Abstract
Background: This phase II trial (Cancer and Leukemia Group B 90102) sought to determine the efficacy of cisplatin, standard infusion of gemcitabine and gefitinib in patients with advanced urothelial carcinoma.
Patients and methods: Eligible patients had previously untreated measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status of zero to two and creatinine clearance >50 ml/min. Treatment consisted of cisplatin 70 mg/m2 day 1 and gemcitabine 1000 mg/m2 on days 1 and 8 given every 3 weeks concurrent with gefitinib 500 mg/day orally for six cycles. Maintenance gefitinib 500 mg/day was continued for responding or stable disease.
Results: Fifty-four of 58 patients were assessable. Twelve patients (22%) had node-only disease, and 25 (46%) had an ECOG performance status of zero. There were 23 objective responses for an overall response rate of 42.6% [95% confidence interval (CI) 29.2% to 56.8%]. The median survival time was 15.1 months (95% CI 11.1–21.7 months) and the median time to progression was 7.4 months (95% CI 5.6–9.2 months).
Conclusions: The combination of cisplatin, gemcitabine and gefitinib is well tolerated and active in advanced transitional cell carcinoma. The addition of gefitinib does not appear to improve response rate or survival in comparison to historical controls of cisplatin and gemcitabine alone.
Keywords: gefitinib, chemotherapy, EGFR, transitional cell carcinoma, urothelial
introduction
The American Cancer Society estimates that urothelial tract (transitional cell) carcinoma (TCC) will be diagnosed in ∼67 160 patients and cause death in 13 750 patients in the United States in 2007 [1]. Among patients with advanced disease, the median survival varies and is dependent upon the prevalence of visceral metastases and performance status in the trial cohort [2].
A recent update of a randomized phase III trial comparing the combination of methotrexate, vinblastine, doxorubicin and cisplatin to the less toxic combination of cisplatin and gemcitabine (GC) suggested similar objective response proportions and 5-year survival for the two regimens [3]. Currently, GC remains a standard treatment regimen for patients with advanced disease. This regimen is frequently administered on a 21-day schedule owing to the degree of myelosuppression seen with the 28-day schedule. A randomized phase II study of a 3- versus 4-week schedule of GC in 96 advanced non-small-cell lung cancer (NSCLC) patients suggested similar response rates (42% versus 38%, respectively), but a lower incidence of grade 3 or 4 thrombocytopenia (5.5% versus 29.5%) with the 3-week schedule [4]. A retrospective analysis of the 3- and 4-week schedules of GC in 212 advanced TCC patients showed very similar response rates and 5-year survival [5].
The epidermal growth factor receptor (EGFR) is a 170-kDa transmembrane receptor tyrosine kinase and EGFR signaling has been shown to regulate cell proliferation, apoptosis, angiogenesis, invasion and spread of TCC in preclinical models [6]. EGFR is expressed in about two-thirds of nonmetastatic muscle-invasive bladder cancer specimens, correlated with primary tumor stage and associated in some studies with tumor recurrence, progression and patient survival [7–10]. Although the patterns and prognostic value of EGFR expression in metastatic urothelial carcinoma have not been extensively studied, strong EGFR immunostaining patterns were observed in the majority (13 of 20) of bladder cancer metastases in one study [11]. Therefore, the EGFR pathway represents a potential therapeutic target in urothelial carcinoma.
Gefitinib (ZD 1839, Iressa®) is an orally active selective EGFR tyrosine kinase inhibitor which has demonstrated synergy with the antitumor activity of platinum and other chemotherapeutic agents in a variety of cell lines and human tumor xenograft models [12, 13]. Antitumor activity was seen at all levels of EGFR expression but correlated with degree of expression of EGFR. In EGFR-expressing human bladder cancer cell lines, gefitinib inhibited extracellular signal-regulated kinase and Akt/protein kinase B phosphorylation as well as EGFR phosphorylation [14]. Furthermore, EGFR targeting by the antibody C225 inhibited angiogenesis in mouse models of TCC, and this activity was enhanced by paclitaxel [15, 16]. Gefitinib's dose-limiting toxicity was diarrhea in single-agent studies but it has been combined safely with GC for the treatment of NSCLC [17].
