Abstract
The central organizing theme of this paper is to discuss the dynamics of the Serengeti grassland ecosystem from the perspective of recent developments in food-web theory. The seasonal rainfall patterns that characterize the East African climate create an annually oscillating, large-scale, spatial mosaic of feeding opportunities for the larger ungulates in the Serengeti; this in turn creates a significant annual variation in the food available for their predators. At a smaller spatial scale, periodic fires during the dry season create patches of highly nutritious grazing that are eaten in preference to the surrounding older patches of less palatable vegetation. The species interactions between herbivores and plants, and carnivores and herbivores, are hierarchically nested in the Serengeti food web, with the largest bodied consumers on each trophic level having the broadest diets that include species from a large variety of different habitats in the ecosystem. The different major habitats of the Serengeti are also used in a nested fashion; the highly nutritious forage of the short grass plains is available only to the larger migratory species for a few months each year. The longer grass areas, the woodlands and kopjes (large partially wooded rocky islands in the surrounding mosaic of grassland) contain species that are resident throughout the year; these species often have smaller body size and more specialized diets than the migratory species. Only the larger herbivores and carnivores obtain their nutrition from all the different major habitat types in the ecosystem. The net effect of this is to create a nested hierarchy of subchains of energy flow within the larger Serengeti food web; these flows are seasonally forced by rainfall and operate at different rates in different major branches of the web. The nested structure that couples sequential trophic levels together interacts with annual seasonal variation in the fast and slow chains of nutrient flow in a way that is likely to be central to the stability of the whole web. If the Serengeti is to be successfully conserved as a fully functioning ecosystem, then it is essential that the full diversity of natural habitats be maintained within the greater Serengeti ecosystem. The best way to do this is by controlling the external forces that threaten the boundaries of the ecosystem and by balancing the economic services the park provides between local, national and international needs. I conclude by discussing how the ecosystem services provided by the Serengeti are driven by species on different trophic levels. Tourism provides the largest financial revenue to the national economy, but it could be better organized to provide more sustained revenue to the park. Ultimately, ecotourism needs to be developed in ways that take lessons from the structure of the Serengeti food webs, and in ways that provide tangible benefits to people living around the park while also improving the experience of all visitors.
Keywords: food web, Serengeti, savanna, complexity, ecotourism, ecosystem service
1. Introduction
In this paper, I will examine the Serengeti grassland ecosystem of Tanzania from the perspective of a number of recent theoretical and empirical studies of food webs. My principal goal is to establish a dialogue between the insights provided by the recent rapid developments into our understanding of food-web structure, and the longer-term empirical understanding of how different processes interact in the East African grasslands. The dialogue will emphasize some areas where theoretical insights sit comfortably with our empirical understanding of how the Serengeti functions; however, I also hope to identify areas where there is a lack of resonance between theory and observation. Hopefully, this will point towards ways in which new theory needs to be developed (or old theory modified!), so that it provides further insights that are useful to both the understanding and conservation of the Serengeti and to the wide diversity of other tropical ecosystems. These ecosystems are increasingly at risk from human population growth, which leads to overexploitation of wildlife populations in parks, land use change around parks and climate change on a global level. The need to conserve tropical ecosystems from these threats is increasingly urgent and food-web studies can provide key insights into how to achieve this.
2. Serengeti food webs
The Serengeti is an ecosystem that is as near pristine as any other on the planet; moreover, it is the ecosystem that bears the closest resemblance to the habitats in which humans first walked upright and began to use their environment in an organized and systematic fashion. There are still hunter-gatherer groups, the Hazda, living within the ecosystem, as well as more recent pastoralists (Maasai) and more sedentary farmers, all of whom interact with a huge diversity of wild animal and plant species. The whole ecosystem also supports a large ecotourism industry that provides the major source of foreign currency to Tanzania's challenged economy. The long-term management policy of the park has been to minimize intervention and allow natural processes to operate (Sinclair & Arcese 1995; Sinclair et al. 2008). The only major exception to this has been continuous attempts to control poaching, which can reach epidemic proportions in northern areas of the park.
The Serengeti has been studied as a fully functioning natural ecosystem for over 50 years (Sinclair & Norton-Griffiths 1979; Sinclair & Arcese 1995; Sinclair et al. 2008); it is one of the largest remaining tropical savannahs and still retains an almost complete set of plant and animal species. The rhinoceros and hunting dogs that almost became locally extinct at the end of the last century have now either been reintroduced, or have naturally recolonized (Burrows et al. 1994; Dye 1996; Metzger et al. 2007). Developing a food web for the Serengeti is a formidable undertaking (especially if it includes invertebrates, birds and smaller mammals); yet, sufficient work has been done on the major plant and mammalian groups that we can begin to see the outlines of the structure of an intact terrestrial food web dominated by vertebrate herbivores and carnivores. We are just beginning to grasp the daunting levels of diversity that are also present in the less-studied invertebrate and fungal groups (McNaughton & Oesterheld 1990; Morell 1997). Insights into the factors that shape the structure of the Serengeti food web should provide insight into the structure of food webs for less-studied and less well-protected natural tropical habitats.
A number of recent theoretical insights have provided a new impetus to food-web studies: (i) recent theoretical work by Allesina & Pascual (2008) has shown that food webs are more likely to be stable if they are dominated by consumer–resource (+/−) links; this work allows us to reconsider Bob May's stage-setting theoretical insight that we should expect an inverse relationship between ecosystem stability and species diversity in food webs with random interaction strength (May 1972, 1973). Allesina & Pascual's (2008) work shows that while the underlying trade-off between diversity and stability remains, if the web is dominated by resource–consumer links, then the number of species and links that permit stability is considerably larger than when it is dominated by competitive or mutualistic links. (ii) Recent work on webs for pollinators and seed dispersers by Bascompte et al. (2003) and Fortuna & Bascompte (2006) has shown that webs with a nested interaction topology are more likely to persist in the presence of habitat loss than webs in which species interactions are ‘non-nested’. This work on nestedness is subtly related to the work on null ‘niche models’ for food-web structure (Williams & Martinez 2000). In particular, the niche model intrinsically predicts that the consumers with the largest body size on each trophic level will have broader niches than smaller ones; thus we would expect the diets of small consumers to be ‘nested’ within the diet of larger bodied consumers. (iii) Rooney et al. (2006) have shown that webs with spatial nesting may be strongly stabilizing in systems where consumers on the upper trophic levels are coupled to lower levels by links that operate at significantly different rates while also coupling resource use across spatially distinct regions of the larger ecosystem. (iv) Olff and Ritchie (Ritchie & Olff 1999; Olff et al. 2002; Balmford & Bond 2005) and Olff et al. (2009) have shown that body size scaling, resource palatability and the presence of decomposer chains are all key to understanding constraints on food-web architecture. In particular, they show that body size and the distribution of food resources are crucial in determining the underlying skeleton of food-web structure. (v) The work of Lafferty et al. (2006), Dobson et al. (2008) and Kuris et al. (2008) has shown that parasites are a huge and underappreciated component of biodiversity that are intimately embedded within the structure of food webs. Furthermore, parasitic species are found to form up to 80 per cent of the links within carefully dissected ecosystems and their biomass may exceed that of the vertebrate taxa that traditionally dominate the upper trophic levels of food webs! It therefore seems essential that food-web theory should be extended to consider parasites (Lafferty et al. 2008). (vi) Finally, work by Balmford & Bond (2005), Kremen (2005) and Dobson et al. (2006) has suggested that the resilience of different ecosystem services provided by natural ecosystems depends upon food-web structure and is likely to be sharply dependent upon the trophic level of the species that deliver these services. Thus, aesthetic and spiritual services that require pristine intact ecosystems will be less resilient to species loss than services such as carbon storage, or prevention of erosion, which may still be accomplished in ecosystems that are reduced to mixtures of native and invasive plants and herbivorous insects (as seems to be the case if two recent detailed studies of plants and ecosystem services in California are compared; Chan et al. 2006; Seabloom et al. 2006).
My initial goal in this paper is to examine the Serengeti from the perspective of these recent theoretical and empirical advances and to examine how these processes and mechanisms are operating in the Serengeti. I will then examine how the different ‘ecosystem services’ provided by the Serengeti are driven by species on different trophic levels, or by interactions between species on sequential trophic levels. I will conclude by briefly examining how the structure of the Serengeti web, and the ecosystem services it provides, are likely to respond to current major threats of poaching, habitat loss and climate change.
3. Consumer–resource links in the Serengeti food web
The Serengeti is perhaps best known as a spectacular predator–prey system. However, the attention paid to lions and hyenas attacking and consuming wildebeest and zebra is only one class of consumer–resource interaction. The consumption of plants by herbivores is equally dramatic and outstanding from an ecological perspective; in some areas of the Serengeti the rates of consumption exceed 90 per cent of primary productivity (Sinclair 1975; McNaughton 1985). This is nearly an order of magnitude higher than has been recorded from any other terrestrial ecosystem, where figures of 1–2% are more characteristic. Ultimately, it is this high level of consumption of primary productivity that creates the huge herbivore population that is in turn consumed by the diverse and abundant carnivore community that epitomizes our mental image of the Serengeti. When the rains fail, plant resources become limiting and ungulate births decline, which in turn leads to a reduction in carnivore births and survival, strongly suggesting that ‘bottom-up’ processes are important in determining the abundance and biomass of vertebrate species in these grassland ecosystems. Parasites and pathogens form a third type of consumer–resource relationship, which have a dramatic impact on the species abundance in the Serengeti. The Pan-African rinderpest pandemic at the end of the nineteenth century caused huge reductions in the abundance of wildebeest, giraffe and buffalo, as well as in the cattle population (Plowright 1982). Outbreaks of distemper and canine parvovirus have more recently reduced the abundance of carnivores (Dye 1996; Roelke-Parker et al. 1996b). In both cases, wide-scale vaccination of domestic livestock, cattle, sheep, goats and dogs has helped reduce the impact of these pathogens on wildlife. There are also endemic pathogens of wildlife, such as malignant catarrhal fever (MCF) that create problems for domestic livestock when hosts share common pastures (Plowright 1968).
All consumer–resource relationships couple groups of species across trophic levels. When converted into a mathematical array of species interactions, with each element of the array defining the population-level impact of each consumer species on each resource species, then for each pair of species the terms describing their interaction will always be of opposite sign; consumption of the resource usually leads to increases in the abundance of the consumer (predator) and concomitant reductions in the abundance of the resource (prey). In most cases, population increases are driven by increased birth rates, while decreases are driven by increased mortality rates; although, at some spatial and temporal scales, increases will be dominated by immigration and decreases by emigration. In the Serengeti, this is best characterized by the annual migration of the large ungulates (and their predators) between different sections of the ecosystem. This means that the majority of interactions between species in the upper trophic levels of the Serengeti will predominantly be spatially transient, consumer–resource (+/−) interactions. Although, grazing facilitation between herbivores may add significant mutualistic (+/+) overtones to this pattern (Bell 1971; McNaughton 1976; McNaughton et al. 1997). By contrast, the plants at the base of the food chain will each compete with a few surrounding neighbours at a multitude of consistent specific locations for nutrient resources and water. This competition for resources will also manifest itself as competition for space and light; many plant species will be aided in their quest for resources by fungal mycorrhizal species with which they will have symbiotic relationships (although these may be very asymmetrical with major benefits of the interaction accrued by the fungi). Similarly, all of the Acacia species in the Serengeti woodlands have symbiotic interactions with nitrogen-fixing bacteria that live in root nodules formed by the plants. Mutualisms will also be common in the detritivore chains of the web, many macrodetrivores, such as termites, are obligatorily dependent upon bacteria and fungi to digest cellulose.
