Abstract
Previous studies demonstrated that the primary antigen presenting cells (APCs) for the hepatitis core antigen (HBcAg) were B cells and not dendritic cells (DC). We now report that splenic B1a and B1b cells more efficiently present soluble HBcAg to naïve CD4+ T cells than splenic B2 cells. This was demonstrated by direct HBcAg-biotin binding studies and by HBcAg-specific T cell activation in vitro in cultures of naïve HBcAg-specific T cells and resting B cell subpopulations.
The inability of DCs to function as APCs for exogenous HBcAg relates to lack of uptake of HBcAg, not to processing or presentation because HBcAg/anti-HBc immune complexes can be efficiently presented by DCs. Furthermore, HBcAg-specific CD4+ and CD8+ T cell priming with DNA encoding HBcAg does not require B cell APCs.
Toll-like-receptor (TLR) activation, another innate immune response, was also examined. Full-length (HBcAg183), truncated (HBcAg149) and the nonparticulate HBeAg were screened for TLR stimulation via NF-Kβ activation in HEK293 cells expressing human TLRs. None of the HBc/HBeAgs activated human TLRs. Therefore, the HBc/HBeAg proteins are not ligands for human TLRs. However, the ssRNA contained within HBcAg183 does function as a TLR-7 ligand as demonstrated at the T and B cell levels in TLR-7 knock-out (KO) mice. Bacterial, yeast and mammalian ssRNA encapsidated within HBcAg183 all function as TLR-7 ligands. These studies indicate that innate immune mechanisms bridge to and enhance the adaptive immune response to HBcAg and have important implications for the use of hepadnavirus core proteins as vaccine carrier platforms.
Keywords: hepatitis B core, antigen presentation, TLR-7, B cell subsets
INTRODUCTION
The HBV core gene encodes two polypeptides. Initiation of translation at the first start codon (AUG) results in a 25-kDa precore protein that is secreted as HBeAg after removal of 19 residues of the leader sequence and 34 C-terminal amino acids. Initiation of translation at the second AUG leads to the synthesis of a 183-aa, 21-kDa protein that assembles to form 27-nm particles that comprise the virion nucleocapsid (HBcAg). Although HBeAg and HBcAg are serologically distinct, these Ag are cross-reactive at the level of Th cell recognition because they are colinear throughout most of their primary sequence. The HBcAg possesses unique immunologic features. For example; HBcAg can function as both a T cell-independent and a T cell-dependent antigen (1); HBcAg is approximately 1000-fold more immunogenic than the HBeAg (2); HBcAg preferentially primes Th1 cells, whereas, HBeAg preferentially primes Th2cells (3); HBcAg-specific Th cells mediate anti-envelope as well as anti-HBc antibody production (4); and HBcAg is an effective carrier platform for heterologous epitopes (5). These characteristics are unique to and require the particulate structure of the HBcAg. A number of hypotheses have been suggested to explain the enhanced immunogenicity of HBcAg relative to HBeAg and to other particulate antigens. We have previously suggested that the orientation of the array of protein spikes distributed over the surface of the HBcAg shell may be optimal for crosslinking B cell membrane receptors (BCR) and upregulation of costimulatory molecules (6), especially because the dominant B cell epitopes are positioned at the tip of the spikes (7, 8). Furthermore, we have previously reported that B cells rather than non-B cell professional APCs such as dendritic cells or macrophages (DC/MØ) act as the primary APC for HBcAg, which may explain its enhanced immunogenicity especially in terms of antibody production (6). Other investigators have suggested that the binding of the protamine-like C-terminus of HBcAg to cell membrane heparan sulfates (HS) or HS and chondroitin sulfates (CS) may play a role in the enhanced immunogenicity of HBcAg (9, 10). An interaction between HBcAg and TLR-2 has been proposed (9). It has also been suggested that contaminating TLR-4 and TLR-2 ligands present in E. coli-derived HBcAg may explain its enhanced immunogenicity (11, 12). Lastly, the presence of encapsidated ssRNA bound to the protamine-like domain within full-length recombinant HBcAg produced in E. coli or yeast expression systems has been shown to enhance Th1-type immune responses (13).
In an attempt to clarify or consolidate these disparate hypotheses, we examined antigen presentation of the HBcAg in vitro and in vivo in detail to determine the ability of B cell subpopulations and DC/MØ cells to function as primary APCs for either exogenous or intracellular HBcAg particles. Furthermore, the possible role of HBcAg-specific TLR-mediated activation of APCs was explored. These studies reveal the involvement of the innate immune system in a rather unique APC pathway for exogenous HBcAg particles which is dependent on the mode of immunization.
MATERIALS AND METHODS
Mice
C57BL/10 (B10), B10.S and 7/16-5 TCR-Tg mice were obtained from the breeding colony of the Vaccine Research Institute of San Diego (VRISD). The B cell knock-out (μMT) mice originally produced by K. Rajewsky (14) were backcrossed onto B10, B10.S and 7/16-5 TCR-Tg backgrounds. The TLR-7KO mice were obtained from Dr. Richard A. Flavell (Yale University) and bred at the VRISD. The C3H.HeJ and C3H.HeSn mice and the CBA/J and CBA/N (xid) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal care was performed according to National Institutes of Health standards as set forth in the Guide for the Care and Use of Laboratory Animals (1996).
Recombinant Proteins and Synthetic Peptide
Recombinant full length HBcAg183 of the ayw subtype was produced in Escherichia coli as described previously (5). Yeast-derived HBcAg183 (ayw) was obtained commercially from Meridian Life (Saco, ME). A truncated version of HBcAg (residues 1 to 149) was produced in E. coli. This material is particulate but expresses both HBcAg and HBeAg antigenicity at the level of antibody recognition and is designated HBcAg149 or HBcAg150C (additional C-terminal cysteine for biotinylation). A recombinant HBeAg corresponding in sequence to serum-derived HBeAg encompassing the 10 precore amino acids remaining after cleavage of the precursor and residues 1 to 149 of HBcAg was produced as described previously (15). The presence of the 10 precore amino acids prevents particle assembly and HBeAg is recognized efficiently by an HBeAg-specific monoclonal antibody (Mab) but displays little HBc antigenicity. Recombinant hybrid-WHcAg (woodchuck hepatitis core antigen) particles containing a P. falciparum malaria circumsporozoite repeat sequence (NANPNVDPNANP3) inserted in the external loop were produced as previously described (16). Anti-HBc Mabs 3105 and 3120 were purchased from the Immunology Institute (Tokyo, Japan).
Purification of core antigens
The core proteins were precipitated from the bacterial lysate by the addition of solid ammonium sulfate to 45% saturation (277 g/liter). The precipitates were collected by centrifugation, redissolved in a minimum of buffer (10 mM sodium phosphate buffer, pH 6.8), and dialyzed extensively against the same buffer. The protein solutions were then applied to a Bio-Rad BioGel HTP hydroxyapatite column (5 cm × 5 to 10 cm, depending on amount of protein) and eluted with 50 mM sodium phosphate buffer, pH 6.8. The core antigens pass through unretained. The proteins were then applied to a Sepharose CL-4B column (5 × 100 cm).
Endotoxin was removed from the core preparations by a modification of a phase separation with Triton X-114 (17). A solution of the protein at a concentration of 5 mg/ml was made with 1% Triton X-114 and incubated at 4° C for 30 min with constant stirring. The solution was then incubated at 37° C for 10 min and then centrifuged at 20,000 x g for 10 min. The protein solution was recovered from above the detergent. This procedure was repeated four times. Finally, the protein was precipitated by lowering the pH to 5. Residual detergent remained in solution. The protein was recovered by centrifugation and dissolved in endotoxin-free buffer. Prior to Triton X-114 treatment, the core preparations contained approximately 10 ng of endotoxin/μg HBcAg; after phase separation with Triton X-114, the endotoxin content was between 0.01 ng/μg HBcAg to an undetectable amount as determined by the QCL-1000 chromogenic Limulus amoebocyte lysate endpoint assay (Cambrex, East Rutherford, NJ). Yeast-derived HBcAg183 contained no measurable endotoxin. Unless otherwise specified, only endotoxin free HBcAg preparations were used.
Synthetic peptides derived from the HBcAg or WHcAg sequences or the P. falciparum repeat sequence were synthesized by the simultaneous peptide synthesis method as previously described (18).
Plasmid DNA and tumor cell line expressing HBcAg
An HBcAg gene fragment (552 nt) of the ayw subtype was amplified and cloned into the eukaryotic expression vector pVAX1 (Invitrogen, San Diego, CA) as previously described (19). The HBcAg expression plasmid was designated HBcAg-pVAX1. Plasmid DNA was grown and purified as described (20). The purified plasmid DNA was dissolved and diluted in sterile PBS to a concentration of 1 mg/ml. A Rauscher virus-induced T cell lymphoma (RBL-5) cell line (H-2b) stably expressing HBcAg (RBL-5/C) (21) was provided by F. Chisari (TSRI, La Jolla, CA).
