Abstract
While food webs have provided a rich vein of research material over the last 50 years, they have largely been the subject matter of the pure ecologist working in natural habitats. While there are some notable exceptions to this trend, there are, as I explain in this paper, many applied questions that could be answered using a food web approach. The paper is divided into two halves. The first half provides a brief review of six areas where food webs have begun to be used as an applied tool: restoration ecology, alien species, biological control, conservation ecology, habitat management and global warming. The second half outlines five areas in which a food web approach could prove very rewarding: urban ecology, agroecology, habitat fragmentation, cross-habitat food webs and ecosystem services.
Keywords: applied ecology, food web, network
1. Introduction
Historically food webs have been used for pure rather than applied research, for example, work investigating network topology, networks stability and robustness, the determinants of food chain length and patterns of generalization. This has been for at least three reasons: first, the people who work on food webs have tended to work in natural habitats, for example rainforests (Lewis et al. 2002), lakes (Havens 1994), deserts (Polis 1991), rivers (Winemiller 1990), rocky shores (Paine 1966), estuaries (Hall & Raffaelli 1991), heathlands (Forup et al. 2008) and meadows (Muller et al. 1999). There are no, or at best very few, urban food webs, landfill food webs, farm food webs, forestry food webs, orchard food webs or motorway embankment food webs, despite the fact that these habitats can be extensive and are much more intimately connected to humans than natural habitats. Second, food webs are perceived as difficult. Thus they require identification of often hundreds of species (most of which are hard to identify and uncharismatic invertebrates) followed by a complex analysis from which few firm practical conclusions can be drawn. Third, food webs are simply not seen as an appropriate tool in applied circles, veering perhaps towards Strong's (1988) tongue-in-cheek description of food webs as ‘a ladder for picking strawberries’.
Food webs can, however, be used to answer significant questions in applied biology and the aim of this paper is to show how the practical and theoretical advances made in food web construction can be used outside of natural habitats to the advantage of pure and applied biologists. This paper is not intended to be a thorough review; rather the intention is to consider the range of questions which my colleagues and I have answered by constructing food webs in a variety of disturbed habitats and then outlining other areas of applied ecology which would benefit from this approach.
2. Part 1: applied food web ecology: six examples illustrating the use of food webs as a tool to answer applied questions
(a) Restoration ecology: assessing the efficacy of ecological restoration by evaluating ecological function and network robustness
When human activities damage ecosystems, it may be possible to repair the damage through ecological restoration. In planning and evaluating restoration projects, it is critical to also consider function and what constituent species do rather than just whether they are present or not (Ehrenfeld & Toth 1997). Direct comparison between restored and reference sites is difficult with a structural focus alone, because species composition will vary from site to site (Williams et al. 1996). Using ecological networks such as food webs or mutalistic (pollination and seed dispersal) webs as a tool to define the target community and to assess the efficacy of a restoration project can provide a powerful tool for asking about the restoration of community structure, of community function and about the resilience of restored communities to future species loss.
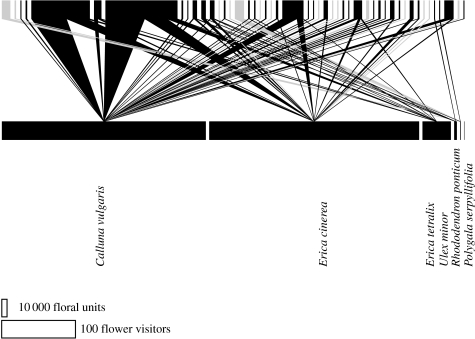
Forup et al. (2008) studied four restored heathlands in 2001 and 2004 and compared their pollination networks with those of four paired ancient heathlands (figure 1). These networks captured the components of the ecosystem service of pollination and used it as a yardstick for judging restoration success. Pollinator function was determined by constructing visitation networks and pollen transport networks, and then using these data to calculate pollinator importance (Gibson et al. 2006). The pollinator importance values identified the possible pollinators on both ancient and restored heathlands, and revealed that these were the same species on both types of sites, i.e. pollination had been restored. In addition to looking at the function of species in the network, Forup et al. (2008) also considered the network overall and compared the robustness of networks from restored sites with those from ancient sites. This assessed the ability of restored sites to withstand future natural and man-made perturbations and they reported a trend of restored networks to be more susceptible to perturbation than ancient networks.
