Abstract
Background
Several studies support a role for cardiovascular risk factors in cognitive aging. The metabolic syndrome, a constellation of cardiovascular risk factors, is common in elderly people. A growing but conflicting body of literature suggests that the metabolic syndrome may be associated with cognitive impairment.
Objective
To investigate the association between the metabolic syndrome, and its components, and incident cognitive impairment in older women.
Design
We prospectively determined if the metabolic syndrome and its components were associated with a 4-year risk of developing cognitive impairment (dementia, mild cognitive impairment or low global cognitive test score).
Setting
The study was conducted at 180 clinical centers in 25 countries.
Participants
A total of 4895 older women (mean age, 66.2 years) with osteoporosis who were part of an ancillary study to determine clinically relevant cognitive impairment were included in this study. These women were free of baseline cognitive impairment and had metabolic syndrome component measures.
Main Outcome Measures
Clinically significant cognitive impairment was defined to include women with clinically adjudicated dementia or MCI and women who had a Short Blessed test score greater than 6 (consistent with impairment), but whose cases were not clinically adjudicated. Logistic regression analysis was used to examine the association between presence of the metabolic syndrome and development of clinically significant cognitive impairment.
Results
A total of 497 women (10.2%) had the metabolic syndrome, and of these, 36 (7.2%) developed cognitive impairment compared with 181 women (of 4398, 4.1%) without the syndrome (age-adjusted odds ratio, 1.66; 95% confidence interval, 1.14–2.41). The mean (SD) number of metabolic syndrome components for all women was 1.0 (1.1); 518 women (10.6%) were obese, 895 (18.3%) had hypertriglyceridemia, 1200 (24.5%) had low high-density lipoprotein cholesterol levels, 1944 (39.7%) had high blood pressure, and 381 (7.8%) had high fasting blood glucose levels. There was a 23.0% age-adjusted increase in the risk of developing cognitive impairment (odds ratio, 1.23, 95% confidence interval, 1.09–1.39), per unit increase in the number of components. Further multivariable adjustment somewhat reduced the effect.
Conclusion
We found an association between the metabolic syndrome and the number of components and risk of developing cognitive impairment in older women. Additional studies are needed to determine if screening and close management of these at-risk elderly women would diminish the incidence of cognitive impairment.
Keywords: dementia, metabolic syndrome, cognitive impairment
Introduction
The lifetime risk of dementia is 33% for women and 20% for men, and lifetime risks for milder forms of cognitive impairment are even higher.1 During the next 50 years, the incidence and prevalence of Alzheimer disease (AD) and other forms of dementia are expected to double in people aged 75 to 85 years, and to quadruple in those older than 85 years, highlighting the importance of prevention.2 Cardiovascular disease risk modification is a promising avenue for preventive strategies. It is widely accepted that cardiovascular risk factors play a role in the development of AD and vascular dementia. Hypertension,3, 4 hyperlipidemia,5–7 obesity,6, 8 diabetes mellitus9, 10 and impaired glucose tolerance10, 11 have all been shown to be related to cognitive decline and risk of dementia.
A collection of cardiovascular disease risk factors comprise the metabolic syndrome, specifically, abdominal obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C) level, high blood pressure, and hyperglyceridemia.12 In the United States, the estimated rate of the metabolic syndrome in adults aged 60 to 70 years is 43.5%.13 With this high rate of metabolic syndrome, even a modest association with cognitive impairment could have large public health implications. Early identification of people with the metabolic syndrome and subsequent treatment of their symptoms could modify or prevent the development of cognitive impairment.
In recent years, although several studies have investigated the association between the metabolic syndrome and the risk of AD or other forms of cognitive impairment,14–19 the role of the metabolic syndrome on the rate of cognitive decline remains controversial.16, 20 Several studies have reported increases in the risk of developing cognitive impairment that range from 2 to 7 times15, 17 among those with the metabolic syndrome, yet other studies have failed to find an increased risk.14 Thus, the association between the metabolic syndrome and the risk of cognitive impairment still remains unclear, especially among older women. This group is of particular importance because previous research has suggested that women were more likely to develop the metabolic syndrome, and women with the metabolic syndrome have increased odds of developing AD, whereas men with the metabolic syndrome may not.16, 17
In the present study, we sought to examine the association in older women between the metabolic syndrome and the risk of developing cognitive impairment. A secondary objective was to assess if each component of the metabolic syndrome and if each unit increase in the number of syndrome components led to an increased risk of cognitive impairment. The Multiple Outcomes of Raloxifene Evaluation (MORE) study offers a unique setting to examine this association because the cases of dementia and mild cognitive impairment were systematically adjudicated. Furthermore, the information to determine the presence of the metabolic syndrome was gathered at baseline, and data for potential confounding factors were carefully collected on all participants.
