Abstract
Dietary intake of long-chain n-3 polyunsaturated fatty acids (n-3 PUFA) has been reported to decrease several markers of lymphocyte activation and modulate monocyte susceptibility to apoptosis. However most human studies examined the combined effect of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) using relatively high daily amounts of n-3 PUFA. The present study investigated the effects of increasing doses of DHA added to the regular diet of human healthy volunteers on lymphocyte response to tetradecanoylphorbol acetate (TPA) plus ionomycin activation, and on monocyte apoptosis induced by oxidized LDL (oxLDL). Eight subjects were supplemented with increasing daily doses of DHA (200, 400, 800 and 1600mg) in a triacylglycerol form containing DHA as the only PUFA, for two weeks each dose. DHA intake dose-dependently increased the proportion of DHA in mononuclear cell phospholipids, the augmentation being significant after 400mg DHA/day. The TPA plus ionomycin-stimulated IL-2 mRNA level started to increase after ingestion of 400mg DHA/day, with a maximum after 800mg intake, and was positively correlated (P<0.003) with DHA enrichment in cell phospholipids. The treatment of monocytes by oxLDL before DHA supplementation drastically reduced mitochondrial membrane potential as compared with native LDL treatment. OxLDL apoptotic effect was significantly attenuated after 400mg DHA/day and the protective effect was maintained throughout the experiment, although to a lesser extent at higher doses. The present results show that supplementation of the human diet with low DHA dosages improves lymphocyte activability. It also increases monocyte resistance to oxLDL-induced apoptosis, which may be beneficial in the prevention of atherosclerosis.
Keywords: DHA enrichment, interleukin-2, mitochondrial membrane potential, oxidized LDL
Introduction
Several human studies have shown that ingesting long-chain n-3 polyunsaturated fatty acids (PUFA) decreases some markers of immune function including lymphocyte proliferative responses and cytokine production(1). This immunosuppressive effect was especially observed with aged people(2, 3). However, studies conducted with younger people suggest that healthy adults are relatively insensitive to immunomodulation with long chain n-3 PUFA (4). Another concern is that most studies examined the combined effects of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) both present in different proportions depending on the source of fish oil administered to the volunteers. Only few human studies examined the effects of either EPA or DHA separately on immune function. In the study of Kew et al (5) examining the effect of dietary supplementation of healthy middle age subjects with 4.7 g/day EPA or 4.9 g/day DHA, it was shown that only DHA, but not EPA, decreased the expression of CD69, an early marker of lymphocyte activation. These results are at variance with those of Thies et al. (6) showing that fish oil, but not highly purified DHA, suppressed lymphocyte proliferation, or with those of Kelley et al (7) showing that consuming 6g DHA/day for 3 months did not alter lymphocyte proliferation. Other studies have even reported quite opposite results. Thus, Schauder et al (8) reported that patients fed on parenteral nutrition supplemented with DHA-enriched fish oil during the postoperative period produced higher levels of IL-2 than those supplemented with soybean oil or unsupplemented patients. The supplementation of healthy volunteers’diet with DHA-enriched oil providing 1.62 g DHA and 0.78 g EPA per day for two months has recently been shown to increase ConA-dependent lymphocyte proliferative responses and IL-10, IFNγ and TNFα production as compared with levels measured before supplementation (9).
The reported effects of DHA and EPA on apoptosis have ranged from inhibition to stimulation, depending on the cell model used. It is generally believed that long chain n-3 PUFA increase the rate of apoptosis of tumour cells, both in vitro when added to cell culture medium, and in tumour-bearing rodents fed DHA- or EPA-enriched diets (10). However, a large variability has been observed in normal cell models including monocytes or monocytic cell lines. Thus, Sweeney et al (11) have recently shown that the treatment of monocytes purified from human umbilical cord blood with DHA or EPA induced a rapid and dose-dependent cell death, due to a loss of mitochondrial membrane potential. In contrast, the preincubation of U937 monocytic cells with DHA has been shown to attenuate apoptosis induced by stimulation with TNFα in the presence or absence of cycloheximide, this antiapoptotic effect being accompanied by an enrichment of DHA in membrane phospholipids (12). However, to our knowledge, the influence of DHA supplementation of the human diet on ex vivo monocyte susceptibility to proapoptotic stimuli has never been investigated.
In the present study we sought to examine the effects of increasing doses of DHA added to the regular diet of human healthy volunteers on some aspects of immune function. We chose to evaluate IL-2 mRNA expression in response to phorbol ester and ionomycin activation as a marker of lymphocyte ex vivo activation and to measure changes in mitochondrial membrane potential induced by oxidized LDL (oxLDL) as a marker of monocyte susceptibility to apoptosis. Results were examined in regard to changes in the fatty acid composition of mononuclear cell phospholipids.
