Abstract
Background: The relationship between serum cholesterol and cancer incidence remains controversial.
Patients and methods: We investigated the association of total serum cholesterol (TSC) with subsequent cancer incidence in a population-based cohort of 172 210 Austrian adults prospectively followed up for a median of 13.0 years. Cox regression, allowing for time-dependent effects, was used to estimate adjusted hazard ratios (HRs) with 95% confidence intervals (95% CIs) for the association of TSC with cancer.
Results: We observed pronounced short-term associations of TSC and overall cancer incidence in both men and women. For malignancies diagnosed shortly (<5 months) after baseline TSC measurement, the highest TSC tertile (>235.0 mg/dl in men and >229.0 in women) compared with the lowest tertile (<194.0 mg/dl in men and <190.0 in women) was associated with a significantly lower overall cancer risk [HR = 0.58 (95% CI 0.43–0.78, Ptrend = 0.0001) in men, HR = 0.69 (95% CI 0.49–0.99, Ptrend = 0.03) in women]. However, after roughly 5 months from baseline measurement, overall cancer risk was not significantly associated with TSC. The short-term inverse association of TSC with cancer was mainly driven by malignancies of the digestive organs and lymphoid and hematopoietic tissue.
Conclusion: The short-term decrease of cancer risk seen for high levels of TSC may largely capture preclinical effects of cancer on TSC.
Keywords: cancer incidence, prospective study, reverse causality, time dependency, total serum cholesterol
introduction
High levels of total serum cholesterol (TSC) are a well-established risk factor for coronary heart disease [1]. The impact of high TSC on cancer incidence is, however, less well understood. Several studies have reported that cancer incidence [2–10] and cancer mortality [7, 8, 11–18] were lower in men with higher baseline levels of TSC. While this inverse association was seen in the majority of previous studies, others found higher cancer risk for those with high TSC concentrations [19], no relation at all [20–28], or a U-shaped association, with both low as well as high TSC levels being significantly related to increased cancer risk [29]. In addition, in women, most previous investigations failed to detect any association of TSC with cancer incidence [22, 23, 30, 31] and/or cancer mortality [22, 25].
Differences in the study populations, length of follow-up, study end points and statistical adjustment for confounding may all have contributed to the conflicting patterns of association seen in earlier studies; consistent evidence may also be lacking due to small sample sizes and infrequent events in several previous investigations. Moreover, it was speculated that rather than reflecting a true causal relationship, the lower cancer risk seen for high TSC levels may be attributable to an effect of preclinical cancer, i.e. the metabolic depression of TSC due to undiagnosed malignant lesions [6, 32]. This hypothesis has been supported by the observation that the inverse association between higher TSC levels and cancer risk attenuated or even disappeared with increasing time lags between TSC measurement and cancer diagnoses [11, 14, 15, 33, 34]. However, the inverse association of high baseline TSC levels with cancer risk was also seen for up to 4 or even more years after baseline measurement in some investigations [6, 8, 17, 30, 35].
Motivated by the inconsistencies in the epidemiological literature, we investigated the association of TSC with overall and site-specific cancer incidence in a prospective, population-based cohort of 172 210 Austrian adults, aged 18–99 years with 9958 incident cancers during 19 years of follow-up. To our knowledge, this is the largest population-based study to date to prospectively explore the association of TSC with overall and site-specific cancer incidence in apparently healthy men and women across a wide age range.
patients and methods
study population
The Vorarlberg Health Monitoring and Promotion Program (VHM&PP) [36–39] is one of the world's largest ongoing population-based risk factor surveillance programs. The cohort was initiated in 1985 and is conducted by the Agency for Social and Preventive Medicine in Vorarlberg, the westernmost province of Austria. All adults in the region are invited to participate by a combination of different measures including written invitations and television, radio and newspaper reports. Active follow-up of study participants is carried out through a recall system of written biennial reinvitation letters. Sociodemographic data are recorded, and a voluntary physical examination is conducted regularly in a standardized manner by trained local physicians and internists. During the exam, a fasting blood sample is taken. Costs are covered by the participant's (compulsory) health insurance. A more detailed description of the program methodology has been reported elsewhere [36].
Between 1985 and 2003, a total of 174 852 Vorarlberg residents (aged >18 years) were enrolled in the VHM&PP. After excluding 2642 (1.5%) participants with either missing measurements on TSC at enrollment, malignant cancer before or at enrollment and/or baseline concentrations of TSC >500 mg/dl, the current analysis was based on 172 210 apparently healthy participants, free of cancer at baseline. All participants signed informed consent to have personal data stored and processed. For this study, institutional review board approval was obtained by the Ethics Committee of the province of Vorarlberg.
data collection
Measurements of height, weight, smoking status (current, former and never) and TSC are routinely obtained for each study participant. Individuals who reported smoking of at least one cigarette per day during the year before examination were classified as current smokers. Occupational status (blue collar, white collar or self-employed) was determined by the insurance number of participants and used as a surrogate measure of socioeconomic status. Participants who were retired at baseline were classified according to their former occupation, and housewives were classified according to their husband's job.