Methods
Study Participants
The MORE trial enrolled 7705 postmenopausal women with osteoporosis to examine the effect of raloxifene hydrochloride on the risk of vertebral fractures.21 The study was conducted at 180 clinical centers in 25 countries, where participants were randomly assigned by site to receive raloxifene hydrochloride (60 or 120 mg/d), or an identical placebo. Details of the study design, study flow, and main results have been reported.21 The human studies review board at each site approved the protocol; all women gave written informed consent. There were 5386 women who were part of an ancillary study to determine clinically relevant cognitive impairment. Of these, 5297 had data on the presence of the metabolic syndrome at baseline. A total of 402 women (7.6%) with potential cognitive impairment at baseline as measured by a Short Blessed Test score higher than 6 were excluded, leaving 4895 women in our analytic subset.
At study onset, information was collected on age, ethnicity, educational level, smoking, health conditions (based on self report or medication use), prior postmenopausal estrogen use, measured height and weight, and calculated body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). Participants completed the 15-item Geriatric Depression Scale, with a score of 6 symptoms or more indicating depression, as previously described.22 Systolic and diastolic blood pressure was measured with standard procedures at baseline. A fasting blood draw was obtained for study participants; measurements included blood glucose and lipid levels. All biochemical assessments were performed at a central laboratory by Covance Central Laboratory Services (Indianapolis, Indiana).
The Metabolic Syndrome
Presence of the metabolic syndrome at baseline was determined using the National Cholesterol Education Program Third Adult Treatment Panel guidelines.12 Guidelines suggest the metabolic syndrome exists if a person has 3 or more of the following criteria: (1) abdominal obesity (we used BMI ≥ 30 because waist circumference was not measured in the MORE study); (2) hypertriglyceridemia (triglycerides level ≥ 150 mg/dL [to convert to millimoles per liter, multiply by 0.0113]); (3) low HDL-C level (HDL-C level <50 mg/dL [to convert to millimoles per liter, multiply by 0.0259]); (4) hypertension (systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥85 mmHg or currently taking an antihypertensive medication); and (5) high fasting glucose level (fasting glucose level ≥110 mg/dL [to convert to millimoles per liter, multiply by 0.0555] or taking antidiabetic medication).
Ascertainment of Cognitive Impairment and Dementia
A complete description of the MORE dementia ancillary study has been reported.23 In brief, the Short Blessed Test 24 was used as a screen for dementia and was administered at baseline and annually, along with a battery of cognitive tests (Trails A and B, Word List Memory and Recall, Word Fluency). After 3 to 4 years of follow-up, women with the worst 10% of the Short Blessed Test scores (by test-taking site) or those with clinical symptoms of cognitive impairment were referred for a clinical dementia evaluation. This evaluation consisted of a standard clinical evaluation by a physician expert in dementia and included interviews with the participant and his or her caregiver, a physical and neurologic examination, and the application of standard cognitive and functional scales.23 Participants with evidence of dementia based on this evaluation were referred for brain computed tomography or magnetic resonance imaging and laboratory tests to diagnose dementia (fluorescent treponemal antibody, vitamin B12, serum folate, and thyroid-stimulating hormone). All brain scans were read by a central neuroradiologist at the University of California, San Francisco.
The clinical dementia evaluation results were presented to a dementia adjudication committee along with scores from all cognitive tests. Two committee members independently judged cognitive status as cognitively normal, mild cognitive impairment,25 AD (in accordance with National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria26), vascular dementia, or other type of dementia. We defined clinically significant cognitive impairment to include women with adjudicated dementia or mild cognitive impairment. There were 63 women who had a Short Blessed Test score greater than 6 consistent with impairment 27 but did not complete the dementia adjudication. These 63 women were also categorized as having cognitive impairment. Mean (SD) length of follow-up to cognitive impairment was 4.0 (0.2) years.
