divided into roots and shoots. The plant materials were then dried at 80°C for 48 h and the dry weight measured. Specific leaf weight was calculated as the ratio of leaf dry weight to leaf area.
Determination of leaf Chl, Car, total soluble protein, and total P
Chl, Chl a, Chl b and Car were assayed according to Lichtenthaler [68]. Total soluble protein was determined according to Bradford [69]. Total P was determined according to Fredeen et al. [44].
Leaf gas exchange measurements
Measurements were made with a CI-301PS portable photosynthesis system (CID, WA, USA) at ambient CO2 concentration with a natural photosynthetic photon flux density of 1500 ± 45 μmol m-2 s-1 between 10:30 and 12:00 on a clear day. During measurements, leaf temperature and ambient vapor pressure were 28.0 ± 1.0°C and 1.8 ± 0.1 kPa, respectively.
Measurements of leaf OJIP transients
OJIP transient was measured by a Handy Plant Efficiency Analyser (Handy PEA, Hansatech Instruments Limited, Norfolk, UK) according to Strasser et al. [26]. The transient was induced by red light of about 3,400 μmol m-2 s-1 provided by an array of three light-emitting diodes (peak 650 nm), which focused on the leaf surface to give homogenous illumination over the exposed area of the leaf. All the measurements were done with 3 h dark-adapted plants at room temperature.
JIP test
OJIP transient was analyzed according to the JIP test. From OJIP transient, the extracted parameters (Fm, F20 μs, F50 μs, F100 μs, F300 μs, FJ, FI etc.) led to the calculation and derivation of a range of new parameters according to pervious authors [27,28,55,70,71] (see Table 1).
Table 1.
Summary of parameters, formulae and their description using data extracted from chlorophyll a fluorescence (OJIP) transient.
| Fluorescence parameters | Description |
| Fluorescence parameters | Description |
| Ft | Fluorescence intensity at time t after onset of actinic illumination |
| F50 μsor F20 μs | Minimum reliable recorded fluorescence at 50 μs with the PEA- or 20 μs with Handy-PEA-fluorimeter |
| F100 μs and F300 μs | Fluorescence intensity at 100 and 300 μs, respectively |
| FJ and FI | Fluorescence intensity at the J-step (2 ms) and the I-step (30 ms), respectively |
| FP (= Fm) | Maximum recorded (= maximum possible) fluorescence at P-step |
| Area | Total complementary area between fluorescence induction curve and F = Fm |
| Derived parameters | |
| Selected OJIP parameters | |
| F0 ≅ F50 μsor F0 ≅ F20 μs | Minimum fluorescence, when all PSII RCs are open |
| Fm = FP | Maximum fluorescence, when all PSII RCs are closed |
| VJ = (F2 ms - Fo)/(Fm - Fo) | Relative variable fluorescence at the J-step (2 ms) |
| VI = (F30 ms - Fo)/(Fm - Fo) | Relative variable fluorescence at the I-step (30 ms) |
| Mo = 4 (F300 μs - Fo)/(Fm - Fo) | Approximated initial slope (in ms-1) of the fluorescence transient V = f(t) |
| Sm = ECo/RC = Area/(Fm - Fo) | Normalized total complementary area above the OJIP (reflecting multiple-turnover QA reduction events) or total electron carriers per RC |
| Yields or flux ratios | |
| φPo = TRo/ABS = 1-(Fo/Fm) = Fv/Fm | Maximum quantum yield of primary photochemistry at t = 0 |
| φEo = ETo/ABS = (Fv/Fm) × (1 - VJ) | Quantum yield for electron transport at t = 0 |
| ψEo = ETo/TRo = 1-VJ | Probability (at time 0) that a trapped exciton moves an electron into the electron transport chain beyond QA- |
| φDo = DIo/ABS = 1-φPo = Fo/Fm | Quantum yield at t = 0 for energy dissipation |