cancer ascertainment
Cancers were identified by the Vorarlberg Cancer Registry, which has been accepted for International Agency for Research on Cancer publication since 1993 and has high completeness of recording [40]. Nearly all cancers (96.7%) were histologically confirmed. Cohort data were linked with the Vorarlberg Death Index to identify deaths and to calculate person-years at risk. For analyses, cancers were grouped into the following subgroups according to the International Classification of Diseases, 9th and 10th Revision (ICD-9, ICD-10) [41]: malignant neoplasms of digestive organs (ICD-9 150–157; ICD-10 C15–C25); respiratory system and intrathoracic organs (ICD-9 160–165; ICD-10 C30–C39); bone, connective tissue, soft tissue and skin (ICD-9 170–173; ICD-10 C40–C49); male genital organs (ICD-9 185–187; ICD-10 C60–C63); breast and female genital organs (ICD-9 174, 179–184; ICD-10 C50–C58); urinary organs (ICD-9 188–189; ICD-10 C64–C68); nervous system and unspecified sites (ICD-9 190–199; ICD-10 C69–C72) and lymphoid, hematopoietic and related tissue (ICD-9 200–208; ICD-10 C81–C96).
laboratory measurements
Two central laboratories undergoing regular internal and external quality procedures determined TSC concentrations on fasting blood samples. Within 60–240 min after venous blood sample collection from a cubital vein, serum was obtained by centrifugation for 15 min at 3309×g at 37° C. Subsequently, TSC concentrations were measured at 37° C and were given as milligrams per deciliter. In order to check calibration, three daily control samples were included. If average values of the control samples of each run were not within 3% of the true value, the run was repeated. Day-by-day variation had to be within 5%.
statistical analyses
Cox proportional hazard models (PROC PHREG, SAS version 9.1; SAS Institute, Cary, NC) were used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for tertiles of the TSC distribution for men and women separately. The TSC categories were <194, 194–235, >235 mg/dl for men and <190, 190–229, >229 mg/dl for women. Age was used as the underlying time metric in all analyses [42]. Follow-up for a participant started at his/her age at enrollment into the cohort and ended at cancer diagnosis or censoring. Censoring events were death, end of study, loss to follow-up and emigration. Adjustment variables included body mass index (BMI, in quartiles with gender-specific cut-offs), year of entry into the cohort (in quartiles), smoking status (never/former/current) and occupational status (three categories) measured at baseline. The proportional hazards assumption was checked visually by plotting hazard curves for men and women for TSC categorized into tertiles using gender-specific cut-off values and for all other adjustment variables (PROC LIFETEST, SAS version 9.1). The proportional hazards assumption was upheld for all covariates except for TSC in both men and women, with crossing hazards within ∼5 months of follow-up. To accommodate the nonproportional effect of TSC in our regression models an interaction term between time of follow-up and TSC was included in each model, allowing for time-varying effects of TSC [43]. To test for differences between the HRs associated with TSC categories at different follow-up periods, we used a three-degrees of freedom Wald test. A test for log-linear trend was carried out across the TSC tertiles. We evaluated whether the TSC–cancer relationship was modified by age or BMI in stratified analyses. In a sensitivity analysis, we repeated all analyses using calendar time as the time scale, additionally adjusting for participant's age, again allowing for different TSC effects before and after 5 months from baseline. We also repeated all analyses using cut-off values of 12 and 24 months instead of 5 months. Two-sided P values <0.05 were considered statistically significant.
results
baseline characteristics of the study population
Demographic and clinical characteristics of the study population are shown in Table 1. A total of 172 210 participants (79 417 men and 92 793 women), free from cancer at enrollment, were included in the analyses. Median follow-up time was 13.0 years with a total of 2 004 174 person-years at risk. Most participants (92.8%) were followed up for at least 2 years after baseline TSC measurement and 63.8% had follow-up times of ≥10 years. Mean age at study entry was 41.6 years. During follow-up, a total of 9958 (5.8%) incident cancers were observed, with 5311 in men and 4647 in women. Numbers of cancer incidence according to anatomic sites are shown in Table 2. Median baseline TSC concentrations were 214.0 mg/dl (interquartile range = 62) in men and 209.0 mg/dl (interquartile range = 60) in women (Table 1).
Table 1.
Characteristics of study population at baseline, VHM&PP, 1985–2003
| Total | Men | Women | |
| All VHM&PP participants 1985–2003, no. | 174 852 | 80 224 | 94 628 |
| Eligible participants for analyses, no.a | 172 210 | 79 417 | 92 793 |
| Age at entry, mean ± SD (range), y | 41.6 ± 15.3 (18–96) | 41.7 ± 14.6 (18–96) | 41.6 ± 15.9 (18–95) |
| Body mass index, mean ± SD (median), kg/m2 | 24.7 ± 4.2 (24.2) | 25.3 ± 3.6 (24.9) | 24.2 ± 4.6 (23.3) |
| Total serum cholesterol, mean ± SD (median), mg/dl | 215.4 ± 46.7 (211.0) | 217.4 ± 47.2 (214.0) | 213.7 ± 46.3 (209.0) |
| Cigarette smoking, % | 25.5 | 30.1 | 21.5 |
| Follow-up, mean ± SD (median), y | 11.6 ± 5.6 (13.0) | 11.3 ± 5.6 (12.5) | 12.0 ± 5.6 (13.5) |
| Total person-years at risk | 2 004 174 | 894 645 | 1 109 529 |
| Incident cancers, no. (%) | 9958 (5.8) | 5311 (6.7) | 4647 (5.0) |
| Age at cancer diagnosis, mean ± SD (range), y | 56.6 ± 12.9 (19–93) | 57.1 ± 12.2 (19–93) | 55.4 ± 13.8 (19–93) |
Participants with (i) missing measurements on total serum cholesterol at enrollment, (ii) malignant cancer before or at enrollment and/or (iii) baseline concentrations of total serum cholesterol >500 mg/dl were excluded.
