Abstract
EphA2 is a receptor tyrosine kinase that has been shown to be overexpressed in a variety of human tumor types. Previous studies demonstrated that agonist monoclonal antibodies targeting EphA2 induced the internalization and degradation of the receptor, thereby abolishing its oncogenic effects. In this study, the in vitro and in vivo antibody-dependent cell-mediated cytotoxicity (ADCC) activity of EphA2 effector-enhanced agonist monoclonal antibodies was evaluated. With tumor cell lines and healthy human peripheral blood monocytes, the EphA2 antibodies demonstrated ∼80% tumor cell killing. In a dose-dependent manner, natural killer (NK) cells were required for the in vitro ADCC activity and became activated as demonstrated by the induction of cell surface expression of CD107a. To assess the role of NK cells on antitumor efficacy in vivo, the EphA2 antibodies were evaluated in xenograft models in severe compromised immunodeficient (SCID) mice (which have functional NK cells and monocytes) and SCID nonobese diabetic (NOD) mice (which largely lack functional NK cells and monocytes). Dosing of EphA2 antibody in the SCID murine tumor model resulted in a 6.2-fold reduction in tumor volume, whereas the SCID/nonobese diabetic model showed a 1.6-fold reduction over the isotype controls. Together, these results demonstrate that the anti-EphA2 monoclonal antibodies may function through at least two mechanisms of action: EphA2 receptor activation and ADCC-mediated activity. These novel EphA2 monoclonal antibodies provide additional means by which host effector mechanisms can be activated for selective destruction of EphA2-expressing tumor cells.
Introduction
EphA2 is a member of the Eph family of receptor tyrosine kinases (RTKs), the largest family of RTKs consisting of at least 15 family members [1,2]. During development, the Eph receptors and ligands are involved in cell-cell interactions and cell migration in morphogenetic processes. In cancer, EphA2 is overexpressed in multiple tumor types including breast, prostate, lung, cervical, colon, esophageal, gastric, ovarian, bladder, renal cell carcinomas, melanoma, and gliomas [2–9]. In ovarian, cervical, and esophageal cancers, high levels of EphA2 expression have been correlated with poor patient survival rates [10–15]. In addition, EphA2 is expressed on tumor endothelial cells in the tumor neovasculature of primary tumor samples, thus presenting another avenue for targeting tumor growth by the inhibition of angiogenesis [1,2].
Several preclinical studies have demonstrated that EphA2 plays a role in cancer. When the nontransformed MCF10A cell line is engineered to express EphA2, the cell line becomes transformed and grows in soft agar and forms tumors in vivo [16]. Furthermore, the down-regulation of EphA2 with small interfering RNA results in an increase of apoptosis in vitro and in vivo and in the suppression of in vivo xenografts [17,18]. Another study used adenoviral-expressed EphrinA1 to down-regulate EphA2 protein in MDA-MB-231 breast tumor cells, and this also decreased their growth in soft agar and in xenograft models [19]. In addition, others have reported similar results whereby EphA2 antibody treatment of tumor cell lines decreased EphA2 protein levels, which resulted in decreased tumor cell growth in soft agar and suppression of tumor growth in xenograft models [20–22].
The development of monoclonal antibody therapeutics for oncology has grown tremendously in the past decade. Currently, there are six monoclonal antibodies and three immunoconjugates approved in the United States for oncology and many more in development [23,24]. The function of the antibodies can vary from Avastin, which binds to vascular endothelial growth factor and blocks its ability to bind to its receptor, to Rituxan, which binds to CD20 present on malignant B cells and functions, in part, through an antibody-dependent cell-mediated cytotoxicity (ADCC)-dependent mechanism [24]. For most of these monoclonal antibodies, several mechanisms of action have been proposed, including receptor down-regulation, induction of apoptosis, ADCC, and complement-dependent cytotoxicity [24,25]. Frequently, the emerging mechanism of action is ADCC. For instance, clinical studies with Rituxan demonstrated that ADCC plays a role in its therapeutic activity and that polymorphisms in the Fc receptor can determine the degree of efficacy achieved [26,27]. Recently, a similar finding was observed for Herceptin where the presence of the FcγRIII 158V/V genotype correlated with response rates and progression-free survival in breast cancer patients [28].
Because ADCC activity may be a significant component of the efficacy of monoclonal antibodies for cancer therapy, we investigated the in vitro and in vivo ADCC activity of an EphA2 agonist antibody that has been modified to enhance ADCC effector activity. The results show that modifications to the Fc portion of the EphA2 antibody enhance binding to FcγRIIIa thereby increasing in vitro ADCC activity and efficacy in xenograft models. Our data strongly suggest that natural killer (NK) cells become activated and are required for ADCC activity and that mutations in the Fc region of an EphA2-targeting antibody enhanced binding to FcγRIIIa and, to a large extent, overrode the effects of FcγRIIIa polymorphism status on effector-mediated cytotoxic activity.
Materials and Methods
Cell Lines and Culture
The human cell lines A549 (non-small cell lung cancer cell (NSCLC)),MDA-MB-231 (breast adenocarcinoma), SKOV3 (ovarian adenocarcinoma), and SKMel28 (malignant melanoma) were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in the recommended media. The M21 cell line was a kind gift from David Cheresh, PhD, Scripps Research Institute, La Jolla, CA.
Antibodies
EphA2 antibodies, B233 and 3F2, were generated as previously described [21,29]. 3F2-3M was generated from the Fc triple substitution of 3F2 and cloned into NS0 cells as an IgG1 isotype. In brief, three amino acid substitutions, namely, S239D, A330L, and I332E, were introduced into the Fc region of 3F2 by QuickChange II XL site-directed mutagenesis kit (Stratagene, San Diego, CA). The Fc mutant was generated by two sequential site-directed mutagenesis using oligo S239DF, S239DR, A330L/I332EF, and A330L/I332ER as primers and 3F2 as template. The mutations were confirmed by DNA sequencing.