Subjects and methods
Subjects and experimental design
The eight subjects recruited for the study were healthy male volunteers aged between 53 and 65 years (mean age 58.7 y). Each of them gave informed consent and the study was approved by the “Comité consultatif de protection des personnes dans la recherche biomédicale de Lyon A”. At the beginning of the study each of them had normal blood cell counts, cholesterol (5.47 ± 0.2 mmol/L), triacylglycerol (1.76 ± 0.4 mmol/L) and glucose (5.25 ± 0.2 mmol/L) blood levels. They ingested successively 200, 400, 800, 1600mg DHA in a triacylglycerol form per day, for two weeks each dose without interruption. DHA supplement was administered in soft gelatine capsules that each contained 200mg DHA and 0.125mg DL-tocopherol plus 0.125mg ascorbyl palmitate (PRO-MIND FORTE, DECOLA Neutraceutics, Maldegem, Belgium). The fatty acid composition of the triacylglycerol was: 22:6n-3: 40%; 18:2n-6: 1.2%; total monounsaturated FA: 22.2% (including 18:1n-9: 20.2% + 16:1n-7: 2%): total saturated FA: 36.6 % (including 16:0: 14% + 14:0: 16% + 12:0: 6.6 %). Blood samples were collected after overnight fasting before DHA supplementation and after each DHA dose, and mononuclear cells were isolated. Blood samples were also collected five weeks after supplementation was arrested. The fatty acid composition of cell phospholipids was analyzed and cell functions were evaluated. Each individual was asked not to deviate from its regular habits during the study period and not to take any drugs at least 10 days before the initial blood sampling and during the test period.
Peripheral blood mononuclear cell (PBMC) and monocyte (PBM) isolation
Venous blood was drawn into ACD anticoagulant. PBMC were separated by dextran sedimentation and density gradient centrifugation through Histopaque 1077 and then washed three times with RPMI 1640 by low speed centrifugation in order to more thoroughly eliminate the contaminating platelets. PBMC were then adjusted to a concentration of 2×107cells/mL in RPMI 1640 (with Hepes and bicarbonate) medium. All steps were carried out at room temperature. Under such conditions, cell viability established by the trypan blue exclusion test, was always greater than 95%. Mononuclear cell suspensions were used to evaluate IL-2 expression and to determine the fatty acid composition of phospholipids. An aliquot of this cell suspension was used for monocyte isolation. Peripheral blood monocytes (PBM) were isolated from mononuclear cell suspensions by adherence (1h). After non-adherent cells were discarded, PBM (106/mL) were incubated in RPMI medium supplemented as previously described (13) and were cultured in presence of native LDL or oxLDL for 4h. PBM preparations were analyzed by flow cytometry using CD14 monoclonal antibody (BD Biosciences, Heildelberg, Germany) and isotype control IgG1 (Serotec, Düsseldorf, Germany) as a negative control. At least 70% of PBMs were CD14+ (not shown).
PBMC treatment, RNA isolation and semi-quantitative RT-PCR analyses
Mononuclear cells suspended at a concentration of 106cells/mL in RPMI were incubated with 200 nmol/L TPA and 500 nmol/L ionomycin for 2h at 37°C in an air-CO2 (95:5) atmosphere. At the end of the incubation period, total RNA was isolated from control and TPA-treated cells using Tri Reagent according to the manufacturer’s instructions. The sense and antisense primers for the amplification of IL-2 were 5′-CACTAAGTCTTGCACTTGTCAC-3′ and 5′-CCTTCTTGGGCATGTAAAACT-3′, respectively (expected size of amplified fragment 186 bp). β-actin primers were used as internal control to normalize the data. RT-PCR were performed on 2 μg RNA for IL-2 and 1 μg RNA for β-actin using QIAGEN® OneStep RT-PCR kit (Courtaboeuf, France) according to the manufacturer’s instructions. Reaction products were resolved by electrophoresis on 1% agarose gel impregnated with ethidium bromide, and visualized by UV transillumination.
