Abstract
Platelet-derived growth factor receptor β (PDGFRβ) is upregulated in most of solid tumors. It is expressed by pericytes/smooth muscle cells, fibroblast, macrophage, and certain tumor cells. Several PDGF receptor-related antagonists are being developed as potential antitumor agents and have demonstrated promising antitumor activity in both preclinical and clinical settings. Here, we produced a fully human neutralizing antibody, IMC-2C5, directed against PDGFRβ from an antibody phage display library. IMC-2C5 binds to both human and mouse PDGFRβ and blocks PDGF-B from binding to the receptor. IMC-2C5 also blocks ligand-stimulated activation of PDGFRβ and downstream signaling molecules in tumor cells. In animal studies, IMC-2C5 significantly delayed the growth of OVCAR-8 and NCI-H460 human tumor xenografts in nude mice but failed to show antitumor activities in OVCAR-5 and Caki-1 xenografts. Our results indicate that the antitumor efficacy of IMC-2C5 is primarily due to its effects on tumor stroma, rather than on tumor cells directly. Combination of IMC-2C5 and DC101, an anti-mouse vascular endothelial growth factor receptor 2 antibody, resulted in significantly enhanced antitumor activity in BxPC-3, NCI-H460, and HCT-116 xenografts, compared with DC101 alone, and the trend of additive effects to DC101 treatment in several other tumor models. ELISA analysis of NCI-H460 tumor homogenates showed that IMC-2C5 attenuated protein level of vascular endothelial growth factor and basic fibroblast growth factor elevated by DC101 treatment. Finally, IMC-2C5 showed a trend of additive effects when combined with DC101/chemotherapy in MIA-PaCa-2 and NCI-H460 models. Taken together, these results lend great support to the use of PDGFRβ antagonists in combination with other antiangiogenic agents in the treatment of a broad range of human cancers.
Introduction
Platelet-derived growth factors (PDGFs), a family of potent mitogens for almost all mesenchymally derived cells, consist of four isoforms, namely, A, B, C, and D [1,2]. These growth factors exert their cellular effects through two structurally related tyrosine kinase receptors: PDGF receptor α (PDGFRα) and PDGF receptor β (PDGFRβ). Two ligands that bind PDGFRβ have been identified including PDGF-B and PDGF-D. Binding of a ligand to the extracellular domain of PDGFRβ results in activation of the intrinsic receptor tyrosine kinase (RTK) activity and subsequent initiation of cytoplasmic signal transduction pathways, in turn, leads to the migration, proliferation, and differentiation of PDGFRβ-expressing cells [1,2]. Platelet-derived growth factor receptor β is expressed on surfaces of connective tissue cells such as fibroblasts and smooth muscle cells (SMCs) and on other cell types. In addition, various studies have showed that PDGF-B and PDGFRβ are also expressed and upregulated in most of solid tumors [3].
Although both autocrine and paracrine PDGF signaling pathways are involved in the development of various cancers, paracrine PDGF signaling is commonly observed in epithelial cancers, especially for PDGF-B/PDGFRβ signaling, where it triggers pericyte/SMC recruitment and leads to maturation of tumor vessels, thereby affecting tumor growth. Early evidences from PDGF-B and PDGFRβ knockout mice revealed the roles of PDGF-B/PDGFRβ signaling pathway in angiogenesis [4,5], an essential process for both tumor growth and metastasis [6]. Through the production of PDGF-B, endothelial cells (ECs) recruit PDGFRβ-expressing pericytes to angiogenic vessels and the process further stimulates vascular SMC development and, therefore, leads to vessel maturation [6,7]. Functional blockade of PDGFRβ, but not PDGFRα, was found to prevent vascular SMC accumulation, induce apoptosis of vascular ECs, and disrupt glomerular capillary formation in neonatal mice [8]. Recent studies indicated that PDGF signaling also regulates the expression of other angiogenic factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), in tumor stroma [9–12].
A number of “spectrum-selective” PDGFRβ kinase inhibitors are currently being developed as potential antitumor agents and have demonstrated promising therapeutic activity in both preclinical and clinical settings, including imatinib mesylate (Gleevec/ST571), sunitinib malate (Sutent/SU11248), and CP-673,451 [13–15]. Most of these PDGFRβ-related inhibitors are multispecific, that is, in addition to their activity on PDGFRβ, they also cross-react to several other kinases, for example, imatinib mesylate to PDGFRα, BCR-ABL, and c-kit, and sunitinib malate to VEGF receptor (VEGFR), c-kit, and FLT3. As a consequence, the contribution of PDGFRβ blockade in tumor growth inhibition could not be clearly defined with these compounds. Previously, we reported identification of an anti-mouse PDGFRβ antibody, 1B3, and evaluation of its efficacy in animal study as monotherapy and its ability to enhance the antitumor and antiangiogenic activities of an antibody to mouse VEGFR2, DC101 [16]. In this study, we described the identification and characterization of a fully human antibody, IMC-2C5, from a naive phage display library. IMC-2C5 binds to both human (hPDGFRβ) and mouse PDGFRβ (mPDGFRβ), thus can be used as an excellent reagent for examining the antitumor efficacy of a specific PDGFRβ antagonist in mouse models of human tumor xenografts because the antibody would block the receptors expressed on both human tumor cells and mouse stromal cells. We studied the antitumor activity of IMC-2C5, used alone or in combination with DC101 and DC101 plus chemotherapy, in several models. We also tested the effects of IMC-2C5 and DC101 on the expression of several tumor-associated growth factors in the treated tumors.Our results indicate that the antitumor efficacy of a specific PDGFRβ antagonist may be primarily due to its effects on tumor stroma rather than on tumor cells directly. Further, an anti-PDGFRβ antibody is most effective when used in combination with other antitumor agents, including antiangiogenic compounds and conventional chemotherapeutics.