Figure 1.
A plant–pollinator network from an ancient heathland field site in Dorset, UK, showing the plant–pollinator interactions that need to be reinstated in a restoration project. In the network, each species of plant and insect is represented by a rectangle: the lower line represents flower abundance, the upper line represents insect abundance. The width of the rectangles and the size of the interaction between them are proportional to their abundance at the field site. The scale bar represents number of floral units and number of insects. The pollen vectors in the network are shown in black, the nectar thieves in grey.
(b) Alien species: the effect of alien species on network structure (the opportunity to manipulate networks)
Alien species pose a significant threat to global biodiversity, second only to habitat loss (Schmitz & Simberloff 1997). The vast majority of alien species research investigates their impact at a single trophic level. Subtle, but highly significant, interactions such as apparent competition and mutualisms can be important forces underlying community structure, yet they are virtually undetectable with the survey techniques traditionally used to study the impact of alien species (Carvalheiro et al. 2008). Moreover, any linked extinctions also remain undetectable—thus if a native plant is driven extinct by an alien plant, the alien plant's specific herbivores and its herbivore's specific parasitoids will also perish. While small and non-charismatic to some, parasitoids along with parasites and pathogens are remarkably common in food webs (Lafferty et al. 2006) and their loss could have a profound effect on community structure and function (Lafferty et al. 2008). Indirect effects and linked extinctions will not be picked up by traditional methods. Food webs, however, can identify the potential for indirect effects such as apparent competition (competition due to shared natural enemies (Holt 1977)) and more generally provide a technique to ask how well alien species are integrated into food web structure. Moreover, alien species provide unparalleled opportunities for manipulative field experiments at the community level. This is because the removal of an alien species rarely elicits any concern from conservationists or the public and so food web ecologists can readily test the effect of node removal in their networks. Indeed such experiments could be run within the many eradication programmes that exist around the world to the advantage of network ecologists, invasion biologists and conservation biologists. For example, Lopezaraiza Mikel et al. (2007) removed the flowers of Himalayan balsam (Impatiens glandifolia Royle, Balsaminaceae) from replicated plots and compared visitation with unmanipulated control plots over a field season. The more generalized insects were more likely to visit the alien plant and their data reveal that generalized native pollinators can provide a pathway of integration for alien plants into native visitation systems. Invaded plots had significantly higher visitor species richness, visitor abundance and flower visitation. However, their pollen transport networks were dominated by alien pollen grains in the invaded plots and consequently higher visitation may not have translated into increased seed set.
(c) Biological control: testing the safety of biological control, using networks for risk assessment
Under current biological control legislation, negative direct non-target impacts of biological control (i.e. the biocontrol agent feeding on a species other than the intended target weed or pest) are rare and when they do occur, predictable (Sheppard et al. 2006). However, the possibility of indirect impacts from introduced agents on native species via apparent competition has rarely been considered in a biocontrol context. A successfully established biocontrol agent can become an abundant food source for generalist natural enemies present in the target ecosystem. Natural enemies (e.g. parasitoids or pathogens) that include the biocontrol agent in their diet may themselves become more abundant. If this happens (and the aim of any biocontrol programme is for the agent to become abundant, at least initially) apparent competition between the biocontrol agent and native species can occur, just as it can between native species.