| δRo = REo/ETo = (1 - VI)/( - VJ) | Efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors |
| φRo = REo/ABS = φPo × ψEo× δRo φ | Quantum yield for the reduction of end acceptors of PSI per photon absorbed |
| Specific fluxes or activities expressed per reaction center (RC) | |
| ETo/RC = (Mo/VJ) × ψEo = (Mo/VJ) × (1-VJ) | Electron transport flux per RC at t = 0 |
| DIo/RC = (ABS/RC) - (TRo/RC) | Dissipated energy flux per RC at t = 0 |
| REo/RC = (REo/ETo) × (ETo/RC) | Reduction of end acceptors at PSI electron acceptor side per RC at t = 0 |
| ETo/CSo = (ABS/CSo) × φEo | Electron transport flux per CS at t = 0 |
| TRo/CSo = (ABS/CSo) × φPo | Trapped energy flux per CS at t = 0 |
| DIo/CSo = (ABS/CSo) - (TRo/CSo) | Dissipated energy flux per CS at t = 0 |
| REo/CSo = (REo/ETo) × (ETo/CSo) | Reduction of end acceptors at PSI electron acceptor side per CS at t = 0 |
| Density of RCs | |
| RC/CSo =φPo × (ABS/CSo) × (VJ/Mo) | Amount of active PSII RCs per CS at t = 0 |
| Performance index | |
| PIabs = (RC/ABS) × (φPo/(1 - φPo)) × (ψo/(1 - ψo)) | Performance index (PI) on absorption basis |
| PItot, abs = (RC/ABS) × (φPo/(1-φPo)) × (ψEo/(1 - ψEo)) × (δRo/(1 - δRo)) | Total PI, measuring the performance up to the PSI end electron acceptors |
Leaf Rubisco activity measurements
Rubisco was extracted according to Chen et al. [72]. Rubisco activity was assayed according to Cheng and Fuchigami [73] with some modifications. For initial activity, 50 μL of sample extract was added to a cuvette containing 900 μL of an assay solution, immediately followed by adding 50 μL of 10 mM RuBP, then mixing well. The change of absorbance at 340 nm was monitored for 40 s. For total activity, 50 μL of 10 mM RuBP was added 15 min later, after 50 μL of sample extract was combined with 900 μL of an assay solution to fully activate all the Rubisco. The assay solution for both initial and total activity measurements, whose final volume was 1 mL, contained 100 mM HEPES-KOH (pH 8.0), 25 mM KHCO3, 20 mM MgCl2, 3.5 mM ATP, 5 mM phosphocretaine, 5 units NAD-glyceraldehyde-3-phosphate dehydrogenase (NAD-GAPDH, EC 1.2.1.12), 5 units 3-phosphoglyceric phospokinase (PCK, EC 2.7.2.3), 17.5 units creatine phosphokinase (EC 2.7.3.2), 0.25 mM NADH, 0.5 mM RuBP, and 50 μL sample extract. Rubisco activation state was calculated as the ratio of initial activity to total activity.
Measurements of leaf nonstructural carbohydrates
Sucrose, fructose, glucose and starch were extracted 3 times with 80% (v/v) ethanol at 80°C and determined according to Jones et al. [74].
Experimental design and statistical analysis
There were 20 pots trees per treatment in a completely randomized design. Experiments were performed with 5–15 replicates (one tree from different pots per replicate). Differences among treatments were separated by the least significant difference (LSD) test at P < 0.05 level.
Abbreviations
Chl: chlorophyll; CS: excited cross section; ETo/ABS: quantum yield of electron transport at t = 0; N: nitrogen; OJIP: Chl a fluorescence; P: phosphorus; PIabs: performance index; PItot, abs: total performance index; RC: reaction center; RC/CSo: amount of active PSII RCs per CS at t = 0; REo/ABS: quantum yield of electron transport from QA- to the PSI end electron acceptors; Rubisco: ribulose-1,5-bisphosphate carboxylase/oxygenase; RuBP: ribulose-1,5-bisphosphate; TRo/ABS or Fv/Fm: maximum quantum yield of primary photochemistry at t = 0; VI: relative variable fluorescence at the I-step; VJ: relative variable fluorescence at the J-step.