Biotinylation of HBcAg150C
Recombinant HBcAg150C (2 mg/ml) in 1.0 ml of PBS containing 1 mM EDTA was mixed with 100 μl of a Biotin-HPDP stock solution (4 mM) (Pierce Chemical Co., Rockford, IL), vortexed and the reaction mixture was incubated for 2 hrs. at room temperature. Excess reagents were removed by dialysis against PBS. Based on the absorbance change at 343 nm, the biotin reacted with between 0.5 and 1 cysteine/monomer (240 monomers/particle).
Immunization protocols
For protein immunization with HBcAg/WHcAgs, groups of 3–5 female mice were injected intraperitoneally (i.p.) with 10–20 μg of protein either in saline or emulsified in complete Freund’s adjuvant (CFA) or incomplete Freund’s adjuvant (IFA) for both antibody production and T cell experiments. For DNA immunization, groups of 5–10 female mice were injected with 100 μg plasmid DNA in PBS intramuscularly (i.m.) in the tibialis cranialis muscle. Mice were boosted at 4 weeks. For immunization with the RBL-5/C tumor cell line, 5 × 106 RBL-5/C tumor cells were given subcutaneously (s.c.).
ELISA for detection of murine antibodies
Sera were collected by retro-orbital bleeding of isofluorane-anesthetized mice and pooled. Antibodies were detected by an indirect solid-phase ELISA with rHBcAg or rHBeAg or P. falciparum repeat peptides as the solid-phase ligands as described previously (2). Serial dilutions of both test and pre-immunization sera were made and the data were expressed as antibody end-point titres, in which the highest dilution of sera required to yield an optical density (492 nm) three times the pre-immunization sera was considered positive. IgG-, IgG1-, IgG2a-, IgG2b-, and IgG3-specific secondary antibodies were purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL).
Determination of CD4+ cytokine production
Spleen cells from either primed wild-type or B cell KO mice were cultured (5 × 106 cells/ml) with various concentrations of a panel of antigens. Culture supernatants were harvested at 48 hours for interleukin-2 (IL-2) determination and at 96 hours for interferon-gamma (IFN-γ) determination. Cytokines were measured using commercial ELISA kits according to the manufacturer’s protocol (eBiosciences).
Detection of HBcAg-specific lytic CTLs
Spleen cells derived from DNA plus tumor cell immunized B10 mice were re-suspended in complete RPMI 1640 medium and in vitro stimulation was carried out for five days in the presence of 0.05 μM of the H-2Kb-restricted HBcAg93–100 peptide (sequence MGLKFRQL) (22). After five days of in vitro stimulation, effector cells were harvested and a four-hour 51Cr assay was performed in 96-well V-bottom plates in a total volume of 200μL. A total of 1 × 106 target cells (e.g. RMA-S) were pulsed with 50 μM of the H-2Kb-restricted HBcAg93–100 peptide for 90 minutes and then subsequently labeled for one hour with 25μL of 51Cr (5 mCi/ml, MP Biomedicals, Solon, OH) and then washed three times in PBS. Different numbers of effectors and 51Cr-labeled target cells (5 × 103 cells/well) were added to wells at effector:target (E:T) ratios of 100:1, 50:1, and 25:1. The level of cytolytic activity was determined after incubation of effectors and targets for four hours.
Fractionation of cell populations
Five to 10-week-old 7/16-5 TCR Tg mice were used as a CD4+ T cell source. Single cell suspensions of splenocytes were labeled with anti-CD4-Microbeads (Miltenyi Biotec, Auburn, CA) and passed through a LS column (Miltenyi Biotec); the cells retained in the column were collected as CD4+ T cells. For fractionation of B cells, splenocytes from B10 mice were labeled with anti-CD90.2-Microbeads to deplete T cells and then passed through the LS column; cell contained in the flow-through were termed enriched B lineage cells. Enriched B cells were then labeled with anti-CD5-Microbeads for positive selection of B1a cells. Similarly, CD5+ B1a cells were collected from peritoneal exudate cells (PEC). Purity of B1a cells was subsequently analyzed by FACS. For isolation of the splenic B1b population (B220+ CD21−/low CD11b+), flow through from the B1a cells were further labeled with CD11b-Microbeads and positively selected as an enriched B1b cell population. FACS analysis was performed to determine the purity and showed > 75% were B220+ CD11b+. Flow through from this procedure was collected and used as B2 cells. Bone marrow derived DC (BMDC) were prepared by an established protocol. Briefly, bone marrow progenitor cells were harvested from femur and tibia bones of B10 mice and negatively depleted of B cells using B220-Microbeads. BM progenitor cells were cultured in RPMI-1640 containing 10% FCS with 20 ng/ml of recombinant mouse (rm) GM-CSF. On day 2, supernatant and non-adherent cells were aspirated and replaced with fresh media containing rmGM-CSF. Adherent immature DCs were harvested by gentle pipetting on day 7.
Flow cytometry analysis
To analyze T cell activation, antigen specific T cells from 7/16-5 TCR-Tg mice (V 11+ CD4+ T cell) were cultured with HBcAg or p120–140 peptide for 4 days. At day 4, cultured cells were stained for CD25. For surface staining, cells were incubated in 3% FBS in PBS containing 10 μg/m l 2.4G2 to block FcR binding followed by the addition of fluorochrome-conjugated antibodies. All fluorochrome-conjugated Abs were obtained from eBioscience (San Diego, CA). Flow cytometry was performed on a LSR flow cytometer (Becton Dickinson, CA) at the Salk Institute (La Jolla, San Diego). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
TLR ligand screening
TLR stimulation was tested by assessing NF-Kβ activation in HEK293 cells ectopically expressing seven different human TLRs including: TLR 2, 3, 4, 5, 7, 8 and 9, and a reporter plasmid, pNiFty, carrying the human secreted embryonic alkaline phosphatase (SEAP) gene controlled by an NF-Kβ inducible ELAM-1 composite promoter (InvivoGen, San Diego, CA). Four HBc/HBeAg preparations were incubated with the TLR-expressing HEK293 cells at a concentration of 2.0 μg/ml. The four HBc/HBeAg preparations varied in the amounts of contaminating LPS ranging from 10 ng/μg to undetectable.
Core protein binding assay
B1a and B2 cells were prepared as described above. Biotinylated HBc150c was added to 1 × 106 B cells and incubated at 4°C for 1 hr. Cells were collected and stained for B220 and sAv-PE for further FACS analysis.
Statistical analysis
Data were analyzed using the student’s t test. SPSS Inc. (Chicago, IL) software was used for all statistical analysis.
RESULTS
Resting B cells but not DC/MØ can efficiently present HBcAg and prime naïve T cells
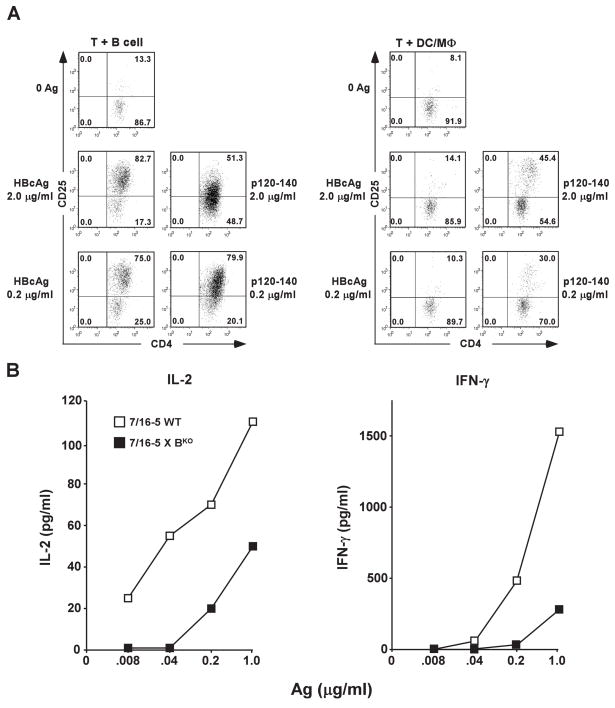
Fractionated B cells (B220+) or DC/MØ cells representing the APC source were co-cultured with naïve HBcAg-specific, splenic CD4+ T cells derived from 7/16-5 TCR-Tg mice (Fig. 1A). Both APC populations are capable of presenting the HBcAg-derived peptidic p120–140 antigen to naïve 7/16-5 CD4+ T cells resulting in T cell activation as demonstrated by up-regulation of cell surface CD25 (Fig. 1A) or T cell proliferation (Fig. 3A). However, only splenic B cells efficiently presented HBcAg183 to naïve 7/16-5 CD4+ T cells resulting in HBcAg dose-dependent T cell activation (Fig. 1A). In a similar experiment, naïve 7/16-5 TCR-Tg spleen cells or naïve spleen cells derived from 7/16-5 TCR-Tg mice bred onto a B cell KO background (7/16-5 × BKO) were cultured with HBcAg183 and HBcAg-specific presentation and T cell activation were measured by IL-2 and IFNγ cytokine production. Efficient HBcAg-specific IL-2 and IFNγ production required the presence of B cells (Fig. 1B). Note that in both experiments the HBcAg-specific CD4+ T cells and the B cell APCs were resting and unprimed (naïve), an advantage of using TCR-Tg mice because the high frequency of HBcAg-specific CD4+ T cells precludes the necessity for immunization.
