Abstract
Interferon gamma (IFN-γ) plays a critical role during the immune response to infection with Listeria monocytogenes. Early in the innate response NK cells are thought to be a primary source of IFN-γ however, protection can be mediated by the presence of significant numbers of primed IFN-γ secreting CD8+ T cells. In this report, we examined the early response to Listeria and found that 18 h after infection spleens contain CD11b+, Gr-1high or Ly6G+ cells that produce significant IFN-γ. Morphological analysis of sorted Gr-1highIFN-γ+ and Gr-1LowIFN-γ+ or Ly6G+IFN-γ+ cells confirmed that these cells were neutrophils. The importance of IFN-γ production by these cells was further tested using adoptive transfer studies. Transfer of purified neutrophils from Ifng+/+ mice lead to increased bacterial clearance in Ifng−/− mice. Transfer of Ifng−/− neutrophils provided no such protection. We conclude that neutrophils are an early source of IFN-γ during Listeria infection and are important in providing immune protection.
Keywords: Mouse, neutrophils, Listeria infection, Interferon gamma (IFN-γ)
INTRODUCTION
Listeria monocytogenes (LM) is an opportunistic pathogen producing severe infections in immunocompromised individuals. LM is a facultative intracellular parasite that infects macrophages and non-phagocytic cells such as hepatocytes. The innate immune response to LM involves recruitment and activation of phagocytic cells such as neutrophils, macrophages, as well as NK cells (1). Treatment of mice with antibodies to deplete neutrophils or NK cells increases LM infection (2, 3) and a number of key cytokines such as TNFα (4), IL-1 (5), IL-12 (6) and IFN-γ (2, 7, 8) are known to be important. LM infection has also been used as a model to study acquired CD8+ T cell immunity (9); however, the early innate response is clearly important to the development of any long lasting acquired immunity (1). T cells are apparently more important to defense against secondary infection and in fact, lymphocyte deficient SCID mice show heightened resistance to LM infection. This is attributed to the immunosuppressive effects of apoptotic lymphocytes that elicit an immunosuppressive IL-10 response (10).
It has long been established that IFN-γ is an important cytokine for clearing infections by LM (2, 7, 8). During the first few days of infection IFN-γ production by NK cells early is thought to be critical to host resistance (1, 2, 7). In addition, memory CD8+ T cells responding to IL-12 and IL-18 can mediate resistance in an antigen non-specific manner (11, 12). Interestingly, mice deficient in IFN-γ can still establish an antigen specific CD8+ T cell response to the bacterium if they were first vaccinated using a non-lethal strain of LM (9, 13). Consequently it is now thought that INF-γ plays a crucial role in establishing the innate response but not necessarily acquired T cell immunity.
In this paper we examine the early response (<24 hr) to LM and identify for the first time a population of neutrophils that produce IFN-γ in response to infection. These cells were critical to resistance to LM as they could provide protection to Ifng−/− mice if adoptively transferred at the time of infection. Thus, neutrophils derived IFN-γ production provides a critical component in anti-Listeria immunity.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from National Cancer Institute (Fredrick, MD). B6.129S7-Ifngtm1ts/J (Ifng−/− mice) and control (C57BL/6J, Ifng+/+) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice maintained in a specific pathogen free facility at Washington University School of Medicine. All animal procedures were performed according to National Institutes ofHealth guidelines and approved by Washington UniversityInstitutional Animal Care and Use Committee (IACUC). Groups consisted of 5 mice and experiments were repeated at least 2 times.
Bacterial infection of mice
ActA− L. monocytogenes that expresses OVA was a gift from Dr. Thomas S. Griffith (University of Iowa, Iowa City, IA). This is an attenuated version of L. monocytogenes created by introducingan in-frame deletion in the actA gene (LM-OVA) (14). While we use this strain throughout, parallel studies with the wildtype strain of LM gave identical results. C57BL/6, Ifng−/− and controllittermates were given 1×106 CFU LM-OVA via i.v. injection. Bacteria were grownand quantified as previously described (9).