Statistical Analyses
Differences in baseline characteristics for the presence or absence of the metabolic syndrome were assessed using t-, Wilcoxon rank sum, chi-square, and Fisher's exact tests as appropriate. We then examined the association between the presence of the metabolic syndrome and the development of clinically significant cognitive impairment by using logistic models. In all 4895 women, we also determined the independent association of each of the 5 components of the metabolic syndrome and cognitive impairment, and whether an association existed between each unit increase in the number of syndrome components and cognitive impairment. Multivariable models included treatment, age, race, and those covariables that were associated with both metabolic syndrome and development of cognitive impairment at the p=0.050 level (eg, educational level or depression; myocardial infarction was not associated with cognitive impairment). All statistical analyses were performed using a commercially available software program (SAS statistical software, version 9.1: SAS Institute Inc, Cary, North Carolina).
Results
The mean (SD) age of women in our study was 66.2 (6.9) years. The mean (SD) number of metabolic syndrome components was 1.0 (1.1); 518 women (10.6%) had a BMI of 30 or higher, 895 (18.3%) had hypertriglyceridemia, 1200 (24.5%) had a low HDL-C level, 1944 (39.7%) had high blood pressure or were taking antihypertensive medications, and 381 (7.8%) had high fasting blood glucose or were taking antidiabetic medications. A total of 497 women (10.2%) met the criteria for having the metabolic syndrome, of whom 222 (44.7%) had obesity, 384 (77.3%) had hypertriglyceridemia, 425 (85.5%) had a low HDL-C, 425 (85.5%) had high blood pressure or were taking antihypertensive medications, and 180 (36.2%) had a high fasting blood glucose level or were taking antidiabetic medications. Women with the metabolic syndrome tended to be older, to be less educated, and had higher rates of depression and a history of myocardial infarction (Table 1).
Table 1.
Baseline Characteristics of the 4895 Women by Metabolic Syndrome Statusa
| Characteristics | No. (%) of Women | P Valueb | |
|---|---|---|---|
| Without the Metabolic Syndrome (N=4398) |
With the Metabolic Syndrome (N=497) |
||
| Age, mean (SD), y | 66.1 (6.9) | 67.6 (6.1) | <0.001 |
| Race, white | 4241 (96.4%) | 472 (95.0%) | 0.10 |
| Educational level, mean (SD), y | 12.1 (3.8) | 11.6 (4.1) | 0.03 |
| Current smoker | 729 (16.6%) | 71 (14.3%) | 0.21 |
| History of myocardial infarction | 71 (1.6%) | 15 (3.0%) | 0.02 |
| History of stroke | 10 (0.2%) | 2 (0.4%) | 0.45 |
| Depression (GDS score ≥6) | 265 (6.0%) | 62 (12.5%) | <0.001 |
| Prior postmenopausal hormone therapy | 1262 (28.8%) | 155 (31.2%) | 0.26 |
Abbreviation: GDS, Geriatric Depression Scale
Data are given as a number (percentage) of women unless otherwise indicated.
P values for categorical data are from χ2 tests. P values for continuous variables are from t tests or Wilcoxon rank sum tests.
During the 4 years of follow-up, 217 women (4.4%) developed cognitive impairment. Of these, 130 (59.9%) were diagnosed as having mild cognitive impairment and 24 (11.1%) were diagnosed as having dementia of any origin (18 with AD). The remaining 63 (29.0%) were classified as having developed cognitive impairment because they had a Short Blessed Test score higher than 6 but were not adjudicated. Thirty-six women (7.2%) with the metabolic syndrome developed cognitive impairment, whereas 181 women (4.1%) without the metabolic syndrome developed cognitive impairment (unadjusted odds ratio [OR], 1.82; 95% confidence interval [CI], 1.26–2.64). After adjustment for age and additional adjustment for educational level, race, depression, and raloxifene hydrochloride treatment, the effect size was attenuated (age-adjusted OR, 1.66; 95% CI, 1.14–2.41; and multivariable-adjusted OR, 1.44; 95% CI, 0.97–2.12; p=0.07).