Figure 1.
Naïve B cells function as APC for naïve, HBcAg-specific CD4+ T cells in vitro. (A). Fractionated, HBcAg-specific CD4+ T cells (1 × 106) derived from three unprimed, TCR-Tg mice (7/16-5) were co-cultured for 4 days with either purified, resting splenic B cells(1 × 106) or splenic DC/MØ (1 × 106) derived from three wild-type mice plus the indicated concentrations of HBcAg183 or the HBcAg-derived peptide (p120–140). CD4+ T cell activation was measured by CD25 expression as determined by FACS analysis. (B). Unprimed spleen cells pooled from either three wild-type (WT) 7/16-5 TCR-Tg mice or three 7/16-5 TCR-Tg mice bred on a B cell knock-out (BKO) background were cultured (5 × 105) with the indicated concentrations of HBcAg183 and IL-2 and IFNγ production were determined by ELISA at day 2 or day 4, respectively. Values are given as pg/ml. This experiment was performed on two occasions and the results are representative.
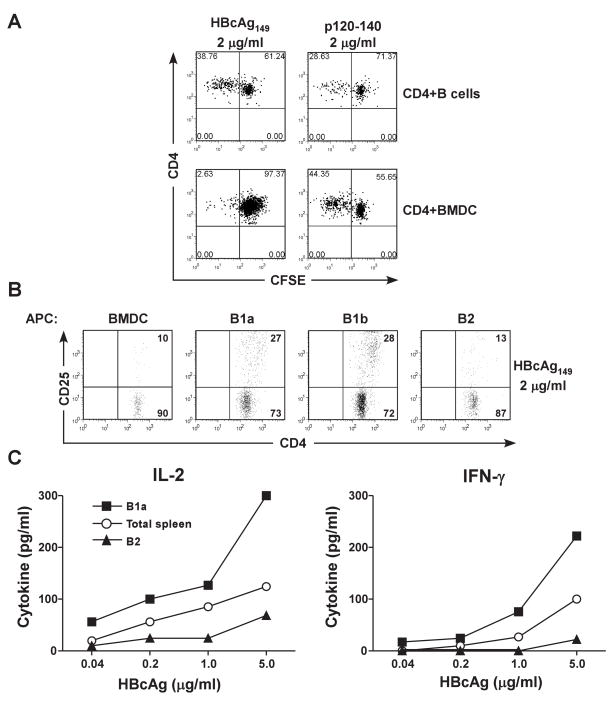
Figure 3.
Preferential antigen presentation of HBcAg by B1a and B1b cells. (A). Fractionated, splenic B cells (4 × 105) or BMDC (4 × 105) were co-cultured with unprimed, CFSE-labeled 7/16-5 TCR-Tg CD4+ T cells (1 × 105) in the presence of 2 μg/ml of HBcAg149 or p120–140 peptide. After 4 days, dilution of CFSE as a measure of CD4+ T cell proliferation was determined by FACS analysis. (B). The indicated fractionated cell populations (4 x 105) (BMDC, splenic B1a, splenic B1b and splenic B2 cells) derived from unprimed B10 mice were cultured with HBcAg149 (2.0 μg/ml) and CD4+ T cells (1 × 105) derived from 7/16-5 TCR-Tg mice. After 4 days of in vitro culture, CD4+ T cell activation was measured by CD25 expression determined by FACS analysis. (C). Unprimed, B10, total spleen cells, fractionated splenic B2 cells or fractionated, splenic B1a cells (4 × 105) were co-cultured with unprimed, 7/16-5 TCR-Tg CD4+ T cells (1 × 105) and HBcAg149 (.04–5.0 μg/ml) and CD4+ T cell activation was measured by IL-2 (day 2) and IFNγ (day 4) production as determined by ELISA. The results are representative of experiments performed on 2 separate occasions.
To determine if the preferential B cell presentation of HBcAg was unique to 7/16-5 CD4+ T cells and/or relevant in vivo, B10.S/+/+ mice or B10.S/BKO mice were immunized with HBcAg183 either emulsified in CFA, IFA, or in saline (only the results of HBcAg/CFA immunization are shown in figure 2). In B10.S/+/+ mice, HBcAg efficiently primed T cells measured by IL-2 and IFNγ production in response to a panel of recall antigens (HBcAg, non-particulate HBeAg and the dominant peptidic T cell site, p120–140). The HBcAg antigen was the superior recall antigen in vitro. In contrast, HBcAg-specific T cells were NOT efficiently primed in B10.S/BKO mice (Fig. 2). This defect was specific for the HBcAg since the particulate envelope protein of HBV, HBsAg, primed HBsAg-specific T cells equally in +/+ and BKO mice (Fig. 2, inset). It was also notable that the low number of T cells primed in B10.S/BKO mice by HBcAg were more efficiently activated in vitro by HBeAg and p120–140 rather than the HBcAg demonstrating that HBeAg and p120–140 are not as dependent on B cells for APC function as the particulate HBcAg.
Figure 2.
Efficient CD4+ T cell priming in vivo with soluble HBcAg requires B cells. Groups of 3 B10.S/+/+ or B10.S/BKO mice were immunized (i.p.) with HBcAg183 (10μg) emulsified in CFA. Ten days after immunization spleen cells were harvested, pooled and cultured with the indicated concentrations of a panel of recall antigens (HBcAg183, HBeAg and p120–140). Culture supernatants were collected at days 2 and 4 for the determination of IL-2 and IFNγ, respectively, by ELISA. Groups of 3 mice each were also immunized with the HBsAg (20 μg, CFA) and spleen cells were cultured with varying concentrations of HBsAg in vitro (INSET). This experiment was performed on at least 3 occasions and the results are representative.
B cell subsets preferentially present HBcAg to naïve CD4+ T cells
We next examined whether a specific B cell subset was responsible for the APC function. Fractionated, naïve HBcAg-specific CD4+ T cells (from 7/16-5 TCR-Tg mice) were labeled with CFSE and co-cultured with fractionated splenic B cells or BMDC as APCs (Fig. 3A). Only CD4+ T cells co-cultured with splenic B cells proliferated efficiently in the presence of HBcAg149. Note that both B cells and BMDC were efficient APC for the HBcAg-derived peptide, p120–140. Importantly, splenic B1a cells and splenic B1b cells were superior APC as compared to splenic B2 cells and BMDC were unable to present HBcAg149 efficiently to naïve HBcAg-specific CD4+ T cells as measured by CD25 up-regulation on CD4+ T cells (Fig. 3B) and IL-2 and IFNγ cytokine production in vitro (Fig. 3C).