Based on promising efficacy observed in previous preclinical studies and clinical trials [18, 19], Cancer and Leukemia Group B (CALGB) undertook a trial of fixed dose rate infusion of gemcitabine at 10 mg/m2/min in combination with cisplatin and gefitinib in advanced urothelial carcinoma patients [20]. However, patients in this first cohort of 25 assessable patients experienced unacceptable toxicity based on predefined trial criteria which did not require a possible causal relationship between grade 4 non-hematologic toxicity or deaths with the treatment regimen. Two deaths were due to internal carotid artery thrombosis and urosepsis. Other grade 4 non-hematologic toxic effects included venous thromboembolism, fatigue, hyperuricemia, hyponatremia, ureteral obstruction and dyspnea. Because the fixed dose rate infusion of gemcitabine was a potential explanation for the observed toxicity, accrual was discontinued to the trial and the protocol was amended to allow accrual of a second cohort of patients treated with the same regimen with the exception of a standard 30-min infusion of gemcitabine.
The rationale for the current trial was based upon the high prevalence of EGFR expression in advanced urothelial carcinoma, experimental evidence for the importance of the EGFR pathway in a variety of neoplastic processes in bladder cancer and synergy of EGFR inhibition with chemotherapy in preclinical studies. The current clinical trial was therefore conducted to determine the efficacy and safety of a combination of gefitinib, cisplatin and standard dose gemcitabine. This report includes only the second cohort of patients since results from the previous cohort have previously been reported [20].
patients and methods
eligibility criteria
The study was approved by the CALGB Executive Committee and by the institutional review boards of each participating site. All patients provided written informed consent. Eligible patients had biopsy-proven TCC arising from the urothelial tract including the bladder, ureter, renal pelvis or urethra. Central pathology review was not required. Tumors with mixed histologies were required to have a dominant transitional cell pattern. Measurable metastatic disease (N2, N3 or M1) by RECIST criteria was required [21]. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of zero to two. There could be no evidence of symptomatic brain metastases, greater than grade 1 peripheral neuropathy, or an active severe gastrointestinal, cutaneous or ocular (especially a corneal or inflammatory) condition. Patients could not have received prior systemic therapy including investigational therapy for advanced urothelial carcinoma. No prior chemotherapy was allowed excepting use as a single agent for radiosensitization. At least 4 weeks had to have elapsed since previous major surgery or radiation. Patients could not be receiving CYP3A4 inducers (e.g. phenytoin, St John's Wort) within 7 days of initiating, or concurrently with, protocol therapy. Patients with a currently active second malignancy other than nonmelanoma skin cancer, or a treated malignancy with a >30% risk of relapse following completion of therapy, were excluded.
Required baseline laboratory parameters included granulocytes ≥1500/cmm, platelets ≥100 000/cmm, bilirubin ≤1.25× normal, AST and ALT ≤2× normal and a calculated creatinine clearance (using the modified Cockroft and Gault formula) of ≥50 ml/min. Assessment of EGFR expression was not required. Baseline evaluation included a history and physical examination which included a visual acuity check, complete blood count and routine serum chemistries. Computed tomography or magnetic resonance imaging scans of the chest, abdomen and pelvis and bone scans were carried out at baseline and repeated every three cycles of treatment of response evaluation.
treatment plan
All patients received chemotherapy and gefitinib concurrently. Patients with responding or stable disease (less than a 30% decrease and less than a 20% increase in the sum of the longest diameters of all target lesions and the appearance of no new lesions) after six cycles were continued on maintenance gefitinib alone until tumor progression. Chemotherapy consisted of gemcitabine administered at a dose of 1000 mg/m2 i.v. over 30 min on days 1 and 8, followed by cisplatin 70 mg/m2 on day 1, given on a 21-day schedule for up to six cycles. Gefitinib was given continuously at a dose of 500 mg/day orally.