Food webs can be used to pinpoint which non-target species biocontrol agents attack in addition to their target weed or pest species (e.g. Henneman & Memmott 2001; Munro & Henderson 2002) and to develop testable hypotheses about their impact on native biodiversity (Willis & Memmott 2005). For example, using 17 replicate food webs Carvalheiro et al. (2008) demonstrated that the use of a highly host-plant specific weed biocontrol agent, recently introduced into Australia, is associated with declines of local insect communities. The agent shares natural enemies (predators and parasitoids) with seed herbivore species from native plants, so apparently competition was the most probable cause for these losses, although manipulative field experiments are still needed to confirm this. Their work indicates that more investment is required in pre-release studies on the effectiveness of biocontrol agents, as well as in post-release studies assessing indirect impacts, to avoid or minimize the release of potentially damaging species.
(d) Conservation ecology: conserving the pollinators of rare plants
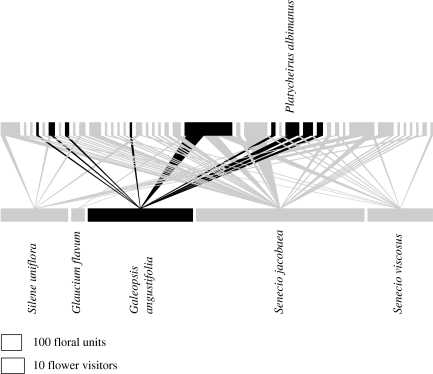
Currently 12.5 per cent of the world's vascular plant species are facing extinction (Wilcock & Neiland 2002). This situation is particularly apparent in Britain, where 40 per cent of the flora is considered to be at risk (Marren 1999). Working on three rare plant species in England, Gibson et al. (2006) used the data provided from visitation webs and pollen transport webs to calculate a pollinator importance index for the pollinators of each plant species (figure 2). In addition to identifying the possible pollinators for each plant at each site, it was obvious from the webs that the rare plants were linked to other plant species in the community via a number of shared pollinators. These other plant species in many cases will be the primary food sources for these shared pollinators. Therefore the long-term survival of rare plant populations is likely to depend on some of the common plant species in the community. Thus, in order to conserve the rare species, the common species also need to be conserved as they support the pollinators of the rare plants. Management recommendations were made for each of the three plant species based on this information.
Figure 2.
Visitation web for the rare plant Galeopsis angustifolia at a coastal site in Norfolk, UK. For an explanation of the network see the legend for figure 1. The rare plant and its insect visitors are shown in black. The probable pollinator, Platycheirus albimanus, is shown. Galeopsis angustifolia interacts indirectly with Silene uniflora and Senecio viscosus via P. albimanus and consequently these two species of plant need to be conserved as part of the conservation of G. angustifolia.
(e) Habitat management: predicting the impact of habitat management on the conservation of a rare plant
The vast majority of conservation practitioners practise some form of habitat management to protect the species they wish to conserve, for example removing any pestiferous species, maintaining the desirable successional stage and or providing particular habitat types for their target species. Removing alien plants is a key part of many habitat management programmes and the untested assumption is that this is good for the habitat concerned.
The plant–pollinator food web and field experiments constructed by Carvalheiro et al. (2008) along with the associated field experiments revealed that their study plant Trinia glauca (Apiaceae) was pollinated by ants and that these ants also fed on alien plants. Carvalheiro et al. (2008) simulated removing the alien species from their network by removing them in silico. In the simulation, the removal of alien plants constituted the primary extinctions and the secondary extinctions were the individual pollinators lost from the network owing to their food supply being removed. By contrast with most previous species removal simulations, host shifting by the remaining species was allowed; thus the ants could shift from feeding on alien species to native species. They concluded that management measures involving removal of alien plants need to consider the possible negative impacts on rare plants as their network simulation models suggested that the sudden removal of aliens could adversely affect T. glauca's pollinators and thereby T. glauca itself.
It would be ironic at best if the conservations measures put in place to save a rare plant inadvertently led to its extinction. While Carvalheiro et al. (2008) did not suggest that removing the aliens is an inappropriate management tool, they did suggest that their impact on pollinators was at least considered, as without the pollinators, the conservation of the rare plants will be an exercise in gardening rather than a sustainable conservation programme. Management plans that involve removal of alien plants should ideally consider the unintended, indirect, short-term negative impact, as well as the intended long-term positive gains.