All of this suggests that the dynamic interactions between species at the base of the Serengeti food web will tend to be symmetrical in ‘sign’ (+/+ or −/−), while interactions between species at higher trophic levels will be dominated by asymmetrical (+/−) resource–consumer links. While the upper trophic levels of the Serengeti food web are almost totally dominated by asymmetric consumer–resource links, it is intriguing that most of the potential symmetrical competitive links occur at the base of the food web (competition between plants for space that allows access to sunlight, nutrients and water). The dynamic consequences of this topographic arrangement need to be explored with the model framework developed by Allesina & Pascual (2008), as this kind of distribution of ‘link signs’ may be a general rule across terrestrial ecosystems.
4. Nestedness in the Serengeti food web
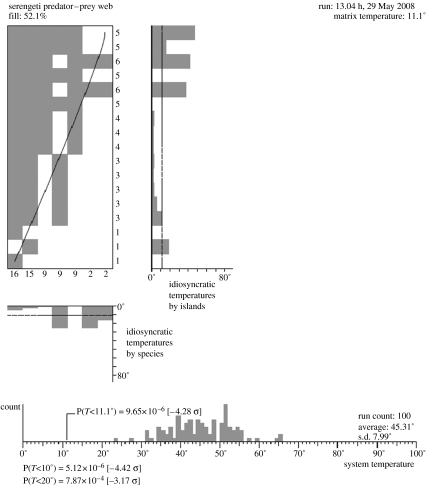
A number of recent papers have suggested that nestedness has important dynamic consequences for webs of plants and their pollinators and seed dispersers; in the systems studied, the links were predominantly mutualistic (Bascompte et al. 2003, 2006; Fortuna & Bascompte 2006). Nestedness can be formally defined as the degree to which the interactions between species can be arranged so that the ‘pairwise’ interspecific interactions of specialist species that interact only with one or a few species are nested as subsets of interactions among generalist species that either consume a diversity of species or are consumed by a diversity of species. Nestedness is also an intrinsic property of the niche model proposed by Williams & Martinez (2000) as a null model for food-web structure; scale-free networks will tend to be nested; this will be much less the case for random networks (Barabasi & Albert 1999). Studies of Serengeti carnivores and their prey provide a classic illustration of this phenomenon (figure 1). Crucially, body size is a strong predictor of the diet width of the carnivores. The largest predators, lions and hyenas, have much broader diets than the smaller carnivores: caracals; jackals; and genets (Sinclair et al. 2003a). Although most of the diet items of the smaller predators are occasionally included in the diets of the larger carnivores, the contrast is not the case; small carnivores do not attack large prey species. The carnivores and their prey can thus be arranged as a two-dimensional array with the diet of the smaller species nested within the diet of the larger species (figure 1). We can use a technique developed from island biogeography to test the degree to which the observed level of nestedness of prey within predator diet differs from a random distribution (Patterson 1987; Atmar & Patterson 1993). Although similar patterns have not been found in studies of carnivores and their prey in the Kruger National Park (Owen-Smith & Mills 2008), the test confirms that the distribution of Serengeti carnivores and their prey species is significantly nested and that the diet width of species with small body sizes are nested within those of species with large body sizes.
Figure 1.
Illustration of the nested relationship between Serengeti carnivores and their prey (data adapted from Sinclair et al. 2003b). The figure in the top left illustrates the prey species on the vertical access and the predators on the horizontal access. The figures to the right and bottom illustrate the degree to which each species of prey and predator fail to fit the underlying pattern of nestedness. The histogram at the bottom compares the probability of obtaining the observed pattern of nestedness (vertical line) with 200 random webs with the same number of predator–prey links, but with prey randomly assigned to each predator.
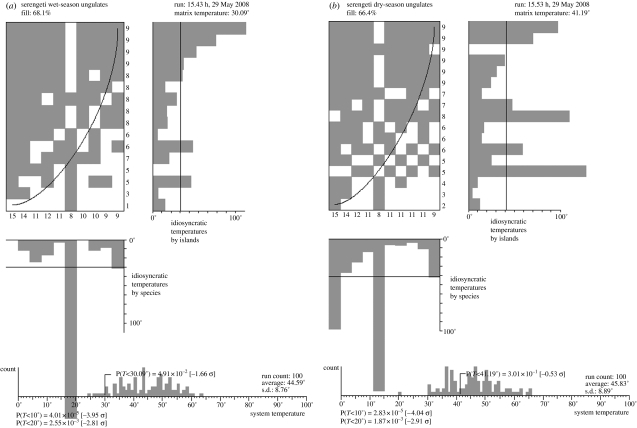
A number of other Serengeti studies provide data that can be used to test for nestedness of resources within the diets of consumers at lower trophic levels: Talbot & Talbot (1962) and Hansen et al. (1985) provide data on the diet of the different major ungulate species: wildebeest, zebra, giraffe, buffalo, and gazelles. In all cases, these diets are significantly nested with the larger ungulates consuming a broader array of vegetation, while the specialist diets of the smaller ungulates are again subsumed within the diets of the larger species (figure 2). It is important to note that there are significant differences in the degree of diet overlap between the wet and dry seasons; the diets are significantly nested during the wet season (when most of the plant species are present in abundant quantities). By contrast, during the dry season, when net plant biomass is significantly lower, there is a significant overlap in the diet of most ungulate species, and diet distributions are not significantly nested. Body size is again a strong predictor of diet breadth, although there are exceptions to this general trend; for example, giraffes have diets very different from the other ungulates, their characteristic morphology permits them access to a variety of plant resources (the tops of Acacia trees) that is unavailable to most other Serengeti ungulates.
Figure 2.
Illustration of the nested relationship between Serengeti herbivores and the plant species they consumed in the (a) wet and (b) dry seasons. The figures are organized in the same way as for figure 1. The wet-season network is significantly nested, the dry-season network is not significantly different from distribution of randomly assembled networks.
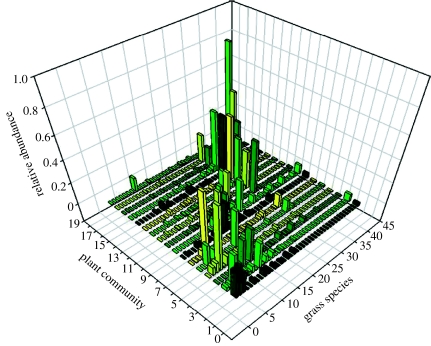
There is a significant spatial structure to the vegetation, which is likely to make an important contribution to the way in which it is used by different ungulate species (Anderson et al. 2008). McNaughton has classified the vegetation of the Serengeti into 17 different types of plant community (McNaughton 1983); these vary from the grasses adapted to dry xeric conditions and shifting sands on the recent volcanic soils in the southeast of the ecosystem, through to the woodlands that are expanding on the laterite soils in the wetter regions of the north and west of the park (figure 3). These plant data illustrate a key difference between the Serengeti as a terrestrial web and the many sets of food-web data collected for fresh-water food webs; there is a considerable spatial structure to the Serengeti web. This is partly because of the huge spatial scale at which the data are collected, but it probably also reflects real differences in the degree of species mixing in terrestrial versus aquatic systems. Both plants and animals are moved by vertical and horizontal water currents in aquatic systems (and there is a significant vertical structure in pelagic webs); in terrestrial systems, animals move readily between patches of resources, but the location of the plants remains fixed. Thus, local competitive interactions for spatially heterogeneous resources, such as soil nutrients and rainfall, determine the composition and relative abundance of the local plant community in the Serengeti. These attract different herbivore species to feed upon them and create the potential structure for the ungulates to be spatially distributed across these plant communities in a nested mosaic of resource use. The larger ungulates (wildebeest, zebra and eland) migrate to the short grass plains during the spring rains and use communities 1–6 during the period of time when these grasses grow rapidly and are most nutritious. The gazelles also move onto the short grass plains and remain there after the larger species have migrated back to the longer grasses and into the woodlands. The woodland species, such as topi, waterbuck, impala and dik-dik, are never seen in the short grass areas, while the buffalo and giraffe move across the edges between the woodland and longer grass plains. This changing spatial distribution of the ungulates will in turn have important implications for the way that ungulates are distributed as prey for the carnivores.
Figure 3.
The relative abundance of different grass species in the 17 plant communities recognized by McNaughton (1983). The communities are listed along a rough gradient that runs from the dry south-eastern boundary of the park, north and west across the plains and woodlands to Lake Victoria and the Kenyan border. This spatial change is matched by an increase in annual rainfall and a decrease in altitude and soil quality. The communities do not follow a distinct well-defined gradient, but instead form a graded mosaic of habitats that interdigitate in ways that reflect local aspects of the topology and underlying soil characteristics.
The plant species effectively form a continuum of plant communities (figure 3), that reflect the underlying rainfall gradient as well as the local soil quality and topological conditions (Anderson & Talbot 1965; McNaughton 1983). The relative abundance of the plant species in each sub-community roughly corresponds to the lognormal distribution of abundance observed in many (plant) communities (although here the distribution is of biomass, rather than individual plants) (May 1975). This may reflect the trade-offs between dispersal and competition that operate as powerfully in tropical grassland as they do in tropical forest (Hubbell & Foster 1986a,b; Hubbell 2001). Consumption of the grasses by the herbivores at the next trophic level also creates a lognormal distribution of relative abundance, with most of the vertebrate biomass (wildebeest and zebra) concentrated in the broad-ranging species that feed on the widest diversity of plants in most of the habitats forming in the ecosystem. These superabundant herbivores are mainly consumed by the larger carnivore species (lions and hyenas); however, the overall interaction across all vertebrate carnivores and herbivores again creates a lognormal distribution of relative abundance, with approximately 10 000 hyenas, 7000 lions, several thousand black-backed jackals and approximately 200 cheetah, but only occasional caracals and servals (hundreds) (Sinclair et al. 2008). The nestedness observed in the coupling of these two ‘vertebrate’ communities to their food supply must contribute in some way to the generation of their lognormal distribution of abundance; in both cases, the most abundant species are those that are most broad ranging and have the widest dietary niches.
This suggests that the relative abundance of the vertebrate species on each trophic level has to be driven by a subtly different trade-off than the one between dispersal and competitive ability that is likely to be driving the relative distribution of abundance observed in the plant communities (Tilman 1988; Hubbell 2001); although see also McGill et al. (2007) for a full list of mechanisms that could produce these patterns of abundance. Primarily, this occurs because animals can move much more frequently between patches of food resources of different quality than can plants, where this is strictly a passive intergenerational phenomenon. However, body size is an important determinant of dispersal and movement throughout the system, with smaller species in general less motile than larger ones. These differences in motility interact with differences in diet breadth; work by Wilmshurst et al. (2000) and Olff et al. (2002) suggests that herbivores of different sizes are trading off quality versus quantity of food, with smaller species increasingly specialized to feed on the most nutritious parts of a few selected plant species. It remains an open question, indeed a challenge, to unify work on consumer–resource communities with ‘neutral theory’ for the relative abundance of plants (Hubbell 1997, 2001), in order to explain how intertrophic couplings produce the observed patterns of relative abundance at higher trophic levels.