Antibodies
The following Abs were obtained from BD Bioscience (San Jose, CA) and used for surface marker analysis or intracellular cytokines: anti-mouse CD4 (clone GK1.5), anti-mouseCD8 (clone 53-6.7), anti-mouse CD11b (clone M1/70), anti-mouse CD11c (clone N418), PE anti-mouse Ly-6G(clone 1A8), anti-mouse IFN-γ (clone XMG1.2). Anti-mouse NK1.1 (clonePK136) and anti-mouse Gr-1 (clone RB6–8C5) used for surface staining were obtained from Biolegend (San Diego, CA). The 7/4 rat anti-mouse neutrophil antibody was obtained from Serotec (Kidington, Oxford, UK).
Ex vivo assay and flow cytometry
Mouse spleens were harvested at various time points postinfection. Single cell suspensions were prepared and the RBC’s were lysed with ACK lysing buffer (Lonza, Walkersville, MD). Five million (5×106) splenocytes were plated in RPMI medium (Gibco-BRL, Gaithersburg, MD) supplemented with 10% FCS, 2mM L-glutamine, 50μm 2-mercaptoethanol, 100U/ml penicillin and 100 μg/ml streptomycin in 24-well plates (2 ml/well) in the presence of GolgiPlug (2 μl/well, BD Biosciences, San Jose, CA). These plates were cultured at 37°C in 5% CO2 for 4h. Spleen cells were stained for 30 min at 4 °C with antibodies to various surface markers. After two washes in PBS, intracellular IFN-γ staining was preformed using the Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) according to manufacturer’s instructions. Fluorescent intensities were measured using a Cytomics FC 500 and analysis was performed using CXP software (Beckman-Coulter, Fullerton, CA). Neutrophils were identified by Wright-Giema stain (Sigma, St. Louis, MO) of sorted Gr-1highIFN-γ+ and Gr-1LowIFN-γ+ or Ly-6G+IFN-γ+ cells. Cells were sorted on a BD FACSVantage SE cell Sorter (BD Biosciences, San Jose, CA).
Isolation of splenic neutrophils and CD8+ T cells
Mouse spleens were harvested and single cell suspensions were prepared. Neutrophils were isolated using a customized negative selection kit (StemCell Technologies, Vancouver, BC, Canada) following manufacturer’s instructions. The cocktail contained antibody to CD5, CD4, CD45R, Ter119, F4/80 and CD19. Purity was confirmed by flow cytometry with Gr-1 and Ly-6G antibody staining as well as Wright-Giema staining. Purity was 90–95%. 1.5×106 neutrophils are recovered from the spleen of single donor mouse. CD8+ T cells were isolated using standard CD8+ T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada) and purity was >95% as determined by flow cytometry.
Real time polymerase chain reaction (PCR)
Total cellular RNA from neutrophils and CD8+ T cells was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. First-strand synthesis was done using high-capacity cDNA archive kit (Applied BioSystems, Foster City, CA). PCR was done with 12.5 μl TaqMan universal master mix, 1.25 μl 20X target or endogenous control primers and probe mix and cDNA at a 25 μl reaction volume. cDNA samples were used at the equivalent of 50ng of total RNA per reaction. PCR thermal cycling condition was 50°C, 2 min; 95°C, 10 min; followed by 40 cycles of 95 °C, 15 sec and 60°C, 1min. Samples were run on an ABI 7500 real time PCR system (Applied Biosystems, Foster City, CA). The data were processed automatically using system’s software based on ΔΔCT method. The primers used were target gene IFN-γ (Assay ID: Mm00801778_m1) and endogenous control mouse GAPDH (Part number: 4352339E) from Applied Biosystems (Foster City, CA).