Fatty acid composition of PBMC phospholipids
20 μg of diheptadecanoyl phosphatidylcholine as an internal standard were added to 1 mL mononuclear cell suspension. The cell suspensions were then extracted according to Bligh and Dyer (14) and cell lipid extracts were separated on silica gel G60 plates (Merck, Darmstadt, Germany) with the solvent system hexane/diethylether/acetic acid (60:40:1, by vol.). The silica gel areas corresponding to phospholipids (PL) were scraped off and transmethylated according to Bowyer et al. (15) as previously described (3). Briefly, 1 vol of 5% H2SO4 in methanol was added to the scraped silica gel and transmethylation was carried out at 100°C for 90 min in screw-capped tubes. The reaction was terminated by the addition of 1.5 vol of ice-cold 5% (w/v) K2CO3, and the fatty acid methyl esters (FAME) were extracted with isooctane and resolved by gas chromatography using a Hewlett Packard chromatograph HP 6890 model, equipped with a capillary column (60 m × 0.25 mm, BPx70 SGE, Bellfonte, USA) and a flame ionization detection. The column was two-step programmed from 135 to 160°C at 2°C/min and from 160 to 205°C at 1.5 °C/min; the detection temperature was maintained at 250°C. The vector gas was helium at a pressure of 0.8 psi (5.52 KPa). FAME were identified by their retention time relative to standards.
LDL isolation and oxidation
LDL fraction was isolated from human plasma by sequential ultracentrifugation (16). LDL (1mg/mL) oxidation was induced for 30 min at 37°C with 4 mmol/L HOCl (corresponding to oxidant/protein molar ratio of 2000/1). Untreated and oxidized LDL were dialyzed overnight against isotonic PBS. Native and oxidized LDL were used at cholesterol concentration of 200 μg/mL in the incubation medium. The lipid peroxide content of native and oxidized LDL was determined by analyzing thiobarbituric acid-reactive substances and expressed as malondialdehyde (MDA) equivalents (17). As compared to previous results obtained after copper-treatment of native LDL, MDA was generated at a lower extent in HOCl-oxLDL than in copper-oxLDL (0.68 nmol MDA/mg proteins for HOCl-oxLDL versus 0.10 for native LDL and 4.20 for copper-oxLDL (18)).
The degree of oxidation was quantified by an increased relative mobility on 0.6 % agarose gels (19), indicating an enhanced negative charge of HOCl-oxLDL. The relative mobility of HOCl-oxLDL on agarose gels as an index for lipoprotein oxidation was 2.5-3.0 compared with that of native LDL.
Analysis of mitochondrial membrane potential (Δψm)
Following individual incubations, cells were loaded with the fluorochrome 3, 3′-dihexyloxacarbocyanine iodide (DiOC6, Molecular Probes, Inc., Eugene, OR, USA), used at 40 nmol/L final concentration for 30 min. The dye accumulates in mitochondria that contain an intact membrane potential, and the fluorescence of DiOC6 can therefore be considered as an indicator of the relative mitochondrial membrane polarization state (20). Relative fluorescence intensities were measured on a FACScan flow cytometer.
Statistical analysis
Data expressed as means ± SEM were analyzed by ANOVA and means were compared by a protected t test. Correlations between changes in parameters induced by DHA were tested by linear regression. P < 0.05 was considered significant.
Results and discussion
DHA supplement was well tolerated throughout the study. No significant variations in systolic and diastolic blood pressure, glucose and triacylglycerol blood concentrations were observed before and after DHA supplementation. DHA treatment was associated with a slight but significant increase in HDL-cholesterol (1.43 ± 0.09 vs 1.60 ± 0.12 mmol/L, +11.9%, P<0.05) and a trend of LDL-cholesterol to increase (3.23 ± 0.24 vs 3.56 ± 0.27 mmol/L, +10.2%, NS) after ingestion of the highest dose of DHA (1600mg/day). Interestingly, the HDL to LDL cholesterol ratio remained unchanged throughout the study. These results are in partial agreement with those of a recent study (21) reporting an increase of both HDL- and LDL-cholesterol after ingestion of 3g/day DHA for 90d. However, at variance with the present results, in the study of Kelley et al. (21) only LDL-cholesterol changes were significant.