The nestedness observed in the different mammalian predator–prey, plant–herbivore relationships will have important dynamic consequences for the structure of the Serengeti food web. Central to this will be the functional response that couples each consumer to its resource. The studies of Brose et al. (2004) and Kondoh (2003a) suggest that food-web models will persist for much longer if these functional responses are predominantly type III, or sigmoidal in shape. Ecologists have long known that type III (sigmoidal) functional responses could potentially stabilize simple two-species predator–prey models (Holling 1965; Hassell & May 1973); however, empirical studies of functional responses in both the laboratory and field are dominated by examples of type II (always asymptotic) functional responses (Hassell 1978). More recently, a number of studies have suggested that the foraging decisions of herbivores and carnivores will cause switches between different food sources that would be important in stabilizing the overall structure of the food web (Kondoh 2003a,b; Beckerman et al. 2006; Petchey et al. 2008).
The nested structure observed in the Serengeti web provides a further important insight into how resource–consumer relationships may be stabilized by prey switching. Although each of the consumers in the web exhibit a type II functional response when feeding on any individual resource, the generalist species will constantly switch between different food species as changes in rainfall and levels of exploitation change the relative abundance of each potential food species in their diets. For example, La Niña rains lead to increases in grass seed production that lead to eruptions of striped grass mice, which lead to increased fecundity and hence increased abundance of rodent predators such as white-shouldered kites and plumed eagles (A. R. E. Sinclair 2008, personal communication). The net effect of this is that the larger, and often most abundant, species on each trophic level will be constantly switching between different prey species, thus creating a series of type III functional responses in the way they are coupled to their food (Wilmshurst et al. 1999; Fryxell et al. 2004). While the more specialist species may be coupled to their resources by type II functional responses, their prey species still experience a type III functional response, as the generalist feeders switch to them whenever they become more abundant. This provides an important layer of detail to the observation by Kondoh (2003b) and Brose et al. (2004) that type III functional responses are crucial for stabilizing resource–consumer relationships in food webs. The nested structure observed in the resource–consumer webs of the Serengeti is also likely to dominate other systems; a key consequence of this for web stability (and resilience) is that it converts the attack rate of generalist consumer species from type II to type III functional responses. Concomitantly, although specialist consumers might not exhibit a sigmoidal (type III) attack rate, it is likely that nearly all their resources experience a type III functional response. These will probably be further enhanced by an effect noted in spatial models for predator–prey interaction on spatial grids where local exhaustion of patches of food by the predators increases the search time until the next patch of food is located; this subtly modifies the spatial attack rate in a way that is again best described by an exponent term that closely resembles a type III functional response (Pascual & Levin 1999).
5. Fast and slow channels of resource exploitation
The nested structure of interactions between Serengeti consumers and their resources is also characterized by interactions that operate at very different speeds. McCaan (McCann et al. 2005) and Rooney (Rooney et al. 2006) have suggested that inequalities in interaction rate are potentially very important in determining the stability of a variety of spatially segregated food webs. If we examine the broad structure of the Serengeti web from this perspective the annual migration of the wildebeest and zebra couple together plant communities whose dynamics operate at very different speeds. During the spring rains, the wildebeest, zebra and gazelles congregate in the southern part of the ecosystem where highly nutritious grasses grow quickly on high-quality soils and provide them with a significant proportion of their annual protein requirement (Anderson et al. 2007). Most of the wildebeest calves are born during a short two-week period (Estes 1966); the zebra births occur over several months that overlap this time, and all of this provides a super abundance of food for the lions, hyenas, cheetahs, jackals and vultures, which in turn allows them to reproduce. When the rains stop, the wildebeest and zebra move north and west into grasslands and woodlands, where the plants are of a lower nutritional quality and competition with the resident herbivores leads to stronger competition for resources and the non-nested distribution of resource consumption described above (figure 2). The dynamics of the woodland plants operate on a slower time scale than in the plains and reproduction of the resident herbivores is more evenly spread throughout the year (Sinclair et al. 2000). The lions and hyenas will switch to these resident species when the migrant herds move away (Cooper et al. 1999; Hopcraft 2002). The resident predators, such as cheetahs, have to find ways to avoid themselves becoming prey or victims at these times (Durant 1998). As with the systems examined by McCann et al. (2005) and Rooney et al. (2006, 2008), the carnivores on the upper trophic levels and the larger herbivore species that are their main prey both integrate their nutritional requirements across several different spatially distinct sets of herbivore–plant communities, each of which has dynamics that tend to operate at different rates and with different energetic efficiencies. Although much of the grass production occurs on an annual seasonal basis, most of the grass species are perennial. When the annual rains arrive they grow very rapidly, but are also rapidly consumed until almost only their below ground parts survive. While the trees, shrubs and grasses of the central and northern woodlands are also perennials, their annual levels of primary productivity are lower than on the ion-rich soils of the short grass plains; similarly the proportion of plant tissue consumed and digested is much lower in the central woodlands than in the grasslands.
6. Spatial structure of the Serengeti food web
A significantly simplified food web of the Serengeti that builds on the ideas described above is sketched in figure 4. The sketch is drawn to illustrate several of the features described for other webs by Rooney et al. (2006, 2008) and by Olff et al. (2009). In particular, the diagram emphasizes how the two major types of habitat in the Serengeti give rise to fast and slow rates of movement of energy up through the web. I envision the grasslands as the fast chain, while the woodland dynamics operate on a slower time scale. The pronounced seasonal variation in rainfall means that use of these two habitat types are often out of synchrony with each other; this cycling resembles in many ways the heteroclinic cycles that Vandermeer has suggested may play a crucial role in maintaining biodiversity (Vandermeer 1989; Vandermeer 2006; Vandermeer & Pascual 2006). A key difference in the Serengeti is the annual cycles of resource use and abundance are not intrinsic but are driven by a strong external force in the form of annual rains. Seasonal use of the short grass plains is dominated by the migratory herds and the predators that commute to exploit the pulse of births that occurs at this time. The woodlands are used more continuously by a great variety of species, but even within this habitat there are likely to be nested fast and slow branches of resource use and energy transfer.
Figure 4.
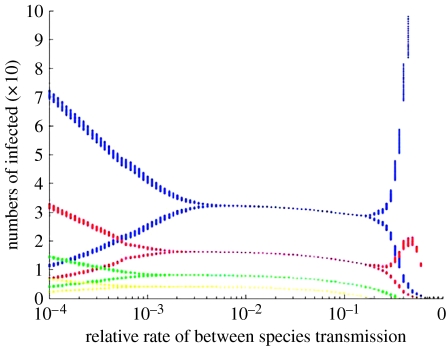
Bifurcation diagram of the relationship between rates of interspecific transmission and the number of infected hosts in each of four host species that potentially share the same pathogen (Dobson 2004b). The abundance (carrying capacity) and birth and death rates of each host species are allometrically scaled to their body size; the smallest species has the fastest birth and death rates and settles to the highest carrying capacity (DeLeo & Dobson 1996). The other three species each illustrate a doubling of body mass and this determines their abundance, birth and death rates, and within-species rates of transmission. Between-species transmission is scaled along the x-axis to be a proportional value of the averaged rates of within-species transmission. When between-species rates of transmission are low, each species exhibits epidemic cycles whose amplitude and frequency are determined by its vital rates (small species have faster larger cycles than larger species). As rates of interspecific transmission increase, the dynamics of all species become coupled and they all settle to relatively constant abundance. As rates of interspecific transmission approach rates of intraspecific transmission, the species that recovers fastest from the disease outbreaks can use the pathogen to drive the other hosts extinct; the surviving species then exhibit the epidemic cycles they exhibited in the absence of between-species transmission.
The two main channels of fast and slow rates of resource usefulness switch as the annual pattern of seasonal rains switches resource use from the southern short grass plains to the central and northern woodlands. The fast dynamics of the short grass plains are transient and occur each year for only a restricted time interval; nevertheless, they supply the majority of the high-quality forage that female wildebeest (and zebra) require when they are lactating during the first months of their calves' lives. The pulse of wildebeest births in turn supplies a crucial pulse of food resources for lions and hyenas. This empirical pattern echoes the spatial food webs explored by Rooney et al. (2006, 2008), but adds the crucial role played by seasonal pulses of resource amplification (Vandermeer 1989; Vandermeer & Pascual 2006). This large-scale alternation of fast and slow pathways is likely to trigger a secondary sequence of fast and slow resource use channels within the woodlands, and between the longer grasslands and the kopjes. The relative intensity with which these channels are exploited switches when the migratory herds leave the short grass plains and enter the longer grass areas and woodlands. This creates a nested hierarchy of resource use at two levels of trophic interaction. The spatial seasonal movement between food resources by the dominant consumers at each trophic level amplifies the behavioural switching to locally abundant prey and ensures that most species are exploited with sigmoidal (type III) functional responses. The mortality rates experienced by most species will thus increase as they become common and decline when they are rare, or when their exploiters have moved to a more productive foraging location. The nested structure of the resource use at each trophic level thus interacts with the pulsed seasonal variation in resource abundance to create a potentially strongly stabilizing set of interactions between the different branches of the web (Rooney et al. 2006; Vandermeer 2006). It seems likely that this pattern will be repeated in a nested fashion at a hierarchy of spatial scales, but we do not yet have the data to test this empirically; it is certainly worth examining from a theoretical perspective and for looking for similar patterns in other ecosystems.
Fire plays a particularly important role by releasing nutrients back into the soil and creating a mosaic of small (single km2) patches of high-quality forage where plants exhibit a highly nutritious ‘green flush’ once the next rainy season has started (Anderson et al. 2007, 2008; Holdo, R. M., Sinclair, A. R. E., Metzger, K. L., Bolker, B. M., Dobson, A. P., Ritchie, M. E. & Holt, R. D. 2009, in review). These patches first attract small, then larger herbivores; as the patches of grass age, the short rapidly growing grasses that use the minerals released by the fire are replaced by larger, more heavily lignified plants that are not a viable source of forage for smaller species (Wilsey 1996). By contrast, increased fire frequency, which occurs outside the park, leads to an increase in the abundance of fire-resistant grass species, such as Themeda triandra, a species that has lower N, P and Na concentrations than are found in the grasses that dominate patches of habitat that have burned less frequently (Anderson et al. 2007). Fire can thus be considered to act as a generalist herbivore that changes the quality of the vegetation on spatially local scales and over time scales of 2–5 years (Bond & Keeley 2005).
Large-scale reduction in the herbivore populations have a very different effect; this occurred when the rinderpest pandemic reduced the wildebeest and buffalo population in the 1890s (Sinclair 1979; Dobson 1995a). The reduction in grazing led to an increase in uneaten grass that in turn leads to larger fires that suppress the establishment of tree species on the soils of the central and northern parts of the Serengeti. This whole process was reversed when rinderpest was eradicated by cattle vaccination in the 1960s; as the wildebeest population expanded, the increased levels of grazing suppressed fire frequency, which led to significant declines in the area of the park that burned each year. This in turn allowed the woodlands to regenerate and slowly replace grasslands in the central areas of the park (Holdo, R. M., Sinclair, A. R. E., Metzger, K. L., Bolker, B. M., Dobson, A. P., Ritchie, M. E. & Holt, R. D. 2009, in review); ironically, this provides better foraging cover for several of the major predators, so their numbers increase, as do their foraging rates on wildebeest and zebra (Hopcraft 2002; Hopcraft et al. 2005; Fryxell et al. 2007).