SD, standard deviation; VHM&PP, Vorarlberg Health Monitoring and Promotion Program.
Table 2.
Incidence of site-specific malignancies in 79 417 men and 92 793 women in the Vorarlberg Health Monitoring and Promotion Program, 1985–2003
| Men | Women | |
| Malignant neoplasms of digestive organs (ICD-9 150–157; ICD-10 C15–C25) | 1244 | 1078 |
| Malignant neoplasms of respiratory system and intrathoracic organs (ICD-9 160–165; ICD-10 C30–C39) | 879 | 226 |
| Malignant neoplasms of bone, connective tissue, soft tissue and skin (ICD-9 170–173; ICD-10 C40–C49) | 448 | 423 |
| Malignant neoplasms of male genital organs (ICD-9 185–187; ICD-10 C60–C63) | 1860 | – |
| Malignant neoplasms of breast and female genital organs (ICD-9 174, 179–184; ICD-10 C50—C58) | – | 2276 |
| Malignant neoplasms of urinary organs (ICD-9 188–189; ICD-10 C64–C68) | 447 | 219 |
| Malignant neoplasms of lymphoid, hematopoietic and related tissues (ICD-9 200–208; ICD-10 C81–C96) | 319 | 325 |
ICD, International Classification of Diseases, 9th and 10th Revision. –, not applicable.
association of TSC with cancer incidence
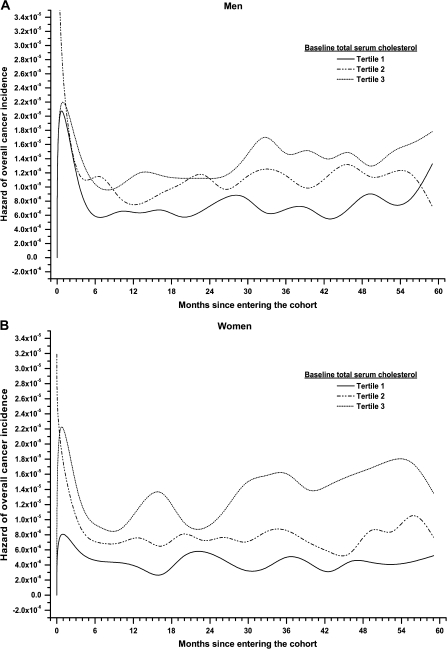
Plots of the hazard function for overall cancer incidence stratified by tertiles of TSC for men and women (Figure 1, panels A and B) revealed a pronounced time-dependent effect of TSC, with effects changing direction after ∼5 months of follow-up. Adjusted HRs with 95% confidence intervals (95% CIs) for the time-dependent association of TSC with overall and site-specific cancer incidence are shown in Table 3 for men and Table 4 for women. Compared with the lowest TSC tertiles, the highest TSC tertiles (>235.0 mg/dl in men and >229.0 mg/dl in women) were associated with a statistically significantly lower overall cancer risk for the first 5 months of follow-up with a HR = 0.58 (95% CI 0.43–0.78, Ptrend < 0.0001) in men and a HR = 0.69 (95% CI 0.49–0.99, Ptrend = 0.03) in women. After roughly 5 months from baseline measurement, however, TSC was not statistically significantly associated with overall cancer risk with a HR = 0.96 (95% CI 0.89–1.03, Ptrend = 0.23) in men and a HR = 0.93 (95% CI 0.85–1.01; Ptrend = 0.07) in women (P for heterogeneity of effects between time periods <0.0001).
Figure 1.
Panels A and B. Hazard function plot for overall cancer incidence by tertiles of total serum cholesterol (TSC) in 79 417 men (Panel A) and 92 793 women (Panel B) in the Vorarlberg Health Monitoring and Promotion Program, 1985–2003.
Table 3.