DHA intake significantly increased the proportion of DHA in mononuclear cell phospholipids (Table 1, Fig. 1), starting from the second dose of 400mg/day (+25% as compared with level measured before DHA intake). DHA incorporation linearly increased (R2=0.99, P<0.003) as a function of DHA doses up to 800mg/day dose, and slowed down thereafter. The increase in DHA proportion reached +76% with respect to baseline (5.67 ± 0.26 vs 3.22 ± 0.28 at baseline) after the highest dose of 1600mg/day. Although several studies have reported that supplementation of the diet with n-3 fatty acids results in an increased n-3 fatty acid content of PBMCs (3, 6), only few studies have examined n-3 fatty acid incorporation in PBMC phospholipids as a function of the ingested dose. In a recent study, Rees et al. (22) have shown that EPA was incorporated in a linear dose-response fashion into PBMC phospholipids following ingestion of EPA doses ranging from 1.35 to 4.05 g/day provided as EPA-rich oil. However, to our knowledge the present study is the first one to describe a dose-response relationship between increased DHA intake and increased DHA incorporation in mononuclear cell phospholipids. After a 5-week wash out period, DHA proportion no longer differed from that measured before DHA supplementation (Table 1). Although the algal oil used in the present study was totally devoid of EPA, the proportion of EPA in mononuclear cell phospholipids was also increased, the rise being significant only after ingestion of the third and fourth DHA doses (0.38 ± 0.06 at baseline vs 0.60 ± 0.07 and 0.61 ± 0.07 after 800 and 1600mg DHA/day, respectively). This result suggests that DHA was efficiently retroconverted to EPA and increased in blood mononuclear cells as previously observed (23, 24). These modifications were accompanied by decreased proportions of long chain n-6 fatty acids, specifically arachidonic acid (20:4n-6) and adrenic acid (22:4n-6). Surprisingly, the proportion of 22:5n-3 was also significantly lowered by DHA treatment, the decrease being significant from the dose of 200mg/day. Such a decrease in 22:5n-3 concentration has already been reported in serum phospholipids of women supplemented with 2.8 g DHA/day for 28 days (24) or in erythrocyte phospholipids of middle-aged people supplemented with 0.7g DHA/day for 3 months (25). This is likely a result of DHA competition for esterification at the sn-2 position of glycerophospholipids.
Table 1.
Fatty acid composition of mononuclear cell phospholipids (mol/100 mol total fatty acids) at baseline (0) and after supplementation with increasing doses of DHA
| Daily DHA dose (mg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 800 | 1600 | wash out | |||||||
| Fatty acids | mean | SE | mean | SE | mean | SE | mean | SE | mean | SE | mean | SE |
| 14 :0 | 0.10a | 0.04 | 0.16a,b | 0.04 | 0.23b,c | 0.03 | 0.26c | 0.26 | 0.22b,c | 0.02 | 0.20a,b,c | 0.04 |
| 16 :0 | 21.45a,b | 0.39 | 18.80a | 2.43 | 22.87b | 0.32 | 22.25b | 0.63 | 22.08a,b | 0.76 | 22.42b | 1.12 |
| 16 :1n-9 | 0.04a | 0.03 | 0.12a,b | 0.04 | 0.13a,b | 0.03 | 0.2b | 0.04 | 0.18b | 0.05 | 0.18b | 0.02 |
| 16 :1n-7 | 0.14a | 0.06 | 0.22a,b | 0.06 | 0.28b | 0.02 | 0.28b | 0.03 | 0.25a,b | 0.03 | 0.29b | 0.03 |
| 18 :0 | 22.10a,b | 0.40 | 21.63a | 0.30 | 22.13a,b | 0.32 | 22.08a,c | 0.47 | 23.21b | 0.47 | 23.07 c,c | 0.56 |
| 18 :1n-9 | 11.60a | 0.75 | 13.25 | 0.97 | 13.45 | 0.56 | 12.83 | 0.45 | 12.83 | 0.47 | 14.83b | 1.44 |
| 18 :1n-7 | 2.78a | 0.30 | 2.37a,b | 0.36 | 2.04b | 0.13 | 2.14b | 0.09 | 2.03b | 0.09 | 2.04b | 0.14 |