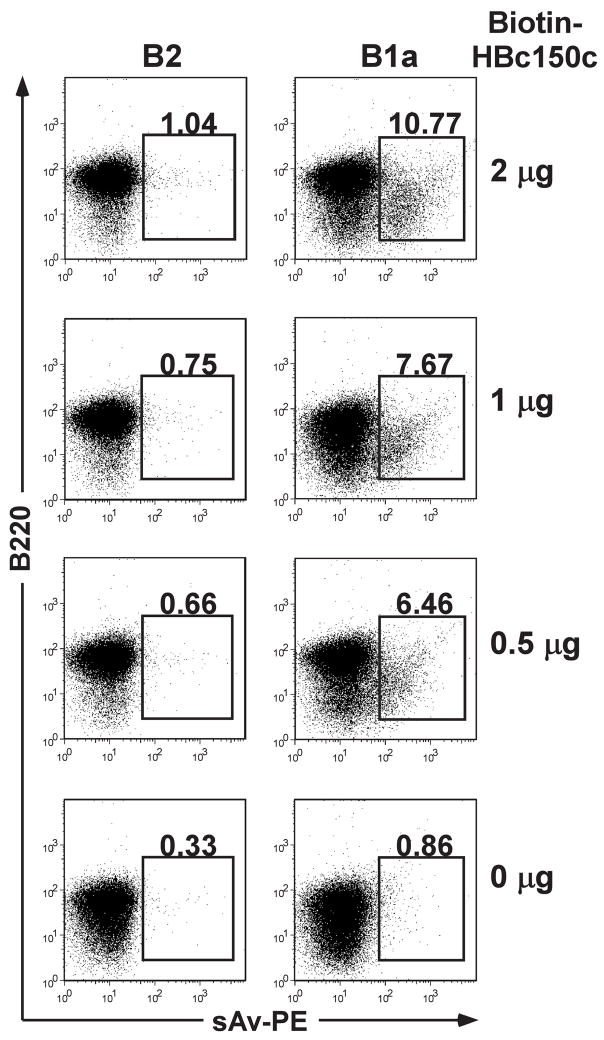
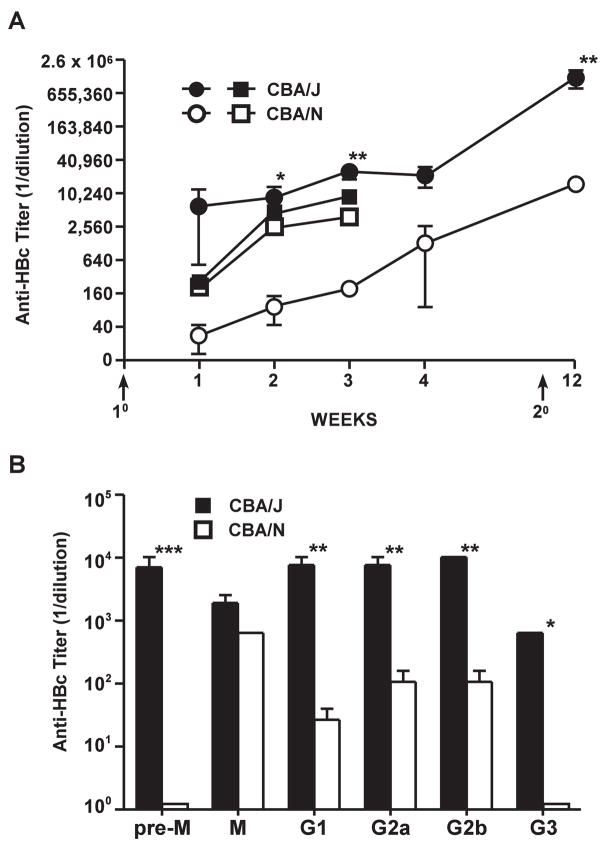
Furthermore, direct binding studies of biotinylated-HBcAg150C particles to separated BMDCs, splenic B2 cells or splenic B1a cells were performed. Figure 4 demonstrates that biotinylated-HBcAg150C binds preferentially to an enriched B1a cell population in a dose-dependent manner and not to a B2 cell population. BMDCs do not bind biotinylated-HBcAg150C (data not shown). Further, to illustrate the consequences of B1a cell APC function in vivo, we immunized CBA/N (xid) mice, which lack B1a cells, with HBcAg149 in saline and compared the response to CBA/J control mice (Fig. 5). Significantly lower and slower developing anti-HBc antibody responses were apparent in CBA/N (xid) mice. Note, however, that CBA/N (xid) mice did demonstrate a moderate secondary anti-HBc antibody response. Therefore, it appears that B1a cells may be responsible for the early and/or initial anti-HBc antibody response. As a further control, these two strains were also immunized with the HBsAg and no significant differences were observed in the anti-HBs antibody responses. We also measured IgM and the various anti-HBc IgG isotype responses in CBA vs. CBA/N mice (Fig. 5B). Note that CBA/J mice possess IgM anti-HBc antibodies prior to immunization (pre-M), whereas, CBA/N mice do not. All murine strains previously tested possess IgM anti-HBc antibodies spontaneously, which likely represents “natural” antibody produced by the innate immune system; in mice at least, this appears to be produced by B1a cells since it is absent in CBA/N mice. However, upon immunization CBA/N mice did produce IgM anti-HBc antibodies in levels equal to CBA/J mice (Fig. 5B). Therefore, pre-existing IgM anti-HBc is produced by B1a cells and IgM anti-HBc produced in response to immunization is produced by another B cell subset, most likely B1b cells or marginal zone B2 cells. CBA/J mice produced significantly more IgG anti-HBc antibodies of all IgG isotypes than CBA/N mice.
Figure 4.
Splenic B1a cells preferentially bind biotinylated HBcAg150C. Enriched B2 cells or B1a cells (1 × 106) were incubated at 4°C for 1 hr. with the indicated concentrations of biotinylated HBcAg150C. Cells were collected and stained for the B cell marker, B220 and for streptavidin binding and analyzed by FACS.
Figure 5.
Anti-HBc antibody production in vivo is influenced by B1a cells. (A). Groups of 5 wild-type CBA/J or CBA/N (xid) mice were immunized (1°) with HBcAg149 (20 μg in saline) and boosted (2°) (i.p.) with 10 μg of HBcAg149 in saline and serum anti-HBc antibody levels determined by ELISA at the indicated times (○-○, ●-●). Serum anti-HBc IgG antibody was quantitated by endpoint dilution of sera (titer). Groups of 3 mice each were also immunized (1°) with HBsAg (20 μg, IFA) and anti-HBs antibody determined by ELISA (□-□, ■- ■). (B). Groups of 3 CBA/J or CBA/N (xid) mice were immunized (i.p.) with HBcAg149 (20 μg insaline) and 4 weeks later serum IgM and IgG isotype- specific anti-HBc antibodies were determined by ELISA. Pre-M, pre-existing IgM anti-HBc (prior to immunization). Results represent the mean values (± SD) of individual mouse sera (*P = 0.02, **P = 0.005, ***P = 0.002).
The requirement for B cell APCs does not apply to immune-complexed HBcAg or HBcAg expressed intracellularly
The preceding experiments were performed using recombinant protein HBcAg as antigen and immunogen and demonstrated that DC/MØ cells do not serve as efficient APC for exogenous HBcAg. However, a single immunization with a DNA plasmid encoding the HBcAg (HBcAg-pVAX1) did not require B cells as APC for efficient CD4+ T cell priming (Fig. 6A). Both wild-type B10 mice and B10/BKO mice immunized with HBcAg-pVAX1 demonstrated efficient HBcAg-, HBeAg- and p120–140-specific T cell cytokine responses in splenic in vitro antigen recall cultures (Fig. 6A). We have previously shown that a prime/boost immunization strategy using HBcAg-pVAX1 and a boost with an HBcAg-expressing tumor cell line (RBL-5/C) elicits an efficient CD8+ CTL response to the HBcAg (23). The induction of an efficient CD8+ CTL response to intracellular HBcAg also does not require B cells as APC (Fig. 6B). It appears that DC/MØ APC can “cross-present” intracellular HBcAg derived from transfected or tumor cells. Furthermore, non-B cell APC can process and present HBcAg when it is complexed to anti-HBc Mabs (Mabs 3105 + 3120) but not free HBcAg. In contrast, B cell APCs can present free HBcAg or immune-complexed HBcAg, as shown in figure 7. Therefore, it appears that DC/MØ can process and present HBcAg to naïve CD4+ and CD8+ T cells but do not efficiently bind and internalize free exogenous HBcAg even though it is a particulate antigen. This most likely reflects the absence of any ligands for DC/MØ endocytic receptors on HBcAg (ie., no carbohydrate or lipid).
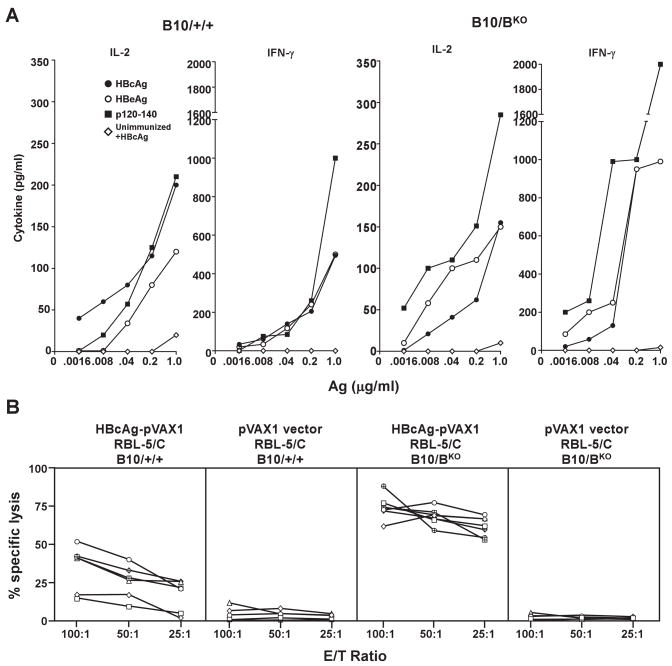
Figure 6.
Plasmid DNA immunization bypasses the requirement for B cell APCs to prime HBcAg-specific CD4+ and CD8+ T cells. (A). Groups of 3 B10/+/+ or B10/BKO mice were immunized i.m. with 100 μg of HBcAg-pVAX1 or unimmunized. Four weeks after immunization spleen cells were harvested and pooled and cultured for 2 days (IL-2) or for 4 days (IFNγ) with the indicated concentrations of HBcAg183, HBeAg or p120–140 as recall antigens and T cell priming was determined by measuring IL-2 and IFNγ production in culture supernatants by ELISA. For unimmunized groups only the response KO to HBcAg183 is shown. Values are given as pg/ml. (B). Groups of 6 B10/+/+ or B10/B mice were immunized i.m. twice with 100 μg of HBcAg-pVAX1 or the empty pVAX1 vector (4 mice/group). All groups were boosted with RBL-5/C (5 × 106) tumor cells given s.c. Six weeks after the tumor cell boost, spleens were harvested and restimulated for 5 days in the presence of the HBcAg93–100 H-2Kb-restricted peptide and thereafter used in a standard 4 hr. Cr51-release assay at the indicated E:T ratios. Each line represents an individual mouse.
