Abstract
Treatment options for pancreatic cancer have shown limited success mainly owing to poor selectivity for pancreatic tumor tissue and to a lack of activity in the tumor. In this study, we describe the ability of the urokinase-type plasminogen activator receptor (uPAR) promoter to efficiently and selectively target pancreatic tumors and metastases, which enables the successful management of pancreatic cancer. We have generated a replication-defective reporter adenovirus, AduPARLuc, and a conditionally replicating adenovirus, AduPARE1A, and we have studied the selectivity and antitumoral efficacy in pancreatic tumors and metastases. Toxicity was studied on intravascular delivery. We demonstrate that the uPAR promoter is highly active in pancreatic tumors but very weak in normal tissues. Tumor specificity is evidenced by a 100-fold increase in the tumor-to-liver ratio and by selective targeting of liver metastases (P < .001). Importantly, the AduPARE1A maintains the oncolytic activity of the wild-type virus, with reduced toxicity, and exhibits significant antitumoral activity (25% tumor eradication) and prolonged survival in pancreatic xenograft models (P < .0001). Furthermore, upon intravascular delivery, we demonstrate complete eradication of liver metastasis in 33% of mice, improving median survival (P = 5.43 x 10-5). The antitumoral selective activity of AduPARE1A shows the potential of uPAR promoter-based therapies in pancreatic cancer treatment.
Introduction
Pancreatic cancer is one of the most aggressive and devastating human malignancies in developed countries. Overall survival rate is less than 4%, with almost all patients dying within the first year after diagnosis as a result of the rapid spreading of the tumor or metastatic dissemination [1].
Up to now, pancreatic cancer treatment has mainly been palliative because of the ineffectiveness of current treatments [1]. Despite the great efforts made to improve therapy, even the very latest therapeutic approaches assayed, such as the combination of gemcitabine with epidermal growth factor receptor-targeting agents, have shown only partial improvement on patients survival [2]. Thus, there is an urgent need for more intensive research seeking ways to treat this devastating disease.
The most important challenge to the development of a safe and effective treatment of pancreatic cancer is the identification of a selective therapeutic agent capable of potent activity both in primary tumors and in tumor metastases with low toxicity when administered systemically. Recently, oncolytic adenovirus therapy, based on restricting viral replication to cancer cells, has emerged as a promising candidate for cancer therapy [3]. The phase 1/2 clinical trials assaying the oncolytic efficacy of ONYX-015 have shown no major toxicity on intravascular infusion [4] but only partial therapeutic benefit when administered intratumorally to pancreatic tumors [5]. Therefore, the remaining issue is still to obtain a virus with sufficient activity and selectivity to the tumor tissue. To achieve tumor-selectivity, several tumor-specific promoters (TSPs) have been assayed for pancreatic cancer. However, they have shown lower activity in comparison to ubiquitously active promoters such as the cytomegalovirus (CMV) [6,7], unless the cholecystokinin type A receptor promoter when engineered with a VP16-GAL4-WPRE integrated systemic amplifier [8]. When used in the context of conditionally replicative adenovirus, although significant responses have been reported for candidate TSP when administered intratumorally, limited antitumor activity has been observed on established xenografts upon intrasvascular delivery, and no studies have been performed on metastatic models [9–11].
The urokinase-type plasminogen activator receptor (uPAR) is a glycosylphosphatydilinositol-anchored protein that binds with high affinity uPA, pro-uPA, amino terminal fragments of urokinase, and other cell receptors. The uPAR has an active role in cancer because it regulates cell migration, adhesion, metastasis, and tumor growth [12]. In pancreatic cancer and in tumor metastasis, uPAR has been found to be overexpressed [13,14].
Several reports have shown that the down-regulation of uPAR expression with uPAR antagonists, small interfering RNA, or antisense genes prevents tumor growth, invasion, and metastasis [15]. Bauer et al. [16] also described how combining gemcitabine and a monoclonal antibody against uPAR reduces pancreatic cancer invasion in orthotopic tumors.
In the present study, we have also targeted uPAR expression in pancreatic cancer but using a completely different approach. We have validated the uPAR promoter to selectively target adenovirus gene expression and replication to primary pancreatic tumors and metastases. To our knowledge, for the first time, the present study demonstrates that upon intravascular injection, uPAR promoter activity is very weak in normal tissues, whereas in pancreatic tumor models and liver metastases, uPAR promoter activity is similar to that of the CMV promoter. Importantly, our results show that the conditionally replicative adenovirus AduPARE1A, in which replication is controlled by the human uPAR promoter, eradicates pancreatic tumors and tumor metastasis.
Materials and Methods
Plasmid Constructs
A 450-bp fragment encompassing the -402/+48 region of the uPAR promoter was excised from pCAT-Basic-uPAR(C2) [17] and subcloned into a pAdTrack to generate pAdTrackuPARp. The cDNA from luciferase was excised from the pGL3-Enhancer (Promega, Madison, WI) plasmid and subcloned into the pAdTrackuPARp to generate pAd-TrackuPARLuc.
Cell Lines
The human pancreatic adenocarcinoma cell lines PANC-1, BxPC-3, RWP1, NP-31, NP-9, and NP-18 were obtained and cultured as previously described [18]. A549 human lung cancer cells, MCF-7 human breast cancer cells, NIH3T3 murine fibroblasts, IMR-90 human lung fibroblasts, and HEK293 were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and maintained as already described [18,19]. U2 OS human osteosarcoma cells were obtained from European Collection of Cell Cultures (Wiltshire, United Kingdom) and were cultured following the manufacturer's description. Human pancreatic ductal epithelial (HPDE) cells were kindly provided by Dr. F.X. Real (IMIM, Barcelona, Spain) and cultured and maintained as reported [20]. Luciferase-expressing cells PANC-1-Luc were established by transducing the parental cells with luciferase recombinant retrovirus. Briefly, pLHCLuc retroviral vector was transfected by the calcium/phosphate-DNA precipitation method into the amphotropic packaging cell line Phoenix Ampho (ATCC, Rockville, MD), and 48 hours after transfection, viral supernatant was collected, passed through 0.45-nm filters, and used for transduction. Twenty-four hours later, cells were selected in 0.2 mg/ml hygromycin. Cells were cloned and tested for luciferase expression.