Materials and Methods
Reagents and Cell Lines
Recombinant human and mouse PDGFRβ extracellular domain-Fc fusion protein, and their ligand, recombinant PDGF-B, were purchased from R&D Systems, Inc. (Minneapolis, MN). Human tumor cell lines, including OVCAR-5 and OVCAR-8 (ovarian), BxPC-3 and MIA-PaCa-2 (pancreatic), HCT-116 (colon), NCI-H460 and NCI-H292 (non-small cell lung carcinoma, NSCLC), Caki-1 (renal), and Detroit-562 (head and neck squamous cell), and murine cell lines, NIH/3T3 (embryonic fibroblasts) and D122 (Lewis lung carcinoma), were obtained from the American Type Culture Collection (Manassas, VA). All cells were maintained in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) containing 10% fetal calf serum (HyClone, Logan, UT) at 37°C in 5% CO2.
Identification of Anti-hPDGFRβ Antibodies from a Phage Display Library
A human Fab phage display library containing 3.7 x 1010 clones [17] was used to screen for anti-hPDGFRβ antibodies following a previously described procedure [16], using hPDGFRβ/Fc as the bait. Individual phage clones recovered after the second and third rounds of selections were examined for binding to immobilized hPDGFRβ/Fc and for blocking of hPDGFRβ/PDGF-B interaction by ELISA previously described [18,19]. Selected clones were also tested for binding to mPDGFRβ/Fc.
Full-length IgG Antibody Cloning and Expression
The DNA sequences encoding the heavy- and light-chain variable genes for the leading clone, IMC-2C5, were amplified by polymerase chain reaction and cloned into an expression vector containing human κ light-chain constant region and human γ1 heavy-chain constant region. The expression vector was transfected into NS0 myeloma cells, and stable clones of IMC-2C5-expressing cells were selected. The cells were grown in serum-free medium, and the full-length IMC-2C5 IgG was purified from the cell culture supernatant by protein A affinity chromatography (Poros A; Applied Biosystems, Foster City, CA).
Quantitative Receptor Binding and Blocking Assays
In the binding assay, various amounts of IMC-2C5 IgG and 1B3 IgG, an antibody specific to mPDGFRβ, were added to hPDGFRβ or mPDGFRβ-coated plates (50 µl at 1 µg/ml) and incubated at room temperature (RT) for 1 hour, after which the plates were washed three times with phosphate-buffered saline containing 0.1% Tween 20 (PBST). The plates were then incubated at RT for an additional 1 hour with a goat anti-human-κ antibody-horseradish peroxidase (HRP) conjugate (Jackson ImmunoResearch, West Grove, PA). The plates were washed and developed as previously described [16]. In the blocking assay, various amounts of purified antibodies were first mixed with a fixed amount of hPDGFRβ or mPDGFRβ (50 ng at 0.5 µg/ml) and incubated at RT for 30 minutes. The mixture was then transferred to 96-well plates precoated with PDGF-B (0.5 µg/ml) and incubated at RT for 1 hour. After washing three times with PBST, the plates were incubated with a goat anti-human Fc antibody-HRP conjugate (Jackson ImmunoResearch) for 1 hour and developed as described. IC50, the antibody concentration that yielded 50% blockade of PDGFRβ from binding to its ligand, was determined.
Antibody Affinity Determination
The binding kinetics of IMC-2C5 to hPDGFRβ and mPDGFRβ were measured using a BIAcore 3000 biosensor (BIACORE, Inc., Uppsala, Sweden). Briefly, hPDGFRβ or mPDGFRβ were immobilized onto a sensor chip, and IMC-2C5 was injected at concentrations ranging from 1.5 to 100 nM. Sensorgrams were obtained at each concentration and were evaluated using the program, BIA Evaluation 2.0. The affinity constant, Kd, was calculated from the ratio of dissociation rate (koff) to association rate (kon).
Flow Cytometric Analysis of PDGFRβ Surface Expression in Tumor Cells
A panel of human tumor cell lines and two murine cell lines were examined for the expression of PDGFRβ through a flow cytometric analysis, including OVCAR-5, OVCAR-8, Caki-1, NCI-H292, NCI-H460, BxPC-3, MIA-PaCa-2, Detroit-562, HCT-116, NIH/3T3, and D122. Briefly, the cells were aliquoted into wells of a 96-well plate at ∼1 x 106 per well, incubated with IMC-2C5 (10 µg/ml) at 4°C for 1 hour, followed by incubation with an anti-human Fc antibody-fluorescein isothiocyanate conjugate (Jackson ImmunoResearch) for an additional hour at 4°C. After several washes with cold phosphate-buffered saline, the cells were analyzed using a Guava EasyCyte Plus flow cytometer (Guava Technologies, Hayward, CA).
Phosphorylation Assay
Caki-1, NIH/3T3, and D122 cells were plated onto 6-cm dishes and grown to 70% to 80% confluence, after which the cells were washed twice with phosphate-buffered saline and cultured overnight in serum-free medium. The cells were first incubated with various amount of IMC-2C5 at RT for 30 minutes, followed by stimulation with PDGF-B at 37°C for 15 minutes. The cells were then lysed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM Na3VO4, 1 µg/ml leupeptin, 1 µg/ml pepstatin, and 1 µg/ml aprotinin) for 1 hour, followed by centrifugation of the lysate at 12,000 rpm for 10 minutes at 4°C. The receptors were immunoprecipitated from the cell lysate supernatant by an anti-hPDGFRβ or anti-mPDGFRβ antibody (R&D Systems, Inc.), followed by the addition of 20 µl of ProA/G sepharose beads. The precipitated receptor proteins were resolved on a 4% to 12% NuPAGE Bis-Tris gel and transferred to a polyvinylidene difluoride membrane. Phospho-PDGFRβ protein was detected on the blot using an anti-phospho tyrosine antibody-HRP conjugate (Santa Cruz Biotech, Santa Cruz, CA). Total receptor proteins loaded on the gel were assayed with an antibody to PDGFRβ. To study the downstream signaling, the cell lysates from the previously mentioned treatment were resolved by a 4% to 12% NuPAGE Bis-Tris gel. Phosphorylated Akt and p44/42 mitogen-activated protein kinase (MAPK) were detected using an anti-phospho-Akt and an anti-phospho-p44/p42 MAPK antibody, respectively (eBioscience, San Diego, CA).