Patients underwent a physical exam at the start of each cycle. Tumor response was evaluated every three cycles using RECIST criteria until disease progression [20]. One cycle in the maintenance phase was represented by a 4-week period. All patients were followed for survival.
dose modification
If chemotherapy was delayed pending hematologic recovery [absolute neutrophil count (ANC) > 1500/μl, platelet count > 100 000] between cycles, gefitinib was continued. In the event of febrile neutropenia or a nadir platelet count <50 000, the gemcitabine dose in subsequent cycles was reduced by 25%. Within a cycle, the day-8 gemcitabine dose was reduced by 25% for a day-8 platelet count of 50 000–75 000/μl or ANC of 500–1000/μl and by ≥50% for lower values. For grade 3 or 4 non-hematologic toxicity, treatment was held for up to 3 weeks to allow resolution to grade ≤ 1 before retreatment at a 20% lower dose of chemotherapy. Specifically for grade 3 or 4 diarrhea, gefitinib and gemcitabine were held and resumed with 50% and 25% dose reductions, respectively. Grade 3 or 4 skin rash required holding gefitinib until resolution to grade ≤ 1 at which time gefitinib could be resumed at the same dose level. A second occurrence of severe skin rash required a 50% dose reduction in the gefitinib dose. Patients were removed from protocol therapy for a >3-week delay in reinstituting protocol therapy or more than two occurrences of the same severe toxicity necessitating dose reduction.
statistical design and data analysis
The primary end point for the trial was objective response rate (ORR) defined as the proportion of patients who had experienced either complete response (CR: the disappearances of all target and non-target lesions and the appearance of no new lesions) or partial response (PR: at least a 30% decrease in the sum of the longest diameters of all target lesions and the appearance of no new lesions) using the RECIST criteria [21]. Other end points were toxicity, duration of response, progression-free survival (PFS) and overall survival (OS). Duration of response was defined as the time between a CR or PR to the date of disease progression or death, whichever occurred first. Survival duration was defined as the time between study entry and death, while PFS was defined as the time between study entry and date of disease progression or death, whichever occurred first. A target sample size of 50 patients was based on the null hypothesis that the ORR among bladder cancer patients treated with cisplatin, gemcitabine and gefitinib was ≤45%. The trial was designed to have 90% power and a type I error rate = 0.07 to reject the null hypothesis if the true ORR was at least 65%. This study was monitored for response and for toxicity using a three-stage design, with two interim analyses to be carried out after 12 and 30 patients had been enrolled to the trial. The stopping rule for unacceptable toxicity was based on a threshold of toxic death or grade 4 non-hematologic toxicity deemed to be possibly, probably or definitely related to treatment.
The ORR and 95% confidence interval (CI) for the ORR were calculated based on the binomial distribution. The Kaplan–Meier product-limit method was used to estimate the PFS, OS and duration of response.
As part of the quality assurance program of the CALGB, members of the Data Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response and outcome in a sample of protocols at each institution. Such on-site review of medical records was carried out for a subgroup of 34 patients (62.96%) of the 54 patients under this study. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were carried out by CALGB statisticians.
results
A total of 58 patients were accrued from August 2003 to April 2005. Two patients were withdrawn from the study before beginning study treatment and two additional patients were deemed ineligible because the creatinine clearance was <50 ml/min. Per protocol, these four patients were excluded from the analysis. Baseline patient characteristics are shown in Table 1. All patients had dominant TCC histology. The median age was 63.6 years and 43 of 54 (80%) assessable patients were male. Utilizing the Bajorin risk criteria (ECOG performance status ≥ 2, visceral metastases), 26% of the patients were in a good-risk group (zero risk factors), 67% in an intermediate-risk group (one risk factor) and 7% in a poor-risk group with two risk factors present.
Table 1.
Baseline patient characteristics
| Characteristic (number evaluable) | n | % |
| Demographics | ||
| Median age in years (interquartile range) | 63.6 | (58.0–70.6) |
| White | 52 | 96 |
| Male | 43 | 80 |
| Location of primary tumor | ||
| Bladder (n = 51) | 34 | 67 |
| Renal pelvis (n = 54) | 15 | 28 |
| Ureter (n = 52) | 13 | 25 |
| Urethra (n = 53) | 2 | 4 |
| Sites of metastases | ||
| Distant metastases | 53 | 98 |
| Sites of metastases | ||
| Nodal/soft tissue | 27 | 50 |
| Liver | 19 | 35 |
| Bone | 9 | 17 |
| Lung/pleura | 27 | 50 |
| Pattern of metastases | ||
| Any visceral metastases | 39 | 72 |
| Nodal disease only | 12 | 22 |
| Neither documented | 3 | 6 |
| ECOG performance status | ||
| 0 | 25 | 46 |
| 1 | 24 | 44 |
| 2 | 5 | 9 |
| Prognostic risk factorsa | ||
| 0 | 14 | 26 |
| 1 | 36 | 67 |
| 2 | 4 | 7 |
ECOG performance status of two or more and presence of visceral metastases count as one risk factor each, adapted from Bajorin et al. [2].