7. Role of pathogens: frequency-dependent regulation of host abundance
There is an increasing realization that parasites play important roles in determining the structural stability of food webs; work by Kuris et al. (2008) has shown that 1–2% of the biomass in salt-marsh food webs may be made up of parasite species; because the parasite species are much smaller than their free-living hosts, then they are also able to exhibit considerable numerical abundance. Work by Lafferty et al. (2006) on salt-marsh food webs has shown that while species diversity increased by at least 50 per cent when parasites are included in free-living food web, this leads to a 400 per cent increase in the number of connections in the food-web network. Parasites have played a crucial role in shaping the dynamics of the Serengeti food web; the rinderpest virus caused a huge reduction in the abundance of wildebeest, buffalo and other artiodactyls for most of the first half of the twentieth century (Sinclair 1979; Plowright 1982; Dobson 1995b). This reduction in food supply in turn led to a reduction in carnivore numbers, which was accompanied by epidemic outbreaks of sleeping sickness as tsetste flies switched from wildebeest and cattle to humans (Ford 1871; Sinclair 1979). More recently, outbreaks of canine distemper, rabies and parvovirus have spilled over from domestic dogs and caused epidemic outbreaks in lions, wild dogs and other carnivores. These spillovers are irregular and unpredictable (Packer et al. 1999), the populations of carnivores in the park do not have any time to develop significant levels of immunity at the population level (‘herd immunity’ Fine 1993), so levels of mortality are high and intermittently lead to significant reductions in abundance (Roelke-Parker et al. 1996a).
Consideration of parasites and pathogens is likely to considerably modify the structure of the Serengeti food web. While generalist pathogens such as rinderpest and distemper can occasionally significantly reduce the abundance of the host species that they infect, chronic pathogens may be host specific and will tend to regulate the abundance of their host populations below levels that are determined purely by available food resources (Anderson 1979). This potentially stabilizing form of top-down regulation has largely been ignored in studies of food webs, predominantly because parasitic species are rarely included in food-web studies. When pathogens infect host species with different vital dynamics then they can potentially stabilize the tendency for each species to individually exhibit violent oscillations in abundance as each epidemic passes (figure 5). However, as the rates of between-species transmission increase to levels similar to those observed within populations of the same species, the shared pathogens can act to significantly reduce the abundance of the most susceptible host species, or the one with the slowest vital rates (usually those with larger body sizes; Dobson 2004a). As pathogens can only be transmitted between species whose ranges and niches overlap, they are likely to play a crucial role in reducing spatial overlap between potential competitors. This may also reinforce spatial subdivisions in resource use between different parts of an ecosystem. This is particularly likely to be important when the pathogen exhibits only mild effects in one host species, but is highly virulent to another. For example, the herpesvirus that causes MCF is almost totally benign to wildebeest, yet it is nearly always fatal to cattle (Plowright 1968). Maasai pastoralists avoid grazing in areas where wildebeest are calving, as this is the peak time for MCF transmission. This effectively excludes cattle from exploiting the highly nutritious grasses produced during the spring rains. From a food-web perspective, it focuses the fast dynamics of this ‘half’ of the web up through the wildebeest, zebra, lion and hyena chain, and away from the cattle-pastoralist chain.
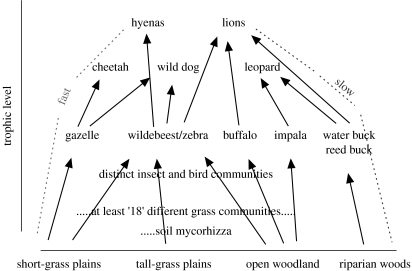
Figure 5.
Sketch of the Serengeti web for the larger species of vertebrates. The x-axis attempts to organize the species along a geographical access that runs from the short grass plains in the south of the park through the long grassland to the woodlands in the north and west; effectively, the plant communities described by McNaughton (1983; figure 3) run along this axis. The y-axis provides an indication or relative trophic level for the vertebrate species that derive the majority of the annual nutritional requirements from the habitats of the x-axis.
The two most important morbillivirus pathogens in the Serengeti ecosystem may operate in a subtly similar fashion; rinderpest and canine distemper are relatively benign when transmitted to young animals but more virulent when transmitted to older individuals. When present in large host populations, transmission rates will be high, and most individuals will be first exposed when they still bear residual maternal antibodies that reduce the impact of the pathogen. They are then resistant for life. Concomitantly, a significant proportion of older hosts will be immunologically resistant due to exposure when young, or constant re-exposure to common pathogens that produce more transient immunity, all of which will considerably reduce the rate of pathogen spread and the size of any disease outbreak within that population. By contrast, pathogen spillover will only occur intermittently to hosts in adjacent habitats, or to sympatric species that live in smaller populations that may be unable to maintain a continuous chain of infection of the pathogen. When pathogen spillover does occur, almost no hosts in the naive population will have had the prior exposure that generates immunological resistance and a more dramatic epidemic outbreak will occur among the high proportion of susceptible young and older breeding individuals. The resultant higher levels of mortality combine with the loss of potential births to significantly reduce the abundance of these occasional ‘spillover’ host species. This is essentially what happened when canine distemper spilled over from domestic dogs into lions (Roelke-Parker et al. 1996b; Craft et al. 2008). From a food web point of view the presence of distemper in domestic dogs significantly reduces the ability of the lions to use habitat and resources converted to pasture and crops by humans. Although the best examples of this effect are those that involve pathogen transmission between wild and domestic host species, there are a number of fairly well-documented examples from natural systems; the nematode Parelaphostrongylus is benign in white-tailed deer in the United States, but highly pathogenic to elk (Schmitz & Nudds 1993); the presence of the parasite effectively prevents elk from encroaching into habitat occupied by white tailed deer, it thus minimizes local competition for food resources between the two species. It seems likely that plant pathogens and fungal mycorrhizae will operate in similar ways at the basal layers of the food web. If so, this will further divide the different plant communities into relatively distinct spatial patches that delineate the bases of different branches of the web.
It will be an interesting empirical exercise to examine how parasites are distributed in spatially structured food webs for the Serengeti and for other ecosystems. I suspect the pathogens will initially appear a lot as Christmas tree ornaments decorating the major branches of the web. However, the pathogens may operate more as the medusae of jelly fishes as they can potentially have a significant impact on infrequently exposed host species from other branches of the food web that try and encroach across the spatial divisions that subdivide the different channels of resource use. If this is the case then these ‘apparently weak’, and only occasionally observed, links may play crucial roles in maintaining the integrity of the spatially distinct, ‘vertical resource–consumer’ channels that McCann, Rooney and Moore (McCann et al. 2005; Rooney et al. 2006) have suggested are crucial for overall web stability. From the perspective of the central dogma of food-web theory, pathogens acting in this way will play a crucial role in partly resolving the dilemma that levels of connectivity and interaction strength need to decline as species diversity increases. If pathogens are important in preventing host species from sharing habitats where they potentially compete with each other, then although the parasites significantly increase the connectivity of the web through their connections to their hosts (Lafferty et al. 2006, 2008), they may simultaneously reduce the strength and numbers of competitive interactions in the web. This indirect effect could enhance web stability if pathogens effectively remove significant numbers of purely competitive (−/−) interactions (that are more destabilizing to overall web structure) and replace them with a few host–parasite (+/−) interactions (Allesina & Pascual 2008; May 2009).
Pathogens also play an important role in creating spatial heterogeneity on a local scale. A series of studies have suggested that outbreaks of anthrax and rinderpest have significantly reduced the abundance of browsers, such as impala; this allows tree species to experience a pulse of recruitment while herbivore numbers are reduced (Prins & Weyerhaeuser 1987; Dobson & Crawley 1994; Sharam et al. 2006). There are many areas of the Serengeti where large even-age cohorts of whistling thorn and Acacia dominate the local landscape; these are likely to reflect past outbreaks of anthrax and the great rinderpest pandemic, respectively.
8. Food-web collapse
A potential way to examine how food webs are assembled is to examine what happens as they collapse or are eroded by human activities. This could be done for East African ecosystems by comparing the Serengeti with parks that are smaller and have experienced local extinction of species. Bill Newmark has made surveys of other Tanzanian parks and shown a strong relationship between park size and extinction rate; smaller parks lose more species and they tend to lose those with larger area requirements, particularly carnivores (Newmark 1996). In some cases, loss of predators leads to increases of potential competitors, so cheetahs do much better when lions are lost (Durant 2000), and wild dogs are less harried when spotted hyenas are removed (Creel & Creel 1996).
Although top trophic levels were massively impacted by hunting and persecution, species on lower trophic levels are now showing marked evidence of decline in many Kenyan and Tanzanian ecosystems (Ottichilo et al. 2000, 2001; Stoner et al. 2006; Newmark 2008). Curiously, smaller bodied herbivores show more pronounced declines than do larger bodied herbivores; if larger herbivores need larger areas to support them, they should be expected to disappear first. Alternatively, if smaller herbivores feed in a more restricted range of habitats, then loss of those habitats could cause their decline. Work in the Laikipia area of Kenya suggests that the nested interactions between predator and prey might also contribute to the decline of smaller bodied herbivores. In smaller reserves, where carnivores do not commute to find prey, then as long as they are not persecuted, they can sustain themselves on larger prey (Georgiadis et al. 2007). However, they also take disproportionate amounts of smaller prey as these species have lost the temporal refuge that allows them to escape from full-time predator persecution (Georgiadis et al. 2007).
9. Ecosystem services and food-web structure
The principal utilitarian reason to worry about the extinction of species from natural systems is that we lose the services that they supply to the human economy (Daily et al. 1997; Carpenter et al. 2005). Before considering this for the Serengeti, let us briefly examine the way species from different trophic levels will contribute to ecosystem services. Several authors have recently noted that the goods and services supplied to the human economy by ecological communities, the ‘ecosystem services’, can be organized in terms of the trophic levels that dominate delivery of specific services (Kremen 2005; Rodríguez et al. 2005, 2006; Dobson et al. 2006). Thus, top trophic levels supply aesthetic goods and services, and in some ecosystems they supply food to the human economy (e.g. tuna and sharks in marine systems). These species also supply less tangible services in the form of regulation of abundance of species at lower trophic levels, which may operate subtly in terrestrial systems by modifying the foraging location of species at lower trophic levels. These services can be classified as brittle, as they are usually quickly degraded or lost as habitat is eroded, or hunting pressure on both predator and prey increases. By contrast, the services supplied by species at the lower trophic levels are more resilient and may well persist, or potentially be amplified in modified habitats. For example, plants drive oxygen production and removal of CO2 from the atmosphere; they also provide fibres from forestry or for firewood and fence construction. Disconcertingly, the species at basal trophic levels will provide services whose value is unappreciated until they are lost, e.g. soil and water retention, nutrient cycling and removal of toxins.