Authors' contributions
ZHL performed most of the experiments and wrote the manuscript. LSC designed and directed the study and revised the manuscript. RBC helped in designing the study. FZZ helped in making nutrient solution and cultivating trees. HXJ and NT helped in measuring CO2 assimilation and Chl a fluorescence. All authors have read and approved the final manuscript.
Contributor Information
Zheng-He Lin, Email: linzhenghe@126.com.
Li-Song Chen, Email: lisongchen2002@hotmail.com.
Rong-Bing Chen, Email: rb_chen@163.com.
Fang-Zhou Zhang, Email: zfz36@163.com.
Huan-Xin Jiang, Email: jianghx@163.com.
Ning Tang, Email: sabrina-0810@hotmail.com.
References
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plant tolerate acid soils? Mechanisms of aluminum tolerate and phosphorous efficiency. Annu Rev Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Yan X, Wu P, Ling H, Xu G, Xu F, Zhang Q. Plant nutriomics in China: an overview. Ann Bot. 2006;98:473–482. doi: 10.1093/aob/mcl116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi SY, Hajiboland R. A high internal phosphorus use efficiency in tea (Camellia sinensis L.) plants. Asian J Plant Sci. 2008;7:30–36. [Google Scholar]
- Guo Y-P, Chen P-Z, Zhang L-C, Zhang S-L. Effects of different phosphorus nutrition levels on photosynthesis in satsuma mandarin (Citrus unshiu Marc.) leaves. Plant Nutr Fert Sci. 2002;8:186–191. [Google Scholar]
- Guo Y-P, Chen P-Z, Zhang L-C, Zhang S-L. Phosphorus deficiency stress aggravates photoinhibition of photosynthesis and function of xanthophyll cycle in citrus leaves. Plant Nutr Fert Sci. 2003;9:359–363. [Google Scholar]
- Fujita K, Kai Y, Takayanagi M, El-Shemy H, Adu-Gyamfi JJ, Mohapatra PK. Genotypic variability of pigeonpea in distribution of photosynthetic carbon at low phosphorus level. Plant Sci. 2004;166:641–649. [Google Scholar]
- Qiu J, Israel DW. Carbohydrate accumulation and utilization in soybean plants in response to altered phosphorus nutrition. Physiol Plant. 1994;90:722–728. [Google Scholar]
- Hart AL, Greer DH. Photosynthesis and carbon export in white clover plants grown at various levels of phosphorus supply. Physiol Plant. 1988;73:46–51. [Google Scholar]
- Rao IM, Terry N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet: I. Changes in growth, gas exchange, and Calvin cycle enzymes. Plant Physiol. 1989;90:814–819. doi: 10.1104/pp.90.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot CC, Boogaard R van den, Marcelis LFM, Harbinson J, Lambers H. Contrasting effects of N and P deprivation on the regulation of photosynthesis in tomato plants in relation to feedback limitation. J Exp Bot. 2003;54:1957–1967. doi: 10.1093/jxb/erg193. [DOI] [PubMed] [Google Scholar]
- Lima JD, Mosquim PR, Da Matta FM. Leaf gas exchange and chlorophyll fluorescence parameters in Phaseolus vulgaris as affected by nitrogen and phosphorus deficiency. Photosynthetica. 1999;37:113–121. [Google Scholar]
- Jacob J, Lawlor DW. Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-biphosphate carboxylase/oxygenase activity and ribulose-1,5-bisphosphate pool size. Plant Physiol. 1991;98:801–807. doi: 10.1104/pp.98.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer MJ, Pallardy SG, Blevins DG, Douglas D, Randall DD. Whole leaf carbon exchange characteristics of phosphate deficient soybeans (Glycine max L.) Plant Physiol. 1989;91:848–854. doi: 10.1104/pp.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. Effect of phosphorus nutrition on ribulose-1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some Calvin cycle metabolites in spinach leaves. Aust J Plant Physiol. 1986;13:221–237. [Google Scholar]
- Brooks A, Woo KC, Wong SC. Effects of phosphorus nutrition on the response of photosynthesis to CO2 and O2, activation of ribulose bisphosphate carboxylase and amounts of ribulose bisphosphate and 3-phosphoglycerate in spinach leaves. Photosynth Res. 1988;15:133–141. doi: 10.1007/BF00035257. [DOI] [PubMed] [Google Scholar]