(f) Global warming: predicting the community level impact of global warming
Anthropogenic climate change is widely expected to drive species extinct by reducing individual fitness or the amount and accessibility of suitable habitat (Thomas et al. 2004), as well as by causing extinction of their partners in ecological interactions (Fitter & Fitter 2002; Stenseth & Mysterud 2002). Less well appreciated is the possibility that climate change may directly disrupt ecological interactions en masse. Memmott et al. (2007) explore the potential disruption of a ubiquitous interaction of terrestrial habitats, between plants and their animal (mostly insect) pollinators, via climate-induced shifts in seasonal timing (phenology) of flowering and pollinator activity. Using a highly resolved empirical network of interactions between 1419 pollinator and 429 plant species, Memmott et al. (2007) simulated the effects of the phenological shifts expected from warming over the next century. Depending on model assumptions, phenological shifts reduced the floral resources available to 17–50% of all pollinator species, by reducing flower availability during the pollinators' flight seasons. Reduced overlap between plants and pollinators also decreased diet breadth and suggested that the disruption of pollination interactions by warming-induced phenological shifts could lead first to extinction of many pollinators and then to loss of the plants they pollinate.
3. Part 2: ecological systems and questions that would benefit from a food web approach
The current epoch has been described as the Anthropocene (Steffen et al. 2007), this term the huge impact of mankind on the planet. In what follows, five areas are proposed that would prove excellent systems both for study by food web ecologists and for which a food web approach could prove enlightening.
(a) Urban ecology
Urban areas are one of the fastest growing habitats in the world. While there are detailed, long-term studies of charismatic vertebrates such as foxes in urban habitats, there are considerably less data available on the plants and invertebrates which make up the vast bulk of biodiversity and practically no data at the food web level. While there are no food webs, there are some excellent examples of projects looking at biodiversity in an urban habitat. The Biodiversity in Urban Gardens project run in the city of Sheffield in England made considerable progress studying the diversity of garden plants and insects (Smith et al. 2006; Loram et al. 2008). Also, driven individuals have listed all the species sampled in a single garden (Owen 1991) or a single town (Price 2002). The next step, however, is to look at the patterns of interactions between species, asking how urbanization changes the patterns of interactions and the ecosystem services that they provide, and asking how best to mitigate adverse effects so that there is space for both wildlife and people in urban habitats.
(b) Agroecology
The intensification of arable agriculture over the last 50 years has been associated with substantial losses of biodiversity and there is considerable concern that intensive agriculture is incompatible with the conservation of biodiversity (Rigby et al. 2001). Given that 77 per cent of the land area in Great Britain is under agricultural production (defra 2002) and that most of its biodiversity is found on farmland (Hole et al. 2005), how we farm has considerable implications for both the maintenance and utilization of biodiversity. To date, information on declines in biodiversity is clustered around particular groups of organisms, particularly farmland birds, farmland mammals, arable weeds, butterflies, bumble-bees and spiders. However, the distribution and abundance of these groups may not provide the data needed for the sustainable management of agriculture. If agroecologists, land managers and policy makers are to include managing for biodiversity as part of their remit, then they also need to understand the ways in which species interact, as these interactions can have a profound impact on a community's response to species loss, stress and ecological restoration. A food web approach has begun here, but progress is slow. Thus de Ruiter et al. (1998) used a food web approach to ask about energy flow and network stability in agroecosystems and Letourneau & Goldstein (2001) looked at pest damage and community structure on tomato farms. However neither of these studies characterized the links between species in a format leading to a standard quantitative network. More recently, Tylianakis et al. (2007) have constructed quantitative networks, which set the standard for assessing the impact of agriculture on food web structure. There remains a huge scope though for asking how different types of agriculture affect biodiversity and how biodiversity provides ecosystem services (which are effectively the product of networks of interactions between species) such as pollination and pest control.