Transfection
Transient transfections were performed in NIH3T3, HPDE, and RWP1 cells, 24 hours after seeding, with SuperFect Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer's protocol using 3 µg of plasmid DNA. Three micrograms of pCMV β-galactosidase plasmid was cotransfected to normalize for transfection efficiency.
Adenoviruses
Replication-defective adenoviruses AdCMVGFPLuc and AduPARLuc express the firefly luciferase gene under the control of CMV or uPAR promoter, respectively. AdCMVGFPLuc has already been described [21]. The AduPARLuc was generated by the homologous recombination of the pAdTrackuPARLuc and the adenoviral genome following a standard protocol.
The oncolytic AduPARE1A was constructed by inserting the uPAR promoter (450-bp fragment) upstream of an E1a adenoviral gene in which the E1a translation start site was replaced by the Kozak sequence. The human DM-1 insulator was cloned upstream the uPAR promoter [9,22]. All cassettes were cloned in a left-to-right orientation. The wild-type virus, Adwt, was obtained from the ATCC (Manassas, VA). Replication-defective AdV has already been described [23]. Replication-defective viruses and Adwt were propagated in HEK293 cells, and the oncolytic AduPARE1A virus was amplified in RWP1 cells. All viruses were purified by standard cesium chloride banding. The physical particle concentration (vp/ml) was determined by optical density reading (OD250), and the plaque-forming units (pfu/ml) were determined by tissue culture infectious dose50 titration on HEK293 cells. AdCMVGFPLuc and AduPARLuc presented an equal ratio of vp and pfu.
Tumor Growth Studies
BxPC-3 cells (2.5 x 106) were injected subcutaneously (SC) into each posterior flank of BALB/c nude mice (Charles River France, Lyon, France). Tumors were measured three times a week, and volumes were calculated according to the formula V (mm3) = larger diameter (mm) x smaller diameter 2 (mm2) / 2. Treatment was initiated when tumors achieved a mean volume of 50 mm3. All animal procedures met the guidelines of European Community Directive 86/609/EEC and were approved by the local ethical committee.
Bioluminescence Assay and Quantification
Animals were anesthetized and the substrate d-Firefly-Luciferin (Xenogen, Alameda, CA) was administered intraperitoneally (32 mg/kg). Luciferase activity was visualized and quantified using an in vivo bioluminescent system (IVIS50; Xenogen) and Living Image 2.20.1 Software overlay on Igor Pro4.06A software (Wavematrics, Seattle, WA) as described [23]. Luciferase activity was quantified from non-saturated images, measuring the total amount of emitted light recorded by the CCD camera.
Immunohistochemistry and Stereological Analysis
Five-micrometer sections were deparaffinized, rehydrated, and treated with 10 mM citrate buffer (pH 6.0) for antigen retrieval. Sections were then incubated overnight with a rabbit anti-luciferase polyclonal antibody (1:500; Sigma, Poole, United Kingdom). Bound antibodies were detected with Universal LSAB+ (DAKO Diagnostics, Denmark). Sections were counterstained with Mayer's hematoxylin. All sections were examined on a Leica DMR microscope (Leica, Madrid, Spain).
A physical dissector method was used to estimate the luciferase-positive cells per squared millimeter in both the liver and tumoral areas, with the aid of a CAST-GRID software package (Olympus, Denmark) adapted to an Olympus BX51 microscope. At least three independent, nonsequential, randomly selected liver sections or three tumor sections were counted from each animal. A mean of 25 dissector probes (dis), of 17,110 µm2 (Sdis), were analyzed per liver area with a x10 lens. A mean of 160 dissector probes of 57,043 µm2 was analyzed per tumoral area with a x40 lens. The total final surface area analyzed was similar for each of the different viral groups. The number of cells per squared millimeter was calculated according to the formula: [N (cells/mm2) = N cells/N dis x Sdis].
Liver Toxicity Studies and Determination of the Adenoviral DNA Content in Liver
For liver toxicity studies, 6- to 8-week-old immunocompetent BALB/c male mice received a single administration of the corresponding virus at a final dose of 2 x 1010 vp (AduPARLuc) or 5 x 1010 vp (AduPARE1A) in a final volume of 0.2 ml injected into the tail vein. Body weight, morbidity, and moribundity were monitored daily. Blood samples were collected by intracardiac puncture under anesthesia. Serum aspartate aminotransferase (AST) and total bilirubin (BLT) were determined on an Olympus AU400 Analyzer. Subsequently, the animals were killed, and liver portions were either frozen for luciferase or viral DNA determination or fixed and embedded in paraffin for hematoxylin and eosin staining or frozen in OCT (Akura Finetek, Zoeterwoude, the Netherlands) for E1A immunodetection.
E1a immunodetection was performed by incubating the OCT-embedded liver sections with a primary polyclonal antibody anti-adenovirus-2 E1a (clone 13 S-5; Santa Cruz Biotechnology, Heidelberg, Germany). AlexaFluor 488-labeled goat anti-rabbit antibody (Molecular Probes, Eugene, OR) was used as a secondary antibody. The nuclei were counterstained with 5 µg/ml bis-benzimide (Hoechst 33342; Sigma) and visualized under a fluorescent microscope (Observer/Z1; Zeiss, Barcelona, Spain). The fluorescent images were captured using a digital camera (AxioCamMRm; Zeiss).
When stated, DNA was obtained from frozen liver tissue by incubating in a buffer containing 0.2 mg/ml RNaseA and 0.1 mg/ml protease overnight at 55°C. The liver adenoviral DNA content was determined with real-time polymerase chain reaction (PCR; 100 ng of DNA) and SYBER Green I Master Plus mix (Roche Diagnostics, Barcelona, Spain). Hexon primer sequences were as follows: forward 5′ GCCGCAGTGGTCTTACATGCACATC 3′ and reverse 5′ CAGCACGCCGCGGATGTCAAAG 3′. The adenovirus copy number was quantified with a standard curve, consisting of adenovirus DNA dilutions (10–107 copies) in a background of liver mouse genomic DNA. Samples and standards were amplified in triplicate, and the average number of total copies was normalized to copies per cell based on the input DNA weight amount and a genome size of 6 x 109 bp/cell. Results are expressed as vp/100 cells.