Human Tumor Xenograft Models
Athymic (nu/nu) mice (female, 7–8 weeks of age) were housed for 7 to 10 days before xenograft implantation. Each mouse was injected subcutaneously with 3 to 10 x 106 cells of various tumor cells. When tumors reached 200 to 400 mm3, mice were randomized into groups of 10 or 12 animals each and treated by intraperitoneal (i.p.) injection of the antibody or antibody combination, twice at 60 mg/kg or three times 40 mg/kg per week, respectively, for a total dose of 120 mg/kg of each antibody per week. Human IgG and/or USP saline was used as controls. Chemotherapy drugs, paclitaxel (20 mg/kg) or gemcitabine (500 mg/kg), were dosed i.p. once a week, which started 1 day before antibody therapy. Tumor sizes and mouse body weight were measured twice a week. Tumor volume was calculated using the formula: π/6 x (length x width2), where length = longest diameter and width = diameter perpendicular to length. A percentage of mean tumor volume of a treatment group versus that of the control group (T/C%) was calculated. Statistic analysis was performed by repeated-measures analysis of variance (ANOVA).
Tumor-Associated Growth Factors in Tumor Homogenates
Athymic nude mice bearing xenografted NCI-H460 tumors of ∼250-mm3 size were randomized and divided into four treatment groups and were treated by i.p. injections three times a week of USP saline, IMC-2C5 (40 mg/kg), DC101 (40 mg/kg), or IMC-2C5 (40 mg/kg) plus DC101 (40 mg/kg). Tumor sizes were measured twice a week. At the days 3, 7, and 14, six animals from each group were killed by CO2 asphyxiation, and their tumors were harvested. Tumors were homogenized with a Kinematica Polytron homogenizer in RIPA Lysis Buffer Kit (Santa Cruz Biotech). After incubation and centrifugation, the supernatants were aliquoted and stored at -80°C. Total protein contents of the homogenates were estimated with the Pierce BCA kit. All enzyme-linked immunosorbent assay (ELISA) analysis was performed by coating capture antibodies (R&D Systems, Inc.) on ELISA plates. After applying the tumor homogenates, captured proteins were detected with biotinylated antibodies (R&D Systems, Inc.) and then streptavidin-HRP conjugate (Pierce). The levels of PDGF-B, VEGF, hypoxia-inducible factor 1α-subunit, and bFGF were estimated from the titrations of standard proteins (R&D Systems, Inc.).
Results
Identification of PDGFRβ Inhibitory Antibody Specific to Both Human and Mouse Receptors from a Fab Phage Display Library
A large naive human Fab phage display library containing 3.7 x 1010 clones from Dyax was used to select antibodies directed against human PDGFRβ. Phage clones from the second and third rounds of affinity selection were randomly picked and tested for hPDGFRβ binding and blocking activities by ELISA. From preliminary screening, IMC-2C5, the clone with the strongest blocking activity was identified and converted into a full IgG and expressed in mammalian cells. Purified IMC-2C5 antibody was then evaluated for its binding activity to hPDGFRβ and mPDGFRβ, and its efficiency in blocking hPDGFRβ and mPDGFRβ from binding to PDGF-B, in comparison with 1B3, an antibody specific to mPDGFRβ. As expected, 1B3 only bound to immobilized mPDGFRβ, but not to hPDGFRβ, whereas IMC-2C5 bound to both human and mouse PDGFRβ (Figure 1, A and B). Binding kinetic analysis by surface plasmon resonance on a BIAcore instrument revealed that the affinities of IMC-2C5 for binding to hPDGFRβ and mPDGFRβ were 0.014 and 0.061 nM, respectively, whereas 1B3 binds to mPDGFRβ with an affinity of 0.09 nM [16]. No cross-reactivity of IMC-2C5 with human and mouse PDGFRα was detected by ELISA (data not shown). In the blocking assay, IMC-2C5 inhibited hPDGFRβ from binding to its ligand PDGF-B with an IC50, the antibody concentration that yielded 50% blocking activity, of 0.55 nM (Figure 1C), whereas it showed an IC50 of 0.35 nM in blocking mPDGFRβ binding to PDGF-B, similar to that of 1B3 (Figure 1D).
Figure 1.
IMC-2C5 binds to human and mouse PDGFRβ and efficiently blocks PDGF-B/PDGFRβ interaction. 1B3, an antibody specific to mouse PDGFRβ, was used as a control. The binding activities of IMC-2C5 to immobilized human and mouse PDGFRβ were assayed by ELISA. (A) Binding to hPDGFRβ. (B) Binding to mPDGFRβ. (C) Blocking PDGF-B binding to hPDGFRβ. (D) Blocking PDGF-B binding to mPDGFRβ. Data represent the mean ± SD of triplicate determinations.
IMC-2C5 Binds to Cell Surface-Expressed PDGFRβ, Inhibits PDGF-B-Stimulated Receptor Phosphorylation and Downstream Signaling in Cell-Based Assay
In a flow cytometric analysis, various human and mouse tumor cell lines were used, including several human cell lines (OVCAR-5, OVCAR-8, Caki-1, NCI-H460, NCI-H292, HCT-116, Detroit-562, MIA-PaCa-2, and BxPC-3) and two murine cell lines (NIH/3T3 and D122). The results showed that Caki-1, OVCAR-8, HCT-116, NIH/3T3, and D122 were strongly stained by IMC-2C5, whereas NCI-H460 and Detroit-562 were weakly stained, and the other cell lines did not yield any staining with IMC-2C5 (Table 1).
Table 1.
Summary of Anti-PDGFRβ Antibodies in Monotherapy.
| Xenograft | Tumor Volume - 1B3 | Tumor Volume - 2C5 | PDGFRβ Expression | ||
| T/C% | P | T/C% | P | MFI* | |
| OVCAR-8 | 43 | .0022 | 39 | .0014 | 5 |
| NCI-H460 | 60 | .0235 | 60 | .0115 | 2 |
| OVCAR-5 | 92 | .7 | 84 | .06 | 1 |
| Caki-1 | 78 | .1004 | 91 | .6303 | 10 |
| BxPC-3 | ND† | ND | 116 | .4377 | 1 |
| MIA-PaCa-2 | ND | ND | 70 | .0554 | 1 |
| HCT-116 | ND | ND | 96 | .5799 | 6 |
| Detroit-562 | ND | ND | 75 | .2405 | 3 |
| NCI-H292 | ND | ND | 75 | .3581 | 1 |
Mean fluorescence intensity (MFI) using IMC-2C5 as the detection agent as determined by FACS analysis. A value of 1 indicates no apparent expression detected when compared with the control sample that was stained by a normal human IgG.