Mouse IgG1 anti-human APC-conjugated CD3, APC-Cy7-conjugated CD19, PerCP-conjugated CD14, PE-Cy5-conjugated CD107a, and PE-conjugated CD4, CD8, and CD56 were purchased from BD Pharmingen (San Jose, CA). Mouse IgG1 anti-human APC-conjugated CD16, Pacific Blue-conjugated CD8, and PE-Cy7-conjugated CD45 were purchased from Biolegend (San Diego, CA). Mouse IgG2 anti-human PE-Cy5.5-conjugated CD3 was purchased from Caltag Laboratories (Burlingame, CA). In addition, EphA2 and isotype control antibodies were conjugated to AlexaFluor488 according to Molecular Probes' AlexaFluor488 Monoclonal Antibody Labeling Kit protocol (Invitrogen, Carlsbad, CA).
EphA2 Antibody Binding Constant—Surface Plasmon Resonance (Biacore)
The interaction of the EphA2 antibody 3F2-3M with immobilized EphA2-Fc was monitored by surface plasmon resonance detection using a Biacore 3000 instrument (Biacore, Pittsburgh, PA). Data were fitted to a 1:1 Langmuir binding model. This algorithm calculates both the kon and the koff, from which the apparent equilibrium dissociation constant, KD, is deduced as the ratio of the two rate constants (koff/kon). Selectivity to EphA2 over other Eph family members was further characterized by ELISA using commercially available Eph fusion proteins, and 3F2-3M demonstrated high affinity and specificity for human EphA2.
Binding of EphA2 Antibodies to Fcγ Receptors—Surface Plasmon Resonance (Biacore)
The interaction of soluble human FcγRIIIA (F158 allotype) and murine FcγRIV with immobilized EphA2 antibodies, 3F2 and 3F2-3M, was monitored by surface plasmon resonance detection using a Biacore 3000 instrument. The different humanized IgG1s were coupled to the dextran matrix of a CM5 sensor chip at a surface density of between 7711 and 9608 RU. Human FcγRIIIA and murine FcγRIV were used in equilibrium binding experiments at concentrations ranging from 16 µM to 0.98 nM at a flow rate of 5 µl/min. Dilutions and binding experiments were carried out in 50 mM Hank's buffered saline buffer containing 0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, and 0.005% P-20. Data were typically collected for 50 minutes, and a 30-second pulse of 5 mM HCl was used to regenerate the surfaces. Human FcγRIIIA and murine FcγRIV were allowed to flow over an uncoated cell, and the sensorgrams from these blank runs were subtracted from those obtained with IgG-coupled cells. Values for KD were determined from the corresponding binding isotherms.
In Vitro EphA2 Activation Assays
The EphA2 activation assays were performed as previously described [21]. Human cancer cells, 3 x 105 per well, were seeded into six-well plates containing medium with 10% fetal bovine serum. After a 24-hour incubation, cells were treated for 15 minutes at 37°C with serum-containing medium including titrated doses (0.1–50 µg/ml) of EphA2 antibody, 10 µg/ml positive control antibody, 10 µg/ml isotype control antibody, or no treatment. After incubation, wells were washed with cold phosphate-buffered saline (PBS) and cells were lysed with 1% triton lysis buffer (TX100; Boston Bioproducts, Boston, MA) containing protease inhibitors. Approximately 100 to 300 µg of protein for each sample was immunoprecipitated with the EphA2 monoclonal antibody, D7 (Upstate, Charlottesville, VA) and rabbit anti-mouse protein A sepharose beads (Chemicon, Temecula, CA; Sigma-Aldrich, St. Louis, MO) for 90 minutes at 4°C. Beads were washed three times with TX100 and stored in 6x gel loading buffer (Boston Bioproducts) at -20°C until analysis by SDS-PAGE and immunoblot analysis. Phosphorylated EphA2 was detected by probing with the antiphosphotyrosine monoclonal antibody, 4G10 (Upstate). Blots were stripped and reprobed with an EphA2 monoclonal antibody (Invitrogen) to determine total EphA2 levels.
Lactate Dehydrogenase Release ADCC Assays
Endogenous EphA2-expressing tumor cell lines were harvested using 0.05% trypsin, washed with 1x PBS, and treated with EphA2 antibodies at 500, 50, and 5 ng/ml on ice for 20 minutes. Peripheral blood monocytes (PBMCs) were isolated from human whole blood obtained from healthy donors using a Lymphocyte Separation Medium (MP BioMedicals, Solon,OH) and washed three times with 1x PBS. Isolated PBMCs were incubated with EphA2-expressing cells that were pretreated with EphA2 antibodies at an effector-to-target ratio of 100:1, 50:1 or 25:1 for 4 hours at 37°C. After the 4-hour incubation period, the cell supernatant was transferred to a 96-well plate to determine the amount of lactate dehydrogenase (LDH) released using a colorimetric assay (Promega, Madison, WI). Percent cytotoxicity was calculated as follows: percent cytotoxicity = (experimental - effector spontaneous - target spontaneous) / (target maximum - target spontaneous) x 100, where “experimental” corresponds to the signal measured in a treated sample, “effector spontaneous” corresponds to the signal measured in the presence of PBMCs alone, “target spontaneous” corresponds to the signal measured in the presence of tumor cells alone, and “target maximum” corresponds to the signal measured in the presence of detergentlysed tumor cells.
Additional studies used purified NK cells in the LDH release assays. Human whole blood was obtained, and PBMCs were isolated as described previously. Natural killer cells were isolated according to the NK cell isolation kit manufacturer's protocol for negative selection (Miltenyi Biotec, Auburn, CA). The magnetically labeled non-NK cells were depleted from the PBMC population by retention on the column. In contrast, non-NK cells were isolated by negative selection using a CD56 MicroBead Kit (Miltenyi Biotec). The magnetically labeled CD56+ cells were depleted from the PBMC population through retention on the column.