Statistical Analysis
The descriptive statistical analysis was performed on SPSS software (SYSTAT software, Inc, Chicago, IL). Results are expressed as mean ± SEM. A Mann-Whitney nonparametric test was used for the statistical analysis (2-tailed) of in vitro and in vivo studies. P < .05 was taken as the level of significance.
The in vivo tumor growth and survival statistical analysis was performed using S-PLUS functions. In line with the experimental design, animal, repeated measures, and site are considered to be nested classification factors. We associated random-effects terms with the animal factor, the day of measurement, and the site of nesting of the animal. Hence, general linear-mixed models were used to estimate the effects of treatment on tumor growth by taking nested and repeated design into account [24]. These models allowed us to analyze the overall effect and the effect of each treatment. The estimation of the coefficients and associated P values was based on restricted maximum likelihood. A plot with residual versus fitted values was used to check the assumptions of the model. With these plots, log transformation of the data was used when homoscedasticity was observed. The variance function structure was used to model heteroscedasticity in the day-to-day errors. P < .05 (Bonferroni correction) was considered statistically significant after performing multiple comparisons of the treatment groups.
Survival analyses were also performed to analyze time-to-event probability. The survival curves obtained were compared for the different treatments. Animals whose tumor size never achieved the threshold or that were alive at the end of the experiment were included as right censored information. A log-rank test was used to determine the statistical significance of the differences in time-to-event. P.05 was considered statistically significant.
Results
uPAR Promoter Drives Adenoviral Transgene Expression to Pancreatic Cancer In Vitro and In Vivo
We analyzed uPAR expression in a panel of pancreatic cancer cell lines, in pancreatic tumors, and in HPDE cells derived from normal ductal epithelium by semiquantitative reverse transcription (RT)-PCR analysis. uPAR expression was weaker in normal ductal HPDE cells when compared with most of the cancer cells (Figure W1A). uPAR expression was also detected in the two different tumor models analyzed (Figure W1B). To initially test the pancreatic cancer selectivity of the uPAR promoter, reporter studies were undertaken. Results showed that a 450-bp fragment of uPAR promoter was significantly more active in RWP1 pancreatic cancer cells than in nontumoral cells HPDE or NIH3T3 (Figure 1A).
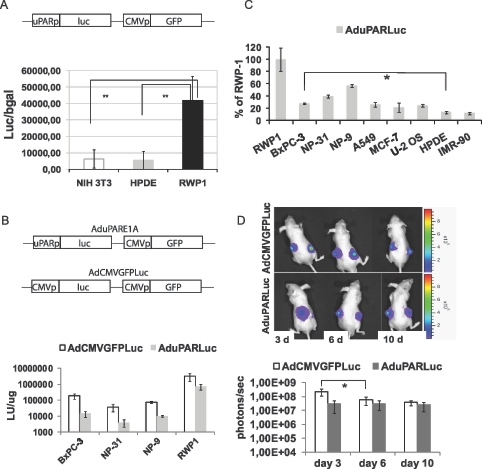
Figure 1.
uPAR promoter activity in pancreatic cancer cell lines and in pancreatic tumors. (A) NIH3T3, HPDE, and RWP1 cells were transfected with the plasmid pAdTrackuPARLuc (shown in the scheme). To normalize for transfection efficiency, pCMVβGal plasmid was used. Forty-eight hours later, luciferase and β-galactosidase activities were determined. Results are expressed as light units (LU) relative to β-galactosidase activity and are shown as the mean ± SEM of three independent experiments. **P = .006, HPDE versus RWP1; **P = .002, NIH3T3 versus RWP1. (B) A total of 20,000 cells/well were seeded in triplicate and infected with either AduPARLuc or AdCMVGFPLuc at 104 vp/cell. Luciferase activity was quantified 72 hours after viral transduction and normalized to total protein levels. Results are expressed as light units per microgram protein. Values are represented as the mean ± SEM of four independent experiments. (C) Percentage of uPAR/CMV luciferase ratio relative to the uPAR/CMV luciferase ratio for RWP1 cells. Values are represented as the mean ± SEM of three or four independent experiments. *P < .05. (D) A total of 3 x 106 BxPC-3 cells were injected SC into each posterior flank of nude mice. When tumors achieved a mean volume of 100 mm3, they were randomized and injected intratumorally with a single 2.5 x 1010 vp dose of AdCMVGFPLuc (n = 9) or AduPARLuc (n = 10). Shown are representative images and quantification of bioluminescent emission from mice receiving AdCMVGFPLuc (upper panel) or AduPARLuc (lower panel) at days 3, 6, and 10 after viral injection. Results are expressed as photons per second. Values are represented as mean ± SEM. *P = .03.
To assess the potential capacity of the uPAR promoter to effectively and selectively target pancreatic cancer, we generated a replication-defective adenovirus, AduPARLuc, which expresses the luciferase reporter gene under the regulation of a 450-bp fragment of the human uPAR promoter (Figure 1B). The AdCMVGFPLuc virus, which expresses the luciferase gene under the CMV promoter, was used as a control.
All pancreatic cancer cells infected with the AduPARLuc virus showed high levels of luciferase activity, indicating that the uPAR promoter was active in pancreatic cancer cells. However, when compared with CMV promoter, uPAR promoter activity resulted in 23% to 27% of that of the CMV (Figure 1B). Interestingly, the 450-bp fragment of the uPAR promoter had relatively higher luciferase activity in pancreatic cancer cells and in cells of other cancer origin (lung, breast, and bone) than in the nontumor cells lines HPDE and IMR-90 (Figure 1C). This then indicates that the uPAR promoter retains its specificity for cancer cells in the context of an E1A-deleted adenoviral genome.