Not done.
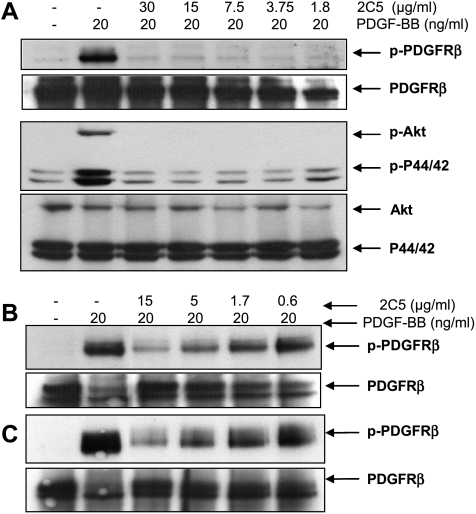
In the receptor phosphorylation assay, IMC-2C5 inhibited PDGFB-stimulated receptor phosphorylation in Caki-1, NIH/3T3 and D122 cells in a dose-dependent manner. At a concentration of 1.8 µg/ml (12 nM), IMC-2C5 antibody completely inhibited PDGF-B-stimulated receptor phosphorylation in Caki-1 cells (Figure 2A). In the two murine cell lines, NIH/3T3 and D122, IMC-2C5 inhibited PDGFRβ phosphorylation with an IC50 of ∼1.7 µg/ml (11 nM; Figure 2, B and C). The antibodies also potently inhibited PDGF-B-stimulated phosphorylation of both Akt and p44/42 MAP kinase in Caki-1 cells (Figure 2A). In the Boyden chamber migration assay, IMC-2C5 significantly inhibited PDGF-B (at 30 ng/ml)-stimulated cell migration of PDGFRβ-expressing NCI-H460, OVCAR-8, WS-1, and U-118MG cells (data not shown).
Figure 2.
IMC-2C5 antibody inhibits PDGF-B-stimulated receptor phosphorylation and downstream signaling. Caki-1 (human), NIH/3T3, and D122 (mouse) cells were treated as described in the Materials and Methods section. The receptors were immunoprecipitated from the cell lysate supernatant by anti-PDGFRβ antibodies and were resolved by SDS-PAGE. The amount of phosphorylated PDGFRβ (p-PDGFRβ) was assessed through immunoblot analysis using an anti-phospho-tyrosine antibody-HRP conjugate. The amounts of total PDGFRβ proteins loaded in each lane were confirmed by detecting with antibodies to PDGFRβ. To analyze the effect of IMC-2C5 on downstream signaling, the cell lysates were resolved by SDS-PAGE. Phosphorylated Akt and p44/42 MAP kinase, as well as total loadings of Akt and p44/42 MAP kinase, were then detected using Western blot analysis. (A) Caki-1. (B) NIH/3T3. (C) D122.
Antitumor Activity of IMC-2C5 as a Monotherapy Agent in Tumor Xenograft Models
Four human tumor xenograft models, including two ovarian carcinomas (OVCAR-5 and OVCAR-8), an NSCLC (NCI-H460), and a renal carcinoma (Caki-1), were used to assess the antitumor activity of IMC-2C5, in comparison with 1B3 antibody. IMC-2C5 demonstrated significant antitumor activity in both OVCAR-8 and NCI-H460 xenograft models: it almost completely blocked the growth of OVCAR-8 tumors by the end of treatment with a T/C of 39% (P = .0014; Figure 3A) and significantly slowed the growth of NCI-H460 tumors with a T/C of 60% (P = .0115; Figure 3B). Conversely, IMC-2C5 failed to show significant antitumor activity in OVCAR-5 xenografts (T/C 84%, P = .06) and Caki-1 xenografts (T/C 91%, P = .6303; Figure 3, C and D). Although IMC-2C5 is capable of blocking PDGFRβ expressed by both tumor and host stroma cells, treatment of IMC-2C5 did not yield better inhibitory effect in tumor growth comparing with 1B3 (Table 1). In addition, antitumor effects of both 1B3 and IMC-2C5 did not seem to correlate directly with the expression level of PDGFRβ by tumor cell lines (Table 1). IMC-2C5 treatment did not cause any overt toxicity, including changes in body weight and animal behavior, in all four models examined.
Figure 3.
IMC-2C5 antibody inhibits tumor growth as a monotherapy agent in xenograft models. nu/nu mice (female, 7–8 weeks) were injected subcutaneously with ∼5 x 106 tumor cells: OVCAR-8 (A), NCI-H460 (B), OVCAR-5 (C), or Caki-1 (D). When tumors reached 250 to 300 mm3, mice were randomized into two treatment groups (n = 10 or 12) following a treatment schedule: antibodies at 60 mg/kg twice weekly (A, C, and D) or three times a week at 40 mg/kg (B) by i.p. injection. Human IgG and USP saline were used as controls. Tumor volumes of mice were recorded twice a week. Results shown are the mean ± SEM.
Enhancement of Antitumor Activity of an Anti-VEGFR2 Antibody, DC101, by IMC-2C5 in Tumor Xenograft Models
IMC-2C5 was evaluated for its ability to enhance the antitumor activity of an anti-mouse VEGFR2 antibody, DC101, in several tumor xenograft models, including BxPC-3, NCI-H460, HCT-116, MIA-PaCa-2, NCI-H292, and Detroit-562. As previously described [20], treatment with DC101 significantly inhibited the growth of all tumor xenografts (Figure 4, A–F). As a monotherapy agent, however, IMC-2C5 showed no effect or very limited effect in the inhibition of tumor growth in five of six tumor models except in NCI-H460 xenografts (T/C 60%, P < .05). However, coadministration of IMC-2C5 and DC101 resulted in a greater inhibition of tumor growth than either of the antibodies alone in all six tumor xenograft models, although only three of them reached statistical significance.
Figure 4.