Antibody Binding to Human Blood Cell Subpopulations
Human whole blood was collected from healthy donors in sodium citrate cell preparation tubes. These were spun at 1700g for 15 minutes at room temperature. The red blood cells were contained beneath the density gradient, and the supernatant, containing PBMCs, was decanted. Peripheral blood monocytes were washed three times in 1x PBS and resuspended in Dulbecco's PBS containing 1% BSA (FACS buffer). Peripheral blood monocytes (1 x 106 cells/well) were treated with 10 µg/ml AlexaFluor488-labeled EphA2 and control antibodies then stained with 5 µl of directly conjugated cell surface markers (including CD3-APC, CD4-PE, CD8-PE, CD19-APC-Cy7, CD14-PerCP, CD56-PE, and CD16-APC) for 30 minutes at 4°C. Cells were washed twice in FACS buffer and either analyzed within 1 hour or fixed in 4% formaldehyde (Tousimis, Rockville, MD) and stored overnight for flow cytometric analysis on a LSRII (BD Biosciences, San Jose, CA).
To include neutrophils in the antibody binding assays, whole blood (100 µl/well) was incubated (30 minutes at 4°C) with 10 µg/ml AlexaFluor488-labeled EphA2 or control antibodies in the presence of 5 µl/well of each cell surface marker (group 1: CD45-PE-Cy7, CD14-PerCP, CD16-APC, and CD56-PE; Group 2: CD3-PE-Cy5.5, CD8-Pacific Blue, CD4-PE, CD16-APC, and CD19-APC-Cy7). Red blood cells were lysed with 200 µl of ammonium chloride (Stem Cell Technologies, Vancouver, British Columbia, Canada) for 10 minutes at 37°C and washed twice with FACS buffer before flow cytometric analysis on a LSRII. FlowJo software (Treestar, Ashland, OR) was used to analyze all data obtained from flow cytometric analysis.
Natural Killer Cell Activation Status—CD107a Assay
Freshly isolated PBMCs (1 x 106 cells/well) were incubated with A549 or SKMel28 cells (1 x 106 cells/well) at a 1:1 ratio in the presence of varying concentrations of unlabeled EphA2 or control antibodies. Peripheral blood monocytes with medium alone served as negative controls and PBMCs stimulated with the leukocyte activation cocktail with GolgiPlug (BD Biosciences) served as positive controls. Effector and target cells were coincubated in 100 µg/ml to 0.1 ng/ml antibody and stained with 5 µl of PE-Cy5-conjugated CD107a (BD Biosciences) for 3 hours at 37°C. Golgi-Stop (BD Biosciences) was added after the first hour of incubation, according to the manufacturer's protocol. Natural killer cell surface markers, 5 µl of PE-conjugated CD56 (US Biological, Swampscott,MA) and 5 µl of FITC-conjugated CD3 (BD Biosciences), were added, and cells weer incubated for an additional 30 minutes at 4°C. Samples were then washed twice in FACS buffer, and flow cytometric analysis was performed on LSRII.
Assessment of FcγRIIIa (CD16) Polymorphisms
To determine the FcγRIIIa genotype of the healthy human donors, heparinized whole blood was processed using the Bio-Fast DNA Extraction Kit according to the manufacturer's instructions (Bio-Synthesis, Inc., Lewisville, TX). Purified genomic DNA was ethanol-precipitated, and the concentration was determined using the Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). Single nucleotide polymorphism analysis was performed using the Plexor System (Promega) where 50 to 100 ng of purified DNA was amplified using the following primer pair: 5′-CCGGAGAAACATTTTTACTCCCAAAAA-3′ for the TTT genotype (F phenotype), 5′-CGTCACGATATTTTTACTCCCAACA-3′ for the GTT genotype (V phenotype), and the anchor primer 5′-TTCTGACTTCTACATTCCAAAAGC-3′. Positive reactions for each genotype were measured, and interpolated homozygous and heterozygous phenotypes were determined.
In Vivo Tumor Xenograft Studies
The MDA-MD-231 human breast adenocarcinoma cell line was grown in monolayer culture and harvested by trypsinization. Athymic nu/nu, severe compromised immunodeficient (SCID; Harlan, Sommerville, NJ) or SCID/nonobese diabetic (NOD) (Harlan; or Taconic, Germantown, MA) female mice 4 to 6 weeks of age were injected subcutaneously either into the right flank or into the mammary fat pad with 5 x 106 or 2 x 106 MDA-MB-231 tumor cells, respectively. Treatments of PBS, isotype control, or anti-EphA2 antibodies were administered intraperitoneally at a dose of 10 mg/kg in PBS. The first dose was given just before or the day after the cell implantation then continued twice weekly. Taxol, a positive control, was administered intravenously at 10 mg/kg diluted in 0.9% aqueous saline solution. Tumor growth was monitored twice weekly by caliper measurements [21]. In addition, only SCID and SCID/NOD mice with less than 100 ng/ml of mouse IgG, as assessed by ELISA (Pierce Biotechnology, Rockford, IL), were used for the study to ensure the animal's compromised immune status.
Statistical Analyses
Two-way analysis of variance using GraphPad PRISM software (GraphPad Software, Inc., San Diego, CA) was used to assess differences in NK cell CD107a expression. Statistical differences in ADCC activity across FcγRIIIa allotypes were determined using a 1-tailed, paired standard t test (SAS, Cary, NC). For all subcutaneous tumor comparisons, groups were analyzed using a 2-tailed Student's t Test assuming equal variance (homoscedastic) and the Mann-Whitney method. Statistical calculations were performed using SigmaStat Version 3.01A 4.0 software (Systat Software, Inc., San Jose, CA).