To identify uPAR activity in pancreatic tumors, BxPC-3 SC xenografts were injected with AduPARLuc or AdCMVGFPLuc at 2.5 x 1010 vp/tumor, and the level and duration of luciferase expression were monitored by optical imaging. Bioluminescence imaging showed that both viruses induced high levels of luciferase expression (Figure 1D). In mice receiving the control AdCMVGFPLuc virus, luciferase activity peaked at day 3 but rapidly decreased, showing a significant four-fold reduction in the initial luciferase activity at day 6 after viral injection (Figure 1D). By contrast, in AduPARLuc-injected tumors, bioluminescence activity was maintained constant, lasting at least 10 days after viral injection. It is worthy of note that from day 6 to the end of the experiment, both promoters presented similar levels of activity. Similar results were obtained with an NP-18 SC tumor model (data not shown). These findings indicated that uPAR promoter activity in pancreatic tumors is as high as that of the constitutively active CMV promoter (Figure 1D).
uPAR Promoter Selectively Targets Pancreatic Cancer Tumors
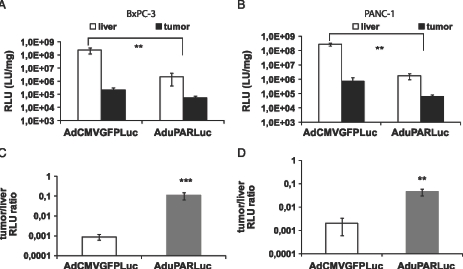
To address the potential selectivity of the AduPARLuc virus for pancreatic cancer tumors, mice bearing BxPC-3 and PANC-1 xenografts were challenged with a single intravenous (IV) injection of 5 x 1010 vp of the AduPARLuc virus. Luciferase expression was then analyzed in the liver and in the tumors. Data showed that the uPAR promoter activity was as high as that of the CMV in the tumor, whereas a significant reduction in transgene expression was detected in the liver of AduPARLuc-injected animals (Figure 2, A and B). The cancer-specific index (established as a tumor-to-liver ratio) was significantly higher in AduPARLuc-injected animals in both BxPC-3 (122-fold, P = .001) and PANC-1 (23-fold, P = .006) SC tumor models (Figure 2, C and D), indicating that the uPAR promoter was more selective for pancreatic tumor tissue in vivo. These results are consistent with the results obtained from the biodistribution studies, where a significantly lower uPAR activity was detected in the liver, spleen, kidneys, heart, and lung, and a very weak activity was observed in the pancreas, intestine, stomach, and testis (Figure W2).
Figure 2.
uPAR promoter tumor-to-liver ratio. A total of 3 x 106 BxPC-3 cells (A and C) or 2.5 x 106 PANC-1 cells (B and D) were injected SC into each posterior flank of nude mice. Once tumors were established, animals received 5 x 1010 vp of AdCMVGFPLuc (n = 4 mice; n = 8 tumors) or AduPARLuc (n = 4 mice; n = 8 tumors) or saline solution (n = 2 mice; n = 4 tumors) through the tail vein. Mice were killed 5 days after virus injection, and tumor and liver extracts were assayed for luciferase activity. (A and B) Luciferase activity from liver and tumor tissues. Luciferase activity from the saline group was considered background and subtracted from the viral groups. Results are expressed as light units per milligram of tissue (LU/mg). Values are represented as mean ± SEM. **P < .01, ***P < .001. (C and D) Tumor-to-liver ratio. Values are represented as mean ± SEM of tumor RLU relative to liver RLU. **P < .01.
AduPARE1A Induces Significant Cytotoxicity in Pancreatic Cancer Cells
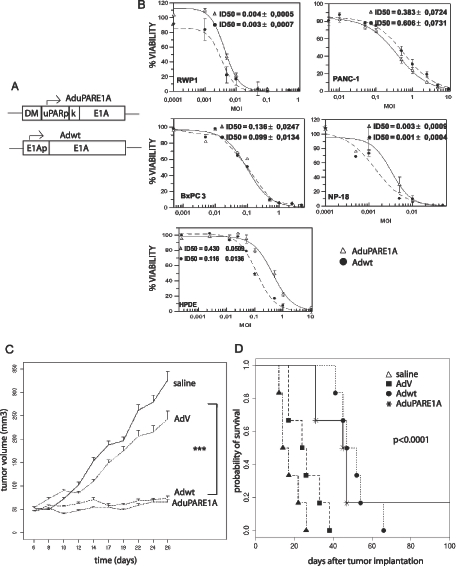
To study whether the uPAR promoter could be a candidate promoter to selectively, efficiently, and safely act against pancreatic cancer, we generated an oncolytic AduPARE1A virus in which the E1A gene expression was regulated by the uPAR promoter. A Kozak sequence was engineered upstream of the E1A gene to increase its replication potency [9]. A DNA fragment from the myotonic dystrophy locus (DM-1), with enhancer-blocking insulator activity, was introduced upstream the uPAR promoter to isolate it from enhancer and transcriptional units from the adenovirus genome [22] (Figure 3A).
Figure 3.
Antitumoral effect of AduPARE1A in pancreatic cancer cells lines, HPDE cells and in BxPC-3 SC xenografts. (A) Schematic representation of the AduPARE1A and Adwt viruses. DM indicates myotonic dystrophy fragment; K, Kozak sequence. (B) A total of 3 x 103 cells/well were seeded in triplicate and infected with a dose range of 0 to 10 multiplicities of infection of Adwt or AduPARE1A. Cell viability was measured by MTT assay 4 days later and is expressed as the percentage of absorbance of treated wells compared with that of mock-infected cultures. Dose-response curves and ID50 values, obtained by a standard nonlinear model based on the Hill equation, are shown as the mean ± SEM of three independent experiments. -●-, Adwt; -Δ-, AduPARE1A. (C) Animals bearing BxPC-3 SC tumors were randomized to four groups: two control groups, namely, saline (n = 11 tumors) and AdV (n = 11 tumors), and two treated groups, namely, Adwt (n = 12 tumors) and AduPARE1A (n = 12 tumors). Animals received intratumoral injections of 107 pfu/tumor at days 6 and 14 after tumor implantation. Tumor growth curves are plotted as mean tumor volume ± SEM. ***P < .0001. (D) Kaplan-Meier survival curves (log-rank test, < .0001). End point (animals with a tumor volume, ≥300 mm3).
We tested the cytotoxic effect of AduPARE1A in four pancreatic cancer cell lines and in HPDE cells infected with different doses of AduPARE1A or Adwt. The results showed that the AduPARE1A dose required to cause a 50%reduction in cell viability (ID50) was equivalent to the ID50 of the Adwt in the cancer cell lines but was higher for the nontumoral cell line HPDE. These data show that AduPARE1A displays an important cytotoxic effect in pancreatic tumor cells, with a potency similar to that of Adwt (Figure 3B). No reduction in cell viability was observed when a nonreplicative empty vector (AdV) was used at similar viral doses (data not shown), demonstrating that the cytotoxic effect observed was specifically due to viral replication and not to nonspecific viral toxicity.