(c) Habitat fragmentation
Nature conservation demands data on ecological patterns and ecological processes, especially when the maintenance of biodiversity is a key issue. The need to give the conservation of ecological processes an equal weighting to the conservation of ecological patterns is repeatedly stressed but much less rarely implemented. Habitat fragmentation is one of the greatest threats to biodiversity and is often followed by invasion of alien species, thereby compounding the problem.
A food web approach to habitat fragmentation is underway, but it has much ground to make up in comparison with species-centric approaches. Two particularly nice studies are Gilbert et al. (1998) who used a field experiment to test the impact of fragmentation on different trophic levels and Valladares et al. (2006) who used food webs to test the effect of fragmentation on plant–herbivore–parasitoid networks. Linking local fragmented communities by dispersal means they can be considered as a metacommunity, ‘a set of local communities linked by dispersal’ (Hanski & Gilpin 1991; Holyoak et al. 2005). A start has been made on a theoretical framework for how food webs respond to fragmentation (Fortuna & Bascompte 2006), but much more work is needed if we are to confidently predict how communities will change under stress and be able to manage natural communities at the landscape level (Holyoak et al. 2005).
(d) Cross-habitat food webs
As mentioned in §1, most food webs are conducted in natural habitats and the field plot almost invariably consists of just one habitat type, for example tropical forests, meadows, heathlands or intertidal zones. Reducing any variation in habitat type reduces the noise in any food web parameters being measured and so makes good sense from an experimental design point of view when testing for a treatment effect such as restoration efficacy (Forup et al. 2008) or impact of alien species (Lopezaraiza Mikel et al. 2007). However, there obviously will be interactions between species in different habitats. For example Knight et al. (2005) demonstrated that ponds with fish had higher seed set in adjacent marginal plants. Clearly fish were not pollinating these plants, rather the fish ate the dragonfly larvae, which then reduced adult dragonflies numbers and thereby reduced the predation pressure on the pollinating bees. Links between habitats are almost certainly widespread and these links could be rather important in community level restoration and management. As an analogy to, say, three habitats within an ecosystem, consider the three components of a desktop computer: the screen, the keyboard and the processor; if the link between any one of these three components is lost, then the computer will not work even if the three components are individually perfectly functional. Given that the techniques used to construct food webs in different habitats can be very different, the fastest way to make progress here would be to increase the discourse and collaboration between terrestrial, aquatic and marine food web ecologists.
(e) Ecosystem services
Ecosystem services, such as pest control and pollination, are ecosystem functions that are useful to humans; many of these are critical to our survival while others enhance it (Kremen 2005). For example organic farms are dependent on natural pest control, and this natural pest control also augments chemical control on conventional farms. Pollination is essential for 15–30% of American food production (Kremen 2005) and more than 150 European crops require pollinators (Williams 1994). The Millienium Ecosystem Assessment (Anon 2005) and defra's guide to valuing ecosystem services (defra 2007) has considerably raised the profile of ecosystem services. However, our understanding of how they work remains both limited (Kremen 2005). Developing a general template that will provide direction for future ecosystem service research is crucial. Ecological networks could provide a quality yardstick for judging both the structure and the function of an ecosystem as they characterize what species actually do and how they interact with one another to produce beneficial outcomes such as ecosystem services. Moreover, research has shown that interaction diversity can decline far more rapidly than the diversity of the species themselves (Albrecht et al. 2007), consequently ecosystem service could well decline before there is any obvious species loss.
4. Discussion
All life on the Earth, whether in natural or managed habitats, exists within food webs that link the species in biological communities to at least one other species in that community. Yet food webs and other ecological networks are not widely used in applied ecology. Given the practical advances being made in food web construction (for example, high-quality replicated networks and ecoinformatics), the theoretical advances (for example models of extinction dynamics) and the past, present and future impact of mankind on the planet, the present is an excellent time to begin using ecological networks as an applied tool. Moreover, the healthy functioning of natural and managed habitats does provide free ecosystem services essential to mankind. Many ecosystem services are the result of interactions between species in a food web context, for example those between pollinators and crop plants or between parasitoids and pest herbivores.