Results
Characterization of an Agonist Monoclonal Antibody Targeting EphA2 with an Engineered Fc Region
The antibody 3F2-3M was generated from the murine parental antibody B233 and humanized antibody 3F2, through three amino acid substitutions in the Fc portion of the molecule (described in Materials and Methods) [21,29]. To verify that modifications to the Fc portion of 3F2 did not alter interaction with EphA2, the antibody dissociation/rate constants were assessed using Biacore (Table 1A). The affinity of 3F2-3M and 3F2-WT to human EphA2 were similar with K D values of 163 and 60 pM, respectively (Table 1A).
Table 1.
3F2-3M Biochemical Properties.
| A) | ||||||
| kon, x 105 (1/Ms) | koff, x105 (1/s) | KD (pM) | ||||
| 3F2-WT | 5.4 (1.32) | 3.0 (2.18) | 60 (53) | |||
| 3F2-3M | 2.7 (1.36) | 4.4 (0.08) | 163 (89) | |||
| B) | ||||||
| IgG | Human FcγRIIIA (KD, nM) | Human FcγRIIIA Fold Improvement | Murine FcγRIV (KD, nM) | Murine FcγRIV Fold Improvement | ||
| 158F | 158V | 158F | 158V | — | — | |
| 3F2-WT | 2878 (153) | 519 (63) | N/A | N/A | 536 (66) | N/A |
| 3F2-3M | 28 (6) | 9 (2) | 103 | 58 | 5 (0) | 107 |
A) The binding of the EphA2 antibody 3F2-3M to recombinant EphA2-Fc was determined by Biacore assessment as described in Materials and Methods. Data are presented as mean (SD).
B) Similar to A), the dissociation constants of the EphA2 antibodies 3F2-WT and 3F2-3M for human CD16 (FcγRIIIa) and murine FcγRIV were determined by Biacore analysis. In the case of human FcγRIIIa, the two CD16 allotypes, 158F and 158V, were evaluated to determine whether this polymorphism may impact binding. Data are presented as mean (SD) from two independent sets of measurements.
To determine whether the 3M mutations altered the interaction of soluble human FcγRIIIA (F158 and V158 allotypes) with immobilized EphA2 antibodies, Biacore binding analysis was performed with 3F2-WT and 3F2-3M. Results demonstrate that dissociation constants for the interaction of 3F2-3M with FcγRIIIA/F158 and FcγRIIIA/V158 were estimated at 28 and 9 nM, respectively (Table 1B). This translated into respective affinity improvements of 103- and 58-fold when compared with the 3F2-WT antibody. In addition, to determine whether the enhanced binding of 3F2-3M and 3F2-WT extended across species to murine FcγRIV, the mouse-equivalent receptor to human FcγRIIIA, additional Biacore studies were performed. As demonstrated in Table 1B, an equivalent ∼100-fold improvement in affinity was demonstrated for the binding of 3F2-3M to mouse FcγRIV compared with the binding of 3F2-WT to mouse FcγRIV.
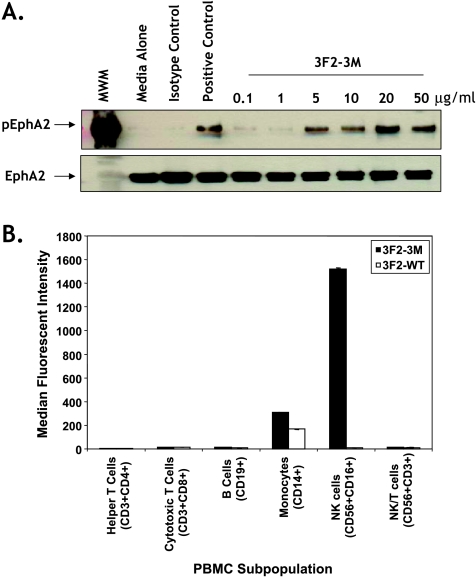
To confirm that the agonist properties of the 3F2-3M antibody were not altered, EphA2 receptor phosphorylation studies were performed [21]. 3F2-3M treatment resulted in a dose-responsive increase in EphA2 protein phosphorylation, which saturated at 20 µg/ml in the MDA-MB-231 breast cancer cell line that express endogenous EphA2 (Figure 1A). These results were similar to data generated with 3F2-WT and the parental antibody, B233 (Figure W1 and data not shown [21]). Furthermore, similar results were obtained with additional cancer cell lines that expressed endogenous EphA2 protein (data not shown; Figure W2).
Figure 1.
Characterization of 3F2-3M. (A) The EphA2 agonist activity of 3F2-3M was determined by evaluating EphA2 phosphorylation in MDA-MB-231 cells at concentrations from 0.1 to 50 µg/ml similar to that previously described [21]. (B) The binding of 3F2-3M to human blood cell subpopulations was determined using healthy human PBMCs and characterizing cell subpopulations as described in Materials and Methods. Binding was demonstrated to NK cells and monocytes where a statistically significant 100- to 250-fold increase in binding to NK cells was observed with 3F2-3M compared to 3F2-WT (P = .002). Error bars, SD.
Because the affinity to FcγRIIIA was improved (Table 1B), we analyzed the binding of 3F2-3M to various T- and B-cell subpopulations in human PBMCs by FACS analyses. Minimal binding of both 3F2-WT and 3F2-3M was observed for helper T, cytotoxic T, B, and NK/T cells (Figure 1B). Binding to monocytes was observed with both 3F2-3M and 3F2-WT. With 3F2-WT, binding could be abolished in the presence of Fc-blocking reagent, which suggests that the binding was mediated through the interaction of the Fc region and not due to the EphA2 expression on this cell population (data not shown). More importantly, 3F2-3M demonstrated an approximately 100-fold increase in binding to NK cells when compared with 3F2-WT (Figure 1B; P = .0002). Given the expression of FcγRIIIA on NK cells and the increased affinity of 3F2-3M for FcγRIIIA shown in Table 1B, the interaction is believed to also occur through the Fc portion of the molecule. Additional studies evaluating cell subpopulations in whole blood revealed 3F2-3M, but not 3F2-WT, bound to neutrophils at comparable levels to NK cells (data not shown).