ECOG, Eastern Cooperative Oncology Group.
treatment
Of the 54 eligible patients, 25 completed the combination treatment phase and initiated maintenance therapy. Among these patients, the median number of gefitinib cycles was 11 cycles including the combination treatment phase. In the maintenance phase of treatment, these patients received a median of 4.2 months (95% CI 2.8–8.0) of gefitinib alone. The maximum number of gefitinib cycles reported was 47 cycles for one patient. A total of 27 of 58 (47%) patients experienced some dose reduction for gefitinib at any time; by definition, this excluded patients who stopped gefitinib abruptly and permanently (e.g. serious toxicity or disease progression) or transiently (dose delays).
clinical outcomes
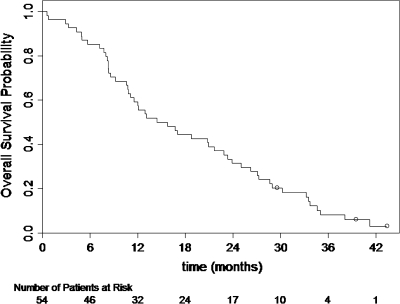
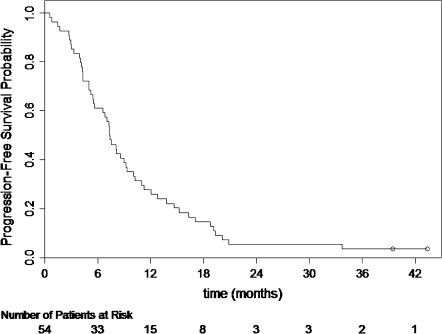
In 54 assessable patients, there were 23 confirmed objective responses (7 CRs and 16 PRs) for an overall objective response proportion of 42.6% (95% CI 29.2% to 56.8%). The response proportion was 57% (8 of 14), 38% (15 of 39) and 25% (1 of 4) in patients with zero, one and two risk factors. The median duration of response for the 23 confirmed responders was 7.1 months (95% CI 5.1–8.9). At the time of this analysis (January 2008) with a median follow-up time of 39.5 months, 51 patients had died. The median OS was 15.1 months (95% CI 11.1–21.7). The Kaplan–Meier plot for OS is presented in Figure 1. The median time to progression was 7.4 months (95% CI 5.6–9.2). The Kaplan–Meier plot for PFS is presented in Figure 2.
Figure 1.
Kaplan–Meier overall survival distribution.
Figure 2.
Kaplan–Meier progression-free survival distribution.
toxicity
Fifty-four patients received at least one dose of protocol therapy and were assessable for toxicity. The median number of cycles of chemotherapy as well as gefitinib administered to patients was 6 (interquartile range 3–6) and 6 (interquartile range 3–10), respectively. Toxic effects with a potential relationship to the treatment regimen are listed in Table 2. Twelve (22%) patients each experienced maximum grade 3 and grade 4 hematologic toxicity, while 35 (65%) and eight (15%) patients experienced maximum grade 3 and grade 4 non-hematologic toxicity, respectively. No fatal toxic effects were observed. Specific unrelated nonmalignant causes of death reported included pulmonary embolism, gastrointestinal hemorrhage, staphylococcal endocarditis and complications of small bowel obstruction.
Table 2.