For food webs to make it into the tool box of applied ecologists, more food webs of applied systems are needed. Food web ecologists should avoid the knee-jerk reaction to work on natural systems, ecological methods books should discuss food webs as a tool rather than a topic and finally there needs to be more of a discourse between applied ecologists and food web ecologists. This could well lead to a win–win situation: food web ecologists would win as they could have network perturbations to order (removal of alien species, application of specific pesticides and the reintroduction of species for example). Likewise, the applied ecologists would win as they would have answers to questions hitherto considered unanswerable, for example the impact of their habitat management at the community level.
Footnotes
One contribution of 15 to a Theme Issue ‘Food-web assembly and collapse: mathematical models and implications for conservation’.
References
- Albrecht M., Duelli P., Schmid B., Muller C.B. Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. J. Anim. Ecol. 2007;76:1015–1025. doi: 10.1111/j.1365-2656.2007.01264.x. doi:10.1111/j.1365-2656.2007.01264.x [DOI] [PubMed] [Google Scholar]
- Anon. World Resources Institute; Washington, DC: 2005. Millenium ecosystem assessment. Ecosystems and human well-being: biodiversity synthesis. [Google Scholar]
- Carvalheiro L.G., Buckley Y.M., Ventim R., Fowler S.V., Memmott J. Apparent competition can compromise the safety of highly specific biocontrol agents. Ecol. Lett. 2008;11:690–700. doi: 10.1111/j.1461-0248.2008.01184.x. doi:10.1111/j.1461-0248.2008.01184.x [DOI] [PubMed] [Google Scholar]
- Carvalheiro L.G., Barbosa E.R.M., Memmott J. Pollinator networks, alien species and the conservation of rare plants: Trinia glauca as a case study. J. Appl. Ecol. 2008;45:1419–1427. doi:10.1111/j.1365-2664.2008.01518.x [Google Scholar]
- defra 2002 Action plan to develop organic farming in England. See http://www.defra.gov.uk/farm/organic/actionplan/indexhtm
- defra. defra; London, UK: 2007. An introductory guide to valuing ecosystem services. [Google Scholar]
- de Ruiter P.C., Neutel A.M., Moore J.C. Biodiversity in soil ecosystems: the role of energy flow and community stability. Appl. Soil Ecol. 1998;10:217–228. doi:10.1016/S0929-1393(98)00121-8 [Google Scholar]
- Ehrenfeld J.G., Toth L.A. Restoration ecology and the ecosystem perspective. Restor. Ecol. 1997;5:307–317. doi:10.1046/j.1526-100X.1997.00544.x [Google Scholar]
- Fitter A.H., Fitter R.S.R. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. doi:10.1126/science.1071617 [DOI] [PubMed] [Google Scholar]
- Fortuna M.A., Bascompte J. Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 2006;9:278–283. doi: 10.1111/j.1461-0248.2005.00868.x. doi:10.1111/j.1461-0248.2005.00868.x [DOI] [PubMed] [Google Scholar]
- Forup M.L., Henson K.E., Craze P.G., Memmott J. The restoration of ecological interactions: plant–pollinator networks on ancient and restored heathlands. J. Appl. Ecol. 2008;45:742–752. doi:10.1111/j.1365-2664.2007.01390.x [Google Scholar]
- Gibson R.H., Nelson I.L., Hopkins G.W., Memmott J. Pollinator webs, plant communities and the conservation of rare plants: arable weeds as a case study. J. Appl. Ecol. 2006;43:246–257. doi:10.1111/j.1365-2664.2006.01130.x [Google Scholar]
- Gilbert F., Gonzalez A., Evans-Freke I. Corridors maintain species richness in the fragmented landscapes of a microecosystem. Proc. R. Soc. B. 1998;265:577–582. doi:10.1098/rspb.1998.0333 [Google Scholar]
- Hall S.J., Raffaelli D. Food-web patterns—lessons from a species-rich web. J. Anim. Ecol. 1991;60:823–842. doi:10.2307/5416 [Google Scholar]