Neutrophil adoptive transfer and calculation of bacterial loads in liver and spleen
Three million (3×106) purified neutrophils were injected i.v. via right retro-orbital plexus and 1×106 CFU actA-LM-OVA were injected i.v. via the left retro-orbital plexus. Both injections were done on the same day. The number of LM-OVA in the spleens and livers of infected mice was determinedon day 3. Spleens and livers were homogenized in PBS by a Tissuemiser (Fisher Scientific, Waltham, MA), the mixtures were serially diluted and 100 μl was spread on Brain Heart Infusion agar plate containing Streptomycin 50 μg/ml) (Sigma-Aldrich, St. Louis, MO). Plates were incubated for 20 h at 37°C and the colonies were counted.
RESULTS
Identification of a Gr-1+, CD11b+ IFN-γ producing neutrophil during LM infection
IFN-γ plays a critical role in the host response to LM infection. NK cells and memory CD8+ T cells can be the source of this cytokine and provide protection against LM infection in adoptive transfer studies (1, 8). In addition, it is established that neutrophils play a critical role early in the infection, presumably from their phagocytic ability that clears infectious organisms (1). We have re-examined IFN-γ production in cell populations of the spleen within the first 24 hrs following infection. Mice were infected with 106 CFU LM-OVA i.v. and 18 h later spleens were removed and cultured in vitro for 4 h with GolgiPlug. Cells were then stained for intracellular IFN-γ along with antibodies to CD4, CD8, CD11b, CD11c, NK1.1 and Gr-1. Data in Figure 1A shows that very few CD4+ and CD8+ T cells producing IFN-γ were detected. In fact, infection did not increase the number of IFN-γ producing T cells at this time point. Infection with LM did, however, increase the number of IFN-γ secreting cells in the non-CD4+ and non-CD8+ population. Further analysis of the non-T cell population revealed that the IFN-γ producing cells were not NK cells or DCs (Figure 1B). Again the IFN-γ secreting cells was noted only in the non-NK and non-DC population. Interestingly, significant IFN-γ producing cells were found in the Gr-1+ and the CD11b+ populations from infected spleens (Figure 1C). Since these markers identify the neutrophil and monocyte/macrophage fractions of spleen, the cells making IFN-γ at 18 h post LM infection likely resided within these populations.
Figure 1. IFN-γ producing cells during LM infection.
C57BL/6 mice were infected i.v. with LM-OVA, control mice received PBS. Mouse spleens were harvested at 18 h postinfection and splenocytes were cultured in 24-well plates in the presence of GolgiPlug for 4 h. Cells were analyzed for cell surface markers and intracellular IFN-γ. A. CD4 and CD8 staining. B. NK1.1 and CD11c staining. C. Gr-1 and CD11b staining. Data presented are representatives from at least 4 experiments with a minimum of 3 mice per each group. Numbers in quadrants indicate percentage of cells.
Characterization of Gr-1+IFN-γ+ and Ly-6G+IFN-γ+ cells
Further characterization of the IFN-γ producing cells in the spleen was carried out by flow cytometry and morphological analysis. Mice were infected with 1×106 CFU LM-OVA i.v. and 18 h later spleens were removed and cultured in vitro for 4h with GolgiPlug. Cells were then stained for Gr-1 and intracellular IFN-γ and sorted based on these markers. Sorted cells were then placed on slides by cytospin and morphological analysis carried out following Wright-Giema staining. The flow cytometry plot in Figure 2A shows that the Gr-1 antibody identified 2 populations of IFN-γ secreting cells. Population 1 was the Gr-1highIFN-γ+ and >98% of these cells had ring-shaped and closed nuclei as shown in the insert for Figure 2A and were therefore neutrophils. The majority (80%) of the Gr-1LowIFN-γ+ (population 2) also with ring-shaped and closed nuclei; however, cells with open nuclei were also observed (20%) suggesting that the latter cells are of the monocyte/macrophage type (15, 16).
Figure 2. Characterization of IFN-γ+ producing cells.