Toxic effects reported as possibly, probably or definitely related to treatment
| Toxicity (n = 54) | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
||||
| n | % | n | % | n | % | n | % | |
| Neutropenia | 4 | 7 | 8 | 15 | 8 | 15 | 11 | 20 |
| Anemia | 12 | 22 | 17 | 31 | 8 | 15 | 1 | 2 |
| Thrombocytopenia | 6 | 11 | 6 | 11 | 13 | 24 | 1 | 2 |
| Hypotension | 1 | 2 | 3 | 6 | 2 | 4 | 0 | 0 |
| Thromboembolism | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Skin rash | 12 | 22 | 17 | 31 | 11 | 20 | 0 | 0 |
| Diarrhea | 18 | 33 | 8 | 15 | 14 | 26 | 1 | 2 |
| Dehydration | 0 | 0 | 6 | 11 | 11 | 20 | 0 | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 4 | 7 | 0 | 0 |
| Documented infection | 0 | 0 | 0 | 0 | 6 | 11 | 0 | 0 |
| Dyspnea | 0 | 0 | 11 | 20 | 4 | 7 | 0 | 0 |
| Elevated serum creatinine | 13 | 24 | 14 | 26 | 2 | 4 | 0 | 0 |
| Auditory loss | 0 | 0 | 3 | 6 | 3 | 6 | 0 | 0 |
| Edema | 4 | 7 | 1 | 2 | 1 | 2 | 0 | 0 |
| Fatigue (asthenia, lethargy, malaise) | 10 | 19 | 26 | 48 | 11 | 20 | 2 | 4 |
| Emesis | 9 | 17 | 13 | 24 | 13 | 24 | 1 | 2 |
| Mucositis/stomatitis | 7 | 13 | 2 | 4 | 4 | 7 | 0 | 0 |
| Melena/Gastrointestinal bleeding | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Hematuria | 2 | 4 | 1 | 2 | 1 | 2 | 0 | 0 |
| Elevated ALT | 7 | 13 | 2 | 4 | 1 | 2 | 0 | 0 |
| Elevated alkaline phosphatase | 6 | 11 | 0 | 0 | 1 | 2 | 0 | 0 |
| Hypokalemia | 13 | 24 | 0 | 0 | 5 | 9 | 2 | 4 |
| Hypomagnesemia | 6 | 11 | 12 | 22 | 3 | 6 | 3 | 6 |
| Hyponatremia | 14 | 26 | 0 | 0 | 4 | 7 | 0 | 0 |
| Neuropathy: motor | 12 | 22 | 5 | 9 | 2 | 4 | 0 | 0 |
| Neuropathy: sensory | 9 | 17 | 2 | 4 | 1 | 2 | 0 | 0 |
| Hypocalcemia | 6 | 11 | 9 | 17 | 2 | 4 | 1 | 2 |
ALT, alanine aminotransferase.
discussion
This multicenter phase II clinical trial of standard dose gemcitabine plus cisplatin chemotherapy with concurrent and maintenance gefitinib has demonstrated activity in advanced urothelial carcinoma. However, the objective response proportion, PFS and OS are not significantly superior to previous results reported with GC alone. The true response rate is unlikely to have been significantly underestimated by the proportion of patients with an unconfirmed partial response (7.4%) or inadequately assessed response status (9.3%). Although the distribution of performance status in the trial cohort is typical of modern clinical trials of advanced urothelial carcinoma, an unusually high proportion of patients (72%) had visceral metastases, which is a consistently powerful predictor of inferior survival [2]. However, based on the Bajorin risk factor distribution of our trial cohort, the observed median survival in our study approximates the predicted median survival [2], suggesting that the addition of gefitinib to the GC regimen does not provide significant additional benefit.
Adverse events observed in this trial were typical for the known toxicity profiles of the individual agents. Unlike the prior cohort treated with fixed dose rate gemcitabine with cisplatin and gefitinib which was prematurely terminated due to dose-limiting toxicity consisting primarily of a vascular, metabolic, infectious or constitutional nature [20], we observed fewer grade 4 non-hematologic toxic effects, especially vascular and thrombotic events. Furthermore, no toxic deaths occurred. Overall, the treatment regimen was tolerable and the vast majority of deaths were secondary to progressive disease or unrelated causes.
The frequent expression of EGFR in urothelial carcinoma makes the addition of gefitinib to GC a reasonable investigational question. The failure to detect a significant improvement in clinical outcomes with this regimen could be due to several reasons. Activating mutations of the fibroblast growth factor receptor 3 tyrosine kinase but not of the EGFR kinase domain have been reported in bladder cancer [22]. Automated sequencing and PCR of the EGFR kinase domain in 11 bladder cancer cell lines and 75 tumor samples found no mutations or evidence of overexpression of EGFR even though 50% of tumor samples were positive for the EGFR kinase domain by routine immunohistochemistry [23]. Other potential molecular mechanism of gefitinib resistance are the uncoupling of EGFR from its downstream effector Ras/mitogen-activated protein kinase [24], as well as the phosphorylation (activation) status of EGFR [25].