- Usuda H, Shimogawara K. Phosphate deficiency in maize. II. Enzyme activities. Plant Cell Physiol. 1991;32:1313–1317. doi: 10.1104/pp.99.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S, Usuda H, Tsukui T. Participation of inorganic orthophosphate in regulation of the ribulose-1,5-biphosphate carboxylase activity in response to changes in the photosynthetic source-sink balance. Plant Cell Physiol. 1992;33:943–949. [Google Scholar]
- Rao IM, Arulanantham AR, Terry N. Leaf phosphate status, photosynthesis and carbon partitioning in sugar beet. II. Diurnal changes in sugar phosphates, adenylates and nicotinamide nucleotides. Plant Physiol. 1989;90:820–826. doi: 10.1104/pp.90.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Lawlor DW. In vivo photosynthetic electron transport does not limit photosynthetic capacity in phosphate-deficient sunflower and maize leaves. Plant Cell Environ. 1993;6:785–795. [Google Scholar]
- Ciereszko I, Johansson H, Hurry V, Kleczkowski LA. Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta. 2001;212:598–605. doi: 10.1007/s004250000424. [DOI] [PubMed] [Google Scholar]
- Pieters AJ, Paul MJ, Lawlor DW. Low sink demand limits photosynthesis under Pi deficiency. J Exp Bot. 2001;52:1083–1091. doi: 10.1093/jexbot/52.358.1083. [DOI] [PubMed] [Google Scholar]
- De Groot CC, Marcelis LFM, Boogaard R van den, Lambers H. Growth and dry-mass partitioning in tomato as affected by phosphorus nutrition and light. Plant Cell Environ. 2001;24:1309–1317. [Google Scholar]
- Plesničar K, Kastori R, Petrović N, Panković D. Photosynthesis and chlorophyll fluorescence in sunflower (Helianthus annuus L.) leaves as affected by phosphorus nutrition. J Exp Bot. 1994;45:919–924. [Google Scholar]
- Usuda H. Phosphate deficiency in maize. V. Mobilization of nitrogen and phosphorus within shoots of young plants and its relationship to senescence. Plant Cell Physiol. 1995;36:1041–1049. [Google Scholar]
- Strasser RJ, Srivastava A, Govindjee Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol. 1995;61:32–42. [Google Scholar]
- Strasser RJ, Srivastava A, Tsimilli-Michael M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P, editor. Probing Photosynthesis: Mechanisms, Regulation and Adaptation. London: Taylor and Francis; 2000. pp. 445–483. [Google Scholar]
- Strasser RJ, Tsimilli-Micheal M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, editor. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Dordrecht: Springer; 2004. pp. 321–362. [Govindjee (Series Editor): Advances in Photosynthesis and Respiration, vol. 19.] [Google Scholar]
- Tóth SZ, Schansker G, Garab G, Strasser RJ. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim Biophys Acta. 2007;1767:295–305. doi: 10.1016/j.bbabio.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biol. 2006;33:9–30. doi: 10.1071/FP05095. [DOI] [PubMed] [Google Scholar]
- Schansker G, Tóth SZ, Strasser RZ. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta. 2005;1706:250–261. doi: 10.1016/j.bbabio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Neubauer C, Klughammer C. Devices and methods for room-temperature fluorescence analysis. Phi Trans R Soc Lond B. 1989;323:241–251. [Google Scholar]
- Schansker G, Tóth SZ, Strasser RJ. Dark-recovery of the Chl-a fluorescence transient (OJIP) after light adaptation: the qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim Biophys Acta. 2006;1757:787–797. doi: 10.1016/j.bbabio.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Abadia J, Rao IM, Terry N. Changes in leaf phosphate status have only small effects on the photochemical apparatus of sugar beet leaves. Plant Sci. 1987;50:49–55. [Google Scholar]
- Ripley BS, Redfern SP, Dames J. Quantificatin of the photosynthetic performance of phosphorus-deficient Sorghum by means of chlorophyll a fluorescence kinetics. South Afr J Sci. 2004;100:615–618. [Google Scholar]
- Tang J-F, Hu K-F, Yin J, Xiong J-W. Distribution of organic matter and available N-P-K in the tea garden soil of Xinyang. Henan Agri Sci. 2007. pp. 81–84.