| 18 :2n-6 | 8.03 | 0.17 | 8.02 | 0.32 | 8.41 | 0.37 | 8.36 | 0.37 | 8.72 | 0.44 | 7.95 | 0.33 |
| 20 :2n-6 | 0.61 | 0.02 | 0.63 | 0.05 | 0.65 | 0.06 | 0.58 | 0.05 | 0.59 | 0.05 | 0.56 | 0.05 |
| 20 :3n-6 | 2.02 | 0.11 | 2.12 | 0.10 | 2.02 | 0.10 | 1.98 | 0.12 | 1.93 | 0.12 | 1.95 | 0.09 |
| 20 :4n-6 | 21.18a | 0.36 | 20.84a | 0.44 | 19.21b | 0.41 | 19.44b | 0.37 | 18.28b | 0.56 | 18.38b | 0.56 |
| 20 :5n-3 | 0.38a | 0.06 | 0.52a,b | 0.03 | 0.46a,b | 0.06 | 0.60b | 0.07 | 0.61b | 0.07 | 0.55a,b | 0.10 |
| 22 :4n-6 | 3.70a | 0.47 | 2.86b | 0.41 | 1.98c | 0.11 | 1.77c | 0.11 | 1.46c | 0.09 | 1.98c | 0.13 |
| 22 :5n-3 | 2.65a | 0.06 | 2.35b | 0.14 | 1.96c | 0.10 | 1.84c | 0.11 | 1.41d | 0.08 | 2.08b,c | 0.13 |
| 22 :6n-3 | 3.22a | 0.28 | 3.64a,b | 0.22 | 4.01b | 0.26 | 5.08c | 0.26 | 5.67c | 0.26 | 3.06a,d | 0.21 |
| SFA | 42.84a | 0.32 | 43.08a,b | 0.43 | 45.23a,c | 0.44 | 44.58a,c | 0.65 | 45.51b,c | 1.20 | 45.68c | 1.61 |
| MUFA | 15.21 | 0.72 | 15.96 | 0.78 | 15.96 | 0.49 | 15.63 | 0.51 | 15.47 | 0.54 | 17.51 | 1.42 |
| n-6 PUFA | 35.38a | 0.76 | 34.46a,b | 0.59 | 32.38c | 0.56 | 32.27c | 0.53 | 31.31c | 0.70 | 31.12c | 0.82 |
| n-3 PUFA | 6.59a | 0.41 | 6.50a,b | 0.21 | 6.43a,b | 0.31 | 7.52c | 0.31 | 7.72c | 0.37 | 5.69a,d | 0.31 |
| n-6 :n-3 PUFA | 5.52a | 0.36 | 5.34a,b | 0.19 | 5.13a,b | 0.27 | 4.33c | 0.16 | 4.11c | 0.17 | 5.46a,d | 0.34 |
Values are means ± SEM of n=8 subjects. Data were analyzed by ANOVA and means were compared by a protected t test. Values in a row not sharing the same superscripts are significantly different, P<0.05. If no superscript appears in a row, the values are not statistically different.
Figure 1.
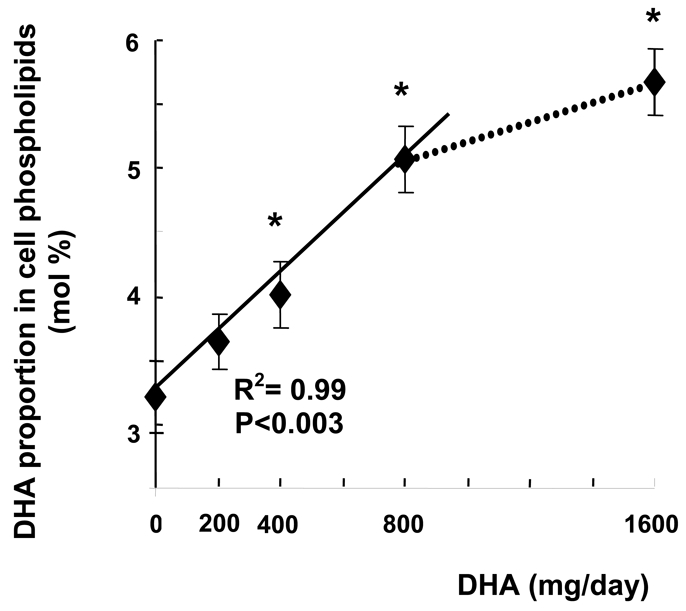
Change in DHA proportion in mononuclear cell phospholipids during supplementation with different dose of DHA-rich oil. Data are mean ± SEM. (n= 8 per treatment group).
An early response of lymphocytes to activation by phorbol ester and ionomycin is the expression of IL-2 mRNA, which starts after 1h of treatment and reaches a maximum after 2h of stimulation (26, 27). Thus, we examined by RT-PCR IL-2 mRNA expression after 2h of TPA and ionomycin treatment of mononuclear cells before DHA ingestion and after each dose intake. In the absence of activators, the basal IL-2 mRNA expression remained very low and unaffected by DHA supplementation (Fig. 2). In contrast, the stimulated mRNA level started to increase after ingestion of 400mg DHA/day, peaked after the dose of 800mg/day and remained elevated after ingestion of the highest DHA dose. After the 5-week wash out period, the stimulated level of mRNA expression returned to values observed before the supplementation period, as did DHA proportion in cell phospholipids. Interestingly, the stimulated mRNA level of IL-2 was positively correlated with DHA enrichment in cell phospholipids (Fig. 3). These results indicate that a moderate DHA intake does not decrease, but rather enhances, IL-2 mRNA expression in lymphocytes from healthy middle-aged volunteers, thus suggesting an improvement of their activability. This is in agreement with recent reports showing that n-3 fatty acids may have different effects on immune function depending on the age and health status of the subjects (4, 28). Because TPA plus ionomycin-induced signals bypass cell membrane receptors, modifications of membrane fluidity or lipid rafts are probably not involved. An alternative mechanism might be a specific effect of DHA or metabolites on the regulation of expression of key lymphocyte genes (9).
Figure 2.