However, it is possible that gefitinib itself, its potentiation of chemotherapy or EGFR targeting is an ineffective strategy in urothelial carcinoma. Single-agent gefitinib given at a dose of 500 mg daily achieved an objective response in only 3% (1 of 29) of patients who had progressed after prior chemotherapy with advanced urothelial carcinoma in a Southwest Oncology Group trial [26]. It remains unknown whether concurrent administration of gefitinib and chemotherapy might lead to antagonism as was suspected for another EGFR inhibitor, erlotinib, and chemotherapy in advanced NSCLC [27, 28]. However, the role of maintenance gefitinib remains worthy of investigation in future clinical trials in urothelial carcinoma in light of the promising results in the maintenance phase of this trial in which some patients experiencing prolonged disease stability on gefitinib alone.
In summary, this multicenter phase II clinical trial of cisplatin, standard dose gemcitabine and concurrent and maintenance gefitinib, demonstrated activity in advanced urothelial carcinoma. However, gefitinib does not appear to add substantial benefit to chemotherapy alone. The treatment was well tolerated without excessive or unexpected toxic effects. Investigation of other treatment schedules of gefitinib (e.g. maintenance only), other EGFR inhibitors (e.g. cetuximab, erlotinib) or other combination strategies may still be of interest because of the strong evidence for a critical role for the EGFR pathway in urothelial carcinoma.
funding
National Cancer Institute (Richard L. Schilsky, CA31946) to the Cancer and Leukemia Group B and to the CALGB Statistical Center (Stephen George, CA33601).
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE—Stephen Grubbs, MD, supported by CA45418; Dana-Farber Cancer Institute, Boston, MA—Eric P. Winer, MD, supported by CA32291; Dartmouth Medical School—Norris Cotton Cancer Center, Lebanon, NH—Marc S. Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC—Jeffrey Crawford, MD, supported by CA47577; Greenville CCOP, Cancer Centers of the Carolinas, Greenville, SC—Jeffrey K. Giguere, MD, supported by CA29165; Illinois Oncology Research Assoc, Peoria, IL—John W. Kugler, MD, supported by CA35113; Hematology–Oncology Associates of Central New York CCOP, Syracuse, NY—Jeffrey Kirshner, MD, supported by CA45389; Illinois Oncology Research Association, Peoria, IL—John W. Kugler, MD, supported by CA35113; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO—Rakesh Gaur, MD; Missouri Baptist Medical Center, St Louis, MO—Alan P. Lyss, MD, supported by CA114558-02; New Hampshire Oncology–Hematology PA, Concord, NH—Douglas J. Weckstein; North Shore University Hospital, Long Island Jewish Health System, Manhasset, NY—Daniel R. Budman, MD, supported by CA35279; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN—Rafat Ansari, MD, supported by CA86726; Rhode Island Hospital, Providence, RI—William Sikov, MD, supported by CA08025; Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC—James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY—Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH—Clara D Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA—Barbara A. Parker, MD, supported by CA11789; University of California at San Francisco, San Francisco, CA—Alan P. Venook, MD, supported by CA60138; University of Chicago, Chicago, IL—Gini Fleming, MD, supported by CA41287; University of Iowa, Iowa City, IA—Daniel A. Vaena, MD, supported by CA47642; University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D., supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO—Michael C Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE—Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC—Thomas C. Shea, MD, supported by CA47559; University of Texas Southwestern Medical Center, Dallas, TX—Debasish Tripathy, MD; Vermont Cancer Center, Burlington, VT—Hyman B. Muss, MD, supported by CA77406; Washington University School of Medicine, St. Louis, MO—Nancy Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY—John Leonard, MD, supported by CA07968.