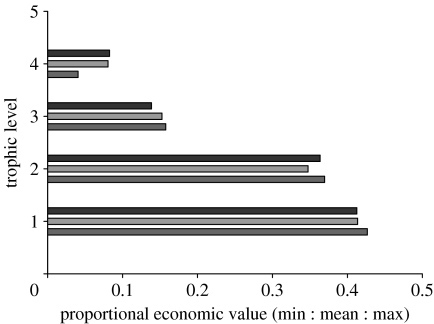
One approach that has been applied to ecosystem services is to estimate the annual net economic value of services supplied by the natural world to the human economy; Costanza et al. (1997) estimate that these services have an annual value of approximately $33 trillion dollars (Costanza et al. 1997). Although this approach is not without its critics (Daily et al. 2000; McCauley 2006), we can use the data presented within the Costanza et al. (1997) study to estimate the relative value of services provided by different trophic levels (figure 6). This admittedly coarse analysis suggests that the lower trophic levels actually supply the largest proportion of services to the human economy, while herbivores and top carnivores together only supply approximately 25 per cent of the net services. If we were to roughly divide the value of the ecosystem services supplied by each trophic level by the relative abundance of species at each level, then the pyramid structure of abundance initially implies that all species are of roughly equal value to the human economy!
Figure 6.
The proportional economic value of ecosystem services provided by species on each trophic level. The original data are provided in the paper by Costanza et al. (1997); the study represents data from a variety of different ecosystems where annual economic benefit to the human economy has been estimated. For each study, I have divided the services into which trophic level dominates their production and then divided the revenue accordingly. I then determine the proportion of revenue provided by each trophic level for each study and determine an average figure across all studies.
This initial coarse calculation assumes that all species are of roughly equal value. It ignores the nested structure observed at each trophic level in the resource–consumer subwebs, which suggests that the larger species at each trophic level are making a disproportionate contribution to the ecosystem processes, such as regulation and consumption that couple trophic levels together and drive ecosystem services. The large carnivores not only consume a disproportionate amount of prey, but their loss from African game parks will be of crucial importance in reducing the parks' huge economic potential as sites for ecotourism. Most tourists come to game parks to photograph lions, leopards and cheetahs (all usually asleep!). Furthermore, it is an ecosystem service whose value can easily be quantified by the magnitude of ‘tips’ to the drivers of tour groups lucky enough to see increasingly rare carnivore species. Unfortunately, excessive levels of tourism may create levels of harassment that drive sensitive species such as cheetah and wild dogs out of parks. This simultaneously erodes the viability of their populations, while increasing the value of the key economic service that these less abundant species provide to the local economy, particularly if people pay more for opportunities to take photographs of rare species.
Unfortunately, large species are also those most valued by humans for food, or hunting trophies. Owing to their large body size, they are also the species with the slowest demographic rates and thus most susceptible to overexploitation (Mduma et al. 1996). There is a constant temptation by a protein-starved local population of humans outside the park to harvest or poach the most abundant herbivore species, particularly wildebeest, but also zebra and other ungulates. In East Africa, the bush meat trade is not catering to an elite market of people who have moved to the city, it is usually food for local people who live on less than a dollar a day. The poaching is essentially indiscriminate as it is based upon snares laid out as trap lines in areas of bush through which wildlife is likely to move. This means that lions, leopards, hyenas and a diversity of other species are killed or maimed by poacher's snares but never enter the human food chain. In economic terms, the tour guides whose passengers fail to obtain photographs of lions, leopards and cheetahs that were killed in snares, pay the externality of poaching in the form of diminished tips. Anti-poaching patrols have a significant effect on reducing poaching, but they form a significant part of the park's financial and staff budget. The budget for anti-poaching patrols have to remain high in order to catch poachers and remove snares (Hilborn et al. 2006; Dobson & Lynes 2008; Holmern et al. 2007). Thus, a second externality of poaching is the opportunity costs of low road maintenance, limited fire control and reduced facilities for tourists. A third and much harder to quantify externality is the diminished role that smaller populations of wildebeest and zebra will play in moving nutrients around the ecosystem and in stabilizing the alternative paths of fast and slow energy flow. Here it is important to recognize that wildebeest and zebra move significant amounts of energy and nutrients around the ecosystem into local aggregations on a fast time scale (digestion and defecation) (McNaughton et al. 1997), while the slower processes of birth and death also move protein on a slower but much larger spatial scale around the whole ecosystem. Poaching of these species will disrupt these processes and potentially change the subtle nutrient and fast–slow energy flow channels that make significant contributions to web stability and biochemical recycling.
10. Conservation Implications
How are species on different trophic levels impacted by poaching, habitat erosion around the park and other external factors? Loss, or reduction in the abundance of top carnivores, can also create a trophic cascade that leads to increases in the abundance of herbivores, which in turn leads to overgrazing. Terborgh et al. (2001) has described the dramatic impact of this on islands of different sizes in Lago Guri in Venezuela. Similar effects will occur when areas set aside for parks are too small, or in those that fail to include corridors that couple wet- and dry-season habitat. The founders of the Serengeti National Park were incredibly long-sighted to expand the boundaries of the park beyond its initial demarcation and include the key wet-season grazing resources within the Ngorongoro Conservation Area and the short grass plains of the Serengeti (Grzimek & Grzimek 1960a,b). These southern areas were matched by crucial wet-season habitat across the Kenyan boundary in the Mara (Darling 1960). Unfortunately, over the last 10–20 years, this crucial dry-season habitat has been compromised by erosion of land around the Mara, and displacement of the water from the Mara river into local crop irrigation schemes (Wolanski et al. 1999). When combined with conversion of the grazing lands beyond the border of the Mara, this has led to a 90 per cent reduction in the abundance of resident wildebeest (Homewood et al. 2001; Ottichilo et al. 2001; Serneels & Lambin 2001), and significant reductions in the abundance of other grazing species (Broten & Said 1995; Ottichilo et al. 2001).
Global climate change also prevents a potential long-term threat to the Serengeti. Analysis of long-term data for the park suggests that the wet season is slowly getting drier, while the dry season is getting wetter (Ritchie, M. E., Anderson, T. M., Dobson, A. P., Mayemba, E. P., Eby, S. L. & McNaughton, S. J. 2009, in review; Serengeti III). Even as I write this on the porch at Seronera, the wildebeest have started to arrive two months early for the wet season. Unusually early rains for the dry season have stimulated the herds to move south into areas that are usually dry and barren at this time of year. In some ways, earlier movement towards more nutritious grazing might be beneficial as they will have had extra time to feed before they arrive at their calving grounds in January. However, their conception times are set by lunar cycles, the rut occurs during the full moon in early summer (Sinclair 1977); if the wet-season rains are diminishing, then there will be less high-quality forage when they most need if when lactating. A drier wet season will ultimately lead to reduced calf survival and the population will slowly decline. This in turn will lead to a reduction in the abundance of the larger predators that depend upon the wildebeest for food.
Intriguingly, the Serengeti and other areas of African savannah potentially hold the part of the key to helping slow climate chain. An additional way to quantify the economic value of the ecosystem is to consider the value of the Serengeti as a carbon sink. The grasslands of the Serengeti produce 664 g dvm m−2 yr−1; 50 per cent of this is carbon; the wildebeest and zebra consume an average of 60 per cent of the grassland primary productivity, although this can range from 15 per cent in the dry season to 94 per cent in the wet season; termites and insects eat the rest. Unfortunately, approximately 6 per cent of their food intake is converted into methane; a figure that is directly compatible with the amount of methane that the Serengeti would produce if it were converted to cattle pasture. The total area of the Serengeti grassland is approximately 20 000 square miles, plus an equal area of woodland, which means that the grasslands remove a net of approximately 5.24 million tons of carbon from the atmosphere each year. Although some will be lost back to the atmosphere due to fire and run-off into the rivers that drain to Lake Victoria, conservative estimates of the net annual build-up in the soil is around half a ton per square kilometre (Holdo, R. M., Sinclair, A. R. E., Metzger, K. L., Bolker, B. M., Dobson, A. P., Ritchie, M. E. & Holt, R. D. 2009, in review; Ritchie, M. E., Anderson, T. M., Dobson, A. P., Mayemba, E. P., Eby, S. L. & McNaughton, S. J. 2009, in review). If this could be amortized and traded on the European ‘Kyoto’-based carbon market, it would easily offset all of the airline flights to East Africa each summer; the current carbon offsets that a subset of people volunteer to pay to offset the airline flights for their safari often go to considerably less well-characterized carbon sinks that are often not even in East Africa. The key point here is that the Serengeti only works as an efficient carbon sink if it is grazed by over a million wildebeest; their abundance is dependent upon both the control of infectious diseases and of poachers. The Tanzanian government has very limited funds available to support these activities; if a carbon tax were charged on each airline seat to East Africa, then it could effectively be used to generate a significant fund to pay for anti-poaching and disease control activities in all of Tanzania, Kenya, Uganda and Rwanda's national parks. It could also be used to pay for schools, health care clinics and other vital human resources in the villages around the parks that receive very little benefit from the revenue generated by the tourists who visit the park.
11. Ecotourism as an ecosystem service
The majority of Tanzania's foreign currency earnings come from ecotourism, around a quarter of a million people visit Tanzania's parks each year and this generates the major source of foreign currency to the nation. This should provide significant funds for park infrastructure, salaries and anti-poaching activities. Unfortunately, this is not the case; at present, less than 2 per cent of the money that tourists spend on their safari goes to the park, even less goes to the local community conservation projects. Instead, most of the funds pass to tour companies and airlines that may not be based in Tanzania. Despite this, the gate fees from Serengeti are expected to supplement the maintenance budgets, not only of the Serengeti, but also of all of Tanzania's less visited national parks! Ecotourism could do a lot more both for the park. It should also provide significant benefits for the local communities who live adjacent to the park and who suffer the opportunity cost of not using it as grazing land, and more direct costs such as crop raiding by animals that live within the park. This creates local resentment towards the presence of the park, which could slowly build as a long-term political threat to the park's viability.
If ecotourism in the Serengeti is to provide a long-term ecosystem service to both the national and local economies, while also making the major contribution to the management of the park, it needs to be reorganized. One way to do this would be to simply encourage more people to visit the park by setting up many more lodges and camps. Unfortunately, this would quickly erode the value of the Serengeti as an almost unique wilderness experience where humans can see the world in its closest form to when their ancestors first walked upright. Alternatively, we can take some insights from the studies of food webs and develop an alternative approach that organizes ecotourism in hierarchical and nested ways that minimize the impact of tourism on the habitat, while maximizing the experience of each individual visitor to the park.
First, we should note that different people pay widely different amounts for their safaris; this ranges from backpackers who share a large bus with around two dozen others and sleep on campsites in tents that they erect themselves through to tourists who have secured a luxury tented camp in an isolated section of the park, where teams of 10 or more cater to their food and logistic needs. Many others will stay in the park's lodges, which are of a diverse range of qualities and prices. All of these tourists pay exactly the same daily fee to spend time in the park, although there are plainly huge differences in the impact they have, and in the quality of visit they experience. An alternative approach would charge fees in direct proportion to the area of the park to which each guest has access, divided by the number of people they are expected to share it with. Large amounts of revenue could be raised from the minority of visitors who have minimal impact on large areas of the park to which they have transient but exclusive access. Significant income could also be raised from the majority of visitors, while their impact was focused and managed in ways that enhance the present experience (for example, more organized schedules for the number of vehicles using each game track that connect to the lodges in the centre of the park). Such a scheme could also be used to create a licensing and incentive scheme for the employees of the safari companies, with different levels of license required to work, or drive, in different areas of the park. Proper licensing and training of drivers, safari guides and cooks will provide them with both a steady income and an incentive to improve their skills and obtain a driver–guide license that allows them access to less seasonally varying employment and to customers who will pay more for their services.