Estimated adjusted hazard ratios with 95% confidence intervals from Cox regression models with time-dependent effects for total and site-specific cancer incidence according to tertiles of baseline TSC and period of follow-up in 79 417 men, Vorarlberg Health Monitoring and Promotion Program, 1985–2003a
| No. of incident cancers | Tertiles of total serum cholesterol (mg/dl) |
P for trend across TSC tertiles | P for heterogeneity of effects between time periods | |||
| <194.0 | 194.0–235.0 | >235.0 | ||||
| Follow-up ≤5 months | ||||||
| Total cancer incidence | 271 | 1.00 (Ref) | 0.77 (0.57–1.04) | 0.58 (0.43–0.78) | <0.0001 | – |
| Malignant neoplasms of digestive organs | 81 | 1.00 (Ref) | 0.54 (0.32–0.92) | 0.42 (0.24–0.72) | 0.002 | – |
| Malignant neoplasms of respiratory system and intrathoracic organs | 41 | 1.00 (Ref) | 0.58 (0.28–1.18) | 0.36 (0.17–0.78) | 0.002 | – |
| Malignant neoplasms of bone, connective tissue, soft tissue and skin | 13 | 1.00 (Ref) | 0.84 (0.22–3.13) | 0.60 (0.15–2.42) | 0.45 | – |
| Malignant neoplasms of male genital organs | 86 | 1.00 (Ref) | 1.15 (0.63–2.10) | 1.12 (0.62–2.00) | 0.88 | – |
| Malignant neoplasms of urinary organs | 31 | 1.00 (Ref) | 1.16 (0.47–2.84) | 0.54 (0.20–1.45) | 0.52 | – |
| Malignant neoplasms of lymphoid, hematopoietic and related tissue | 15 | 1.00 (Ref) | 0.72 (0.24–2.16) | 0.09 (0.01–0.75) | 0.005 | – |
| Follow-up >5 months | ||||||
| Total cancer incidence | 5040 | 1.00 (Ref) | 0.96 (0.89–1.04) | 0.96 (0.89–1.03) | 0.23 | <0.0001 |
| Malignant neoplasms of digestive organs | 1163 | 1.00 (Ref) | 0.85 (0.73–1.56) | 0.76 (0.65–0.89) | 0.001 | <0.0001 |
| Malignant neoplasms of respiratory system and intrathoracic organs | 838 | 1.00 (Ref) | 1.05 (0.86–1.29) | 1.30 (1.07–1.57) | 0.004 | <0.0001 |
| Malignant neoplasms of bone, connective tissue, soft tissue and skin | 435 | 1.00 (Ref) | 1.19 (0.91–1.56) | 1.12 (0.86–1.47) | 0.81 | 0.07 |
| Malignant neoplasms of male genital organs | 1774 | 1.00 (Ref) | 1.01 (0.88–1.16) | 1.00 (0.88–1.14) | 0.25 | <0.0001 |
| Malignant neoplasms of urinary organs | 416 | 1.00 (Ref) | 1.07 (0.80–1.44) | 1.18 (0.89–1.56) | 0.02 | <0.0001 |
| Malignant neoplasms of lymphoid, hematopoietic and related tissue | 304 | 1.00 (Ref) | 0.82 (0.61–1.10) | 0.69 (0.51–0.93) | 0.71 | <0.0001 |
Estimated from Cox regression models with time-dependent effects and adjustment for body mass index, smoking status (current, former and never), occupational status (blue collar, white collar and self-employed) and year of entry into the cohort. Age was used as the underlying time metric for all analyses.
TSC, total serum cholesterol. –, not applicable.
Table 4.
Estimated adjusted hazard ratios with 95% confidence intervals from Cox regression models with time-dependent effects for total and site-specific cancer incidence according to tertiles of baseline TSC and period of follow-up in 92 793 women, Vorarlberg Health Monitoring and Promotion Program, 1985–2003a
| No. of incident cancers | Tertiles of total serum cholesterol (mg/dl) |
P for trend across TSC tertiles | P for heterogeneity of effects between time periods | |||
| <190.0 | 190.0–229.0 | >229.0 | ||||
| Follow-up ≤5 months | ||||||
| Total cancer incidence | 236 | 1.00 (Ref) | 0.88 (0.60–1.28) | 0.69 (0.49–0.99) | 0.03 | – |
| Malignant neoplasms of digestive organs | 63 | 1.00 (Ref) | 0.63 (0.32–1.24) | 0.37 (0.19–0.70) | 0.001 | – |
| Malignant neoplasms of respiratory system and intrathoracic organsb | 5 | 1.00 (Ref) | – | – | – | – |
| Malignant neoplasms of bone, connective tissue, soft tissue and skin | 13 | 1.00 (Ref) | 1.63 (0.43–6.16) | 0.25 (0.04–1.52) | 0.08 | – |
| Malignant neoplasms of breast and female genital organs | 134 | 1.00 (Ref) | 1.17 (0.67–2.03) | 1.09 (0.65–1.84) | 0.88 | – |
| Malignant neoplasms of urinary organs | 9 | 1.00 (Ref) | 0.37 (0.02–5.91) | 1.15 (0.14–9.38) | 0.52 | – |
| Malignant neoplasms of lymphoid, hematopoietic and related tissue | 11 | 1.00 (Ref) | 0.24 (0.06–0.96) | 0.08 (0.02–0.39) | 0.001 | – |
| Follow-up >5 months | ||||||
| Total cancer incidence | 4411 | 1.00 (Ref) | 0.97 (0.89–1.07) | 0.93 (0.85–1.01) | 0.07 | <0.0001 |
| Malignant neoplasms of digestive organs | 1015 | 1.00 (Ref) | 0.88 (0.67–1.19) | 0.87 (0.71–1.06) | 0.25 | <0.0001 |
| Malignant neoplasms of respiratory system and intrathoracic organsb | 221 | 1.00 (Ref) | – | – | – | – |
| Malignant neoplasms of bone, connective tissue, soft tissue and skin | 410 | 1.00 (Ref) | 0.89 (0.67–1.19) | 0.86 (0.65–1.16) | 0.36 | 0.0005 |
| Malignant neoplasms of breast and female genital organs | 2142 | 1.00 (Ref) | 0.97 (0.86–1.10) | 0.94 (0.83–1.07) | 0.32 | <0.0001 |
| Malignant neoplasms of urinary organs | 210 | 1.00 (Ref) | 1.14 (0.71–1.86) | 1.13 (0.71–1.80) | 0.69 | 0.05 |
| Malignant neoplasms of lymphoid, hematopoietic and related tissue | 314 | 1.00 (Ref) | 1.28 (0.89–1.83) | 0.88 (0.62–1.27) | 0.12 | <0.0001 |
Estimated from Cox regression models with time-dependent effects and adjustment for body mass index, smoking status (current, former and never), occupational status (blue collar, white collar and self-employed) and year of entry into the cohort. Age was used as the underlying time metric for all analyses.