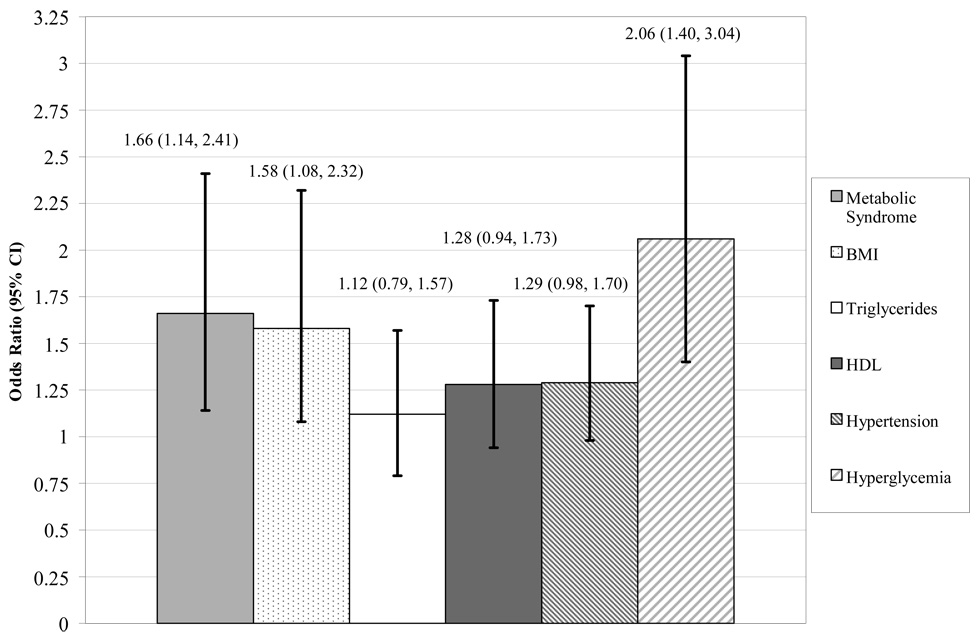
Among all 4895 women, we also individually analyzed the 5 components that make up the metabolic syndrome to assess their effect on the risk of development of cognitive impairment. The age-adjusted ORs (95& CI) of developing cognitive impairment for each component were as follows: obesity: 1.58; 1.08–2.32; hypertriglyceridemia: 1.12; 0.79–1.57; low HDL-C level: 1.28; 0.94–1.73; high blood pressure or taking antihypertensive medications: 1.29; 0.98–1.70; and high fasting blood glucose level or taking antidiabetic medications: 2.06; 1.40–3.04 (Figure). The only association that remained statistically significant after multivariable adjustment was high fasting blood glucose level or taking antidiabetic medications (OR, 1.66; 95% CI, 1.10–2.49). In addition, in all women, for every 1-unit increase in the number of metabolic syndrome components, there was a 29.0% unadjusted increase in risk (unadjusted OR, 1.29; 95% CI, 1.14–1.45) and a 23.0% age-adjusted increase in risk of developing cognitive impairment (age-adjusted OR, 1.23; 95% CI, 1.09–1.39) (Table 2). Further adjustment for educational level, race, depression, and raloxifene treatment lessened the magnitude of the association slightly, but was still statistically significant (OR=1.17, 95% CI=1.04, 1.33) (Table 2).
Figure 1.
The age-adjusted association (odds ratios and 95% confidence intervals (CI)) between the metabolic syndrome components and risk of developing cognitive impairment among all 4895 women throughout 4 years. BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol.
Table 2.
Likelihood of Developing Cognitive Impairment among Women With the Metabolic Syndrome
| Metabolic Syndrome Status |
No. (%) with cognitive impairment |
Unadjusted OR (95% CI) |
Age Adjusted OR (95% CI) |
Multivariable Adjusteda OR (95% CI) |
|---|---|---|---|---|
| No Metabolic Syndrome (N=4398) | 181 (4.1%) | - | - | - |
| Metabolic Syndrome (N=497) | 36 (7.2%) | 1.82 (1.26, 2.64) | 1.66 (1.14, 2.41) | 1.44 (0.97, 2.12) |
| Association per 1 unit increase in number of components | - | 1.29 (1.14, 1.45) | 1.23 (1.09, 1.39) | 1.17 (1.04, 1.33) |
Adjusted for age, educational level, race, depression, and raloxifene hydrochloride treatment.