12. Conclusions
The multiple ecosystem services provided by the Serengeti are entirely dependent upon the ecosystem continuing to function as a complex interactive system. Central to this will be the control of poaching and infectious disease outbreaks, while ensuring the park maintains its present size and potentially expanding beyond its current boundaries. Economic pressure to expand might arise if the carbon offsets that the park generates are recognized and start producing significant revenue to the park and surrounding community. The most significant pressure to reverse these trends comes from a rapidly expanding human population on the park's boundary, particularly along its western edge. The only way to minimize the threat provided by an expanding and often sick and hungry human population is to ensure that revenue generated by the park is used to develop schools, health care clinics and access to jobs that are all directly associated with the presence of the park.
The food web of the Serengeti exhibits several features that are tantalizingly similar to those suggested by recent theoretical studies to provide stability to large networks of interacting populations. In particular, the forced annual oscillations of wet and dry seasons couple the major consumers to their resources in a way that sequentially enhances and reduces competition between species. This seems to echo the heteroclinic cycles explored by Vandermeer (1989, 2006) in his studies of forced oscillators and the maintenance of biodiversity. However, the cycles in the Serengeti are externally driven by climate and operate at a large spatial scale; they are coupled together by the migratory herds of wildebeest, zebra and their predators, this in turn echoes the recent work of McCann, Rooney and their colleagues. The couplings of the herbivores to the plants, and the carnivores to the herbivores, seem to be significantly nested in ways that closely resemble the patterns suggested by the niche models of Williams & Martinez (2000). The nestedness also matches those observed by Bascompte and colleagues for communities of pollinators and seed dispersers. In the Serengeti, the nestedness of consumers and resources has a strong underlying spatial component, and there are fairly clear relationships between the degree of nestedness and body size. The asymmetries created by the interaction between level of polyphagy and body size will also create structure for energy flow in the web that echoes that suggested by McCann and Rooney. The nested structure that couples consumer and resources in the Serengeti is intimately linked with a hierarchy of fast and slow channels of nutrient flow, which operate at a variety of different spatial scales. I suspect that these patterns will also be observed in food webs for other systems if we collect data at a sufficiently large spatial scale. Unfortunately, all too few natural systems have been observed and conserved on the comparable temporal and spatial scales to the Serengeti.
Parasites and pathogens present a major challenge to food-web ecologists; they add a significant amount of species to the system and significantly increase levels of connectedness (Lafferty et al. 2006, 2008). The Serengeti has long provided the ‘textbook’ example of how a single species of pathogen, the rinderpest virus, can have impacts that modify the relative abundance of all other species in the ecosystem (Dobson 1995a; Sinclair 1979). There are a large diversity and abundance of other pathogens in the Serengeti; these parasite species will instantly falsify the niche and cascade models that have provided important insights into the way links are organized for free-living species (Williams & Martinez 2000; Cohen et al. 2003). So, ways need to be found to develop a next generation of niche and cascade models that can accommodate parasites and pathogens. A key step that may be important here is to examine the role that parasites may play in preventing potentially competitive species from coexisting in the same patches of habitat. The presence of such pathogens may ultimately create subdivisions of habitat into a hierarchical mosaic that reinforces the spatial subdivisions of vertical energy flow up through the web where it is ultimately coupled by motile species at higher trophic levels.
Finally, it is clear that body size is crucial in determining the rates and spatial scale of interactions between species at all trophic levels of the web. Recent theoretical advances in allometry have provided a whole suit of important insights into the structure of food webs and distributions of natural abundance (Ritchie & Olff 1999; Haskell et al. 2002; Olff et al. 2002; Cohen et al. 2003; Petchey et al. 2008). The insights gained from these approaches need to be used to scale the rates of interaction in the next generation of food-web models. All of this will be non-trivial and technically challenging, but I remain optimistic that a continual dialogue between theory and long-term ecosystem studies will ultimately unravel the key to understanding the factors that determine the structure of food webs.
Acknowledgments
All of my work in the Serengeti has been sponsored by the National Science Foundation, Wildlife Conservation Society and Frankfurt Zoological Society. I am particularly grateful to all the members of the Serengeti BioComplexity Project (an NCEAS working group) for many helpful discussions. All my thoughts about food webs ecosystem services, and the Serengeti have benefitted hugely from discussions with Markus Borner, Richard Beattie, Sara Cleaveland, Meggan Craft, John Fryxell, Nick Georgiadis, Katie Hampson, Ricardo Holdo, Bob Holt, Grant Hopcraft, Kevin Lafferty, Simon Mduma, Sam McNaughton, Paul Milton, Craig Packer, Mercedes Pascual and particularly Han Olff, Tony Sinclair and Mark Ritchie.
Footnotes
One contribution of 15 to a Theme Issue ‘Food-web assembly and collapse: mathematical models and implications for conservation’.
References
- Allesina S., Pascual M. Network structure, predator–prey modules, and stability in large food webs. Theor. Ecol. 2008;1:55–64. doi:10.1007/s12080-007-0007-8 [Google Scholar]
- Anderson R.M. Parasite pathogenicity and the depression of host population equilibria. Nature. 1979;279:150–152. doi:10.1038/279150a0 [Google Scholar]
- Anderson G.D., Talbot L.M. Soil factors affecting the distribution of the grassland types and their utilization by wild animals on the Serengeti plains, Tanganyika. J. Ecol. 1965;53:33–56. doi:10.2307/2257564 [Google Scholar]
- Anderson T.M., Ritchie M.E., Mayemba E., Eby S., Grace J.B., McNaughton S.J. Forage nutritive quality in the Serengeti ecosystem: the roles of fire and herbivory. Am. Nat. 2007;170:343–357. doi: 10.1086/520120. doi:10.1086/520120 [DOI] [PubMed] [Google Scholar]
- Anderson T.M., Dempewolf J., Metzger K.L., Reed D.N., Serneels S. Generation and maintenance of heterogeneity in the Serengeti ecosystem. In: Sinclair A.R.E., Packer C., Mduma S.A.R., Fryxell J.M., editors. Serengeti III. Huamn impacts on ecosystem dynamics. University of Chicago Press; Chicago, IL: 2008. pp. 135–182. [Google Scholar]
- Atmar W., Patterson B.D. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia. 1993;96:373–382. doi: 10.1007/BF00317508. doi:10.1007/BF00317508 [DOI] [PubMed] [Google Scholar]
- Balmford A., Bond W.J. Trends in the state of nature and their implications for human well-being. Ecol. Lett. 2005;8:1218–1234. doi: 10.1111/j.1461-0248.2005.00814.x. doi:10.1111/j.1461-0248.2005.00814.x [DOI] [PubMed] [Google Scholar]
- Barabasi A.-L., Albert R. Emergence of scaling in networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. doi:10.1126/science.286.5439.509 [DOI] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Melian C.J., Olesen J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. doi:10.1073/pnas.1633576100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Olesen J.M. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. doi:10.1126/science.1123412 [DOI] [PubMed] [Google Scholar]
- Beckerman A.P., Petchey O.L., Warren A. Foraging biology predicts food web complexity. Proc. Natl Acad. Sci. USA. 2006;103:137 45–137 49. doi: 10.1073/pnas.0603039103. doi:10.1073/pnas.0603039103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.H.V. A grazing ecosystem in the Serengeti. Am. Sci. 1971;224:86–93. [Google Scholar]
- Bond W.J., Keeley J.E. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005;20:387–394. doi: 10.1016/j.tree.2005.04.025. doi:10.1016/j.tree.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Brose U., Ostling A., Harrison K., Martinez N.D. Unified spatial scaling of species and their trophic interactions. Nature. 2004;428:167–171. doi: 10.1038/nature02297. doi:10.1038/nature02297 [DOI] [PubMed] [Google Scholar]
- Broten M.D., Said M. Population trends of ungulates in and around Kenya's Masai Mara reserve. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, IL; London, UK: 1995. pp. 169–193. [Google Scholar]
- Burrows R., Hofer H., East M.L. Demography, extinction and intervention in a small population—the case of the Serengeti wild dogs. Proc. R. Soc. B. 1994;256:281–292. doi: 10.1098/rspb.1994.0082. doi:10.1098/rspb.1994.0082 [DOI] [PubMed] [Google Scholar]
- Carpenter S.R., Pingali P.L., Bennett E.M., Zurek M.B. Island Press; Washington, DC: 2005. Ecosystems and human well-being: scenarios. Millennium ecosystem assessment. [Google Scholar]
- Chan K.M.A., Shaw R.M., Cameron D.R., Underwood E.C., Daily G.C. Conservation planning for ecosystem services. PLoS Biol. 2006;4:e379. doi: 10.1371/journal.pbio.0040379. doi:10.1371/journal.pbio.0040379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.E., Jonsson T., Carpenter S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA. 2003;100:1781–1786. doi: 10.1073/pnas.232715699. doi:10.1073/pnas.232715699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S.M., Holekamp K.E., Smale L. A seasonal feast: long-term analysis of feeding behaviour in the spotted hyaena (Crocuta crocuta) Afr. J. Ecol. 1999;37:149–160. doi:10.1046/j.1365-2028.1999.00161.x [Google Scholar]
- Costanza R., et al. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253–260. doi:10.1038/387253a0 [Google Scholar]
- Craft M.E., Hawthorne P.L., Packer C., Dobson A.P. Dynamics of a multihost pathogen in a carnivore community. J. Anim. Ecol. 2008;77:1257–1264. doi: 10.1111/j.1365-2656.2008.01410.x. doi:10.1111/j.1365-2656.2008.01410.x [DOI] [PubMed] [Google Scholar]
- Creel S., Creel N.M. Limitation of African wild dogs by competition with larger carnivores. Conserv. Biol. 1996;10:526–538. doi:10.1046/j.1523-1739.1996.10020526.x [Google Scholar]
- Daily G.C., et al. Ecosystem services: benefits supplied to human societies by natural ecosystems. Issues Ecol. 1997;2:1–16. [Google Scholar]