Effect of increasing DHA intake on IL-2 mRNA expression in mononuclear cells. PBMC suspended at a concentration of 106cells/mL in RPMI were incubated with 200 nmol/L TPA and 500 nmol/L ionomycin for 2h at 37°C. At the end of the incubation period, total RNA was isolated from control and TPA-treated cells, and submitted to RT-PCR. Amounts of IL-2 amplification products were normalized by the amounts of β-actin amplification products. Ratio values are expressed relative to IL-2/β-actin ratio of unstimulated cells at baseline taken as 1 and are means ± SEM (n= 8 per treatment group). *, significantly different from TPA + ionomycin stimulated cells at baseline; P<0.05.
Figure 3.

Positive relationship between DHA enrichment in mononuclear cell phospholipids and IL-2 mRNA expression. For each subject normalized IL-2/β-actin ratio is plotted as a function of the relative DHA enrichment (with respect to baseline value taken as 1) in cell phospholipids.
In a previous study (18), we showed that HOCl-oxLDL was able to induce apoptosis of human cultured U937 monocytic cell line in a concentration-dependent manner, via the mitochondrial apoptotic pathway. Based on this, we chose to expose human primary monocytes to a HOCl-oxLDL concentration of 200 μg/mL. As shown in Fig. 4, the treatment of monocytes freshly isolated from volunteers before DHA supplementation by oxLDL drastically reduced mitochondrial membrane potential, as compared with native LDL treatment (negative control). After DHA intake monocytes became more resistant to oxLDL toxic effect starting from the second DHA dose of 400mg/day (62.8 ± 1.8 % of cells with ΔΨm disruption for oxLDL treatment and DHA 400mg/day vs. 77.1 ± 1.2 % for oxLDL treatment before DHA supplementation). The protective effect of DHA supplementation was maintained throughout the experiment although it tended to decline after ingestion of the two highest DHA doses (68.3 ± 2.0 and 69.3 ± 1.8 % of cells with low ΔΨm after 800 and 1600mg DHA/day). However, whatever the DHA doses ingested, oxLDL still induced monocyte mitochondrial membrane depolarisation, which may promote apoptosis. At the present time, it is unclear whether apoptosis induced by various stimuli is harmful or beneficial in various physiological and pathological circumstances. Indeed, apoptosis of mature macrophage is thought to promote plaque destabilization and vessel occlusion in the late stages of atherosclerosis, whereas monocyte apoptosis may be beneficial in the initial stages of the atheromatous process (29). After the wash out period, the susceptibility of monocytes to oxLDL apoptosis was no longer different from that observed at baseline (oxLDL alone before DHA ingestion). Some nutritional studies have shown that LDL isolated from fish oil supplemented volunteers, thus enriched in n-3 fatty acids, induced less apoptosis in U937 cells after oxidation compared to LDL isolated from sunflower oil supplemented subjects (30, 31). On the other hand, the effects of n-3 fatty acid enrichment of monocyte phospholipids on susceptibility to apoptosis seem to be largely dependent on experimental conditions (11, 12). Thus, in umbilical cord monocytes the pro-apoptotic effect of DHA involved a loss of mitochondrial membrane potential accompanied by caspase-3 activation (11), whereas in U937 monocytic cell line the anti-apoptotic effect of DHA was attributed to inhibition of cPLA2 through esterification in cell phospholipids (12).
Figure 4.

Effect of increasing DHA intake on oxLDL-treated monocyte mitochondrial membrane potential. Human monocytes were treated with 200 μg/mL native LDL or 200 μg/mL oxLDL for 4h at 37°C. Cells were then incubated for 30 min with 40 nmol/L DiOC6 at 37°C, and analyzed by flow cytometry. The vertical axis shows DiOC6 relative fluorescence intensity. Means ± SEM, n = 4 independent experiments, † p < 0.0001, oxLDL versus nLDL; ** p < 0.05, oxLDL versus oxLDL+DHA.