- Hanski I., Gilpin M. Metapopulation dynamics—brief-history and conceptual domain. Biol. J. Linn. Soc. 1991;42:3–16. doi:10.1111/j.1095-8312.1991.tb00548.x [Google Scholar]
- Havens K.E. Experimental perturbation of a fresh-water plankton community—a test of hypotheses regarding the effects of stress. Oikos. 1994;69:147–153. doi:10.2307/3545295 [Google Scholar]
- Henneman M.L., Memmott J. Infiltration of a Hawaiian community by introduced biological control agents. Science. 2001;293:1314–1316. doi: 10.1126/science.1060788. doi:10.1126/science.1060788 [DOI] [PubMed] [Google Scholar]
- Hole D.G., Perkins A.J., Wilson J.D., Alexander I.H., Grice F., Evans A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005;122:113–130. doi:10.1016/j.biocon.2004.07.018 [Google Scholar]
- Holt R.D. Predation, apparent competition and the structure of prey communities. J. Theor. Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. doi:10.1016/0040-5809(77)90042-9 [DOI] [PubMed] [Google Scholar]
- Holyoak M., Leibold M.A., Holt R.D. University of Chicago Press; Chicago, IL: 2005. Metacommunities: spatial dynamics and ecological communities. [Google Scholar]
- Knight T.M., McCoy M.W., Chase J.M., McCoy K.A., Holt R.D. Trophic cascades across ecosystems. Nature. 2005;437:880–883. doi: 10.1038/nature03962. doi:10.1038/nature03962 [DOI] [PubMed] [Google Scholar]
- Kremen C. Managing ecosystem services: what do we need to know about their ecology? Ecol. Lett. 2005;8:468–479. doi: 10.1111/j.1461-0248.2005.00751.x. doi:10.1111/j.1461-0248.2005.00751.x [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Dobson A.P., Kuris A.M. Parasites dominate food web links. Proc. Natl Acad. Sci. USA. 2006;103:11 211–11 216. doi: 10.1073/pnas.0604755103. doi:10.1073/pnas.0604755103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D., et al. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. doi:10.1111/j.1461-0248.2008.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau D.K., Goldstein B. Pest damage and arthropod community structure in organic vs. conventional tomato production in California. J. Appl. Ecol. 2001;38:557–570. doi:10.1046/j.1365-2664.2001.00611.x [Google Scholar]
- Lewis O.T., Memmott J., Lasalle J., Lyal C.H.C., Whitefoord C., Godfray H.C.J. Structure of a diverse tropical forest insect–parasitoid community. J. Anim. Ecol. 2002;71:855–873. doi:10.1046/j.1365-2656.2002.00651.x [Google Scholar]
- Lopezaraiza Mikel M.E., Hayes R.B., Whalley M.R., Memmott J. The impact of an alien plant on a native plant–pollinator network: an experimental approach. Ecol. Lett. 2007;10:539–550. doi: 10.1111/j.1461-0248.2007.01055.x. doi:10.1111/j.1461-0248.2007.01055.x [DOI] [PubMed] [Google Scholar]
- Loram A., Thompson K., Warren P.H., Gaston K.J. Urban domestic gardens (XII): the richness and composition of the flora in five UK cities. J. Veg. Sci. 2008;19:321–330. doi:10.3170/2007-8-18373 [Google Scholar]
- Marren P. Natural History; London, UK: 1999. Britain's rare flowers T & AD Poyser. [Google Scholar]
- Memmott J., Craze P.G., Waser N.M., Price M.V. Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. doi:10.1111/j.1461-0248.2007.01061.x [DOI] [PubMed] [Google Scholar]
- Muller C.B., Adriaanse I.C.T., Belshaw R., Godfray H.C.J. The structure of an aphid–parasitoid community. J. Anim. Ecol. 1999;68:346–370. doi:10.1046/j.1365-2656.1999.00288.x [Google Scholar]
- Munro V.M.W., Henderson I.M. Nontarget effect of entomophagous biocontrol: shared parasitism between native Lepidopteran parasitoids and the biocontrol agent Trigonospila brevifacies (Diptera: Tachinidae) in forest habitats. Environ. Entomol. 2002;31:388–396. [Google Scholar]