Potent ADCC Activity by 3F2-3M
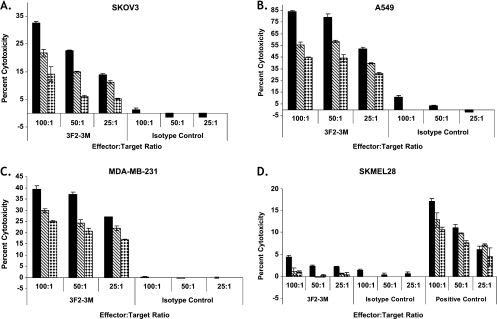
The ADCC activity of 3F2-3M was assessed using a panel of human solid tumor cell lines that express endogenous EphA2 as target cells and healthy donor human PBMCs as effector cells in an in vitro assay based on LDH release on cell lysis. As shown in Figure 2, 3F2-3M induced significant cytotoxicity in the SKOV3 (Figure 2A), A549 (Figure 2B), and MDA-MB-231 (Figure 2C) cell lines. At concentrations from 5 to 500 ng/ml and effector-to-target ratios from 25:1 to 100:1, 3F2-3M elicited 10% to 80% cytotoxicity in a dose-dependent manner when compared with the isotype control (Figure 2). Although the ADCC activity did not directly correlate to EphA2 expression levels, it may be related to the inherent sensitivity of the cancer cells to ADCC-mediated killing and to the overall amounts of LDH within each cell type (Table W1). In addition, minimal cytotoxicity (0%–4%) was observed using an EphA2-negative cancer cell line, SKMel28 (Figure 2D), suggesting the specificity of 3F2-3M for EphA2-expressing target cells.
Figure 2.
EphA2 antibodies elicit ADCC activity in vitro. The ADCC activity of 3F2-3M was assessed using healthy donor PBMCs as effectors and SKOV3 ovarian cancer (A), A549 NSCLC (B) and MDA-MB-231 breast cancer (C) cell lines as targets as described in Materials and Methods. Cytotoxicity was observed in a dose-dependent and effector-to-target ratio manner. Concentrations of 3F2-3M or isotype control antibodies used were 5 ng/ml (diamonds), 50 ng/ml (hatched lines), or 500 ng/ml (solid bars). Little to no cytotoxicity was observed when 3F2-3M was evaluated in the SKMel28, a non-EphA2-expressing melanoma cell line (D). Error bars, SEM. Data are representative of three independent experiments.
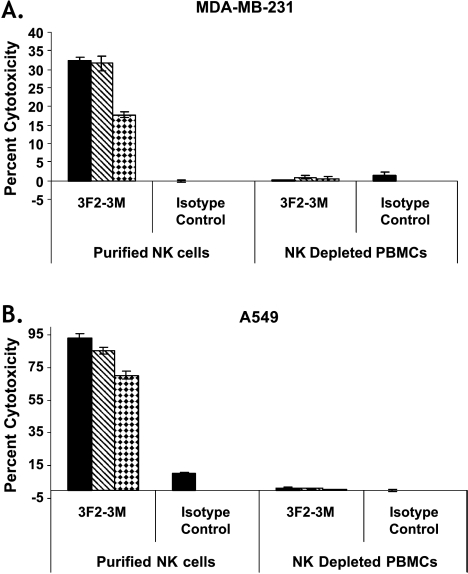
Next, we assessed if NK cells played a predominant role in the ADCC activity of 3F2-3M. EphA2-expressing cell lines, pretreated with 3F2-3M, were incubated with purified NK and PBMCs lacking NK cells from healthy donors in vitro. Results demonstrated that 3F2-3M elicited a potent and reproducible ADCC activity in the presence of human purified NK cells but not NK-depleted PBMCs (Figure 3). In the presence of NK cells, 3F2-3M was able to elicit 17% to 33% cytotoxicity in a breast carcinoma cell line, MDA-MB-231 (Figure 3A), and 79% to 93% cytotoxicity in a NSCLC cell line, A549 (Figure 3B), at concentrations ranging from 5 to 500 ng/ml and an effector-to target ratio of 10:1 and 5:1 when compared with the isotype control. In the absence of NK cells, minimal cytotoxicity was observed with 3F2-3M (Figure 3).
Figure 3.
NK cells are required for ADCC activity in vitro. The ADCC activity of 3F2-3M was evaluated in purified NK and non-NK cells isolated from healthy donors as described in Materials and Methods. Potent and reproducible ADCC activity against MDA-MB-231 cells (A) and A549 NSCLC cells (B) in the presence of human purified NK cells but not purified NK-depleted PBMCs. Error bars, SEM. Antibody concentrations tested were 5 ng/ml (diamonds), 50 ng/ml (hatched lines), and 500 ng/ml (solid bars) at an effector-to-target ratio of 10:1 for the MDA-MB-231 study and 5:1 for the A549 study. Data are representative of two independent experiments.