IMC-2C5 enhances the antitumor activity of an anti-VEGFR2 antibody, DC101. Athymic nude mice bearing xenografted tumors of 200 to 300 mm3 size were randomized and divided into four treatment groups (n = 12 per group) and were treated by i.p. injections three times a week with USP saline, IMC-2C5 (40 mg/kg), DC101 (40 mg/kg), or IMC-2C5 plus DC101 (40 mg/kg each). (A) BxPC-3. (B) NCI-H460. (C) MIA-PaCa-2. (D) NCI-H292. (E) Detroit-562. (F) HCT-116. Results shown are the mean ± SEM.
In the BxPC-3 xenografts (Figure 4A), combination of IMC-2C5 and DC101 led to significantly enhanced tumor inhibition on day 31: T/C was 21% in mice that received antibody combination (P = .0001) compared with that of 47% in mice treated with DC101 alone (P = .0027). Treatment of USP saline and IMC-2C5 groups was terminated at day 31, and treatments of DC101 and IMC- 2C5 plus DC101 groups were continued up to day 56. Repeated-measures ANOVA through day 52 showed that the efficacy of the combination group was significantly higher than that of the DC101 alone (P < .0001). In addition, three partial tumor regressions were observed in the combination group versus only one regression with DC101 monotherapy at the end of treatment. In the NCI-H460 xenografts (Figure 4B), the T/C of IMC-2C5/DC101 treatment at day 15 was 22% (P < .0001), whereas it was 32% for DC101 monotherapy (P = .0003). Repeated-measures ANOVA through day 21 showed that the efficacy of the combination therapy was significantly higher than DC101 monotherapy (P < .0001). In the HCT-116 xenografts (Figure 4F), the T/C of the combination group at the end of study (day 29) was 36% (P = .0001) compared with that of 44% in mice treated with DC101 alone (P = .0001). Statistical analysis showed that the efficacy of the antibody combination was significantly higher than that of DC101 alone (P = .0010). Greater inhibitions of tumor growth by the combination therapy over DC101 monotherapy were also observed in MIA-PaCa-2, NCI-H292, and Detroit-562 xenografts (Figure 4, C–E), although the differences between two groups at the end of the study failed to reach statistical significance, with P values of .4164 on day 46 for MIA-PaCa-2 tumors, .3832 on day 36 for NCI-H292 tumors, and .1146 on day 74 for Detroit-562 tumors.
Effects in Expression of Tumor-Associated Growth Factors by IMC-2C5 in NCI-H460 Tumor Xenografts
To evaluate the effects of antibody treatment on the expression of tumor-associated growth factors during tumor development, six tumor xenografts of NCI-H460 from each treatment group were harvested after 3, 7, and 14 days of treatment and were homogenized. Tumor sizes on day 12 were 1442 ± 171, 1492 ± 274, 749 ± 119, and 631 ± 85 mm3 for saline, IMC-2C5, DC101, and DC101/IMC-2C5, respectively. The difference of tumor growth inhibition between DC101 and IMC-2C5/DC101 was not significant. Tumor lysates were then tested for the total protein contents and the levels of PDGF-B, VEGF, HIF-1α, and bFGF (Figure 5, A–D). The results showed that IMC-2C5 treatment elevated the protein levels of PDGF-B compared with the saline control on days 7 and 14 (Figure 5A). In addition, IMC-2C5 plus DC101 group had a significantly higher level of PDGF-B compared with DC101 alone on day 14. As previously observed, DC101 treatments, either alone or in combination, elevated the expression of VEGF at all time points (Figure 5B). However, on day 14, the antibody combination-treated tumors had a much-reduced level of VEGF compared with DC101 alone treatment. The contents of proteins that are not directly related to the antibodies used in the study, including HIF-1α and bFGF, were also evaluated. The HIF-1α levels were significantly higher in DC101 treatment groups on days 7 and 14 compared with that in the saline control (Figure 5C). DC101 alone group also showed a significantly higher level of bFGF on day 7 compared with the saline and the combination groups (Figure 5D).
Figure 5.
IMC-2C5 affects the levels of expression of several tumor-associated growth factors in the antibody treated tumors. NCI-H460 cell suspension (5 x 106 cells per mouse), was subcutaneously injected into female athymic mice. When tumors reached ∼250 mm3, mice were randomized and divided into four treatment groups and were treated by i.p. injection three times a week with USP saline, IMC-2C5 (40 mg/kg), DC101 (40 mg/kg), or IMC-2C5 plus DC101 (40 mg/kg each). Six tumors from each treatment group were harvested at days 3, 7, and 14 and were homogenized. ELISA was performed to estimate the levels of PDGF-B (A), VEGF (B), HIF-1α (C), and bFGF (D) in each treated tumors. Results shown are the mean ± SEM. *P < .05 of treatment group versus saline at the same time point, unless indicated otherwise.
Additive Effect of IMC-2C5 on Antitumor Activity of DC101 Plus Chemotherapy in Tumor Xenograft Models
IMC-2C5 was further evaluated for its ability to enhance the antitumor activities of DC101/chemotherapy combination in two tumor models, including NCI-H460 and MIA-PaCa-2 xenografts. In the triple combination studies, larger tumors, at sizes of 350 to 400 mm3, were used to reduce the tumor sensitivity to DC101/chemotherapy. In the NCI-H460 tumor model, DC101/paclitaxel and DC101/paclitaxel plus IMC-2C5 treatments both significantly inhibited the tumor growth with P values of .0038 and .0018 on day 19, respectively (Figure 6A). The control group was terminated on day 19, and treatments of DC101/paclitaxel and DC101/paclitaxel plus IMC-2C5 were continued up to day 43. A separation of the tumor growth between the two groups was observed with smaller tumor sizes for DC101/paclitaxel plus IMC-2C5 group, although repeated-measures ANOVA through day 43 showed that the improvement in tumor inhibition of the triple combination was not significant compared with that of the DC101/paclitaxel treatment (P = .2693).
Figure 6.