C57BL/6 mice were infected i.v. with LM-OVA while control mice received PBS. Mouse spleens were harvested at 18 h and splenocytes were cultured in 24-well plates in the presence of GolgiPlug for 4 h. They were then stained for cell surface markers and intracellular IFN-γ. A. The cells were sorted based on expression of these markers into Gr-1highIFN-γ+ and Gr-1LowIFN-γ+. Cells were placed on slides and stained with Wright-Giema solution. Insert: representative Gr-1highIFN-γ+ cells (population 1), Gr-1lowIFN-γ+ cells (population 2). B. The cells were sorted based on expression of Ly-6G and these cells were placed on slides and stained with Wright-Giema solution. Insert: representative Ly-6G+IFN-γ+ cells. C. Spleens were stained with antibody to 7/4 and Gr-1 (left 2 panels) or 7/4 and IFN-γ (right 2 panels). Uninfected control mice (top 2 panels) were compared to LM-OVA infected mice (bottom 2 panels). Numbers in quadrants indicate percentage of cells.
Antibody to Gr-1 (Clone RB6–8C5) reacts with both Ly-6G and Ly-6C and identifies neutrophils; however, this antibody can also react with subsets of monocytes, dendritic cells, macrophages, and lymphocytes (15, 17, 18). Although we did not detect IFN-γ producing cells in these latter populations, we wanted to be sure that we had in fact identified neutrophils as the source of IFN-γ during LM infection. Consequently we further analyzed IFN-γ producing cells with the Ly-6G and 7/4 antibodies. Spleens from mice infected as above were stained for Ly-6G and intracellular IFN-γ followed by sorting and morphological analysis. The flow cytometry plot in Figure 2B shows that the majority of cells making IFN-γ were found in the Ly-6G+ population and 95 % of these cells had ring-shaped and closed nuclei (Figure 2B insert). Figure 2C explores expression of 7/4; an allotypic marker expressed on neutrophils, but absent on macrophages (19, 20). Dual staining with 7/4 and Gr-1 LM shows that infection increased the number of Gr-1+, 7/4+ cells from 3.4% to 14.4% of total spleen cells. Co-staining for 7/4 and IFN-γ revealed that these cells were virtually absent in uninfected but were increased to 12.8% of total spleen cells 18 h following LM infection.
Based on our data in Figures 1 and 2 we can calculate the percentage of neutrophils producing INF-γ. In uninfected mice 8% of the Gr-1+ cells produce the cytokine; this increases to ~60% 18h following infection. When cells are gated on the 7/4 marker, INF-γ producing cells increased from 0% in uninfected mice to >75% 18 h following LM infection. Thus, the vast majority of neutrophils in infected mice producing IFN-γ protein.
The highest numbers of IFN-γ producing neutrophils were found at 18h post infection (Figure 3). This number declined by day 3 and the cell counts were near control numbers 7 days following LM infection. This contrasts IFN-γ producing cells in the NK, and T cell populations. We never observed more than 1% NK1.1+ cells producing IFN-γ in the spleen at 18h following infection (Figure 3) and in most other cases IFN-γ producing cells represented less that 0.1% of the spleen (see Figure 1B). T cells producing IFN-γ were at low numbers 18 h and 3 days following infection. Higher numbers of CD4+ T cells were found on day 7 while the number of CD8+ T cells increased slightly over the course of the experiment (Figure 3).
Figure 3. IFN-γ production by spleen cells from LM-infected mice.