AduPARE1A Represses Tumor Growth and Prolongs Mouse Survival in BxPC-3 SC Xenografts
Next, we evaluated the antitumoral effect of the AduPARE1A oncolytic virus in pancreatic tumors. BxPC-3 SC xenografts received two injections, weekly separated, of 107 pfu/tumor of AduPARE1A or Adwt or AdV or saline solution. As shown in Figure 3C, a significant reduction in tumoral growth was observed in both treated groups (AduPARE1A and Adwt) compared with the saline or AdV control groups (P < .0001). Importantly, 25% (3/12) of AduPARE1A-injected tumors and 16% (2/12) of Adwt-injected tumors were completely eradicated and no tumor burden detected when some of the animals were killed owing to the growth of the contralateral tumor. The median survival time for mice receiving AdV control was 32 days. In contrast, the median survival time for mice receiving AduPARE1A was 51 days (P < .01; Figure 3D). Moreover, it is worth mentioning that one of the six mice treated with AduPARE1A did not show any tumor burden in either of the two injected tumors 6 months after treatment, indicating that there was no recurrence of the tumors eradicated.
AduPARE1A Represses Tumor Growth and Prolongs Mouse Survival in a Metastasis Tumor Model
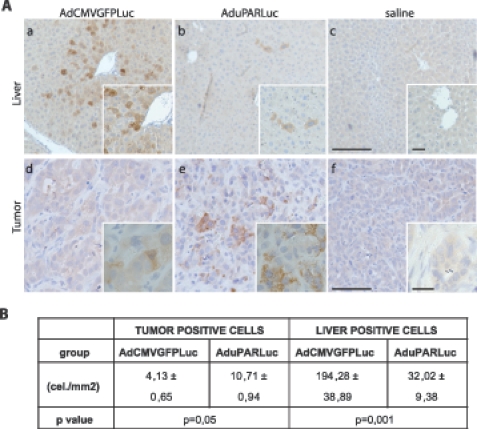
The uPAR is a key factor in invasion and metastasis. In this respect, high levels of uPAR expression have been described in human pancreatic cancer metastases [14]; therefore, we hypothesized that the uPAR promoter could also selectively target pancreatic tumor metastasis. To ascertain this, we first established a metastasis model by inoculating PANC-1 cells into the spleen of nude mice (Figure W3). By day 10 of tumor cell inoculation, when metastatic liver nodules were present, mice were injected IV with 5 x 1010 vp of AduPARLuc or AdCMVGFPLuc. Luciferase immunostaining in liver sections revealed strong immunoreactivity in PANC-1 tumor cells in both AduPARLuc and AdCMVGFPLuc-treated mice (Figure 4A). The proportion of luciferase-positive tumor cells in AduPARLuc-treated mice was significantly higher than that in AdCMVGFPLuc mice (Figure 4B). Interestingly, in the liver parenchyma, luciferase expression was detected at very low levels and in a limited number of cells in the AduPARLuc-treated mice, whereas strong luciferase staining and a significant increased proportion of luciferase-positive hepatocytes were detected in the AdCMVGFPLuc group (Figure 4A), providing new evidence of the selectivity of the uPAR promoter.
Figure 4.
AduPARLuc expression in liver metastasis model. Animals bearing PANC-1 tumor metastases received saline solution (n = 3) or 5 x 1010 vp of AdCMVGFPLuc (n = 3) or AduPARLuc (n = 3) IV. Three days later, animals were killed, livers were excised, and luciferase expression was determined by immunohistochemistry with a polyclonal anti-luciferase antibody. (A) Representative images of luciferase expression in liver (a, b, c) and tumor areas (d, e, f) of animals injected with AdCMVGFPLuc or AduPARLuc or saline. (a, b, c) Scale bar, 200 µm; original magnifications, 100 µm. (d, e, f) Scale bar, 50 µm; original magnifications, 20 µm. (B) Stereological analysis. Quantification of luciferase-positive cells in the liver and tumor. Values are shown as the mean ± SEM of three animals. Results are expressed as cells per total area analyzed (cells/mm2).
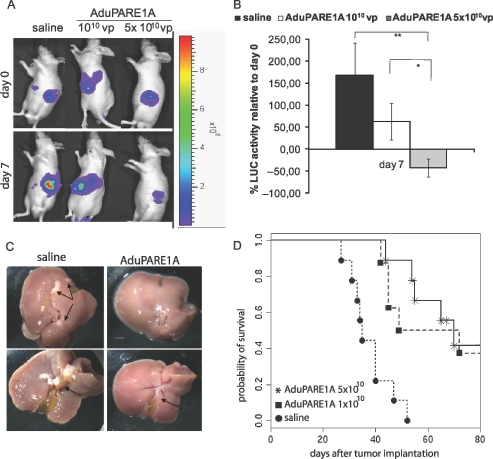
To evaluate the effectiveness of AduPARE1A on the metastatic model, PANC-1 luciferase-expressing cells were inoculated into the spleen of nude mice. Six days later, once the metastases were formed (Figure 5A), the animals were treated IVwith saline or with a single dose of 1010 vp or 5 x 1010 vp of AduPARE1A. Seven days after virus administration, both treated groups presented lower luciferase activity than control animals did. A significant 50% reduction in initial luciferase activity was detected in animals receiving the highest viral dose (P = .005; Figure 5B). To better address whether this reduction in luciferase-positive cells correlated with a reduction in liver tumors, representative animals from each group were killed at day 21 after treatment. No tumor nodules were found in the liver parenchyma of the 5 x 1010 vp AduPARE1A-treated mice (Figure 5C). The number of nodules found was also lower in the animals treated with 1010 vp AduPARE1A than in saline-treated mice (data not shown). The median survival time of the saline injected mice was 35 days, whereas the median survival time of both treated groups was significantly longer, that is, 60 days for the 1010 vp viral dose and 70 days for the 5 x 1010 vp dose (Figure 5D). Surviving mice (33%) were alive for more than 5 months with no symptoms of morbidity. Then, they were killed and no tumors were observed in any abdominal organ indicating tumor eradication.