Hypochlorous acid is known to preferentially modify the protein moiety of human LDL, but HOCl-modified LDL can in turn induce lipid peroxidation and antioxidant depletion as secondary events subsequent to aminoacid modifications (32). Thus, we have recently shown that HOCl-modified oxLDL induced the generation of Reactive Oxygen Species (ROS) in U937 monocytic cells (29). Although highly susceptible to peroxidation, DHA may have antioxidant properties especially when used at low concentrations. This proposal is supported both by nutritional studies in humans, and by studies involving in vitro DHA enrichment of different cell models. Indeed, Vericel et al. (33) have shown a significant increase of α- and γ-tocopherol accompanied by a decrease in malondialdehyde in platelets of elderly people supplemented for 6 weeks with low doses of marine oil. Bechoua et al. (34) have reported that low concentrations of DHA decrease malondialdheyde production induced by H2O2 in human mononuclear cells. Similarly, the pretreatment of RAW264 macrophages with DHA has been shown to prevent the accumulation of intracellular peroxides induced by IFNγ plus LPS in a dose-dependent manner by a mechanism involving upregulation of intracellular glutathione level (35). Finally, low doses of DHA have been shown to strengthen cellular antioxidant defences by increasing glutathione peroxidase activity/expression in various types of cells including platelets (36), mononuclear cells (37) or neuronal cells (38). Thus, the antioxidant properties of low DHA doses might explain the antiapoptotic effects observed in monocytes. Furthermore, they could also explain the stimulating effects on lymphocytes as both prooxidant and antioxidant states are required sequentially during lymphocyte activation (39).
Acknowledgments
This work was supported by Inserm and a grant from the Groupe Lipides et Nutrition (GLN) (2005)
Abbreviations used
- DHA
docosahexaenoic acid
- DiOC6
3,3′-dihexyloxacarbocyanine iodide
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl ester
- PBM
peripheral blood monocyte
- PBMC
peripheral blood mononuclear cell
- IL-2
interleukine-2
- oxLDL
oxidized LDL
- TPA
tetradecanoylphorbol acetate
Footnotes
The authors accept the conditions laid down in the Direction to Contributors. This submission represents original work that has not been published previously, that is not currently being considered by another journal. If accepted for the British Journal of Nutrition it will not be published elsewhere in the same form, in English or in any language, without the written consent of the Nutrition Society.
The contribution of each author was as follows: SM, NE, AB, MD: doing experiments; SV, GN, BL, AFP: writing of the manuscript, M Laville: promotor, investigator, coordonator; EV, M Lagarde, AFP: designing the study.
All the authors have seen and approved the contents of the submitted manuscript. All the authors stated that there are no conflicts of interest and all the authors adhere to the Committee on Publication Ethics guidelines on research and publication ethics.
References
- 1.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Bechoua S, Dubois M, Vericel E, Chapuy P, Lagarde M, Prigent AF. Influence of very low dietary intake of marine oil on some functional aspects of immune cells in healthy elderly people. Br J Nutr. 2003;89:523–531. doi: 10.1079/BJN2002805. [DOI] [PubMed] [Google Scholar]
- 4.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–259. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 5.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 6.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131:1918–1927. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids. 1998;33:559–566. doi: 10.1007/s11745-998-0240-8. [DOI] [PubMed] [Google Scholar]
- 8.Schauder P, Röhn U, Schäfer G, Korff G, Schenk HD. Impact of fish oil enriched total parenteral nutrition on DNA synthesis, cytokine release and receptor expression by lymphocytes in the postoperative period. Br J Nutr. 2002;87:S103–S110. doi: 10.1079/bjn2001463. [DOI] [PubMed] [Google Scholar]
- 9.Gorjão R, Verlengia R, Lima TM, et al. Effect of docosahexaenoic acid-rich fish oil supplementation on human leukocyte function. Clin Nutr. 2006;25:923–938. doi: 10.1016/j.clnu.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Field CJ, Schley PD. Evidence for potential mechanisms for the effect of conjugated linoleic acid on tumor metabolism and immune function: lessons from n-3 fatty acids. Am J Clin Nutr. 2004;79:1190S–1198S. doi: 10.1093/ajcn/79.6.1190S. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney B, Puri P, Reen DJ. Induction and modulation of apoptosis in neonatal monocytes by polyunsaturated fatty acids. J Pediatr Surg. 2007;42:620–628. doi: 10.1016/j.jpedsurg.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Yano M, Kishida E, Iwasaki M, Kojo S, Masuzawa Y. Docosahexaenoic acid and vitamin E can reduce human monocytic U937 cell apoptosis induced by tumor necrosis factor. J Nutr. 2000;130:1095–1101. doi: 10.1093/jn/130.5.1095. [DOI] [PubMed] [Google Scholar]