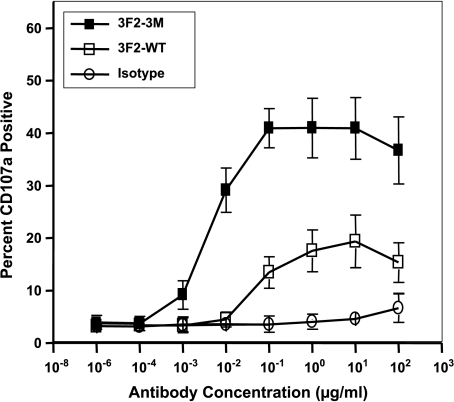
Because 3F2-3M binds to NK cells, which play significant roles in mediating the ADCC activity of 3F2-3M, we wanted to determine whether specific NK cell markers of activation were induced. In the presence of EphA2-expressing tumor cell lines and healthy donor NK cells, both 3F2-WT and 3F2-3M were capable of activating NK cells as measured by the expression of the NK cell surface marker CD107a (Figure 4). The increase in CD107a occurred in a dose-responsive manner where 3F2-3M demonstrated approximate a two-fold and eight-fold increase in CD107a expression when compared with 3F2-WT and isotype control, respectively (Figure 4). Detectable cell surface CD107a expression was observed at a 10-fold lower concentration of 3F2-3M antibody compared with 3F2-WT. In addition, EphA2 expression on the tumor cells was required for NK cell activation (data not shown).
Figure 4.
CD107a expression is increased on NK cells in the presence of EphA2 antibodies. The expression of the activation marker CD107a was evaluated on the surface of healthy donor NK cells by flow cytometry. Isolated NK or non-NK cells were incubated in the presence of tumor cells expressing or lacking EphA2, EphA2 antibodies, or isotype control antibodies. When EphA2-expressing tumor cells are present, a dose-responsive increase in CD107a protein occurs with both the 3F2-WT (open squares) and 3F2-3M (solid squares) antibodies, where 3F2-3M > 3F2-WT. At high concentrations of antibody, CD107a expression increases in a nonspecific fashion as observed with the isotype control antibody (open circles) and with EphA2-negative cell lines (data not shown). Error bars, SEM. 3F2-3M was statistically different from both 3F2-WT and isotype control antibody at all concentrations greater than 0.010 µg/ml (P < .001).
Given that the FcγRIIIA allotype can impact the ability of other monoclonal antibodies, such as Rituxan, to elicit ADCC activity and effect patient outcomes [26,27], we assessed ADCC activity elicited by 3F2-3M using effector cells isolated from healthy donors expressing the FcγRIIIA 158 allotypes of VF, FF, or VV and A549 cells as the target tumor cell line (Figure 5). With the FcγRIIIa 158 V/F and F/F allotypes the 3F2-WT antibody demonstrated very low (10% or less) ADCC activity, whereas the 3F2-3M molecule demonstrated 20% ADCC activity; this translated into a two-fold to four-fold improvement over 3F2-WT in tumor cell killing at the highest concentration examined (Figure 5, A, C, and D). With the FcγRIIIa 158 V/V allotype, both the 3F2-WT and 3F2-3M molecules were capable of eliciting ADCC activity Figure 5B. In addition, there was a shift in the dose-response curve to the left, which reflected that a lower dose of 3F2-3M antibody was required to initiate the ADCC-mediated cytotoxic response (Figure 5). Overall, these data demonstrate that 3F2-3M is capable of eliciting in vitro ADCC-mediated cytotoxicity regardless of the FcγRIIIa allotype present in the donor PBMCs.
Figure 5.
Impact of FcγRIIIA polymorphisms on ADCC activity. Natural killer cells isolated from healthy human donors expressing the FcγRIIIA 158 V/F, F/F, or V/V polymorphisms were evaluated in the LDH release cytotoxicity assay using A549 NSCLC cells as targets (E/T = 2:1). 3F2-3M (solid triangles) elicited robust cytotoxicity independent of the FcγRIIIa allotype. The 3F2-WT (solid squares) antibody demonstrated higher cytotoxicity using the V/V allotype compared with either the V/F or the F/F allotype: (A) V/F, (B) V/V, (C) F/F. Isotype control antibody is represented as open squares. Net cell lysis across independent experiments is represented in (D) where *P < .0022, comparing 3M to WT for each allotype.
3F2-3M Is Efficacious In Vivo
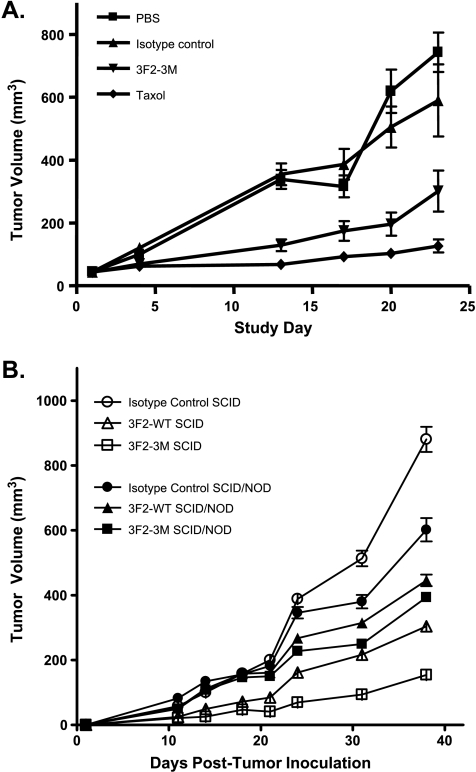
To determine whether the in vitro activity of 3F2-3M translates into in vivo antitumor activity and to understand the role that NK cells may play in its activity, several tumor xenograft models using nude, SCID, and SCID/NOD mice were evaluated. To verify that 3F2-3M retained efficacy comparable to the parental antibody B233, an orthotopic MDA-MB-231 breast cancer model was evaluated in female nude mice (Figure 6A). When 3F2-3M was administered twice per week in a breast tumor xenograft orthotopic model using nude mice, a significant inhibition of tumor growth was demonstrated compared with the animals treated with isotype control antibody or PBS (Figure 6A; P < .009).
Figure 6.