Insufficient events for analyses.
TSC, total serum cholesterol. –, not applicable.
Analyses of site-specific cancers revealed that for both men and women the short-term inverse association of high TSC levels on overall cancer risk was mainly due to cancers of the digestive organs and lymphoid and hematopoietic tissue (Tables 3 and 4), for which the risk-lowering effects of high baseline TSC lasted past 5 months, albeit less strong, and in men also due to cancers of the respiratory system and intrathoracic organs (Table 3). For cancers of the digestive organs, the HR for the highest TSC tertile compared with the lowest TSC tertile was HR = 0.42 (95% CI 0.24–0.72, Ptrend = 0.02) in men and HR = 0.37 (95% CI 0.19–0.70, Ptrend = 0.001) in women within the first 5 months of follow-up, and HR = 0.76 (95% CI 0.65–0.89, Ptrend = 0.001) in men and HR = 0.87 (95% CI 0.71–1.06, Ptrend = 0.25) in women after 5 months. The TSC effects before and after 5 months were statistically significantly different for both men and women (Pheterogeneity < 0.0001). The corresponding HRs for neoplasms of lymphoid, hematopoietic and related tissue were HR = 0.09 (95% CI 0.01–0.75, Ptrend = 0.005) in men and HR = 0.08 (95% CI 0.02–0.39, Ptrend = 0.001) in women for the highest compared with the lowest TSC tertile within the first 5 months of follow-up, and after 5 months HR = 0.69 (95% CI 0.51–0.93, Ptrend = 0.71) in men and HR = 0.88 (95% CI 0.62–1.27, Ptrend = 0.12) in women. Again, the estimates for the two time periods were statistically significantly different (Pheterogeneity < 0.0001) for both men and women. For men, we further found a short-term protective effect of high TSC on risk of respiratory cancers, with a HR = 0.36 (95% CI 0.17–0.78, Ptrend = 0.002) for the highest versus lowest TSC tertile within the first 5 months of follow-up and a significant reversal in the direction of this association afterward (Table 3). We found no association of baseline TSC neither for cancers of the bone, connective tissue, soft tissue and skin as well as urinary organs in both men and women nor for breast cancer or cancers of the female or male genital organs. Results were very similar when we repeated all analyses using calendar time as the time scale and when data were stratified by the BMI categories <20, 20–30, >30 kg/m2 (data not shown).
sensitivity analyses
When we used a cut-off value of 12 instead of 5 months, the inverse short-term association of TSC with overall cancer incidence remained statistically significant in both men and women. Compared with the lowest TSC tertiles, the HRs for the highest TSC tertiles were 0.56 (95% CI 0.44–0.72) and 0.62 (95% CI 0.47–0.82), in men and women, respectively. For follow-up periods >12 months, TSC was not statistically significantly associated with overall cancer risk, with HR = 0.97 (95% CI 0.90–1.05] in men and 0.95 (95% CI 0.86–1.04) in women; P for heterogeneity of effects between time periods, both <0.0001. Similar results were obtained when we used a cut-off value of 24 months. The inverse short-term associations of TSC with malignancies of digestive organs, the respiratory system and lymphoid, hematopoietic and related tissue, however, were somewhat attenuated in both men and women, but remained statistically significant. Notably, for men, the inverse association of TSC with malignancies of the digestive organs was seen also for follow-up periods >24 months [HR for the highest versus lowest TSC tertile 0.80 (95% CI 0.68–0.94)], while we found no association of TSC with overall and/or other site-specific cancer incidence after 24 months of follow-up in men nor in women.
discussion
The present study, including 172 210 Austrian adults, to our knowledge, is the largest investigation, to prospectively assess the association between baseline TSC levels and subsequent cancer risk in a population-based cohort of healthy men and women across a wide age range. We found that in both men and women overall cancer risk was significantly decreased for those in the highest TSC tertile for malignancies diagnosed shortly after baseline TSC measurement, while after some time (e.g. 5, 12 or 24 months) overall cancer risk was not significantly associated with TSC.