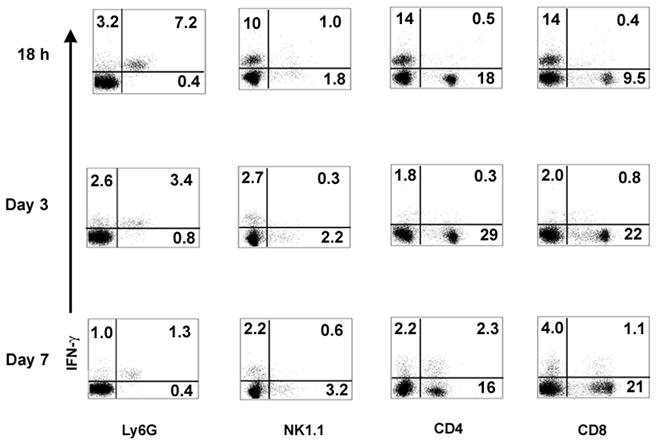
Mouse spleens were harvested at 18 h, 3 days and 7 days postinfection and stained for IFN-γ along with Ly-6G, NK1.1, CD4, or CD8. For Ly6G, NK1.1 and CD8 staining, splenocytes were cultured and treated as in Fig. 1. For CD4 and IFN-γ staining, splenocytes were cultured overnight and restimulated with LLO190 peptide (NEKYAQAYPNVS, Bio-Synthesis, Inc, Lewisville, TX) in 24-well plates. GolgiPlug was added during the last 4 h incubation. Data presented are representatives of two independent experiments. Numbers in quadrants indicate percentage of cells.
Further examination of IFN-γ production by neutrophils in the spleen of infected mice was carried by qRT-PCR with magnetic bead purified cell populations. Splenic neutrophils and CD8+ T cells were isolated from LM-OVA-infected 18 h post infection and mRNA levels were evaluated in comparison to uninfected control mice. Table 1 shows that while CD8+ T cells produced significant levels of IFN-γ mRNA; purified splenic neutrophils make at nearly 70 fold more. In addition, stimulation of isolated Ly-6G+ cells with LM in vitro resulted in a 53.3 fold increase in IFN-γ mRNA vs. unstimulated cells. We conclude from our data in Figure 1, Figure 2, Figure 3, and Table 1 that the vast majority of splenic IFN-γ producing cells 18 h post-infection were in the neutrophil fraction of spleen.
Table I.
IFN-γ mRNA expression at 18 h following LM-OVA infectiona
C57/BL6 mice were infected with LM-OVA and 18 h later CD8+ T cells and neutrophils were isolated using magnetic beads (see Material and Methods). CD8+ T cells and neutrophils were also isolated from uninfected (Control) mice. Quantitative real time PCR was performed and values were normalized to an internal GAPDH control. Numbers represent the means of 3 replicates ± S.E. and expressed as fold change vs. control mice.
P < 0.01.
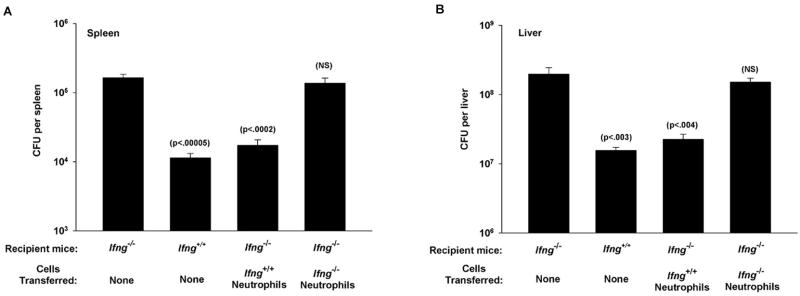
Adoptive transfer of neutrophils to Ifng−/− mice increases bacterial clearance
IFN-γ production during the early stages of the innate response is critical to resistance to LM, consequently Ifng−/− mice are highly susceptible to infection (9, 13). These mice have difficulty clearing the bacterium from infected organs and this can lead to a lethal infection. To evaluate the importance of IFN-γ producing neutrophils in this model we used an adoptive transfer system with Ifng+/+ (wild type C57BL/6) and Ifng−/− mice (also C57BL/6 background). Neutrophils were purified from the spleens of normal (not infected) Ifng+/+ mice at 18 h and transferred to Ifng−/− mice (recipient mice) who were infected with LM on the same day. Three days later bacterial loads in the spleen and liver were quantified. As shown in Figure 4 Ifng−/− mice had very high bacterial loads in the spleen (Figure 4A) and liver (Figure 4B) compared to Ifng+/+ mice. In fact, in several experiments at least 50% of infected Ifng−/− mice succumbed to the infection before day 3 (not shown). In contrast, Ifng−/− mice that receive Ifng+/+ neutrophils had reduced bacterial loads in both the spleen and liver. In addition no mortality in the Ifng−/− recipients of Ifng+/+ neutrophils was observed (not shown). In sharp contrast, purified neutrophils from Ifng−/− mice provided no such protection when transferred to Ifng−/− mice and mortality remained at 50% for these animals (not shown). Thus, neutrophil derived IFN-γ is critical to controlling bacterial colonization of the spleen and liver.