IMC-2C5 has an additive effect to DC101/chemotherapy in xenograft models. Athymic nude mice bearing xenografted tumors of 350 to 400 mm3 size were randomized and divided into four treatment groups (n = 12 per group) and were treated by i.p. injections three times a week with USP saline, DC101 (40 mg/kg), or IMC-2C5 plus DC101 (40 mg/kg each). Chemotherapy drugs were dosed i.p. once a week, which started 1 day before the antibody treatment as indicated. (A) NCI-H460 and paclitaxel at 20 mg/kg. (B) MIA-PaCa-2 and gemcitabine at 500 mg/kg. Results shown are the mean ± SEM.
In the MIA-PaCa-2 model, the analysis on day 37 showed that DC101/gemcitabine treatment, with T/C of 44%, was not statistically significant (P = .0769) compared with the saline control group. DC101/gemcitabine plus IMC-2C5, however, yielded a T/C of 34%, which reached statistical significance (P = .0270; Figure 6B). The control group was terminated on day 37, and treatments of DC101/gemcitabine and DC101/gemcitabine/IMC-2C5 were continued up to day 49. At the end of the study, the average tumor volume in DC101/gemcitabine/IMC-2C5 group was only approximately 50% of the size of that in the DC101/gemcitabine group. Repeated-measures ANOVA through day 43, however, showed that the difference between the two groups was not statistically significant (P = .2399).
Discussion
Recent studies have shown the complex interplay between various tumor-associated growth factor/receptor pathways in promoting tumor angiogenesis, growth, and metastasis [9,11]. As a consequence, the field of targeted cancer therapy is being rapidly expanded from simply targeting cancer cells directly to also targeting their supporting cells in tumor microenvironment, such as EC, pericytes/vascular SMC, and stromal fibroblasts [21]. Early antiangiogenic therapy in cancer drug development focused mainly on ECs, such as a blockade of VEGFR/VEGF signaling. For example, a variety of animal studies have shown that treatment with an anti-VEGF antibody, bevacizumab, or an anti-mouse VEGFR2 antibody, DC101, was efficacious in antitumor and antiangiogenesis in xenograft models in mice, including a significant life span extension [22–24]. Extended treatment with these antibodies, however, has been shown to result in a phenotypic resistance to VEGF/VEGFR inhibition owing to the up-regulation of other proangiogenic growth factors including HIF-1α, PDGF, and bFGF [22,25–27]. The resistance of tumor blood vessels to VEGF/VEGFR inhibition was also observed in tumors treated with the tyrosine kinase inhibitors such as SU5416 [28]. To this end, a number of studies have revealed that a blockade of PDGF/PDGFR signaling with multi-tyrosine kinase inhibitor such as imatinib led to downregulate bFGF expressed by stromal fibroblasts [10] and abrogate VEGF production in gastrointestinal stromal tumors [29]. Taken together, these findings demonstrate the complexity of multiple processes in tumor microenvironment during the tumor development and the complex survival mechanisms of tumors to treatment.
Accumulating evidence suggests that simultaneously targeting cancer as well as their supporting (co-opted) cells may lead to enhanced anticancer efficacy [21,28,30,31]. Among various strategies to target tumor co-opted cells, agents direct against molecules (receptors) expressed on pericyte/vascular SMC and stromal fibroblasts, such as PDGFRβ, are being actively pursued [32–34]. Platelet-derived growth factor receptor β has been shown primarily responsible for pericyte recruitment to capillaries, development of SMCs in vessels and of mesangial cells in the kidney [4,35]. Under pathological conditions, the PDGF-B/PDGFRβ signaling pathway has been implicated in several tumor-associated processes including stimulation and regulation of tumor angiogenesis and recruitment and regulation of tumor fibroblasts (for details, see review by Ostman [3]). Recently, Rolny et al. [36] showed that PDGFRβ is expressed on CD31+CD41+ VEGFR2+ hematopoietic/endothelial precursors, or hemangioprecursors. Activation of PDGFRβ on these hemangioprecursors accelerates differentiation of ECs and vascular remodeling. In another report, Song et al. [37] identified a subset of PDGFRβ+/sca1+ progenitor perivascular cells that could be recruited from bone marrow to perivascular sites in tumors. Through paracrine mechanism involving growth factors, such as PDGF-B, produced by ECs, such precursors could differentiate into mature pericytes and regulate vessel stability and survival in tumors. Specific inhibition of PDGFRβ signaling with a neutralizing antibody to the receptor eliminates PDGFRβ+ progenitor perivascular cells and mature pericytes around tumor vessels, leading to increased EC apoptosis [8,37,38]. Taken together, these findings suggest that PDGF-B/PDGFRβ pathway may represent an excellent target for cancer therapy through both direct antitumor and antiangiogenesis mechanisms. To this end, a number of selective PDGFRβ kinase inhibitors, including imatinib mesylate and sunitinib malate [13,14,39], have demonstrated promising therapeutic activity in both preclinical and clinical settings. However, because these small molecule antagonists are not truly (or restrictively) specific to the receptor, it is not possible to dissect out the specific contribution to the overall antitumor activity as well as the potential toxicities that may be associated with PDGFRβ inhibition.
Here, we generated a high-affinity fully human neutralizing antibody that binds to both human and mouse PDGFRβ and efficiently neutralizes PDGF-B-induced receptor activation and downstream signaling (Figures 1 and 2). This unique species cross-reactive feature of the antibody enabled us to investigate the antitumor activity of a specific PDGFRβ blockade approach in mouse models of human tumor xenografts because the antibody would inhibit the receptor activity on both human tumor cells and host stromal cells, a situation similar to that in the treatment of patients in clinical practice. We previously reported the generation of an anti-mPDGFRβ antibody, 1B3, and demonstrated that 1B3 modestly inhibited the growth of a certain type of human tumor xenografts [16]. Here, we compared the antitumor effect of targeting host PDGFRβ alone (by 1B3) with that of targeting PDGFRβ on both host and human tumor cells in four human xenograft models. To our surprise, the IMC-2C5 was not more efficacious than 1B3, in all the four models tested, including those tumors with a high-level expression of human PDGFRβ (Figure 3). Further, there seems no direct correlation between the level of PDGFRβ expression on tumor cells and the antitumor activity of IMC-2C5 (Table 1). Conversely, it was unclear if PDGFRβ expressed by the tumor cells was activated in the xenografts. Kim et al. [40] found that PDGFR activation occurs only on PC-3MM2-MDR cells growing in the bone but not on tumor cells growing in the muscles, indicating that PDGFR activation depends on the cross talk between tumor cells and host microenvironment. It is pertinent to note that, whereas PDGF-B strongly activates PDGFRβ and its downstream signaling pathways (including MAPK and Akt) and stimulates migration of PDGFRβ-expressing cells, it did not manifest any significant mitogenic activity in vitro in promoting growth and proliferation of the same types of tumor cells (our unpublished data). In the animal study with PC-3MM2-MDR model, imatinib treatment increased apoptosis of tumor-associated ECs but not tumor cells, although inhibited phosphorylation of PDGFR was obtained on both cell types, indicating a complex downstream signaling of PDGFRβ [40]. Taken together, these observations suggest that the antitumor activity of the anti-PDGFRβ antibodies is perhaps primarily due to their effects on PDGFRβ-expressing cells in tumor stroma rather than on tumor cells directly.