Previous reports of high TSC levels lowering cancer risk raised concerns that an increase in cancer rates might outweigh benefits of decreasing cardiovascular disease risk by cholesterol-lowering drugs [44]. The associations seen in our study, however, support the hypothesis that the cancer risk-lowering effect of high TSC may largely be due to mechanisms of reverse causality, i.e. be caused by cancers in preclinical stages [14, 15, 32–34], e.g. through protean physiological effects, which include the metabolic depression of blood cholesterol [6]. Additionally, leukemic blood and bone marrow cells were shown to have an elevated low-density lipoprotein (LDL) receptor activity that inversely correlated with plasma cholesterol levels [45]. Further evidence that low levels of TSC are the effect rather than the cause of cancer comes from several clinical trials that correlated low cholesterol levels with disease activity and showed that TSC concentrations increased after therapy that reduced tumor mass [32]. Still another explanation for the inverse cancer–cholesterol relationship in the literature subsumes subjects with high TSC to be more likely to be censored due to cardiovascular deaths before developing cancer, which might result in an inverse association of high TSC with cancer risk [6, 10]. While in our cohort high baseline TSC levels increased risk of death from coronary heart disease in men of all ages and in women under the age of 50, low TSC levels were significantly associated with all-cause mortality, showing significant associations with death from cancer, liver diseases, and mental diseases in men across the entire age range and in women aged 50 or older [46].
Previous epidemiological investigations of cancer incidence and/or cancer mortality have produced inconsistent results on the effects of TSC. Similar to our findings, Knekt et al. [34] reported the association between TSC and risk of cancer incidence in a Finish cohort of 39 268 men and women, to strongly vary from negative to slightly positive according to anatomic sites of cancer. The strongest inverse associations were seen during the first years of follow-up, especially for rapidly developing cancers. Similarly, in the largest study of cancer mortality, Sherwin et al. [15] found a significant excess of cancer death in >360 000 men from the United States in the lowest decile of TSC during the early years of follow-up, which attenuated over time. In another large-scale investigation that combined data from 11 studies from eight countries [14], men who died from cancer within 1 year of cholesterol measurement had lower mean cholesterol levels than the rest of the study population; however, this inverse association markedly diminished with time. Finally, Rose and Shipley [33] found the inverse association between plasma cholesterol and non-coronary heart disease deaths to be confided to the first 2 years of follow-up; beyond this time, total mortality and cholesterol levels were positively correlated. Törnberg et al. [19] reported a consistently positive association between TSC and incidence of rectal cancer in a cohort of Swedish men. On the other hand, Schatzkin et al. [35] found lower cancer risk for high baseline TSC levels among 5125 men in the National Health and Nutrition Examination Survey even for malignancies diagnosed ≥5 years after baseline TSC measurement. Similar long-term protective effects of high TSC levels were seen in the Framingham study [30].
Our study had several strengths and some potential limitations. Major strengths are the prospective design, the large sample size, length of follow-up and the standardized protocol carried out by experienced physicians. Limitations are that we were unable to analyze lipid subfractions including LDL cholesterol and high-density lipoprotein cholesterol and to account for some risk factors including alcohol consumption, physical activity and diet that might have residually confounded the relationship between TSC and cancer incidence. We were also not able to examine the effect of cholesterol-lowering drugs on the TSC–cancer relationship. However, in 75% of the VHM&PP study participants, serum for TSC measurement was drawn before 1995, the year of the implementation of statin therapy in Austria. Additionally, recent clinical trials have shown no differences in rates of cancer incidence between subjects undergoing statin treatment and those with no such treatment [47], indicating a minor role of cholesterol-lowering drugs on our risk estimates. Finally, the choice of a cut-off at 5 months of follow-up, used in the main analyses to accommodate the nonproportional, time-dependent effect of TSC on cancer incidence, was data driven and therefore may not apply in a more general fashion.
In summary, the present 19-year follow-up study prospectively investigated the association of TSC with overall and site-specific cancer incidence in a population-based cohort of >172 000 Austrian adults, free of cancer at baseline. Our results indicate a strong time-dependent association of TSC with overall cancer incidence and several site-specific malignancies in both men and women, with a significant risk excess in the lowest TSC tertile for malignancies diagnosed shortly after baseline TSC measurement. While further research is needed to shed light on the underlying pathophysiological mechanisms, the pattern of association seen in the present study supports the hypothesis that the inverse association of high TSC levels with cancer risk may largely be attributable to reverse causation due to preclinical malignancies.
funding
Supported by Austrian National Bank Grant OENB-12737 (to HU) and National Institute on Aging Intramural Research Program (to LJB).
Acknowledgments
Members of the VHM&PP study group are Guntram Hinteregger, MD, Karin Parschalk, MD, Wolfgang Metzler, MD, Elmar Stimpfl (Agency for Preventive and Social Medicine, Bregenz, Austria), Jochen Klenk, MSc, and Stephan K Weiland,† MD, MSc (Institute of Epidemiology, Ulm University, Germany). We would like to thank all the participants and physicians of the VHM&PP. We are grateful to the Government of the State of Vorarlberg, Austria, for funding the program and thank Elmar Bechter, MD, and Hans-Peter Bischof, MD, at the Health Department of the Vorarlberg State Government.