Figure 5.
Antitumoral effect of AduPARE1A in PANC-1-Luc liver metastasis model. PANC-1-Luc cells were injected intrasplenically into BALB/c male mice. Six days after tumor implantation bioluminescent activity was recorded (day 0). The day after, animals received a single IV injection, of either saline (n = 10) or AduPARE1A at 1010 vp/animal (n = 8) or AduPARE1A at 5 x 1010 vp/animal (n = 10) in a Vf = 0.2 ml. (A) Bioluminescent images of liver metastases-bearing mice at days 0 and 7 after adenoviral treatment. (B) Bioluminescent emission quantification at day 7 relative to day 0. **P = .005, *P = .02. (C) Representative images of livers excised at day 21 after treatment. Tumor nodules and bile duct obstruction are shown in the saline group (arrows, left panels). Normal bile duct in the 5 x 1010 AduPARE1A-injected animals (arrow, right panels). Scale bar, 1.7 cm. (D) Kaplan-Meier survival curves. AduPARE1A 1010 vp versus saline log-rank test = .003; AduPARE1A 5 x 1010 vp versus saline log-rank test = 5.43 x 10-5. End point (decreased body weight ≥20% or animal experiencing jaundice).
uPAR Promoter Presents Low Liver Toxicity on IV Injection
After intravascular injection, adenoviruses liver tropism leads to liver toxicity, caused by an immune response to the viral proteins as well as transgene expression.
First, we evaluated the potential toxicity of AduPARLuc after IV injection in immunocompetent mice and compared it to that of AdCMVGFPLuc. Minimal liver histopathologic changes were observed in AduPARLuc animals in contrast to the hepatocytotoxicity found in all AdCMVGFPLuc liver sections. Importantly, all animals that received the AduPARLuc virus presented AST levels within the reference range (Figure W4). To discard differences in liver viral transduction between the AduPARLuc- and AdCMVGFPLuc-injected mice, we assessed the amount of viral particles in the liver by quantitative real-time PCR. Quantification showed a similar amount of viral particles in both groups (AdCMVGFPLuc 13.14 ± 1.33 vp/100 cells and AduPARLuc 10.82 ± 0.92 vp/100 cells) indicating that the toxicity elicited by transgene expression in the liver, in the context of a replication-defective adenovirus, can be minimized by the uPAR promoter.
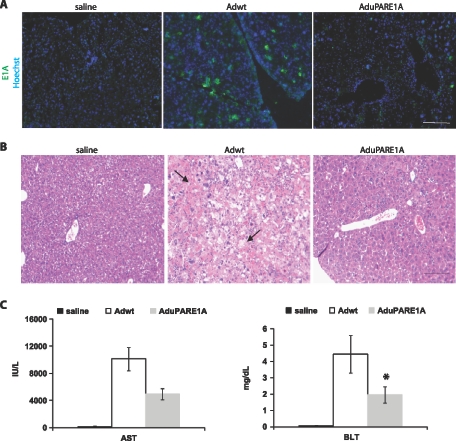
Next, we studied the potential liver toxicity of the oncolytic AduPARE1A and compared it to that of Adwt. E1A Immunostaining revealed negligible E1A expression in the liver sections of AduPARE1A-injected animals, whereas strong immunoreactivity was detected in the liver of Adwt mice (Figure 6A). Consistently, lower areas of hepatocyte swelling and necrotic hepatocytes were observed in the liver sections of AduPARE1A-injected mice compared with Adwt-injected mice (Figure 6B). Moreover, serum levels of AST and BLT were significantly lower in AduPARE1A-injected mice than those in Adwt-injected mice (Figure 6C). Furthermore, a 50% mortality rate was detected in the 5 x 1010 vp Adwt-injected animals, whereas all the animals injected with this dose of AduPARE1A survived. The data thus indicate that the uPAR promoter provided reduced toxicity to the replication-competent AduPARE1A virus.
Figure 6.
AduPARE1A toxicity studies. BALB/c immunocompetent mice received IV saline solution (n = 6) or 5 x 1010 vp of Adwt (n = 6) or 5 x 1010 vp of AduPARE1A (n = 6). Five days later, blood samples were collected. Next, animals were killed, and the liver tissue excised was frozen in OCT or fixed and embedded in paraffin. (A) E1A expression in the liver tissue. Nuclei were stained with Hoechst. Scale bar, 100 µm. (B) Hematoxylin and eosin staining of liver tissue. Necrotic and swollen hepatocytes (arrows). Scale bar, 1.3 cm. (C) AST and BLT determination. *P < .05.
Discussion
Oncolytic virotherapy is a promising approach for the systemic treatment of cancers, such as pancreatic cancer, characterized by the rapid dissemination of the tumor and the lack of any effective treatment. However, the need of highly selective and efficient viruses is a requisite for a safe and significant therapeutic benefit. Transcriptional targeting strategies based on using TSP to redirect adenoviral gene expression and replication have proved the importance of such approaches to achieve tumor specificity. uPAR expression has been shown to be transcriptionally upregulated in pancreatic ductal adenocarcinoma, compared with normal pancreatic cells, suggesting that the factors driving uPAR transcription may be highly active in a tumoral context. In this study, we have demonstrated that the uPAR promoter in an adenovirus vector delivers potent activity in all the pancreatic cancer cell lines studied, although lower than that of the CMV promoter (10%–22% of the latter). Interestingly, such differences were no longer found on intratumoral or intravascular viral administration in mice bearing pancreatic SC xenografts. Thus, in vivo, the uPAR promoter activity in the tumors was similar to that of the CMV promoter, suggesting that in the tumor either CMV promoter activity is reduced or uPAR promoter activity is favored. Most probably, both phenomena take place. In fact, a comparative analysis of uPAR and CMV promoter activity in vivo showed persistent luciferase expression in the AduPARLuc-injected tumors, in contrast to a significant decrease in luciferase expression 1 week after AdCMVGFPLuc injection. This effect could be related to the susceptible epigenetic inactivation of robust but exogenous promoters such as CMV, whereas such effects may be less relevant when using endogenous promoters [25]. On the other hand, one could hypothesize that tumor environment factors could positively regulate uPAR expression. In line with this, a lower oxygen pressure within the tumor environment has been described to positively regulate uPAR expression in several types of tumor [26]. Furthermore, uPAR has been found to be upregulated in the tumor stroma [27] and in the invasive front of pancreatic tumors [28].