- 13.Xu XP, Meisel SR, Ong JM, Kaul S, Cercek B, Rajavashisth TB, Sharifi B, Shah PK. Oxidized low-density lipoprotein regulates matrix metalloproteinase-9 and its tissue inhibitor in human monocyte-derived macrophages. Circulation. 1999;99:993–998. doi: 10.1161/01.cir.99.8.993. [DOI] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Bowyer DE, Leat WM, Howard AN, Gresham GA. The determination of the fatty acid composition of serum lipids separated by thin-layer chromatography; and a comparison with column chromatography. Biochim Biophys Acta. 1963;70:423–431. doi: 10.1016/0006-3002(63)90772-8. [DOI] [PubMed] [Google Scholar]
- 16.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti M, Morand PC, Levillain P, Lemonnier A. Improved fluorometric determination of malonaldehyde. Clin Chem. 1991;37:1273–1275. [PubMed] [Google Scholar]
- 18.Ermak N, Lacour B, Drüeke TB, Vicca S. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocytes. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.12.052. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Lin KY, Pan JP, Yang DL, Huang KT, Chang MS, Ding PY, Chiang AN. Evidence for inhibition of low density lipoprotein oxidation and cholesterol accumulation by apolipoprotein H (beta2-glycoprotein I) Life Sci. 2001;69:707–719. doi: 10.1016/s0024-3205(01)01164-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr. 2007;86:324–33. doi: 10.1093/ajcn/86.2.324. [DOI] [PubMed] [Google Scholar]
- 22.Rees D, Miles EA, Banerjee T, Wells SJ, Roynette CE, Wahle KW, Calder PC. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83:331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 23.Joulain C, Guichardant M, Lagarde M, Prigent AF. Influence of polyunsaturated fatty acids on lipid metabolism in human blood mononuclear cells and early biochemical events associated with lymphocyte activation. J Lipid Mediat Cell Signal. 1995;11:63–79. doi: 10.1016/0929-7855(94)00028-b. [DOI] [PubMed] [Google Scholar]
- 24.Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- 25.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr. 2007;137:973–978. doi: 10.1093/jn/137.4.973. [DOI] [PubMed] [Google Scholar]
- 26.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28:109–123. doi: 10.1016/j.cyto.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Diaz O, Mébarek-Azzam S, Benzaria A, Dubois M, Lagarde M, Némoz G, Prigent AF. Disruption of lipid rafts stimulates phospholipase d activity in human lymphocytes: implication in the regulation of immune function. J Immunol. 2005;175:8077–8086. doi: 10.4049/jimmunol.175.12.8077. [DOI] [PubMed] [Google Scholar]
- 28.Miles EA, Banerjee T, Wells SJ, Calder PC. Limited effect of eicosapentaenoic acid on T-lymphocyte and natural killer cell numbers and functions in healthy young males. Nutrition. 2006;22:512–519. doi: 10.1016/j.nut.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Ermak N, Lacour B, Drüeke TB, Vicca S. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocyte. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.12.052. In the Press. [DOI] [PubMed] [Google Scholar]
- 30.Wu T, Geigerman C, Lee YS, Wander RC. Enrichment of LDL with EPA and DHA decreased oxidized LDL-induced apoptosis in U937 cells. Lipids. 2002;37:789–796. doi: 10.1007/s11745-002-0962-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Wander RC. Reduced effect on apoptosis of 4-hydroxyhexenal and oxidized LDL enriched with n-3 fatty acids from postmenopausal women. J Nutr Biochem. 2005;16:213–221. doi: 10.1016/j.jnutbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Malle E, Marsche G, Arnhold J, Davies MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochim Biophys Acta. 2006;1761:392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Véricel E, Calzada C, Chapuy P, Lagarde M. The influence of low intake of n-3 fatty acids on platelets in elderly people. Atherosclerosis. 1999;147:187–192. doi: 10.1016/s0021-9150(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 34.Bechoua S, Dubois M, Dominguez Z, Goncalves A, Némoz G, Lagarde M, Prigent AF. Protective effect of docosahexaenoic acid against hydrogen peroxide-induced oxidative stress in human lymphocytes. Biochem Pharmacol. 1999;57:1021–1030. doi: 10.1016/s0006-2952(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med. 2003;34:1006–1016. doi: 10.1016/s0891-5849(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 36.Lemaitre D, Véricel E, Polette A, Lagarde M. Effects of fatty acids on human platelet glutathione peroxidase: possible role of oxidative stress. Biochem Pharmacol. 1997;53:479–486. doi: 10.1016/s0006-2952(96)00734-4. [DOI] [PubMed] [Google Scholar]
- 37.Joulain C, Prigent AF, Némoz G, Lagarde M. Increased glutathione peroxidase activity in human blood mononuclear cells upon in vitro incubation with n-3 fatty acids. Biochem Pharmacol. 1994;47:1315–1323. doi: 10.1016/0006-2952(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi F, Attorri L, Benedetto RD, Biase AD, Sanchez M, Tregno FP, Nardini M, Salvati S. Docosahexaenoic acid supplementation induces dose and time dependent oxidative changes in C6 glioma cells. Free Radic Res. 2007;41:748–756. doi: 10.1080/10715760701324067. [DOI] [PubMed] [Google Scholar]
- 39.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]