References
- 1.Huxley R, Lewington S, Clarke R. Cholesterol, coronary heart disease and stroke: a review of published evidence from observational studies and randomized controlled trials. Semin Vasc Med. 2002;2:315–323. doi: 10.1055/s-2002-35402. [DOI] [PubMed] [Google Scholar]
- 2.Pearce ML, Dayton S. Incidence of cancer in men on a diet high in polyunsaturated fat. Lancet. 1971;1:464–467. doi: 10.1016/s0140-6736(71)91086-5. [DOI] [PubMed] [Google Scholar]
- 3.Rose G, Blackburn H, Keys A, et al. Colon cancer and blood cholesterol. Lancet. 1974;1:181–183. doi: 10.1016/s0140-6736(74)92492-1. [DOI] [PubMed] [Google Scholar]
- 4.Williams RR, Sorlie PD, Feinleib M, et al. Cancer incidence by levels of cholesterol. JAMA. 1981;245:247–252. [PubMed] [Google Scholar]
- 5.Stemmerman GN, Nomura AM, Heilbrun LK, et al. Serum cholesterol and colon cancer incidence in Hawaiian Japanese men. J Natl Cancer Inst. 1981;67:1179–1182. [PubMed] [Google Scholar]
- 6.Schatzkin A, Hoover NR, Taylor PR, et al. Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiological Follow-up study. Cancer Res. 1988;48:452–458. [PubMed] [Google Scholar]
- 7.Tornberg SA, Holm LE, Carstensen JM, Eklund GA. Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst. 1989;81:1917–1921. doi: 10.1093/jnci/81.24.1917. [DOI] [PubMed] [Google Scholar]
- 8.Isles CG, Hole DJ, Gillis CR, et al. Plasma cholesterol, coronary heart disease, and cancer in the Renfrew and Paisley survey. Br Med J. 1989;298:920–924. doi: 10.1136/bmj.298.6678.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–664. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 10.Asano K, Kubo M, Yonemoto K, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. Int J Cancer. 2008;122:909–914. doi: 10.1002/ijc.23191. [DOI] [PubMed] [Google Scholar]
- 11.Cambien F, Ducimitiere P, Richard J. Total serum cholesterol and cancer mortality in a middle-aged population. Am J Epidemiol. 1980;112:388–394. doi: 10.1093/oxfordjournals.aje.a113004. [DOI] [PubMed] [Google Scholar]
- 12.Kagan A, McGee DL, Vano K, et al. Serum cholesterol and mortality in a Japanese-American population: the Honolulu Heart Program. Am J Epidemiol. 1981;114:11–20. doi: 10.1093/oxfordjournals.aje.a113157. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Palmieri MR, Soglie PD, Costas R, Havlik RJ. An apparent inverse relationship between serum cholesterol and cancer mortality in Puerto Rico. Am J Epidemiol. 1981;114:29–40. doi: 10.1093/oxfordjournals.aje.a113171. [DOI] [PubMed] [Google Scholar]
- 14.International Collaborative Group. Circulating cholesterol level and risk of death from cancer in men aged 40 to 69 years: experience of an international collaborative group. JAMA. 1982;248:2853–2859. doi: 10.1001/jama.1982.03330210035031. [DOI] [PubMed] [Google Scholar]
- 15.Sherwin RW, Wentworth DN, Cutler JA, et al. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA. 1987;257:943–948. [PubMed] [Google Scholar]
- 16.Schuit AJ, Van Dijk CE, Dekker JM, et al. Inverse association between serum total cholesterol and cancer mortality in Dutch civil servants. Am J Epidemiol. 1993;137:966–976. doi: 10.1093/oxfordjournals.aje.a116769. [DOI] [PubMed] [Google Scholar]
- 17.Eichholzer M, Stahelin HB, Gutzwiller F, et al. Association of low plasma cholesterol with mortality for cancer at various sites in men: 17-y follow-up of the prospective Basel study. Am J Clin Nutr. 2000;71:569–574. doi: 10.1093/ajcn/71.2.569. [DOI] [PubMed] [Google Scholar]
- 18.Panagiotakos DB, Pitsavos C, Polychronopoulos E, et al. Total cholesterol and body mass index in relation to 40-year cancer mortality (the Corfu cohort of the seven countries study) Cancer Epidemiol Biomarkers Prev. 2005;14:1797–1801. doi: 10.1158/1055-9965.EPI-04-0907. [DOI] [PubMed] [Google Scholar]
- 19.Törnberg SA, Holm LE, Carstensen JM, Eklund GA. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N Engl J Med. 1986;315:1629–1633. doi: 10.1056/NEJM198612253152601. [DOI] [PubMed] [Google Scholar]
- 20.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, et al. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland) Cancer Causes Control. 2002;13:417–426. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 21.Keys A, Aravanis C, Blackburn H, et al. Serum cholesterol and cancer mortality in the seven countries study. Am J Epidemiol. 1985;121:870–883. doi: 10.1093/oxfordjournals.aje.a114057. [DOI] [PubMed] [Google Scholar]
- 22.Wingard DL, Criqui MH, Holdbrook MJ, Barrett-Connor E. Plasma cholesterol and cancer morbidity and mortality in an adult community. J Chronic Dis. 1984;37:401–406. doi: 10.1016/0021-9681(84)90107-3. [DOI] [PubMed] [Google Scholar]
- 23.Hiatt RA, Fireman BH. Serum cholesterol and the incidence of cancer in a large cohort. J Chronic Dis. 1986;39:861–870. doi: 10.1016/0021-9681(86)90034-2. [DOI] [PubMed] [Google Scholar]