Comment
In this prospective study, we found that in older women, the metabolic syndrome is associated with an increased risk of developing cognitive impairment during a 4-year period. In addition to the overall association, for every unit increase in the number of syndrome components, there was a 23% age-adjusted increase in the risk of developing cognitive impairment. These findings support the hypothesis that the metabolic syndrome is linked to an increased risk of developing cognitive impairment and dementia in older women. Finally, of the 5 components, hyperglycemia was the only component with a higher age-adjusted risk than the metabolic syndrome. These findings suggest that contrary to previous findings,28 it is important, from a clinical perspective to diagnose the syndrome, rather than just its individual components.
Our findings add to a growing body of literature that suggests the metabolic syndrome is associated with accelerated cognitive aging and risk of cognitive impairment among ethnically diverse groups of elderly people. In the Health, Aging, and Body Composition Study, the metabolic syndrome was associated with greater 5-year cognitive decline among elderly blacks and whites.19 Elderly Latinos enrolled in the Sacramento Area Latino Study of Aging who had the metabolic syndrome also had greater cognitive decline compared with those without the metabolic syndrome.18 As part of the Honolulu-Asia Aging Study, a composite score of 7 cardiovascular risk factors was associated with increased risk of vascular dementia in older Japanese-American men.29 The metabolic syndrome was also found to increase the risk of developing AD in a case-control and a population based sample.15, 17
Not all studies, however, have reported a significant association between the metabolic syndrome and cognitive decline.16, 17 This discrepancy may be explained by study population differences. For example, Vanhanen and colleagues found no significant association between the metabolic syndrome and risk of AD among men but found an association among women.17 Another study that focused on the oldest of the elderly (≥85 years) failed to find a link between the syndrome and cognitive decline, but this result may have been related to a survival bias.16
The mechanisms underlying an association between the metabolic syndrome and cognitive impairment are not completely understood, but there are several plausible pathways. One mechanism is increased cerebrovascular disease, on a microlevel and macrolevel, which in turn could be a risk for development of both AD and vascular mediated dementia.4, 30 Another possible mechanism is a direct effect on the pathogenesis of AD. For example, neurofibrillary tangles develop more often in hypertensive individuals without dementia,31 and in the setting of insulin resistance, β-amyloid aggregation is accelerated.15, 32 The metabolic syndrome, a condition closely linked to insulin resistance, could increase cognitive aging by alterations in β-amyloid deposition or clearance. Furthermore, increased neuronal degradation and brain atrophy have been linked to obesity, indicating connections between obesity and the pathologic manifestations of cognitive decline.8, 33 Another probable mechanism is the effect of the elevated inflammation often seen in the setting of the metabolic syndrome. Markers of inflammation have been associated with an increased risk of developing dementia and cognitive decline.19 Although we did not have the ability to measure inflammation in the MORE study, we previously have demonstrated that elderly people with both the metabolic syndrome and elevated inflammation are at an increased risk for cognitive decline.18, 19, 34
Our study has many strengths, including the large sample size and inclusion of high-functioning, community-dwelling women who agreed to participate in the trial. We performed an extensive clinical evaluation for cognitive impairment. Finally, we were able to adjust for possible confounders, such as age, educational level, depression, and race. Our study has several limitations that may limit the interpretation of the results. The rate of mild cognitive impairment and dementia occurrence in our study was not sufficient to allow us to analyze the effect of the metabolic syndrome on the risk of developing each outcome alone or one of the subtypes of dementia. Also, the rate of the metabolic syndrome present in our study population (10.2%) was considerably lower than that found in a representative sample of 60- to 69-year-old women in the United States (40–45%). 13 This finding may be due to our participants’ ability and willingness to participate in a trial or because our participants were from 25 countries, where prevalence of the syndrome may be lower. This finding could also be partially attributable to the women in our study having osteoporosis because having a low BMI is associated with increased risk for osteoporosis but it decreases the risk for the metabolic syndrome.35 We also had to substitute BMI for waist circumference which may have an effect on the overall results. Among women, the metabolic syndrome rates of those with a BMI of 30 or higher are lower than rates of those with a waist circumference of 88 cm or greater.36 Thus, we may have underestimated the presence of abdominal adiposity. Finally, our study was composed of mostly white women with osteoporosis, and we do no know if our findings apply to men or women of other ethnic groups.