3F2-3M is efficacious in vivo. The in vivo efficacy of 3F2-WT and 3F2-3M was evaluated in nude, SCID, and SCID/NOD mice. 3F2-3M demonstrated significant suppression of MDA-MB-231 tumor growth when administered twice per week in the nude mouse mammary fat pad model (A) (P < .009): isotype control (solid triangles), 3F2-3M (solid upside-down triangles), taxol positive control (solid diamonds). In addition, the role of NK cells on antitumor activity was evaluated using SCID and SCID/NOD mouse models (B). Significant suppression of tumor growth was observed with the 3F2-3M-treated SCID mice compared with the 3F2-3M-treated SCID/NOD mice and control-treated animals (P < .001). The 3F2-WT-treated SCID mice also showed significant tumor suppression compared with the 3F2-WT-treated SCID/NOD mice (P < .031). In addition, there was a significant difference between the 3F2-3M- and 3F2-WT-treated SCID mice (P <.001): isotype control (open circles), 3F2-WT (open triangles), and 3F2-3M (open squares). In SCID/NOD animals: isotype control (solid circles), 3F2-WT (solid triangles), and 3F2-3M (solid squares). Data are representative of two independent experiments. Error bars, SEM.
To determine the potential contribution of the ADCC mechanism of action, we compared the activity of 3F2-3M and 3F2-WT in SCID mice, which possess NK cells, with that in SCID/NOD mice, which lack NK cells. As shown in Figure 6B, a significant suppression of tumor growth was observed in the 3F2-3M-treated SCID mice compared with the 3F2-3M-treated SCID/NOD mice or the PBS or isotype control groups in either mouse strain (P < .001). The 3F2-WT-treated SCID mice also showed significant tumor suppression compared with the 3F2-WT-treated SCID/NOD mice (P < .031). In addition, there was a significant difference between the 3F2-3M- and 3F2-WT-treated SCID mice (P < .001), which suggests that the in vivo antitumor activity of 3F2-3M is dependent, in part, on the presence of NK cells.
Discussion
In this study, we report the development of an EphA2 agonist antibody, which, through three mutations in the Fc region, has enhanced ADCC activity and antitumor efficacy. We demonstrate that 3F2-3M retains agonist activity, is active both in vitro and in vivo, and, in part, requires NK cells for tumor cell killing. Thus, 3F2-3M has two potential mechanisms for targeting EphA2 on cancer cells and hence provides a new therapeutic strategy for the treatment of EphA2-expressing cancers.
The results from this study create an interesting paradigm for the role of antibodies in targeting EphA2 as a cancer therapeutic; an agonist EphA2 monoclonal antibody that is capable of decreasing cell surface expression of EphA2 but can also drive ADCC, which requires EphA2 on the cell surface. One possibility is that the agonist EphA2 antibody, by mimicking the action of the ligand, pushes the tumor cells toward a more “normal cell” phenotype by initiating a proposed normal cell signaling cascade where by there is activation, downstream signaling, EphA2 degradation, and de novo synthesis of EphA2 (Figures W3 and W4). This is supported in part by studies demonstrating that small interfering RNA-mediated down-regulation of EphA2 expression, as well as other approaches, results in decreased tumor growth in vivo [16–20]. However, a decrease in EphA2 expression or the initiation of downstream signaling alone may not be sufficient as demonstrated in the study by Kiewlich et al. [30], where their EphA2 agonist antibodies, although able to downregulate EphA2 protein, failed to demonstrate antitumor activity. By having the additional potency of an ADCC effector-enhanced antibody, direct tumor cell targeting by activation of NK cells may overcome the resistance to normal EphA2 signaling cascades and regulation.
Antibody-dependent cell-mediated cytotoxicity is a predominant mechanism of action for a number of therapeutic monoclonal antibodies, including Rituxan and Herceptin [24]. Furthermore, clinical data suggest that the FcγRIIIa polymorphism status of cancer patients plays a key role in the clinical outcome of patients receiving Rituxan or Herceptin [26–28]. In the case of non-Hodgkin lymphoma, the FcγRIIIa 158 V/V genotype was associated with both clinical and molecular positive responses to Rituxan [27]. In further studies on indolent lymphoma [31] and extended to Waldenstrom macroglobulinemia in autoimmune disease, there is a correlation between FcγRIIIa 158 V/V polymorphism status and patient responses to Rituxan [32]. Thus, antibody engineering approaches to enhance ADCC have resulted in improved in vitro ADCC activity mediated by NK cells expressing the various FcγRIIIa polymorphisms that has translated into increased efficacy [33,34]. It has been demonstrated that with the same three mutations in the Fc region of alemtuzumab and trastuzumab, a 2- to 3-log increase in affinity to both FcγRIIIa variants, 158V and 158F, occurred, which translated into a similar 2- to 3-log improvement in potency in vitro across the various 158 V/V, V/F, and F/F allotypes [34]. An alternative approach using a low-fucose version of Rituxan, KM3065, also demonstrated increased affinities and in vitro ADCC activity to the FcγRIIIa allotypes [35]. In our current study, the three modifications to the Fc portion of our 3F2-3M EphA2 agonist monoclonal antibody increased the affinity to both the FcγRIIIa 158V and 158F allotypes, and this resulted in increased in vitro ADCC activity using effector cells from donors expressing the V/V, V/F, and F/F allotypes (Figures 1 and 5). Given the prevalence of FcγRIIIA 158 F allele (40% V/F and 40% F/F) in the general population, our results suggest that 3F2-3M has the potential to impact a much larger and heterogeneous cancer patient population and thus may have an advantage over current non-effector-enhanced monoclonal antibody therapies.