- 24.Yaari S, Goldbourt U, Evan-Zohar S, Neufeld HN. Associations of serum high density lipoprotein and total cholesterol with total, cardiovascular, and cancer mortality in a 7-year prospective study of 10000 men. Lancet. 1981;1:1011–1015. doi: 10.1016/s0140-6736(81)92184-x. [DOI] [PubMed] [Google Scholar]
- 25.Dyer AR, Stamler J, Paul O, et al. Serum cholesterol and risk of death from cancer and other causes in three Chicago epidemiological studies. J Chronic Dis. 1981;34:249–260. doi: 10.1016/0021-9681(81)90030-8. [DOI] [PubMed] [Google Scholar]
- 26.Hiatt RA, Friedman GD, Bawol RD, Ury HK. Breast cancer and serum cholesterol. J Natl Cancer Inst. 1982;68:885–889. [PubMed] [Google Scholar]
- 27.Salonen JT. Risk of cancer and death in relation to serum cholesterol: a longitudinal study in an eastern Finnish population with high overall cholesterol level. Am J Epidemiol. 1982;116:622–630. doi: 10.1093/oxfordjournals.aje.a113445. [DOI] [PubMed] [Google Scholar]
- 28.Lim U, Gayles T, Katki HA, et al. Serum high-density lipoprotein cholesterol and risk of non-Hodgkin lymphoma. Cancer Res. 2007;67:5569–5574. doi: 10.1158/0008-5472.CAN-07-0212. [DOI] [PubMed] [Google Scholar]
- 29.Andreotti G, Chen J, Gao YT, et al. Serum lipid levels and the risk of biliary tract cancers and biliary stones: a population-based study in China. Int J Cancer. 2008;122:2322–2329. doi: 10.1002/ijc.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorlie PD, Feinleib M. The serum cholesterol-cancer relationship: an analysis of time trends in the Framingham Study. J Natl Cancer Inst. 1982;69:989–996. [PubMed] [Google Scholar]
- 31.Wallace RB, Rost C, Burmeister LF, Pomrehn PR. Cancer incidence in humans: relationship to plasma lipids and relative weight. J Natl Cancer Inst. 1982;68:915–918. [PubMed] [Google Scholar]
- 32.Kritz H, Zielinski C, Sinzinger H. Low cholesterol and cancer. J Clin Oncol. 1996;14:3043–3048. doi: 10.1200/JCO.1996.14.11.3043. [DOI] [PubMed] [Google Scholar]
- 33.Rose G, Shipley MJ. Plasma lipids and mortality, a source of error. Lancet. 1980;1:181–183. doi: 10.1016/s0140-6736(80)92775-0. [DOI] [PubMed] [Google Scholar]
- 34.Knekt P, Reunanen A, Aromaa A, et al. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41:519–530. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 35.Schatzkin A, Hoover RN, Taylor PR, et al. Serum cholesterol and cancer in the NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Lancet. 1987;2:298–301. doi: 10.1016/s0140-6736(87)90890-7. [DOI] [PubMed] [Google Scholar]
- 36.Ulmer H, Kelleher C, Diem G, Concin H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system: the Vorarlberg Health Monitoring & Promotion Programme. Eur Heart J. 2003;24:1004–1013. doi: 10.1016/s0195-668x(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 37.Strasak AM, Rapp K, Brant LJ, et al. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–3977. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strasak AM, Rapp K, Hilbe W, et al. The role of serum uric acid as an antioxidant protecting against cancer: prospective study in more than 28 000 older Austrian women. Ann Oncol. 2007;18:1893–1897. doi: 10.1093/annonc/mdm338. [DOI] [PubMed] [Google Scholar]
- 39.Strasak AM, Rapp K, Hilbe W, et al. Serum uric acid and risk of cancer mortality in a large prospective male cohort. Cancer Causes Control. 2007;18:1021–1029. doi: 10.1007/s10552-007-9043-3. [DOI] [PubMed] [Google Scholar]
- 40.Oberaigner W, Vittadello F. Cancer mapping in alpine regions 1996–2000. Mammendorf, Germany: Pro Literature Verlag; 2006. [Google Scholar]
- 41.World Health Organization. International Classification of Diseases (ICD) http://www.who.int/classifications/icd/en (6 November 2008, date last accessed) [Google Scholar]
- 42.Thiébaut AC, Bénichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 43.Lehr S, Schemper M. Parsimonious analysis of time-dependent effects in the Cox model. Stat Med. 2007;26:2686–2698. doi: 10.1002/sim.2742. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein MR, Mascitelli L. Do statins decrease cardiovascular disease at the expense of increasing cancer? Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.11.020. (doi: 10.1016/J. IJCARD.2007.11.020) [DOI] [PubMed] [Google Scholar]
- 45.Vitols S, Bjorkkholm M, Gahrton G, et al. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: evidence from studies in patients with leukemia. Lancet. 1985;2:1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- 46.Ulmer H, Kelleher C, Diem G, et al. Why Eve is not Adam: prospective follow-up in 149650 women and men of cholesterol and other risk factors related to cardiovascular and all-cause mortality. J Womens Health (Larchmt) 2004;13:41–53. doi: 10.1089/154099904322836447. [DOI] [PubMed] [Google Scholar]
- 47.Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]