In this study, we found that women with the metabolic syndrome have a greater risk of developing cognitive impairment. As the obesity and sedentary lifestyle epidemic escalates throughout the world, identification of the role of these modifiable behaviors in increasing risk for developing deleterious outcomes such as cognitive impairment is critical. Future research should assess whether identification of cognitive impairment among patients with the metabolic syndrome or more aggressive clinical control of the factors that compose the metabolic syndrome might lessen the risk of developing cognitive impairment in elderly people.
Acknowledgements
Kristine Yaffe takes responsibility for the integrity of the data and the accuracy of the data analysis. The parent trial was funded by Eli Lilly and Company. Dr. Yaffe is supported in part by AG031155. Dr. Krueger receives full salary from Eli Lilly and Company. Ms. Blackwell receives partial salary support from Eli Lilly and Company.
References
- 1.Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam Study. American Journal of Epidemiology. 1998;147(6):574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer Disease in the US population. Prevalence estimates using the 2000 census. Archives of Neurology. 2003 August;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiology of aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 5.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County Study. Alzheimer's Disease and Associated Disorders. 2006 April–June;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 6.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Archives of Neurology. 2002 March;59(3):378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 8.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360–1365. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease. Neurology. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Blackwell TL, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004 August 24;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 11.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: Potential mechanisms and implications for treatment. Current Alzheimer Research. 2007;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 12.Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Journal of the American Medical Association. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Dietz WH. Prevalence of the Metabolic Syndrome Among US Adults. Journal of the American Medical Association. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 14.Muller M, Ming-Xin Tang H, Schupf HN, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dementia and Geriatric Cognitive Disorders. 2007;24(3):185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer's disease. Archives of Neurology. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg E, Biessels GJ, de Craen AJM, Gussekloo J, Westendorp RGJ. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology. 2007;69:979–985. doi: 10.1212/01.wnl.0000271381.30143.75. [DOI] [PubMed] [Google Scholar]
- 17.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease, a population-based study. Neurology. 2006;67:843–847. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Haan MN, Blackwell TL, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly latinos: findings from the Sacramento Area Latino Study of Aging study. Journal of the American Geriatrics Society. 2007;55(5):758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K, Kanaya AM, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Journal of the American Medical Association. 2004 November 10;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 20.Yaffe K. Metabolic syndrome and cognitive disorders: Is the sum greater than its parts? Alzheimer's Disease and Associated Disorders. 2007 April–June;21(2):167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trail. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Selective estrogen receptor modulators: a look ahead. Journal of the American Medical Association. 1999;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh J, Yesavage J. Geriatric Depression Scale: Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–173. [Google Scholar]
- 23.Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention of dementia and cognitive impairment in older women: The Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. American Journal of Psychiatry. 2005 April;162:683–690. doi: 10.1176/appi.ajp.162.4.683. [DOI] [PubMed] [Google Scholar]
- 24.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Blessed G, Tomlinson BE, Roth M. Blessed-Roth Dementia Scale (DS) Psychopharmacology Bulletin. 1988;24(4):705–708. [PubMed] [Google Scholar]
- 28.Lawlor DA, Ebrahim S, Davey Smith G. The metabolic syndrome and coronary heart disease in older women: findings from the British Women's Heart and Health Study. Diabetic Medicine. 2004;21:906–913. doi: 10.1111/j.1464-5491.2004.01245.x. [DOI] [PubMed] [Google Scholar]
- 29.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arteriosclerosis, thrombosis, and vascular biology. 2000 October;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 30.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Aging Research Reviews. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 31.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 32.Gasparini L, Gouras GK, Wang R, et al. Stimulation of β-amyloid precursor protein trafficking by insulin reduces intraneuronal β-amyloid and requires mitogen-activated protein kinase signaling. The Journal of Neuroscience. 2001;21(8):2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagust W, Harvey D, Mungas D, Haan MN. Central obesity and the aging brain. Archives of Neurology. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 34.Dik MG, Jokner C, Comijs HC, et al. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30(10):2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- 35.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. Journal of Womens Health. 2006;15(9):1028–1034. doi: 10.1089/jwh.2006.15.1028. [DOI] [PubMed] [Google Scholar]
- 36.Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. Journal of Internal Medicine. 2003;254:555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]