Significant uPAR tumoral activity would be in line with the remarkable antitumor response achieved with the intratumoral administration of the conditionally replicating adenovirus AduPARE1A, which completely eradicates 25% of the BxPC-3 SC-treated tumors and offers a significant increase in survival rates. The significant response achieved might also be accounted for additional mechanisms, such as the fact that the AduPARE1A virus demonstrated a strong in vitro cytocidal effect, similar to that of Adwt. Such similar in vitro potency is highly relevant because a loss of potency has been reported with some oncolytic adenoviruses that use TSP. The introduction of a Kozak sequence in the E1A gene may have facilitated E1A translation. Indeed, it has been proposed that achieving near wild-type levels of E1A could be required to maintain efficient adenoviral replication [29].
One of the major challenges was to demonstrate cancer selectivity of the uPAR promoter for pancreatic cancer and metastasis. The first evidence emerged from the in vitro data, which showed that uPAR activity was significantly higher in pancreatic cancer cells than in normal pancreatic or embryonic lung cells. Notably, the cancer-specific index of the uPAR promoter was significantly higher than that of CMV, as shown by the 2-log increase in the tumor-to-liver ratio. In this respect, biodistribution studies of tumor-free mice confirmed that uPAR promoter activity was very weak in normal tissue. This is not surprising because uPAR expression in the normal tissue of adult mice has been reported to be restricted to the placenta and epididymis [30]. More importantly, our results demonstrate that upon intravascular delivery, uPAR promoter activity is significantly higher than that of the CMV promoter in tumor metastases in the liver, resulting in a significant increase in tumor selectivity. Accordingly, AduPARE1A shows a potent antitumoral effect in metastasis bearing mice, resulting in a significant reduction of tumor burden at day 7, eradication of metastasis in 33% of mice, and a significant increase in survival rates. The specificity of the uPAR promoter was further demonstrated by the systemic toxicity studies of immunocompetent mice. It is worth mentioning that, at the highest therapeutic viral dose used (5 x 1010), whereas a 50% mortality rate was recorded for Adwt, as previously reported [9], all the animals receiving the AduPARE1A virus survived the course of the study, indicating that AduPARE1A presents a wider therapeutic window.
The uPAR promoter could also be potentially suited to targeting other types of cancer, as shown by increased uPAR activity in breast and lung cancer cell lines, compared with normal cells. Along such lines, uPAR has been found to be overexpressed in several types of cancer, such as breast [18], prostate [31], and colon cancer [32]. Moreover, the 0.4-kb uPAR promoter used in this study is positively regulated by the Sp1 transcription factor [17], which has been shown to activate the uPAR promoter in colon cancer [32], suggesting the ubiquitous cancer-specific activity of the uPAR promoter.
Altogether, our results indicate that we have developed a potent IV-administered therapeutic agent, capable of great activity and selectivity for pancreatic tumor tissue, yet with low toxicity in normal tissue. Thus, AduPARE1A is a promising therapeutic agent for the treatment of pancreatic cancer and has the potential to be effective in other types of cancer. Future studies on arming this therapeutic virus with candidate transgenes may lead to further enhancing its antitumoral activity.
Supplemental Data
uPAR Messenger RNA Determination
RNA was extracted from semiconfluent cell cultures, from BxPC-3 xenografts, and from PANC-1 liver metastases using the RNeasy Mini RNA Extraction Kit (Qiagen). One microgram of total RNA was reverse-transcribed using Moloney Murine Leukemia Virus reverse transcriptase (Ambion, Austin, TX) and 1/10 of this reaction was linearly amplified for 23 cycles (cell lines) or 28 cycles (tumor samples) after denaturation (30 seconds at 95°C), annealing (30 seconds at 60°C), and extension (30 seconds at 72°C) in a thermal cycler (GeneAmp PCR System 9700; Applied Biosystems, London, UK). The following primers were used: forward 5′ CAGGACCTCTGCAGGACCAC 3′, reverse 5′ CCTTGCAGCTGTAACACTGG 3′. Total RNA was normalized using QuantumRNA 18S Internal Standards at a 1:4 ratio (Ambion).
Biodistribution Studies
BALB/c nude male mice (Charles River France, Lyon, France) received 2 x 1010 vp of AdCMVGFPLuc or AduPARLuc or saline solution through tail vein injection in a final volume of 200 µl. At days 3, 5, 11, 20, 31 and 50 after adenoviral transduction, luciferase activity was visualized and quantified using an in vivo bioluminescent imaging system (IVIS; Xenogen). At day 50, immediately after imaging, mice were killed and organs were removed and frozen. When stated, frozen tissues were mechanically homogenized, 100 mg was used for protein extraction using the Cell Culture Lysis Reagent (Promega) for 15 minutes at 25°C, and 10 µl was assayed for luciferase activity.
Metastasis Model by Intrasplenic Injection of Tumor Cells
Metastatic pancreatic adenocarcinoma animalmodels were generated following the protocol previously described [1]. Briefly, BALB/c nude mice were anesthetized with isoflurane and placed in the right lateral decubitus. Thereafter, a left subcostal 1-cm incision was made, and 4 x 106 PANC-1 or PANC-1-Luc cells in 50-µl saline solution were injected into the spleen with a 29-G needle. To maintain hemostasis and prevent leakage of tumor cells outside the splenic capsule, a cottontipped applicator was applied to the puncture site. After surgery, mice were monitored daily for health and survival.
Acknowledgments
The authors thank Núria Andreu and Meritxell Carrió for technical assistance. The authors thank Francesco Blasi for providing the plasmid pCAT-Basic-uPAR(C2).
Footnotes
This work was supported by the Spanish Ministry of Education and Science, BIO2005-08682-C03-02/01 and received partial support from the Generalitat de Catalunya SGR0500008 and European Commission Theradpox contract LSHB-CT-2005-018700. M. Huch was supported by a fellowship (BEFI) granted by the Instituto de Salud Carlos III, Spain.