As a single agent, PDGFR-selective RTK inhibitor (such as imatinib) has not yet been proven efficacious in PDGFR-expressing solid tumors in several phase 2 clinical trails [41]. However, combined inhibition of both VEGF and PDGF signaling pathways with so-called “spectrum-selective” RTK inhibitors, such as sunitinib malate, has demonstrated good antitumor efficacy in animal models and in patients by causing tumor vessel regression through inducing EC apoptosis [28]. In a previous study, we also demonstrated that 1B3 antibody significantly enhanced the antitumor and the antiangiogenic activity of an anti-VEGFR2 antibody, DC101, in xenograft models [16]. In the current study, we evaluated the ability of IMC-2C5 antibody in potentiating the antitumor activity of DC101 in various human tumor xenografts. Consistent with our observation with 1B3, in three of six xenograft models tested, the IMC-2C5/DC101 combination was significantly more efficacious than DC101 alone (given at the optimum dose), whereas IMC-2C5 by itself only had a modest antitumor effect (NCI-H460 model) or no effect at all (BxPC-3 and HCT-116 models). The combination of IMC-2C5 and DC101 also led to a greater tumor inhibition in three other models tested, albeit the difference did not reach statistical significance when compared with DC101 alone. Similarly, addition of IMC-2C5 to DC101/chemotherapy combinations also demonstrated an additional antitumor activity.
Because pericyte recruitment to coat nascent vessels during angiogenesis is essential for the stabilization and further establishment of the vascular network, vessels lacking adequate pericyte coverage are more vulnerable to VEGF inhibition [4,22]. In a previous study, we showed that 1B3 treatment alone significantly reduced matured vessel density (CD31+/αSMA+) compared with the control group, whereas the total CD31+ vessels of the 1B3 group were not affected, indicating that PDGFRβ+ pericytes were selectively inhibited [16]. Furthermore, addition of 1B3 to DC101 treatment led to a significantly more drastic reduction of vessel density compared with DC101 alone. This was in consistency with an earlier report showing that combination treatment with imatinib and metronomic chemotherapy significantly reduced pericyte coverage on tumor vessels [23], leading to decreased percentage of pericyte-covered mature vessels. In this study, we demonstrated that the PDGFRβ inhibition not only decreased PDGFRβ expression (data not shown) but also attenuated the expression of VEGF and bFGF elevated by VEGFR2 inhibition. Several different effects on tumor stroma by a PDGFRβ antagonist, such as imatinib, have also been demonstrated. Imatinib relieved interstitial hypertension and increased tumor uptake of chemotherapy, which led to enhance the antitumor effects of chemotherapy in KAT-4 and PROb tumors [42]. In an NSCLC, A549 xenograft, imatinib treatment resulted in a significant reduction of p-PDGFRβ and VEGF expression, which in turn reduced tumor vessel density, interstitial fluid pressure, and improved tumor oxygenation [43]. Recently, Kim et al. [40] showed that imatinib sensitized PDGFRβ-expressing tumor-associated ECs to chemotherapy in a chemoresistant tumor model, PC-3MM2-MDR, and led to apoptosis of the ECs in bone lesions. In addition, multiple abnormalities in various tumor tissues have been reported, including the relationship between ECs and pericytes, endothelial sprouting, and altered expression of marker proteins, possibly depending on different contexts of tumors [6,44]. Taken together, these observations indicate the complex mechanisms of targeting PDGFRβ in cancer treatment.
In summary, here we produced a fully human antagonistic anti-PDGFRβ antibody, IMC-2C5, and demonstrated that the antibody could significantly enhance the antitumor activity of DC101, an antibody directed against VEGFR2, and further inhibit tumor growth when in combination with DC101 plus chemotherapy in various tumor xenograft models. In addition, we demonstrated that adding IMC-2C5 led to the attenuation of the expression of several tumor-associated growth factors elevated by DC101 before a significant inhibition of the combination therapy was achieved compared with DC101 treatment alone. A number of previous studies have shown that PDGFRβ inhibition could also provide additive/synergistic antitumor activity to a number of chemotherapeutic agents [13,42,45] as well as selective kinase inhibitors including those to VEGFR2 kinase [13,28,30]. The enhanced antitumor effect usually correlates well with the increase in tumor uptake of the concurrently administered cytotoxic agents, a benefit that likely resulted from the reduction of interstitial fluid pressure in tumor after treatment with PDGFRβ antagonist [42,46]. Further, coinhibition of PDGFRβ activity has been shown to increase antitumor efficacy of radiotherapy [47,48]. Taken together, all these findings should lend great support to use PDGFRβ antagonists in combination with other antitumor and/or antiangiogenic agents in the treatment of a broad range of human cancers [49].