References
- 1.Jemal A, Seigel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 3.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 4.Soto PH, Cavina R, Latteri F, et al. Three-week versus four-week schedule of cisplatin and gemcitabine: results of a randomized phase II study. Ann Oncol. 2002;13(7):1080–1086. doi: 10.1093/annonc/mdf186. [DOI] [PubMed] [Google Scholar]
- 5.Als AB, Sengelov L, von der Maase H. Gemcitabine and cisplatin in locally advanced and metastatic bladder cancer; 3- or 4-week schedule? Acta Oncol. 2008;47:110–119. doi: 10.1080/02841860701499382. [DOI] [PubMed] [Google Scholar]
- 6.Bellmunt J, Hussain M, Dinney CP. Novel approaches with targeted therapies in bladder cancer. Therapy of bladder cancer by blockade of the epidermal growth factor receptor family. Crit Rev Oncol Hematol. 2003;46(Suppl):S85–S104. doi: 10.1016/s1040-8428(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 7.Lipponen P, Eskelinen M. Expression of epidermal growth factor receptor in bladder cancer as related to established prognostic factors, oncoprotein (c-erbB-2, p53) expression and long-term prognosis. Br J Cancer. 1994;69(6):1120–1125. doi: 10.1038/bjc.1994.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal DE, Sharples L, Smith K, et al. The epidermal growth factor receptor and the prognosis of bladder cancer. Cancer. 1990;65(7):1619–1625. doi: 10.1002/1097-0142(19900401)65:7<1619::aid-cncr2820650728>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Ravery V, Colombel M, Popov Z, et al. Prognostic value of epidermal growth factor-receptor, T138 and T43 expression in bladder cancer. Br J Cancer. 1995;71(1):196–200. doi: 10.1038/bjc.1995.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkeri LN, Erton ML, Cevik I, Akdas A. Impact of the expression of epidermal growth factor, transforming growth factor alpha, and epidermal growth factor receptor on the prognosis of superficial bladder cancer. Urology. 1998;51(4):645–649. doi: 10.1016/s0090-4295(97)00648-1. [DOI] [PubMed] [Google Scholar]
- 11.Bue P, Wester K, Sjostrom A, et al. Expression of epidermal growth factor receptor in urinary bladder cancer metastases. Int J Cancer. 1998;76(2):189–193. doi: 10.1002/(sici)1097-0215(19980413)76:2<189::aid-ijc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6(5):2053–2063. [PubMed] [Google Scholar]
- 13.Sirotnak FM, Zakowski MF, Miller VA, et al. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6(12):4885–4892. [PubMed] [Google Scholar]
- 14.Dominguez-Escrig JL, Kelly JD, Neal DE, et al. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin Cancer Res. 2004;10(14):4874–4884. doi: 10.1158/1078-0432.CCR-04-0034. [DOI] [PubMed] [Google Scholar]
- 15.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5(2):257–265. [PubMed] [Google Scholar]
- 16.Inoue K, Salton JW, Perrotte P, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6:4874–4884. [PubMed] [Google Scholar]
- 17.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi V, Plunkett W, Du M, et al. Prolonged infusion of gemcitabine: clinical and pharmacodynamic studies during a phase I trial in relapsed acute myelogenous leukemia. J Clin Oncol. 2002;20(3):665–673. doi: 10.1200/JCO.2002.20.3.665. [DOI] [PubMed] [Google Scholar]
- 19.Tempero M, Plunkett W, Ruiz VH, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21(18):3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 20.Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), fixed dose rate gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. BJU Int. 2007;101:20–25. doi: 10.1111/j.1464-410X.2007.07226.x. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer: National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Cappellen D, De Oliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23(1):18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 23.Blehm KN, Spiess PE, Bondaruk JE, et al. Mutations within the kinase domain and truncations of the epidermal growth factor receptor are rare events in bladder cancer: implications for therapy. Clin Cancer Res. 2006;12:4671–4677. doi: 10.1158/1078-0432.CCR-06-0407. [DOI] [PubMed] [Google Scholar]
- 24.Kassouf W, Dinney CP, Brown G, et al. Uncoupling between epidermal growth factor receptor and downstream signals defines resistance to the antiproliferative effect of gefitinib in bladder cancer cells. Cancer Res. 2005;65:10524–10535. doi: 10.1158/0008-5472.CAN-05-1536. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs MA, Wotkowicz C, Baumgart ED, et al. Epidermal growth factor receptor status and the response of bladder carcinoma cells to erlotinib. J Urol. 2007;178:1510–1514. doi: 10.1016/j.juro.2007.05.113. [DOI] [PubMed] [Google Scholar]
- 26.Petrylak DP, Faulkner JR, Van Veldhuizen PJ, et al. Evaluation of ZD1839 for advanced transitional cell carcinoma (TCC) of the urothelium: A Southwest Oncology Group Trial. Proc Am Soc Clin Oncol. 2005;22:403. [Google Scholar]
- 27.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]