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Rustgi AK. The molecular pathogenesis of pancreatic cancer: clarifying a complex circuitry. Genes Dev. 2006;20:3043–3053. doi: 10.1101/gad.1501106. [DOI] [PubMed] [Google Scholar]
- 2.Saif MW. Pancreatic cancer: are we moving forward yet? Highlights from the Gastrointestinal Cancers Symposium. Orlando, FL, USA. January 20th, 2007. JOP. 2007;8:166–176. [PubMed] [Google Scholar]
- 3.Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, Maples P, Tong AW. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–893. doi: 10.1038/sj.cgt.7701080. [DOI] [PubMed] [Google Scholar]
- 5.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 6.Ohashi M, Kanai F, Tanaka T, Lan KH, Shiratori Y, Komatsu Y, Kawabe T, Yoshida H, Hamada H, Omata M. In vivo adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing pancreatic cancer. Jpn J Cancer Res. 1998;89:457–462. doi: 10.1111/j.1349-7006.1998.tb00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesseling JG, Yamamoto M, Adachi Y, Bosma PJ, van Wijland M, Blackwell JL, Li H, Reynolds PN, Dmitriev I, Vickers SM, et al. Midkine and cyclooxygenase-2 promoters are promising for adenoviral vector gene delivery of pancreatic carcinoma. Cancer Gene Ther. 2001;8:990–996. doi: 10.1038/sj.cgt.7700403. [DOI] [PubMed] [Google Scholar]
- 8.Xie X, Xia W, Li Z, Kuo HP, Liu Y, Ding Q, Zhang S, Spohn B, Yang Y, Wei Y, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Cascallo M, Alonso MM, Rojas JJ, Perez-Gimenez A, Fueyo J, Alemany R. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol Ther. 2007;15:1607–1615. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez PJ, Vickers SM, Ono HA, Davydova J, Takayama K, Thompson TC, Curiel DT, Bland KI, Yamamoto M. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. Am J Surg. 2008;195:481–490. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, Curiel DT. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 12.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 13.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- 14.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Interleukin-1alpha enhances the aggressive behavior of pancreatic cancer cells by regulating the alpha6beta1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–39. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, Bucana CD, Callahan J, Parry GC, Evans DB, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65:7775–7781. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 17.Soravia E, Grebe A, De Luca P, Helin K, Suh TT, Degen JL, Blasi F. A conserved TATA-less proximal promoter drives basal transcription from the urokinase-type plasminogen activator receptor gene. Blood. 1995;86:624–635. [PubMed] [Google Scholar]
- 18.Carrio M, Arderiu G, Myers C, Boudreau NJ. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 2005;65:7177–7185. doi: 10.1158/0008-5472.CAN-04-1717. [DOI] [PubMed] [Google Scholar]
- 19.Bayo-Puxan N, Cascallo M, Gros A, Huch M, Fillat C, Alemany R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J Gen Virol. 2006;87:2487–2495. doi: 10.1099/vir.0.81889-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol. 1998;153:263–269. doi: 10.1016/S0002-9440(10)65567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- 22.Majem M, Cascallo M, Bayo-Puxan N, Mesia R, Germa JR, Alemany R. Control of E1A under an E2F-1 promoter insulated with the myotonic dystrophy locus insulator reduces the toxicity of oncolytic adenovirus Ad-Delta24-RGD. Cancer Gene Ther. 2006;13:696–705. doi: 10.1038/sj.cgt.7700940. [DOI] [PubMed] [Google Scholar]
- 23.Huch M, Abate-Daga D, Roig JM, Gonzalez JR, Fabregat J, Sosnowski B, Mazo A, Fillat C. Targeting the CYP2B1/cyclophosphamide suicide system to fibroblast growth factor receptors results in a potent antitumoral response in pancreatic cancer: models. Hum Gene Ther. 2006;17:1187–1200. doi: 10.1089/hum.2006.17.1187. [DOI] [PubMed] [Google Scholar]
- 24.Heitjan DF, Manni A, Santen RJ. Statistical analysis of in vivo tumor growth experiments. Cancer Res. 1993;53:6042–6050. [PubMed] [Google Scholar]
- 25.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 26.Trisciuoglio D, Iervolino A, Candiloro A, Fibbi G, Fanciulli M, Zangemeister-Wittke U, Zupi G, Del Bufalo D. bcl-2 induction of urokinase plasminogen activator receptor expression in human cancer cells through Sp1 activation: involvement of ERK1/ERK2 activity. J Biol Chem. 2004;279:6737–6745. doi: 10.1074/jbc.M308938200. [DOI] [PubMed] [Google Scholar]
- 27.Giannopoulou I, Mylona E, Kapranou A, Mavrommatis J, Markaki S, Zoumbouli C, Keramopoulos A, Nakopoulou L. The prognostic value of the topographic distribution of uPAR expression in invasive breast carcinomas. Cancer Lett. 2007;246:262–267. doi: 10.1016/j.canlet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Tan X, Egami H, Nozawa F, Abe M, Baba H. Analysis of the invasion-metastasis mechanism in pancreatic cancer: involvement of plasmin(ogen) cascade proteins in the invasion of pancreatic cancer cells. Int J Oncol. 2006;28:369–374. [PubMed] [Google Scholar]
- 29.Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- 30.Wang H, Hicks J, Khanbolooki P, Kim SJ, Yan C, Wang Y, Boyd D. Transgenic mice demonstrate novel promoter regions for tissue-specific expression of the urokinase receptor gene. Am J Pathol. 2003;163:453–464. doi: 10.1016/S0002-9440(10)63675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavrilov D, Kenzior O, Evans M, Calaluce R, Folk WR. Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer. 2001;37:1033–1040. doi: 10.1016/s0959-8049(01)00077-6. [DOI] [PubMed] [Google Scholar]
- 32.Allgayer H, Wang H, Gallick GE, Crabtree A, Mazar A, Jones T, Kraker AJ, Boyd DD. Transcriptional induction of the urokinase receptor gene by a constitutively active Src. Requirement of an upstream motif (-152/-135) bound with Sp1. J Biol Chem. 1999;274:18428–18437. doi: 10.1074/jbc.274.26.18428. [DOI] [PubMed] [Google Scholar]
References
- 1.Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857–865. doi: 10.1067/msy.2002.127680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.