Figure 7.
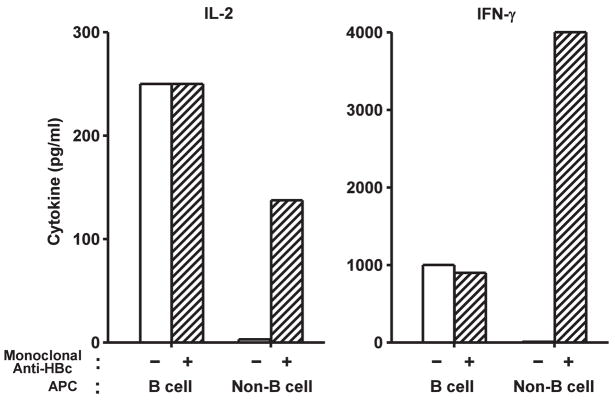
Non-B cell APCs can process and present immune-complexed HBcAg to naïve, CD4+ T cells but not free HBcAg. Either free HBcAg183 (1 μg/ml) or HBcAg183- complexed to HBcAg-specific Mabs (3105 + 3120) (2 μg/ml) were added to 7/16-5 TCR-Tg spleen cells (B cell+) or 7/16-5/BKO spleen cells (B cell−) and CD4+ T cell activation was determined by IL-2 (day 2) and IFNγ (day 4) cytokine production in culture supernatants measured by ELISA. Values are express as pg/ml. The results are representative of 3 separate experiments.
Potential role of TLRs in antigen presentation of the HBcAg
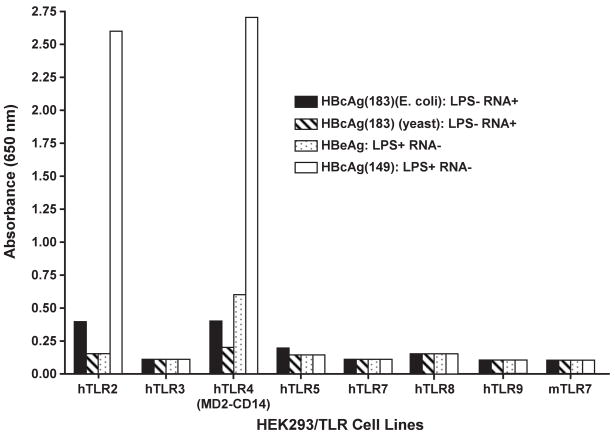
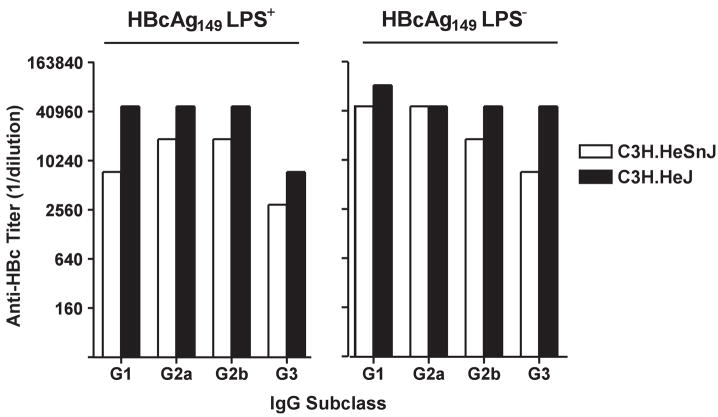
Various preparations of HBcAg and the nonparticulate HBeAg were examined for the ability to signal through a number of human TLRs (Fig. 8). Only a truncated HBcAg149 preparation, which contained E. coli-derived LPS as a contaminant (10ng/μg HBcAg), signaled through TLR-2 and TLR-4, whereas, the “cleanest” HBcAg183 preparation, which was produced in yeast, demonstrated zero TLR signaling. Therefore, it appears that the HBcAg/HBeAg proteins do not possess TLR-binding capability, but the LPS that can co-purify with rHBcAg produced in E. coli can bind and signal through TLRs-2 and 4. Recently, a number of publications have suggested that this contaminating LPS may explain the enhance immunogenicity of HBcAg in mice and that researchers have been unaware of this problem (11, 12). Although we disagree that researchers have not been aware of this issue and, in fact, have rigorously controlled for the presence of LPS, it was important to address the possible effects contaminating LPS may have on HBcAg immunogenicity studies in mice. Therefore, LPS-sensitive (C3H.HeSn) and congenic LPS-insensitive (C3H.HeJ) mice, which possess a mutation preventing TLR-4 signaling, were immunized with either a highly LPS-contaminated (10ng/μg) HBcAg149 preparation or an LPS-negative HBcAg149 preparation and anti-HBc antibody IgG isotype distribution was then determined. As shown in figure 9, in LPS-sensitive C3H.HeSn mice the LPS-negative HBcAg149 preparation elicited equal or greater anti-HBc antibodies of all IgG isotypes than the LPS-positive HBcAg149 preparation. Reciprocally, in LPS-insensitive mice the LPS-negative and LPS-positive HBcAg149 preparations elicited equal anti-HBc IgG isotype responses with the exception of a higher IgG3 anti-HBc response elicited by the LPS-negative HBcAg149 (Fig. 9). Therefore, the level of LPS contamination likely to occur in E. coli-derived HBcAg preparations is not determinative in terms of the humoral anti-HBc response in vivo and cannot explain the enhanced immunogenicity of HBcAg in mice.
Figure 8.
The HBcAg protein is not a TLR ligand. Four HBc/HBeAg preparations were screened for TLR ligand activity on a panel of human TLR expressing HEK293 cells. TLR ligand activity was determined by NF-Kβ activation as described in Methods and Materials. The 2 full length HBcAg183 preparations contained no endotoxin, the HBeAg preparation contained low level endotoxin (1.0 ng/ml) and the truncated HBcAg149 preparation was contaminated with 10 ng/ml of endotoxin.
Figure 9.
Endotoxin contamination of HBcAg does not enhance anti-HBc antibody production in vivo. Groups of 3 LPS-sensitive (C3H.HeSnJ) or LPS-insensitive (C3H.HeJ) mice were immunized with either a highly (LPS+) HBcAg149 preparation (10 ng/ml) or an HBcAg149 preparation free of LPS contamination (LPS−). Mice were immunized i.p. with 10 μg of HBcAg149 in saline and 5 weeks later serum anti-HBc IgG isotypes were measured by ELISA.