Figure 4. Adoptive transfer of neutrophils toIfng−/− mice.
Mouse splenic neutrophils were isolated by negative selection from uninfected Ifng+/+ (C57Bl/6) mice at 18 h. Ifng−/− mice (Recipient mice) received 3×106 purified neutrophils (Cells transferred) or no cells (None) along with LM-OVA i.v. The number of LM-OVA in the spleens (A) and livers (B) was determinedon day 3. Data are derived from two independent experiments (A and B) with 5 mice per group. p values represent comparisons to infected Ifng−/− mice by Student’s t-test using a 95% confidence interval. NS denotes not significant.
DISCUSSION
The immune response to pathogenic microorganisms involves both the innate and acquired immune responses. Innate immunity involves the rapid, antigen non-specific response of phagocytic cells and serum components while acquired immunity is delayed, often requiring days or weeks to develop. LM is an intracellular parasite infecting macrophages of immunocompromised hosts. Resistance to LM requires both the innate and acquired immunity with both arms of the response being required for successful clearage of the most virulent strains (1, 7). Key cytokines such as TNFα (4), IL-1 (5), IL-12 (6) and IFN-γ (2, 7, 8) are also critical to the response to LM. IFN-γ is very important during the innate phase being required for clearing the organism, but it plays a lesser role in the establishment of the T cell response to LM as shown in studies with Ifng−/−mice (9, 13).
In this report we identify for the first time IFN-γ producing neutrophils early in the response to LM. The cell was identified by cell surface staining and morphological analysis of splenic IFN-γ producing cells. The importance of these cells to LM resistance was demonstrated by adoptive transfers to Ifng−/− mice. These mice succumb to LM infection, however, transfer of neutrophils from Ifng+/+ mice eliminated mortality and increased bacterial clearance.
To our knowledge this is the first demonstration of IFN-γ producing neutrophils in LM infection and one of only a few reports showing that neutrophils can make this cytokine. Myeloid cells (including neutrophils and monocytes/macrophages) are typically viewed as targets of IFN-γ rather than producers, however, IFN-γ producing neutrophils have been observed in human tissue and in cultured human peripheral blood cells (21, 22). It was also observed that human neutrophils can contain preformed INF-γ that is rapidly released in response to granulating agents (23). IFN-γ producing neutrophils have also been shown in the mouse by intracellular staining of the Gr-1high population during infections with Salmonella typhimurium (24) and Norcardia asteroides (25). Our data demonstrated IFN-γ is rapidly made in response to LM infection coming predominantly from the induction of mRNA transcription. This is because we observe a small percentage of neutrophils containing IFN-γ protein in uninfected mice (Figure 1C, 8% of GR-1+ cells, Figure 2C, 0% 7/4+ cells). This number increases to ~80% following LM infection.