The activation of NK cells can also be a surrogate marker of ADCC activity, and this can be measured by an increase in cell surface expression of CD54, CD69, or CD107a. Several studies have evaluated NK cell activation with ADCC-enhanced antibodies as well as the effects of FcγRIIIA polymorphisms on the activation potential [35–37]. A recent study by Fischer et al. using Rituxan and alemtuzumab in chronic lymphocytic leukemia patients demonstrated that increased CD107a expression on peripheral blood NK cells correlated with ADCC activity and suggested a possible means to measure ADCC activity in the clinical setting. Our in vitro data support the use of CD107a as a marker for NK cell activation and ADCC activity where, at very low concentrations (10−2 µg/ml), 3F2-3M demonstrated robust increases in NK cell surface CD107a expression, which was dependent on the presence of EphA2-expressing tumor cells lines (Figure 4). These combined results provide a potential avenue to monitor the ADCC activity of monoclonal antibodies, which could act as a clinical pharmacodynamic marker, facilitate dose and scheduling optimization, and minimize clinical trial durations. Furthermore, use of CD107a in conjunction with other NK cell activation markers, such as CD54 and CD69, may further increase the specificity of monitoring ADCC activity and have broad applicability to monoclonal antibody therapies for oncology.
In summary, our preclinical evaluations of the EphA2 agonist antibody 3F2-3M demonstrate robust ADCC activity and the potential to overcome the FcγRIIIa polymorphism obstacles that have challenged other therapeutic monoclonal antibodies. Thus, by combining the activation of EphA2 receptor-mediated signaling events with enhanced ADCC activity in 3F2-3M, we have generated a novel and potent therapeutic approach for the potential treatment of cancer patients with tumors that overexpress EphA2.
Supplementary Material
Acknowledgments
The authors thank the members of the laboratory animal resource group for their technical assistance with the in vivo studies and Andrew Chen and Lanju Zhang for their assistance with statistical analyses.
Footnotes
This article refers to supplementary materials, which are designated by Table W1 and Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–461. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
- 2.Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 4.Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–360. doi: 10.1158/1078-0432.CCR-05-1505. [DOI] [PubMed] [Google Scholar]
- 5.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 6.Wang LF, Fokas E, Bieker M, Rose F, Rexin P, Zhu Y, Pagenstecher A, Engenhart-Cabillic R, An HX. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep. 2008;19(1):151–156. [PubMed] [Google Scholar]
- 7.Yuan WJ, Ge J, Chen ZK, Wu SB, Shen H, Yang P, Hu B, Zhang GW, Chen ZH. Overexpression of EphA2 and ephrin A1 in human gastric adenocarcinoma and its prognostic value for postoperative patients. Dig Dis Sci. 2008 Dec. doi: 10.1007/s10620-008-0649-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Shao Z, Zhang WF, Chen XM, Shang ZJ. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: correlation with the angiogenesis and clinical outcome. Oral Oncol. 2008;44(12):1110–1117. doi: 10.1016/j.oraloncology.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9(2):613–618. [PubMed] [Google Scholar]
- 10.Wu D, Suo Z, Kristensen GB, Li S, Troen G, Holm R, Nesland JM. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol Oncol. 2007;94:312–319. doi: 10.1016/j.ygyno.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 13.Merritt WM, Thaker PH, Landen CN, Jr, Deavers MT, Fletcher MS, Lin YG, Han LY, Kamat AA, Schmandt R, Gershenson DM, et al. Analysis of EphA2 expression and mutant p53 in ovarian carcinoma. Cancer Biol Ther. 2006;5:1357–1360. doi: 10.4161/cbt.5.10.3225. [DOI] [PubMed] [Google Scholar]
- 14.Meade-Tollin L, Martinez JD. Loss of p53 and overexpression of EphA2 predict poor prognosis for ovarian cancer patients. Cancer Biol Ther. 2007;6:288–290. doi: 10.4161/cbt.6.2.4024. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Dong Z, Qiao Y, Kristensen GB, Holm R, Nesland JM, Suo Z. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol Oncol. 2005;99:278–286. doi: 10.1016/j.ygyno.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 17.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 18.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–1456. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 19.Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, Mittal SK. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 20.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 21.Coffman KT, Hu M, Carles-Kinch K, Tice D, Donacki N, Munyon K, Kifle G, Woods R, Langermann S, Kiener PA, et al. Differential EphA2 epitope display on normal versus malignant cells. Cancer Res. 2003;63:7907–7912. [PubMed] [Google Scholar]
- 22.Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–2847. [PubMed] [Google Scholar]
- 23.Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 2007;254:165–177. doi: 10.1016/j.canlet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 25.Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 28.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with Her-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1778–1780. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 29.Dall'Acqua WF, Damschroder MM, Zhang J, Woods RM, Widjaja L, Yu J, Wu H. Antibody humanization by framework shuffling. Methods. 2005;36:43–60. doi: 10.1016/j.ymeth.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Kiewlich D, Zhang J, Gross C, Xia W, Larsen B, Cobb RR, Biroc S, Gu JM, Sato T, Light DR, et al. Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia. 2006;8:18–30. doi: 10.1593/neo.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatjiharissi E, Hansen M, Santos DD, Xu L, Leleu X, Dimmock EW, Ho AW, Hunter ZR, Branagan AR, Patterson CJ, et al. Genetic linkage of Fc gamma RIIa and Fc gamma RIIIa and implications for their use in predicting clinical responses to CD20-directed monoclonal antibody therapy. Clin Lymphoma Myeloma. 2007;7:286–290. doi: 10.3816/clm.2007.n.004. [DOI] [PubMed] [Google Scholar]
- 32.Treon SP, Hansen M, Branagan AR, Verselis S, Emmanouilides C, Kimby E, Frankel SR, Touroutoglou N, Turnbull B, Anderson KC, et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:474–481. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 33.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, Yokoi H, Nakamura K, Shitara K. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 34.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, Nakamura K, Shitara K. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res. 2005;11:2327–2336. doi: 10.1158/1078-0432.CCR-04-2263. [DOI] [PubMed] [Google Scholar]
- 36.Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Exp Hematol. 2006;34:753–759. doi: 10.1016/j.exphem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods. 2005;304:88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.