- Wei G, Zhang Q, Feng P. Soil fertility status of tea plantation in Guizhou. Guizhou Agri Sci. 1996. pp. 22–26.
- Mills HA, Jones JB., Jr . Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide. Georgia: Micromacro Publishing; 1996. p. 186. [Google Scholar]
- Halsted M, Lynch J. Phosphorus responses of C3 and C4 species. J Exp Bot. 1996;47:497–505. [Google Scholar]
- Brahim MB, Loustau D, Gaudillère JP, Saur E. Effects of phosphate deficiency on photosynthesis and accumulation of starch and soluble sugars in 1-year-old seedlings of maritime pine (Pinus pinaster Ait) Ann Sci For. 1996;53:801–810. [Google Scholar]
- Hammond JP, White PJ. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J Exp Bot. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- Hernández G, Ramírez M, Valdés-López O, Tesfaye M, Graham MA, Czechowski T, Schlereth A, Wandrey M, Erban A, Cheung F, Wu HC, Lara M, Town CD, Joachim Kopka J, Udvardi MK, Vance CP. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007;144:752–767. doi: 10.1104/pp.107.096958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciereszko I, Gniazdowska A, Mikulska M, Rychter AM. Assmilatie translocation in bean plants (Phaseolus vulgaris L.) during phosphate deficiency. J Plant Physiol. 1996;149:343–348. [Google Scholar]
- Fredeen AL, Rao IM, Terry N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989;89:225–230. doi: 10.1104/pp.89.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T. Regulation of phosphate transport and homeostasis in plant cells. Int Rev Cytol Cell Biol. 1999;191:149–200. [Google Scholar]
- Qiu J, Israel DJ. Diurnal starch accumulation and utilization in phosphorus-deficient soybean plants. Plant Physiol. 1992;98:316–323. doi: 10.1104/pp.98.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, Spencer C. The relationship between phosphate status and photosynthesis in leaves. Planta. 1986;167:369–373. doi: 10.1007/BF00391341. [DOI] [PubMed] [Google Scholar]
- Nanamori M, Shinano T, Wasaki J, Yamamura T, Rao IM, Osaki M. Low phosphorus tolerance mechanisms: Phosphorus recycling and photosynthate partitioning in the tropical forage grass, Brachiaria hybrid cultivar Mulato compared with rice. Plant Cell Physiol. 2004;45:460–469. doi: 10.1093/pcp/pch056. [DOI] [PubMed] [Google Scholar]
- Usuda H, Shimogawara K. Phosphate deficiency in maize. III. Changes in enzyme activities during the course of phosphate deprivation. Plant Physiol. 1992;99:1680–1685. doi: 10.1104/pp.99.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciereszko I, Barbachowska A. Sucrose metabolism in leaves and roots of bean (Phaseolus vulgaris L.) during phosphate deficiency. J Plant Physiol. 2000;156:640–644. [Google Scholar]
- Rao IM, Fredeen AL, Terry N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar Beet: III. Diurnal changes in carbon partitioning and carbon export. Plant Physiol. 1990;92:29–36. doi: 10.1104/pp.92.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JH, Duncan LW, Eissenstat DM. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997;135:335–343. [Google Scholar]
- Sheen J. Feedback control of gene expression. Photosynth Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux J-P, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H-X, Chen L-S, Zheng J-G, Han S, Tang N, Smith BR. Aluminum-induced effects on photosystem II photochemistry in citrus leaves assessed by chlorophyll a fluorescence transient. Tree Physiol. 2008;28:1863–1871. doi: 10.1093/treephys/28.12.1863. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Baker NR, Eva Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J Exp Bot. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- Chen L-S, Li P, Cheng L. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta. 2008;228:745–756. doi: 10.1007/s00425-008-0776-3. [DOI] [PubMed] [Google Scholar]