Role of TLR-7
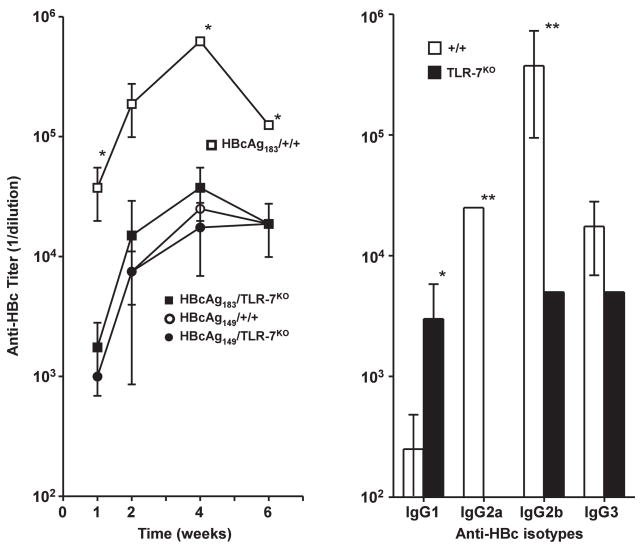
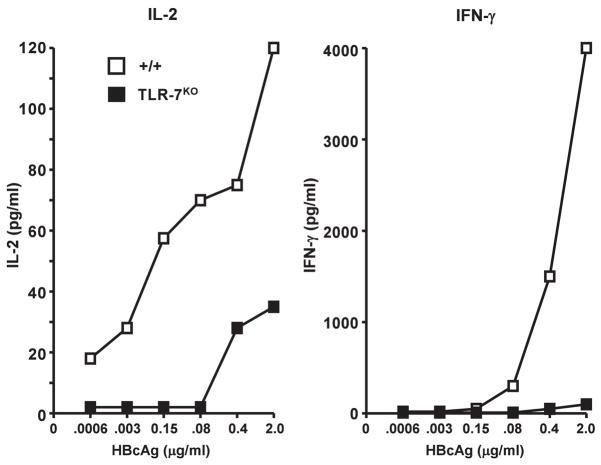
Full-length HBcAg183, which contains a protamine-like C-terminus, encapsidates pregenomic RNA during natural infection and recombinant HBcAg encapsidates ssRNA when produced in prokaryotic or eukaryotic expression systems. Further, the enhanced immunogenicity of full-length versus truncated HBcAg is well known and has been attributed to the encapsidated ssRNA (13). However, formal proof that the encapsidated ssRNA acts as a TLR-7 ligand is lacking. Therefore B10/+/+ and B10/TLR-7KO mice were immunized with either truncated HBcAg149 or full-length HBcAg183 and anti-HBc antibody production was then determined. As shown in figure 10, immunization (10μg in saline) with full-length HBcAg183 in B10/+/+ mice elicited significantly higher titer (approximately 25-fold) anti-HBc antibody at every time point compared to truncated HBcAg149. Full-length HBcAg183 also elicited significantly higher titer anti-HBc antibody in B10/+/+ mice as compared to B10/TLR7KO mice. Truncated HBcAg149 was equally immunogenic in B10/+/+ and B10/TLR7KO mice. Also note that the humoral response to full-length HBcAg183 in B10/TLR7KO mice was identical to the response to the truncated HBcAg149 in either B10/+/+ mice or B10/TLR7KO mice. Furthermore, the anti-HBc IgG isotype distribution was dramatically influenced by the absence of TLR-7 signaling (Fig. 10). Anti-HBc IgG1 was enhanced in TLR-7KO mice, IgG2a anti-HBc antibodies were totally absent in TLR-7KO mice and IgG2b anti-HBc antibodies were significantly reduced in TLR-7KO mice as compared to wild-type mice. Anti-HBc IgG3 antibodies were not significantly different in wild-type versus TLR-7KO mice. These results indicate that the ssRNA of bacterial origin that is encapsidated within full-length HBcAg183 acts as a TLR-7 ligand and enhances anti-HBc antibody production while shifting IgG isotype distribution via signaling through endosomal TLR-7 within B cells or other APCs. These results also indicate that the difference in immunogenicity between full-length HBcAg183 and truncated HBcAg149 resides solely in ssRNA signaling through TLR-7 because HBcAg183 and HBcAg149 are equivalently immunogenic in TLR-7KO mice. Recombinant HBcAg183 expressed in E. coli or yeast behaves similarly, therefore, both prokaryotic and eukaryotic ssRNA within HBcAg can function as TLR-7 ligands. Immunization with a pVAX1 DNA vector encoding full length HBcAg183 elicits greater anti-HBc antibody production than a pVAX1 vector encoding truncated HBcAg149 suggesting that mammalian ssRNA can also function as a TLR-7 ligand (data not shown). The ssRNA-TLR-7 pathway predictably affected HBcAg-specific CD4+ T cell priming as well as in vivo anti-HBc antibody production as shown in Figure 11. Splenic T cells derived from B10/TLR7KO mice immunized with HBcAg183 (ie., ssRNA+) produced significantly less IL-2 and IFNγ than T cells derived from B10/+/+ immunized mice in HBcAg recall spleen cultures.
Figure 10.
Enhanced immunogenicity of full length HBcAg183 is mediated by TLR-7 signaling. (A). Groups of 3 wild-type B10/+/+ or B10/TLR-7KO mice were immunized i.p. with 10 μg in saline of either full length HBcAg183 (ssRNA+) or HBcAg149 (ssRNA−). At 1, 2, 4 and 6 weeks after immunization IgG anti-HBc antibodies in pooled sera were measured by ELISA. (B). Groups of 3 wild-type B10/+/+ mice or B10/TLR-7KO mice were immunized i.p. with 10 μg in saline of full length HBcAg183 (ssRNA+). Two weeks after immunization anti-HBc antibodies of the indicated IgG isotypes were measured in pooled sera by ELISA. The results depicted represent mean values (± SD) of experiments performed using pooled serum samples on 2 separate occasions (*P = 0.05, **P = 0.005).
Figure 11.
HBcAg-specific T cell cytokine production is regulated by TLR-7 signaling. Groups of 3 B10/+/+ and B10/TLR-7KO mice were immunized i.p. with 10μg of full length HBcAg183 (ssRNA+) in saline and 4 weeks later in vitro splenic recall cultures were performed in the presence of the indicated concentration of HBcAg183. HBcAg- specific IL-2 (day 2) and IFNγ (day 4) production was measured by ELISA and expressed as pg/ml. The results are representative of 2 separate experiments.
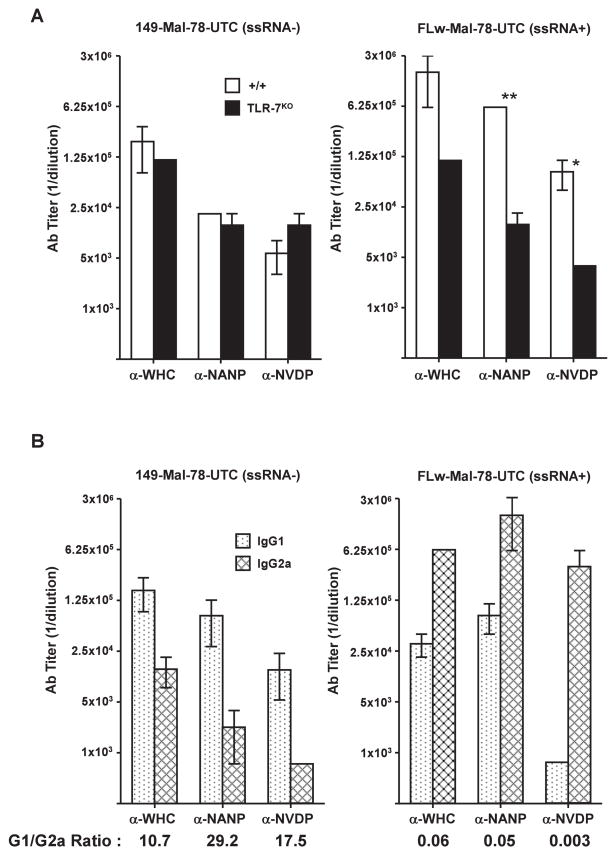
Because the woodchuck hepadnavirus core protein (WHcAg) has been developed as a vaccine carrier platform (16), it was of interest to determine if TLR-7 signaling was also relevant to immunization with hybrid-WHcAg particles. For this purpose, full-length WHcAg187 (ssRNA+) carrying two P. falciparum malaria neutralizing B cell epitopes (ie., NANP and NVDP) in the loop region at position 78 and a truncated WHcAg149 (ssRNA−) particle carrying the identical malaria B cell inserts were used to immunize B10/+/+ and B10/TLR7KO mice; anti-WHc and anti-insert antibody responses were then compared. As shown in Figure 12A, the anti-WHc, anti-NANP and anti-NVDP antibody responses were significantly higher (13–25 fold) in wild-type (+/+) mice immunized with the ssRNA+ hybrid-WHcAg particle as compared to the ssRNA− particle. Furthermore, the ssRNA+ hybrid-WHcAg particle elicited 25-fold higher anti-insert antibody responses in B10/+/+ mice as compared to B10/TLR7KO mice demonstrating the positive effect of signaling through TLR-7. Note also that the immunogenicity of ssRNA+ and ssRNA−hybrid-WHcAg particles were virtually identical in TLR-7KO mice, again illustrating that only TLR-7 signaling distinguished between the full-length and truncated hybrid-WHcAg particles. Furthermore, immunization of B10/+/+ mice with ssRNA+ hybrid-WHcAg particles elicited IgG2a-predominant anti-WHc, anti-NANP and anti-NVDP antibodies, whereas, ssRNA− hybrid-WHcAg particles elicited IgG1-predominant antibody responses (Fig. 12B).
Figure 12.
Antibody production to hybrid-WHcAg particles is influenced by TLR-7 signaling. (A). Groups of 3 B10/+/+ or B10/TLR-7KO mice were immunized i.p. with 20 μg of either truncated hybrid-WHcAg (149-Mal-78-UTC) (ssRNA−) or full length hybrid-WHcAg (FLW-Mal-78-UTC) (ssRNA+) particles emulsified in IFA. Six weeks after immunization anti-WHc (carrier), anti-NANP (insert) and anti-NVDP (insert) antibodies were measured in pooled sera by ELISA. (B). Groups of 3 B10 wild-type mice were immunized i.p. with 20 μg of either truncated hybrid-WHcAg (149-Mal-78-UTC) (ssRNA−) or full-length hybrid-WHcAg (FLW-Mal-78-UTC) (ssRNA+) particles emulsified in IFA. Six week sera were collected, pooled and IgG1 and IgG2a anti-WHc, anti-NANP and anti-NVDP antibodies were measured by ELISA. G1/G2a ratio’s are also shown. The results represent mean values (± SD) of experiments performed using pooled serum samples on 2 separate occasions (*P = 0.05, **P = 0.005).