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- bFGF
basic fibroblast growth factor
- PDGF
platelet-derived growth factor
- PDGFRβ
PDGF receptor β
- RT
room temperature
- RTK
receptor tyrosine kinase
- SMC
smooth muscle cell
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
References
- 1.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 3.Ostman A. PDGF receptors—mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15:275–286. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl P, Hellstrom M, Kalen M, Betsholtz C. Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice. Curr Opin Lipidol. 1998;9:407–411. doi: 10.1097/00041433-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103:2955–2960. doi: 10.1161/01.cir.103.24.2955. [DOI] [PubMed] [Google Scholar]
- 9.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokuda H, Takai S, Hanai Y, Harada A, Matsushima-Nishiwaki R, Kato H, Ogura S, Kozawa O. Potentiation by platelet-derived growth factor-BB of FGF-2-stimulated VEGF release in osteoblasts. J Bone Miner Metab. 2008;26:335–341. doi: 10.1007/s00774-007-0829-x. [DOI] [PubMed] [Google Scholar]
- 12.Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14:285–294. doi: 10.1177/107327480701400312. [DOI] [PubMed] [Google Scholar]
- 13.Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, Cherrington JM, Pryer NK. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 14.Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, Rahman A, Chen G, Staten A, Griebel D, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034–3038. [PubMed] [Google Scholar]
- 15.Roberts WG, Whalen PM, Soderstrom E, Moraski G, Lyssikatos JP, Wang HF, Cooper B, Baker DA, Savage D, Dalvie D, et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res. 2005;65:957–966. [PubMed] [Google Scholar]
- 16.Shen J, Vil MD, Zhang H, Tonra JR, Rong LL, Damoci C, Prewett M, Deevi DS, Kearney J, Surguladze D, et al. An antibody directed against PDGF receptor beta enhances the antitumor and the anti-angiogenic activities of an anti-VEGF receptor 2 antibody. Biochem Biophys Res Commun. 2007;357:1142–1147. doi: 10.1016/j.bbrc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 17.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruine AP, Arends JW, Hoogenboom HR. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, Jimenez X, Zhang H, Bohlen P, Witte L, Zhu Z. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393–399. doi: 10.1002/ijc.1634. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Rockwell P, Lu D, Kotanides H, Pytowski B, Hicklin DJ, Bohlen P, Witte L. Inhibition of vascular endothelial growth factor-induced receptor activation with anti-kinase insert domain-containing receptor single-chain antibodies from a phage display library. Cancer Res. 1998;58:3209–3214. [PubMed] [Google Scholar]
- 20.Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 21.Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, Hicklin DJ. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006;12:2197–2207. doi: 10.1158/1078-0432.CCR-05-1682. [DOI] [PubMed] [Google Scholar]
- 22.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Miller DW, Vosseler S, Mirancea N, Hicklin DJ, Bohlen P, Volcker HE, Holz FG, Fusenig NE. Rapid vessel regression, protease inhibition, and stromal normalization upon short-term vascular endothelial growth factor receptor 2 inhibition in skin carcinoma heterotransplants. Am J Pathol. 2005;167:1389–1403. doi: 10.1016/S0002-9440(10)61226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 25.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 26.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco M, Man S, Chen L, Emmenegger U, Shaked Y, Cheung AM, Brown AS, Hicklin DJ, Foster FS, Kerbel RS. Targeted anti-vascular endothelial growth factor receptor-2 therapy leads to short-term and long-term impairment of vascular function and increase in tumor hypoxia. Cancer Res. 2006;66:3639–3648. doi: 10.1158/0008-5472.CAN-05-3295. [DOI] [PubMed] [Google Scholar]
- 28.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 29.McAuliffe JC, Lazar AJ, Yang D, Steinert DM, Qiao W, Thall PF, Raymond AK, Benjamin RS, Trent JC. Association of intratumoral vascular endothelial growth factor expression and clinical outcome for patients with gastrointestinal stromal tumors treated with imatinib mesylate. Clin Cancer Res. 2007;13:6727–6734. doi: 10.1158/1078-0432.CCR-07-0895. [DOI] [PubMed] [Google Scholar]
- 30.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Lee FY, Bhalla KN, Wu J. Potent inhibition of platelet-derived growth factor-induced responses in vascular smooth muscle cells by BMS-354825 (dasatinib) Mol Pharmacol. 2006;69:1527–1533. doi: 10.1124/mol.105.020172. [DOI] [PubMed] [Google Scholar]
- 33.George DJ. Receptor tyrosine kinases as rational targets for prostate cancer treatment: platelet-derived growth factor receptor and imatinib mesylate. Urology. 2002;60:115–121. doi: 10.1016/s0090-4295(02)01589-3. discussion 122. [DOI] [PubMed] [Google Scholar]
- 34.Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR, Blake RA, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 35.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 36.Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, Lindblom P, Norlin J, Betsholtz C, Heuchel R, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108:1877–1886. doi: 10.1182/blood-2006-04-014894. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano H, Ueda Y, Takakura N, Takemura G, Doi T, Kataoka H, Murayama T, Xu Y, Sudo T, Nishikawa S, et al. Blockade of platelet-derived growth factor receptor-beta pathway induces apoptosis of vascular endothelial cells and disrupts glomerular capillary formation in neonatal mice. Am J Pathol. 2002;161:135–143. doi: 10.1016/s0002-9440(10)64165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ, Uehara H, Yazici S, Busby JE, Nakamura T, He J, Maya M, Logothetis C, Mathew P, Wang X, et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
- 41.Jain RK, Lahdenranta J, Fukumura D. Targeting PDGF signaling in carcinoma-associated fibroblasts controls cervical cancer in mouse model. PLoS Med. 2008;5:e24. doi: 10.1371/journal.pmed.0050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- 43.Vlahovic G, Rabbani ZN, Herndon JE, II, Dewhirst MW, Vujaskovic Z. Treatment with imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer. 2006;95:1013–1019. doi: 10.1038/sj.bjc.6603366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 46.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 47.Baranowska-Kortylewicz J, Abe M, Pietras K, Kortylewicz ZP, Kurizaki T, Nearman J, Paulsson J, Mosley RL, Enke CA, Ostman A. Effect of platelet-derived growth factor receptor-beta inhibition with STI571 on radioimmunotherapy. Cancer Res. 2005;65:7824–7831. doi: 10.1158/0008-5472.CAN-04-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell JS, Brady K, Burgan WE, Cerra MA, Oswald KA, Camphausen K, Tofilon PJ. Gleevec-mediated inhibition of Rad51 expression and enhancement of tumor cell radiosensitivity. Cancer Res. 2003;63:7377–7383. [PubMed] [Google Scholar]
- 49.Zhu Z. PDGFRβ: a multifaceted player in vascular and hemotopoietic development. Blood. 2006;108:788–789. [Google Scholar]