Studies by North and colleagues over a decade ago established that neutrophils and NK cells were important to protection from LM infection (1–3, 26). More recent reports have confirmed the role of NK cells and revealed a role for INF-γ producing CD8+ T cells early in the response to the bacterium (7, 11, 12). Neutrophils are seen as critical to phagocytosis and destruction of the bacterium while NK cells are the source of IFN-γ. Both of these innate response cells can be detected in the first 3 days following infection and various experiments involving antibody depletion and adoptive transfers were used to define their importance. For example, depletion of NK cells reduced the number of IFN-γ producing cells in the spleen of infected mice (2). In addition, NK cells producing IFN-γ were identified in the spleens of mice 3 days following infection (7). We do not believe our data contradict these finding as we have observed the presence of INF-γ+ neutrophils earlier (18 h) in the infection. By day 3 this number had fallen dramatically (See Figure 3). In addition, it was reported by Andersson et al. (27) that mice deficient in NK cells mounted a normal innate immune response to LM infection and these mice had increased blood levels of IFN-γ. The authors concluded that the response in this case was not NK dependent; however, they did not identify the IFN-γ source in vivo. We would suggest that these cells may have been neutrophils although in vitro analysis by these authors suggested T cells may be an important source. It is important to note that these studies were carried out on day 2 following infection while our studies were performed at 18 h. However, we did not observe significant increases in NK1.1 cells at anytime point post-LM infection supporting a lesser role for NK cells in this infection. It is clear from our adoptive transfer studies that the rapid neutrophil derived IFN-γ is a critical component in resistance to this infection. Thus, we propose that the neutrophilic response to LM may be more rapid than the NK or T cell response, and may promote the development of the innate immune response to LM.
Footnotes
This work was supported by National Institutes of Health Grants EY06765, EY015570, EY02687 (Dept. of Ophthalmology and Visual Science Core Grant), a Department of Ophthalmology and Visual Science grant from Research to Prevent Blindness, New York, NY, and the Macular Vision Research Foundation.
References
- 1.North RJ, Dunn PL, Conlan JW. Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunn PL, North RJ. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancroft GJ, Sheehan KC, Schreiber RD, Unanue ER. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- 5.Rogers HW, Tripp CS, Schreiber RD, Unanue ER. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J Immunol. 1994;153:2093–2101. [PubMed] [Google Scholar]
- 6.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol. 2005;175:1751–1757. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier NA, Schreiber RD. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985;82:7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 10.Carrero JA, Calderon B, Unanue ER. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J Exp Med. 2006;203:933–940. doi: 10.1084/jem.20060045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messingham KA, V, Badovinac P, Jabbari A, Harty JT. A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes. J Immunol. 2007;179:2457–2466. doi: 10.4049/jimmunol.179.4.2457. [DOI] [PubMed] [Google Scholar]
- 14.Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, Ratliff TL. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood. 2008;111:3684–3691. doi: 10.1182/blood-2007-05-091728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 16.Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkotter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65:217–231. doi: 10.1002/jlb.65.2.217. [DOI] [PubMed] [Google Scholar]
- 17.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 18.Jutila MA, Kroese FG, Jutila KL, Stall AM, Fiering S, Herzenberg LA, Berg EL, Butcher EC. Ly-6C is a monocyte/macrophage and endothelial cell differentiation antigen regulated by interferon-gamma. Eur J Immunol. 1988;18:1819–1826. doi: 10.1002/eji.1830181125. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 20.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 21.Bogdan C, Schleicher U. Production of interferon-gamma by myeloid cells--fact or fancy? Trends Immunol. 2006;27:282–290. doi: 10.1016/j.it.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–5153. [PubMed] [Google Scholar]
- 23.Ethuin F, Gerard B, Benna JE, Boutten A, Gougereot-Pocidalo MA, Jacob L, Chollet-Martin S. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 24.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169:4450–4459. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 25.Ellis TN, Beaman BL. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J Leukoc Biol. 2002;72:373–381. [PubMed] [Google Scholar]
- 26.Conlan JW, North RJ. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991;174:741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161:5600–5606. [PubMed] [Google Scholar]