Evidence that early TLR-7 signaling occurs in B cells
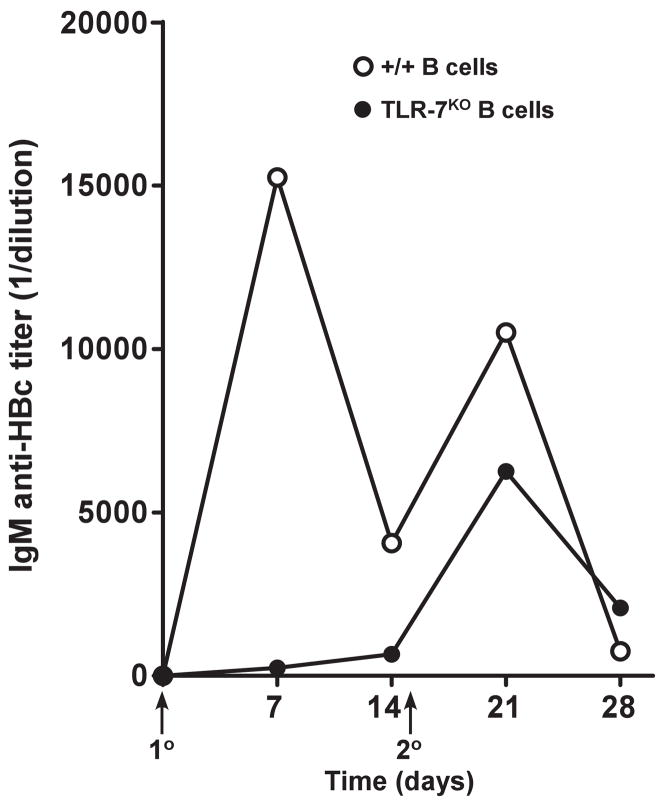
The preceding experiments indicate that B cells, and preferentially B1a and B1b cells act as the primary APC for exogenous HBcAg and that TLR-7 signaling enhances anti-HBc antibody production and HBcAg-specific T cell cytokine production. This suggests but does not prove that TLR-7 signaling occurs, at least initially, in B cells. To determine if TLR-7 signaling occurs in HBcAg-specific B cells and is relevant to in vivo anti-HBc antibody production, a B cell transfer experiment was employed. Recipient, B cell KO mice were adoptively transferred with purified, splenic B cells (30 × 106) derived either from wild-type (+/+) mice or from TLR-7KO mice, in which case only B cells lacked TLR-7 expression and non-B cell APC populations were wild-type with respect to TLR-7. Three days after the B cell transfer, mice were immunized (20μg) and boosted (10μg) with HBcAg183 (ssRNA+) in saline and IgM anti-HBc antibodies were measured by ELISA (using this protocol IgG anti-HBc is not efficiently produced). As shown in Figure 13, TLR-7 expression in B cells was required for an efficient primary IgM anti-HBc response in vivo indicating the relevance of TLR-7 signaling to this response. After the boost, IgM anti-HBc antibody production was less dependent on B cell TLR-7 expression. An in vitro correlate assay also indicated the importance of B cell TLR signaling for IgM anti-HBc antibody production. As noted in Figure 1, unprimed B cells present HBcAg to and activate naïve, 7/16-5 TCR-Tg CD4+ T cells in culture and HBcAg149 as well as HBcAg183 can activate T cells. However, for production of IgM anti-HBc antibody to occur in this primary in vitro culture system the HBcAg must be associated with a TLR signal (Fig. 14). Naïve, HBcAg-specific 7/16-5 spleen cells cultured with HBcAg in vitro for 4–5 days produced IgM anti-HBc antibodies in the supernatant only if the HBcAg possessed ssRNA as a TLR-7 ligand or if HBcAg was associated with LPS as a contaminant, which is a TLR-4 ligand (Fig. 14). The LPS does not have to be physically associated with the HBcAg because HBcAg plus soluble LPS will elicit IgM anti-HBc antibody production as well (data not shown). It appears that primary in vitro anti-HBc antibody production requires three signals including: BCR crosslinking, cognate T cell help and a TLR signal.
Figure 13.
TLR-7 signaling occurs in B cells. Two groups of 6 B10/BKO mice served as recipients for the adoptive transfer of 30 × 106 fractionated splenic B cells derived from either B10/+/+ mice or B10/TLR-7KO mice. Successful B cell grafting was confirmed by the presence of serum IgG 3 days after adoptive transfer. At 7 days after adoptive transfer mice were immunized i.p. with HBcAg183 (ssRNA+) (20 μg in saline) and boosted with 10 μg of HBcAg183 in saline. Sera was collected, pooled and assayed for IgM anti-HBc antibodies at the indicated time points by ELISA.
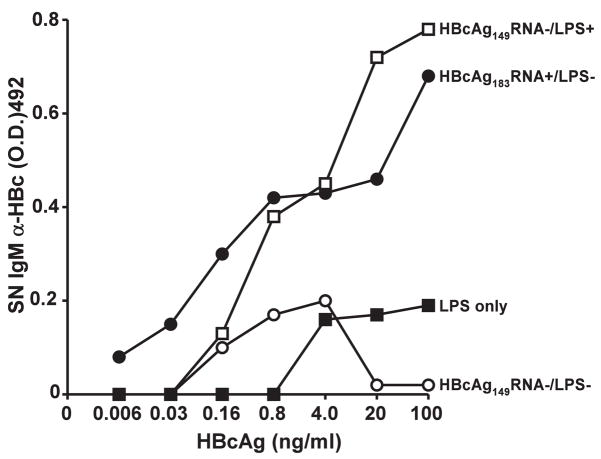
Figure 14. In vitro.
IgM anti-HBc antibody production requires 3 signals. TCR-Tg 7/16-5 spleen cells were cultured with varying concentrations of HBcAg preparations, which contained LPS (10 ng/μg) HBcAg149 RNA−/LPS+; ssRNA, HBcAg183 RNA+/LPS−; neither LPS or ssRNA, HBcAg149 RNA−/LPS− or LPS without HBcAg particles. After 5 days of culture, supernatants (SN) were collected and IgM anti-HBc antibodies measured by ELISA. This experiment was performed on 3 separate occasions and the results are representative.
DISCUSSION
Although previous studies indicated that HBcAg-specific B cells were the primary APC for HBcAg, the readout system consisted of presentation to HBcAg-specific T cell hybridomas, which do not require co-stimulatory signals for T cell activation (6, 24). In the current study, it was demonstrated that naïve HBcAg-specific CD4+ T cells, derived from unprimed 7/16-5 TCR-Tg mice, were activated by HBcAg presented by resting, naïve B cells in vitro and furthermore that DC/MØ APC could not serve as efficient APC for exogenous HBcAg. The ability of resting B cells to prime naïve CD4+ T cells remains a controversial subject with opinions ranging from B cells being essential as APC (25–27); B cells being unable to function as APC (28, 29); and B cells inducing anergy in naïve T cells (30). This lack of consensus may relate to the nature of the antigens under study. For example, the HBcAg is a particulate, multivalent protein antigen capable of crosslinking BCRs sufficiently to induce B7.2 and B 7.1 costimulatory molecules on resting, HBcAg-specific B cells (6). Furthermore, in the present study it was demonstrated that B1a cells are superior APCs for the HBcAg as compared to B2 cells and a relatively high frequency of B1a cells bind biotinylated HBcAg150C. Furthermore, in mice lacking B1a cells the in vivo anti-HBc response was delayed as compared to wild-type mice. B1a cells are members of the innate immune system that produce IgM natural antibodies and play a role in defence against bacterial infection (31). The specificities characteristic of B1a cells are encoded by distinctive germ line genes, possibly with preferential Ig heavy-chain-light-chain combinations, resulting in a restricted or “hard-wired” antibody repertoire (32). In this regard, it is interesting that a linear motif that binds HBcAg has been identified in the framework region 1 (FR1)-complementarity-determining region 1 (CDR1) junction of an IgM anti-HBc hybridoma (9C8). This motif is present in heavy chains of the mouse VH1 family and human VH1 and VH7 families (33). A cryo-EM reconstruction of an immune-complex between HBcAg and an Fab fragment of 9C8 indicates binding via the VL chain rather than the VH chain; however, the VL chain FR1-CDR1 junction region contains a motif with 73% similarity to the VH1 consensus motif (34). These studies suggest interaction between HBcAg and Igs derived from restricted V region germ line genes, which is consistent with preferential recognition of HBcAg by B1 cells. Because WHcAg as well as HBcAg is preferentially presented by B cell APC (data not shown) and these hepadnavirus core proteins are not crossreactive at the antibody level, we suggest that structural features of the spikes on the capsids surface rather than specific amino acids per se may serve as recognition sites for B1 cell Ig receptors. Although not extensively studied, B1a cells have been reported to be very efficient APCs (35).