- Yamane Y, Kashino Y, Koike H, Satoh K. Increases in the fluorescence Fo level and reversible inhibition of Photosystem II reaction center by high-temperature treatments in higher plants. Photosynth Res. 1997;52:57–64. [Google Scholar]
- Bukhov NG, Sabat SC, Mohanty P. Analysis of chlorophyll a fluorescence changes in weak light in heat treated Amarenthus chloroplasts. Photosynth Res. 1990;23:81–87. doi: 10.1007/BF00030066. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Debrunner PG, Govindjee Comparative time-resolved photosystem II chlorophyll a fluorescence analyses reveal distinctive differences between photoinhibitory reaction center damage and xanthophyll cycle-dependent energy dissipation. Photochem Photobiol. 1996;64:552–563. doi: 10.1111/j.1751-1097.1996.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Setlik I, Allakhveridiev SI, Nedbal L, Setlikova E, Klimov VV. Three types of Photosystem II photoinactivation. I. Damaging process on the acceptor side. Photosynth Res. 1990;23:39–48. doi: 10.1007/BF00030061. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Guisse B, Greppin H, Strasser RJ. Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta. 1997;1320:95–106. [Google Scholar]
- Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim Biophys Acta. 2005;1706:68–80. doi: 10.1016/j.bbabio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Streusand VJ, Portis AR., Jr Rubisco activase mediates ATP-dependent activation of ribulose bisphosphate carboxylase. Plant Physiol. 1987;85:152–154. doi: 10.1104/pp.85.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Cheng L. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are up-regulated in grape (Vitis labrusca BL. cv. Concord) leaves in response to N limitation. J Exp Bot. 2003;54:2165–2175. doi: 10.1093/jxb/erg220. [DOI] [PubMed] [Google Scholar]
- Song YG, Liu B, Wang LF, Li MH, Liu Y. Damage to the oxygen-evolving complex by superoxide anion, hydrogen peroxide, and hydroxyl radical in photoinhibition of photosystem II. Photosynth Res. 2006;90:67–78. doi: 10.1007/s11120-006-9111-7. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Tsimilli-Michael M, Strasser RJ. In vivo assessment of stress impact on plant's vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A, editor. Mycorrhiza: Genetics and Molecular Biology, Eco-function, Biotechnology, Eco-physiology, and Structure and Systematics. Berlin: Springer; 2008. pp. 679–703. [Google Scholar]
- Smit MF, Krüger GHJ, van Heerden PDR, Pienaar JJ, Weissflog L, Strasser RJ. Effect of trifluoroacetate, a persistent degradation product of fluorinated hydrocarbons, on C3 and C4 crop plants. In: Allen JF, Gantt E, Golbeck JH, Osmond B, editor. Photosynthesis Energy from the Sun: 14th International Congress on Photosynthesis. Dordrecht: Springer; 2008. pp. 1501–1504. [Google Scholar]
- Chen L-S, Qi Y-P, Smith BR, Liu XH. Aluminum-induced decrease in CO2 assimilation in citrus seedlings is unaccompanied by decreased activities of key enzymes involved in CO2 assimilation. Tree Physiol. 2005;25:317–324. doi: 10.1093/treephys/25.3.317. [DOI] [PubMed] [Google Scholar]
- Cheng L, Fuchigami LH. Rubisco activation state decreases with increasing nitrogen content in apple leaves. J Exp Bot. 2000;51:1687–1694. doi: 10.1093/jexbot/51.351.1687. [DOI] [PubMed] [Google Scholar]
- Jones MGK, Outlaw WJ, Lowery OH. Enzymic assay of 10-7 to 10-14 moles of sucrose in plant tissues. Plant Physiol. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]