- Daily G.C., et al. The value of nature and the nature of value. Science. 2000;289:395–396. doi: 10.1126/science.289.5478.395. doi:10.1126/science.289.5478.395 [DOI] [PubMed] [Google Scholar]
- Darling F.F. An ecological reconnaissence of the Mara plains in Kenya colony. Wildl. Monogr. 1960;5:1–41. [Google Scholar]
- DeLeo G.A., Dobson A.P. Allometry and simple epidemic models for microparasites. Nature. 1996;379:720–722. doi: 10.1038/379720a0. doi:10.1038/379720a0 [DOI] [PubMed] [Google Scholar]
- Dobson A. The ecology and epidemiology of rinderpest virus in Serengeti and Ngorongoro conservation area. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, IL: London, UK: 1995a. pp. 485–505. [Google Scholar]
- Dobson A.P. The ecology and epidemiology of rinderpest virus in Serengeti and Ngorongoro crater conservation area. In: Sinclair A.R.E., Arcese P., editors. Serengeti II: research, management and conservation of an ecosystem. University of Chicago Press; Chicago, IL: 1995b. pp. 485–505. [Google Scholar]
- Dobson A. Population dynamics of pathogens with multiple host species. Am. Nat. 2004a;164:S64–S78. doi: 10.1086/424681. doi:10.1086/424681 [DOI] [PubMed] [Google Scholar]
- Dobson A., Lafferty K.D., Kuris A.M., Hechinger R.F., Jetz W. Homage to Linnaeus: how many parasites? How many hosts? Proc. Natl Acad. Sci. USA. 2008;105:11482–11489. doi: 10.1073/pnas.0803232105. doi:10.1073/pnas.0803232105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P. Population dynamics of pathogens with multiple hosts. Am. Nat. 2004b;164:S64–S78. doi: 10.1086/424681. doi:10.1086/424681 [DOI] [PubMed] [Google Scholar]
- Dobson A.P., Crawley M.J. Pathogens and the structure of plant communities. Trends Ecol. Evol. 1994;9:393–398. doi: 10.1016/0169-5347(94)90062-0. doi:10.1016/0169-5347(94)90062-0 [DOI] [PubMed] [Google Scholar]
- Dobson A.P., Lynes L. How does poaching effect teh size of National Parks? Trends Ecol. Evol. 2008;23:177–180. doi: 10.1016/j.tree.2007.08.019. doi:10.1016/j.tree.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Dobson A.P., et al. Habitat loss, trophic collapse and the decline of ecosystem services. Ecology. 2006;87:1915–1924. doi: 10.1890/0012-9658(2006)87[1915:hltcat]2.0.co;2. doi:10.1890/0012-9658(2006)87[1915:HLTCAT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Durant S.M. Competition refuges and coexistence: an example from Serengeti carnivores. J. Anim. Ecol. 1998;67:370–386. doi:10.1046/j.1365-2656.1998.00202.x [Google Scholar]
- Durant S.M. Living with the enemy: avoidance of hyenas and lions by cheetahs in the Serengeti. Behav. Ecol. 2000;11:624–632. doi:10.1093/beheco/11.6.624 [Google Scholar]
- Dye C. Serengeti wild dogs: what really happened? Trends Ecol. Evol. 1996;11:188–189. doi: 10.1016/0169-5347(96)30021-9. doi:10.1016/0169-5347(96)30021-9 [DOI] [PubMed] [Google Scholar]
- Estes R.D. Behaviour and life history of the wildebeest (Connochaetes taurinus Burchell) Nat. Lond. 1966;212:999. doi:10.1038/212999a0 [Google Scholar]
- Fine P.E.M. Herd immunity: history, theory, paractice. Epidemiol. Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- Ford J. Clarendon Press; Oxford, UK: 1871. The role of trypanosomiasis in African ecology. [Google Scholar]
- Fortuna M.A., Bascompte J. Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 2006;9:281–286. doi: 10.1111/j.1461-0248.2005.00868.x. doi:10.1111/j.1461-0248.2005.00868.x [DOI] [PubMed] [Google Scholar]
- Fryxell J.M., Wilmshurst J.F., Sinclair A.R.E. Predictive models of movement by Serengeti grazers. Ecology. 2004;85:2429–2435. doi:10.1890/04-0147 [Google Scholar]
- Fryxell J.M., Mosser A., Sinclair A.R.E., Packer C. Group formation stabilizes predator–prey dynamics. Nature. 2007;449:1041–1044. doi: 10.1038/nature06177. doi:10.1038/nature06177 [DOI] [PubMed] [Google Scholar]
- Georgiadis N.J., Ihwagib F.J.G., Olweroc N., Roman S.S. Savanna herbivore dynamics in a livestock-dominated landscape II: ecological, conservation, and management implications of predator restoration. Biol. Conserv. 2007;137:473–483. doi:10.1016/j.biocon.2007.03.006 [Google Scholar]
- Grzimek B., Grzimek M. Hamish Hamilton; London, UK: 1960a. Serengeti shall not die. [Google Scholar]
- Grzimek M., Grzimek B. Census of plains animals in the Serengeti National Park, Tanganyika. J. Wildl. Manage. 1960b;24:27–37. doi:10.2307/3797353 [Google Scholar]
- Hansen R.M., Mugambi M.M., Bauni S.M. Diets and trophic rankings of Ungulates of the Northern Serengeti. J. Wildl. Manage. 1985;49:823–829. doi:10.2307/3801717 [Google Scholar]
- Haskell J.P., Ritchie M.E., Olff H. Fractal geometry predicts varying body size scaling relationships for mammal and bird home ranges. Nature. 2002;418:527–530. doi: 10.1038/nature00840. doi:10.1038/nature00840 [DOI] [PubMed] [Google Scholar]
- Hassell M.P. Princeton University Press; Princeton, NJ: 1978. Dynamics of arthropod predator–prey systems. [PubMed] [Google Scholar]
- Hassell M.P., May R.M. Stability in insect host-parasite models. J. Anim. Ecol. 1973;42:693–726. doi:10.2307/3133 [Google Scholar]
- Hilborn R., Arcese P., Borner M., Hando J., Hopcraft G., Loibooki M., Mduma S., Sinclair A.R.E. Effective enforcement in a conservation area. Science. 2006;314:1266. doi: 10.1126/science.1132780. doi:10.1126/science.1132780 [DOI] [PubMed] [Google Scholar]
- Holling C.S. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965;45:1–60. [Google Scholar]
- Holmern T., Muya J., Roskaft E. Local law enforcement and illegal bushmeat hunting outside the Serengeti National Park, Tanzania. Environ. Conserv. 2007;34:1–9. doi:10.1017/S0376892907003554 [Google Scholar]
- Homewood K., Lambin E.F., Coast E., Kariuki A., Kikula I., Kivalia J., Said M., Serneels S., Thompson M. Long-term changes in Serengeti-Mara wildebeest and land cover: pastoralism, population, or policies? Proc. Natl Acad. Sci. USA. 2001;98:12 544–12 549. doi: 10.1073/pnas.221053998. doi:10.1073/pnas.221053998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopcraft G. Department of Zoology, University of British Columbia; Vancouver, BC: 2002. The role of habitat and prey density on foraging by Serengeti lions. [Google Scholar]
- Hopcraft G., Sinclair A.R.E., Packer C. Planning for success: Serengetil lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005;74:559–566. [Google Scholar]
- Hubbell S.P. A unified theory of biogeography and relative species abundance and its application to tropical rain forests and coral reefs. Coral Reefs. 1997;16:S9–S21. doi:10.1007/s003380050237 [Google Scholar]
- Hubbell S. Princeton University Press; Princeton, NJ: 2001. The unified theory of biodiversity and biogeography. Monographs in population biology. [Google Scholar]
- Hubbell S.P., Foster R.B. Biology, chance and history and the structure of tropical rain forest tree communities. In: Diamond J.M., Case T.J., editors. Community ecology. Harper & Row; New York, NY: 1986a. pp. 314–329. [Google Scholar]
- Hubbell S.P., Foster R.B. Commonness and rarity in a neotropical forest: implications for tropical tree conservation. In: Soulé M.E., editor. Conservation biology: the science of scarcity and diversity. Sinauer Associates, Inc; Sunderland, MA: 1986b. pp. 205–232. [Google Scholar]
- Kondoh M. Foraging adaptation and the relationship between food web complexity and stability. Science. 2003a;299:1388–1391. doi: 10.1126/science.1079154. doi:10.1126/science.1079154 [DOI] [PubMed] [Google Scholar]
- Kondoh M. Response to comment on ‘foraging adaptation and the relationship between food-web complexity and stability’. Science. 2003b;301:918c. doi: 10.1126/science.1079154. doi:10.1126/science.1087539 [DOI] [PubMed] [Google Scholar]
- Kremen C. Managing ecosystem services: what do we need to know about their ecology? Ecol. Lett. 2005;8:468–479. doi: 10.1111/j.1461-0248.2005.00751.x. doi:10.1111/j.1461-0248.2005.00751.x [DOI] [PubMed] [Google Scholar]
- Kuris A.M., et al. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature. 2008;454:515–518. doi: 10.1038/nature06970. doi:10.1038/nature06970 [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Dobson A.P., Kuris A.M. Parasites dominate food web links. Proc. Natl Acad. Sci. USA. 2006;103:11 211–11 216. doi: 10.1073/pnas.0604755103. doi:10.1073/pnas.0604755103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K.D., et al. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. doi:10.1111/j.1461-0248.2008.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R.M. Will a large complex system be stable? Nature. 1972;238:413–414. doi: 10.1038/238413a0. doi:10.1038/238413a0 [DOI] [PubMed] [Google Scholar]
- May R.M. Princeton University Press; Princeton, NJ: 1973. Stability and complexity in model ecosystems. [Google Scholar]
- May R.M. Patterns of species abundance and diversity. In: Cody M.L., Diamond J.M., editors. Ecology and evolution of communities. Harvard University Press; Cambridge, MA: 1975. pp. 81–120. [Google Scholar]
- May R.M. Food-web assembly and collapse: mathematical models and implications for conservation. Phil. Trans. R. Soc. B. 2009;364:1643–1646. doi: 10.1098/rstb.2008.0280. doi:10.1098/rstb.2008.0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K., Rasmussen J.B., Umbanhowar J. The dynamics of spatially coupled food webs. Ecol. Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. doi:10.1111/j.1461-0248.2005.00742.x [DOI] [PubMed] [Google Scholar]
- McCauley D.J. Selling out on nature. Nature. 2006;443:27–28. doi: 10.1038/443027a. doi:10.1038/443027a [DOI] [PubMed] [Google Scholar]
- McGill B.J., et al. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007;10:995–1015. doi: 10.1111/j.1461-0248.2007.01094.x. doi:10.1111/j.1461-0248.2007.01094.x [DOI] [PubMed] [Google Scholar]
- McNaughton S.J. Serengeti migratory wildebeest: facilitation of energy flow by grazing. Science. 1976;191:92–94. doi: 10.1126/science.191.4222.92. doi:10.1126/science.191.4222.92 [DOI] [PubMed] [Google Scholar]
- McNaughton S.J. Serengeti grassland ecology: the role of composite environmental factors and contingency in community organization. Ecol. Monogr. 1983;53:291–320. doi:10.2307/1942533 [Google Scholar]
- McNaughton S.J. Ecology of a grazing ecosystem: the Serengeti. Ecol. Monogr. 1985;55:259–294. doi:10.2307/1942578 [Google Scholar]
- McNaughton S.J., Oesterheld M. Extramatrical mycorrhizal abundance and grass nutrition in a tropical grazing ecosystem the Serengeti National Park Tanzania. Oikos. 1990;59:92–96. doi:10.2307/3545127 [Google Scholar]
- McNaughton S.J., Banyikwa F.F., McNaughton M.M. Promotion of the cycling of diet-enhancing nutrients by African grazers. Science. 1997;278:1798–1800. doi: 10.1126/science.278.5344.1798. doi:10.1126/science.278.5344.1798 [DOI] [PubMed] [Google Scholar]
- Mduma, S., Hilborn, R. & Sinclair, A. R. E. 1996 Limits to exploitation of Serengeti wildebeest and implications for management. In Dynamics of tropical communities, vol. 37th Symposium (eds D. M. Newbery, H. H. T. Prins & N. D. Brown), pp. 243–265. Oxford, UK: Blackwell Scientific.