- Owen J. Cambridge University Press; Cambridge, UK: 1991. The ecology of garden: the first fifteen years. [Google Scholar]
- Paine R.T. Food web complexity and species diversity. Am. Nat. 1966;100:65–75. doi:10.1086/282400 [Google Scholar]
- Polis G.A. Complex trophic interactions in deserts—an empirical critique of food-web theory. Am. Nat. 1991;138:123–155. doi:10.1086/285208 [Google Scholar]
- Price J.M. Gem Publishing; Wallingford, UK: 2002. Stratford-upon-avon—a flora and Fauna. [Google Scholar]
- Rigby D., Young T., Burton M. The development of and prospects for organic farming in the UK. Food Policy. 2001;26:599–613. doi:10.1016/S0306-9192(01)00023-9 [Google Scholar]
- Schmitz D.C., Simberloff D. Biological invasions: a growing threat. Issues Sci. Technol. 1997;13:33–40. [Google Scholar]
- Sheppard A.W., Shaw R.H., Sforza R. Top 20 environmental weeds for classical biological control in Europe: a review of opportunities, regulations and other barriers to adoption. Weed Res. 2006;46:93–117. doi:10.1111/j.1365-3180.2006.00497.x [Google Scholar]
- Smith R.M., Warren P.H., Thompson K., Gaston K.J. Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodivers. Conserv. 2006;15:2415–2438. doi:10.1007/s10531-004-5014-0 [Google Scholar]
- Steffen W., Crutzen P.J., McNeill J.R. The Anthropocene: are humans now overwhelming the great forces of nature. Ambio. 2007;36:614–621. doi: 10.1579/0044-7447(2007)36[614:taahno]2.0.co;2. doi:10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stenseth N.C., Mysterud A. Climate, changing phenology, and other life history and traits: nonlinearity and match–mismatch to the environment. Proc. Natl Acad. Sci. USA. 2002;99:13 379–13 381. doi: 10.1073/pnas.212519399. doi:10.1073/pnas.212519399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D.R. Food web theory—a ladder for picking strawberries. Ecology. 1988;69:1647. doi:10.2307/1941140 [Google Scholar]
- Thomas C.D., et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Tylianakis J.M., Tscharntke T., Lewis O.T. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature. 2007;445:202–205. doi: 10.1038/nature05429. doi:10.1038/nature05429 [DOI] [PubMed] [Google Scholar]
- Valladares G., Salvo A., Cagnolo L. Habitat fragmentation effects on trophic processes of insect–plant food webs. Conserv. Biol. 2006;20:212–217. doi: 10.1111/j.1523-1739.2006.00337.x. doi:10.1111/j.1523-1739.2006.00337.x [DOI] [PubMed] [Google Scholar]
- Wilcock C., Neiland R. Pollination failure in plants: why it happens and when it matters. Plant Sci. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. doi:10.1016/S1360-1385(02)02258-6 [DOI] [PubMed] [Google Scholar]
- Williams I.H. The dependence of crop production within the European Union on pollination by honeybees. Agric. Zool. Rev. 1994;6:229–257. [Google Scholar]
- Williams R.J., Duff G.A., Bowman D., Cook G.D. Variation in the composition and structure of tropical savannas as a function of rainfall and soil texture along a large-scale climatic gradient in the Northern Territory, Australia. J. Biogeogr. 1996;23:747–756. doi:10.1111/j.1365-2699.1996.tb00036.x [Google Scholar]
- Willis A.J., Memmott J. The potential for indirect effects between a weed, one of its biocontrol agents and native herbivores: a food web approach. Biol. Control. 2005;35:299–306. doi:10.1016/j.biocontrol.2005.07.013 [Google Scholar]
- Winemiller K.O. Spatial and temporal variation in tropical fish trophic networks. Ecol. Monogr. 1990;60:331–367. doi:10.2307/1943061 [Google Scholar]