- Metzger K.L., Sinclair A.R.E., Campbell K.L.I., Hilbornc R., Hopcraft G.C., Mduma S.A.R., Reich R.M. Using historical data to establish baselines for conservation: the black rhinoceros (Diceros bicornis) of the Serengeti as a case study. Biol. Conserv. 2007;139:358–378. doi:10.1016/j.biocon.2007.06.026 [Google Scholar]
- Morell V. Counting creatures of the Serengeti, great and small. Science. 1997;278:2058. doi:10.1126/science.278.5346.2058 [Google Scholar]
- Newmark W.D. Insularization of Tanzanian parks and the local extinction of large mammals. Conserv. Biol. 1996;10:1549–1556. doi:10.1046/j.1523-1739.1996.10061549.x [Google Scholar]
- Newmark W.D. Isolation of African protected areas. Front. Ecol. 2008;6:321–328. doi:10.1890/070003 [Google Scholar]
- Olff H., Ritchie M.E., Prins Herbert H.T. Global environmental controls of diversity in large herbivores. Nature. 2002;415:901–904. doi: 10.1038/415901a. doi:10.1038/415901a [DOI] [PubMed] [Google Scholar]
- Olff H., Alonso D., Berg M.P., Eriksson B.K., Loreau M., Piersma T., Rooney N. Parallel ecological networks in ecosystems. Phil. Trans. R. Soc. B. 2009;364:1755–1779. doi: 10.1098/rstb.2008.0222. doi:10.1098/rstb.2008.0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottichilo W.K., de Leeuw J., Skidmore A.K., Prins H.H.T., Said M.Y. Population trends of large non-migratory wild herbivores and livestock in the Masai Mara ecosystem, Kenya, between 1977 and 1997. Afr. J. Ecol. 2000;38:202–216. doi:10.1046/j.1365-2028.2000.00242.x [Google Scholar]
- Ottichilo W.K., de Leeuw J., Prins H.H.T. Population trends of resident wildebeest [Connochaetes taurinus hecki (Neumann)] and factors influencing them in the Masai Mara ecosystem, Kenya. Biol. Conserv. 2001;97:271–282. doi:10.1016/S0006-3207(00)00090-2 [Google Scholar]
- Owen-Smith N., Mills M.G.L. Predator–prey size relationships in an African large-mammal food web. J. Anim. Ecol. 2008;77:173–183. doi: 10.1111/j.1365-2656.2007.01314.x. doi:10.1111/j.1365-2656.2007.01314.x [DOI] [PubMed] [Google Scholar]
- Packer C., Altizer S., Appel M., Brown E., Martenson J., O'Brien S.J., Roelke-Parker M., Hofmann-Lehmann R., Lutz H. Viruses of the Serengeti: patterns of infection and mortality in African lions. J. Anim. Ecol. 1999;68:1161–1178. doi:10.1046/j.1365-2656.1999.00360.x [Google Scholar]
- Pascual M., Levin S. From individuals to population densities: searching the intermediate scale of nontrivial determinism. Ecology. 1999;80:2225–2236. doi:10.2307/176905 [Google Scholar]
- Patterson B.D. The principle of nested subsets and its implications for conservation biology. Conserv. Biol. 1987;1:323–334. doi:10.1111/j.1523-1739.1987.tb00052.x [Google Scholar]
- Petchey O.L., Beckerman A.P., Riede J.O., Warren P.H. Size, foraging, and food web structure. Proc. Natl Acad. Sci. USA. 2008;105:4191–4196. doi: 10.1073/pnas.0710672105. doi:10.1073/pnas.0710672105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright W. Malignant catarrhal fever. J. Am. Vet. Med. Assoc. 1968;152:795–804. [Google Scholar]
- Plowright W. The effects of rinderpest and rinderpest control on wildlife in Africa. Symp. Zool. Soc. Lond. 1982;50:1–28. [Google Scholar]
- Prins H.H.T., Weyerhaeuser F.J. Epidemics in populations of wild ruminants: anthrax and impala, rinderpest and buffalo in Lake Manyara National Park, Tanzania. Oikos. 1987;49:28–38. doi:10.2307/3565551 [Google Scholar]
- Ritchie M.E., Olff H. Spatial scaling laws yield a synthetic theory of biodiversity. Nature. 1999;400:557–560. doi: 10.1038/23010. doi:10.1038/23010 [DOI] [PubMed] [Google Scholar]
- Rodriguez, J. P. et al 2005 Interactions among ecosystem services. In Ecosystems and human well-being: scenarios, vol. 2 (eds S. R. Carpenter, P. L. Pingali, E. M. Bennett & M. B. Zurek), pp. 431–448. Washington, DC: Island Press.
- Rodríguez J.P., Beard T.D.J., Bennett E.M., Cumming G.S., Cork S.J., Agard J.B.R., Dobson A.P., Peterson G.D. Trade-offs across space, time, and ecosystem services. Ecol. Soc. 2006;11:28. [Google Scholar]
- Roelke-Parker M.E., et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996a;379:441–445. doi: 10.1038/379441a0. doi:10.1038/379441a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelke-Parker M.E., et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996b;381:172. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney N., McCann K., Gellner G., Moore J.C. Structural asymmetry and the stability of diverse food webs. Nature. 2006;442:265–269. doi: 10.1038/nature04887. doi:10.1038/nature04887 [DOI] [PubMed] [Google Scholar]
- Rooney N., McCaan K.S., Moore J.C. A landscape theory for food web architecture. Ecol. Lett. 2008;11:867–881. doi: 10.1111/j.1461-0248.2008.01193.x. doi:10.1111/j.1461-0248.2008.01193.x [DOI] [PubMed] [Google Scholar]
- Schmitz O.J., Nudds T.D. Parasite mediated competition in deer and moose: how strong is the effect of meningeal worm on moose? Ecol. Appl. 1993;4:91–103. doi:10.2307/1942118 [Google Scholar]
- Seabloom E.W., Williams J.W., Slayback D., Stoms D.M., Viers J.H., Dobson A.P. Human impacts, plant invasion, and imperiled species in California. Ecol. Appl. 2006;16:1338–1350. doi: 10.1890/1051-0761(2006)016[1338:hipiai]2.0.co;2. doi:10.1890/1051-0761(2006)016[1338:HIPIAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Serneels S., Lambin E.F. Impact of land-use change on the wildebeest migration in the Northern part of the Serengeti-Mara ecosystem. J. Biogeogr. 2001;28:391–407. doi:10.1046/j.1365-2699.2001.00557.x [Google Scholar]
- Sharam G., Sinclair A.R.E., Turkington R. Establishment of broad-leaved thickets in Serengeti, Tanzania: the influence of fire, browsers, grass competition, and elephants. Biotropica. 2006;38:599–605. doi:10.1111/j.1744-7429.2006.00195.x [Google Scholar]
- Sinclair A.R.E. The resource limitation of trophic levels in tropical grassland ecosystems. J. Anim. Ecol. 1975;44:497–520. doi:10.2307/3608 [Google Scholar]
- Sinclair A.R.E. Lunar cycle and timing of mating season in Serengeti wildebeest. Nature. 1977;267:832–833. doi:10.1038/267832a0 [Google Scholar]
- Sinclair A.R.E. The eruption of the ruminants. In: Sinclair A.R.E., Norton-Griffiths M., editors. Serengeti: dynamics of an ecosystem. University of Chicago Press; Chicago, IL: 1979. pp. 82–103. [Google Scholar]
- Sinclair A.R.E., Arcese P. Chicago University Press; Chicago, IL: 1995. Serengeti II. Dynamics, management, and conservation of an ecosystem. [Google Scholar]
- Sinclair A.R.E., Norton-Griffiths M. University of Chicago Press; Chicago, IL: 1979. Serengeti; dynamics of an ecosystem. [Google Scholar]
- Sinclair A.R.E., Mduma Simon A.R., Arcese P. What determines phenology and synchrony of ungulate breeding in Serengeti? Ecology. 2000;81:2100–2111. doi:10.2307/177099 [Google Scholar]
- Sinclair A.R.E., Mduma S., Brashares J.S. Patterns of predation in a diverse predator–prey system. Nature. 2003a;425:288–290. doi: 10.1038/nature01934. doi:10.1038/nature01934 [DOI] [PubMed] [Google Scholar]
- Sinclair A.R.E., Mduma S., Brashares J.S. Patterns of predation in a diverse predator–prey system. Nature. 2003b;425:288–290. doi: 10.1038/nature01934. doi:10.1038/nature01934 [DOI] [PubMed] [Google Scholar]
- Sinclair A.R.E., Packer C., Mduma Simon A.R., Fryxell J.M., editors. Serengeti IIII. Human impacts in ecosystem dynamics. University of Chicago Press; Chicago, IL: 2008. [Google Scholar]
- Stoner C., Caro T., Mduma S., Mlingwa C., Sabuni G., Borner M., Schelten C. Changes in large herbivore populations across large areas of Tanzania. Afr. J. Ecol. 2006;45:202–215. doi:10.1111/j.1365-2028.2006.00705.x [Google Scholar]
- Talbot L.M., Talbot M.H. Food preferences of some East African Wild Ungulates. East Afr. Agric. For. J. 1962;27:131–138. [Google Scholar]
- Terborgh J., et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. doi:10.1126/science.1064397 [DOI] [PubMed] [Google Scholar]
- Tilman D. Princeton University Press; Princeton, NJ: 1988. Plant strategies and the dynamics and structure of plant communities. [Google Scholar]
- Vandermeer J.H. Loose coupling of predator–prey cycles: entrainment, chaos, and intermittency in the classic MacArthur predator–competitor equations. Am. Nat. 1989;141:687–716. doi:10.1086/285500 [Google Scholar]
- Vandermeer J. Oscillating populations and biodiversity maintenance. Bioscience. 2006;56:967–975. doi:10.1641/0006-3568(2006)56[967:OPABM]2.0.CO;2 [Google Scholar]
- Vandermeer J.H., Pascual M.M. Competitive coexistence through intermediate polyphagy. Ecol. Complex. 2006;3:37–43. doi:10.1016/j.ecocom.2005.05.005 [Google Scholar]
- Williams R.J., Martinez N.D. Simple rules yield complex food webs. Nat. (London) 2000;404:180–183. doi: 10.1038/35004572. doi:10.1038/35004572 [DOI] [PubMed] [Google Scholar]
- Wilmshurst J.F., Fryxell J.M., Farm B.P., Sinclair A.R.E., Henschel C.P. Spatial distribution of Serengeti wildebeest in relation to resources. Can. J. Zool. 1999;77:1223–1232. doi:10.1139/cjz-77-8-1223 [Google Scholar]
- Wilmshurst J.F., Fryxell J.M., Bergman C.M. The allometry of patch selection in ruminants. Proc. R. Soc. B. 2000;267:345–349. doi: 10.1098/rspb.2000.1007. doi:10.1098/rspb.2000.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B.J. Variation in use of green flushes following burns among African ungulate species: the importance of body size. Afr. J. Ecol. 1996;34:32–38. doi:10.1111/j.1365-2028.1996.tb00591.x [Google Scholar]
- Wolanski E., Gereta E., Borner M., Mduma S. Water, migration and the Serengeti ecosystem. Am. Sci. 1999;87:526–533. doi:10.1511/1999.6.526 [Google Scholar]