As interesting as the HBcAg-B1 cell APC pathway may be, the more surprising observation is the inability of non-B cell professional APCs (ie., DC/MØ) to efficiently present exogenous HBcAg to CD4+ T cells in vitro or in vivo. DC/MØ cells are known to be highly efficient APCs due to constitutive expression of costimulatory molecules and are also known to be able to cross-present virus-like-particles (VLPs) and other large particulate antigens (36–38). Nevertheless, immunization of B cell KO mice even with HBcAg emulsified in CFA, a very strong APC activator, was unable to efficiently prime CD4+ T cells (Fig. 2). The fact that immunization in CFA was unable to prime HBcAg-specific CD4+ T cells in B cell knock-out mice indicated that the defect in APC function was not at the level of DC/MØ activation and suggested that the uptake of free HBcAg particles by DC/MØ was the limiting factor. Because the HBcAg is non-glycosylated and contains no lipid and apparently no other ligands recognized by DC/MØ endocytic receptors, it appears that HBcAg particles are “ignored” by DC/MØ APCs at lease at concentrations of 1 μg/ml or less. The finding that DC/MØ cells were efficient APC for HBcAg immune-complexed to HBcAg-specific Mabs established that once the HBcAg is internalized, presumably through FcR-mediated uptake, processing of HBcAg by DC/MØ APC and subsequent presentation to naïve CD4+ T cells occurs efficiently. Furthermore, immunization with a DNA plasmid encoding HBcAg elicited very efficient CD4+ T cell and CD8+ CTL responses in B cell KO mice. This indicates that B cells are not required as APCs for intracellular HBcAg, which can apparently be “cross-presented” by DC/MØ to both HBcAg-specific CD4+ and CD8+ T cells. Because HBcAg exists as an exogenous antigen as well as an intracellular antigen within hepatocytes during a natural infection, B cell presentation of exogenous HBcAg to CD4+ T cells and DC/MØ cross-presentation of intracellular HBcAg to CD4+ and CD8+ T cells are both relevant APC pathways during HBV infection. Predictably, B cell presentation of exogenous HBcAg leads to significantly greater anti-HBc antibody production as compared to DC/MØ presentation of intracellular HBcAg; and, reciprocally, HBcAg-specific CD8+ T cells and possibly CD4+ T cells are more efficiently primed by DC/MØ presentation of intracellular HBcAg.
The HBcAg and WHcAg have been proposed as vaccine carrier platforms for heterologous T and B cell epitopes (16, 39). Vaccination with hybrid-HBcAg/WHcAg particles would at least initially rely on B cells for primary APC function, however, once anti-HBc and anti-insert antibodies are produced immune-complexed hybrid-HBcAg/WHcAg particles would be “recognized” by DC/MØ APCs as well.
Historically, naïve B cell activation, maturation and antibody production have been assumed to be dependent on the sequential integration of two signals: BCR crosslinking by antigen followed by cognate interaction with Th cells through an immunological synapse involving CD40-CD40L interaction and cytokines (40, 41). More recent studies suggest a three signal pathway for naïve B cell activation that includes TLR ligands acting directly on B cells resulting in coligation of the BCR and TLRs (42, 43). Although the absolute requirement for TLR signaling in naïve B cell activation has been questioned (44), there is no disagreement that coligation of BCRs and TLRs augments antibody responses. There has been some debate as to whether HBcAg can function directly as a TLR ligand or whether LPS or some other contaminant indirectly activates TLR-2/4 (45). The current study did not find any evidence that the HBcAg protein possessed TLR activating activity, which was restricted to HBcAg particles that were contaminated with LPS (ie., TLR 2/4 activation). However, the ssRNA encapsidated in either E. coli-derived or yeast-derived full-length HBcAg stimulated significant TLR-7 activation. For example, full-length HBcAg183 (ssRNA+) elicited 25-fold higher anti-HBc responses in wild-type mice as compared to TLR-7KO mice. Furthermore, in the absence of TLR-7 (TLR-7KO mice) full length HBcAg183 (RNA+) and truncated HBcAg149 (ssRNA−) were equally immunogenic. Therefore, the significantly greater immunogenicity of HBcAg183 as compared to HBcAg149 in wild-type mice is mediated entirely through ssRNA-TLR-7 signaling within B cell APCs as demonstrated by adoptive transfer of TLR-7 negative B cells into B cell KO mice immunized with HBcAg183 (Fig. 13). Predictably, the ssRNA encapsidated within HBcAg183 also enhanced HBcAg-specific T cell cytokine production. Furthermore, ssRNA-TLR-7 signaling skewed the anti-HBc antibody isotype distribution towards IgG2a, IgG2b and away from IgG1 indicative of a Th1-type response.
Because of our interest in using the WHcAg as a vaccine carrier platform for heterologous B and T cell epitopes, hybrid-WHcAg carrying P. falciparum malaria protective B cell epitopes (NANP, NVDP) were used to immunize wild-type and TLR-7KO mice. Hybrid-WHcAg particles containing ssRNA were significantly more immunogenic and elicited IgG2a/2b-predominant anti-WHc and anti-NANP and anti-NVDP antibody responses as compared to ssRNA-negative hybrid-WHcAg particles in wild-type mice and equivalent antibody responses in TLR-7KO mice. The finding that anti-insert as well as anti-WHc antibody titers and IgG isotype distribution were affected by the presence of ssRNA within hybrid-WHcAg particles indicates that all three B cell specificities are regulated by TLR-7 signaling. It appears that hybrid-WHcAg particles are excellent delivery vehicles for nucleic acid TLR ligands because the ssRNA is protected from nucleases within the WHcAg capsid and is delivered intracellularly to the APC; in this case, antigen-specific B cells. The ssRNA present in hybrid-WHcAg can also be removed by RNase treatment and replaced with other TLR ligands such as CpG, a TLR-9 ligand (46). The adverse systemic effects reported for TLR ligands such as CpG mixed with antigen as an adjuvant should not be problematic because TLR ligands encapsidated within hybrid-WHcAg particles are present in relatively low amounts and are delivered intracellularly to antigen-specific B cells or other APCs.
Given the spatial segregation between the BCR on the plasma membrane and both TLR-7 and TLR-9, which are endosomal receptors, it has not been clear if enhanced B cell responses to ssRNA or dsDNA-containing antigens is achieved by synergistic BCR-TLR coligation and/or whether coligation occurred in the same cellular compartment. However, a recent study clarified this point by demonstrating that as internalized BCR-antigen traffics to autophagosomes, it continues to signal through a phospholipase-D-dependent pathway to recruit TLR-9-containing endosomes to autophagosomes where they colocalize with BCR-antigen complexes (47). The recruitment of TLR-9 to BCR-containing compartments permits TLR-9 to “sample” BCR-bound antigen for the presence of its ligand (47). Although not experimentally demonstrated, it is likely that the synergistic interaction between BCRs and TLR-9 also occurs between BCRs and TLR-7 because coligation of BCR and TLR-7 within B cells has been described (48) and TLR-7 and TLR-9 are both localized in endosomes.
Although the relevance of TLR ligands encapsidated within hybrid-HBcAg/WHcAg particles used for vaccine purposes is clear, the relevance of the viral RNA/DNA incorporated in HBcAg to TLR signaling in a natural HBV infection is less obvious. The HBV has been characterized as a “stealth” virus in terms of innate immunity because in the liver of acutely HBV-infected chimpanzees, HBV does not induce an intrahepatic innate immune response during the lag phase of infection or the log phase of viral spread (49). It was suggested that HBV RNA/DNA species may be shielded from recognition by TLR or other nucleic acid sensors because HBV replication occurs within HBcAg particles (49). However, this would not preclude TLR signaling in APCs during processing of HBcAg, which may enhance both innate and adaptive immune responses to the HBcAg during a natural infection. Furthermore, recent studies of very early acute HBV infections in humans indicate early development of NK and NT cell responses, suggesting that the innate immune system is able to sense HBV infection (50, 51).
The ability of the HBcAg to interact with the innate immune system was demonstrated by the preferential binding and presentation of HBcAg to CD4+ T cells by B1a and B1b APCs and TLR-7 signaling within B cell APCs. These interactions serve to bridge the innate and adaptive immune responses to the HBcAg. However, it is important to emphasize that these innate immune interactions enhance but are not necessary for an adaptive immune response to the HBcAg. For example, in CBA/N (xid) mice, which lack efficient B1a cell function, pre-existing IgM anti-HBc antibodies are not present in serum and the induced IgG anti-HBc antibody response is lower and delayed as compared to wild-type CBA mice. Nevertheless, a secondary IgG anti-HBc antibody response occurs after boosting in CBA/N (xid) mice although less efficient than the wild-type response (Fig. 5). Furthermore, truncated HBcAg149 which lacks encapsidated ssRNA is still a relatively strong immunogen in the absence of TLR-7 signaling and TLR-7KO mice are still capable of producing anti-HBc antibodies (Fig. 10).
The suggestions that HBcAg protein binding to TLR-2 (9) or full length HBcAg183 binding to cell membrane HS (9, 10), or the activity of contaminating LPS may contribute to the enhanced immunogenicity of the HBcAg in vivo (11, 12) could not be substantiated in this study (see Figs. 8 and 9). These previous suggestions were based solely on in vitro systems, whereas, the present study relied heavily on evaluation of in vivo immune responses to the HBcAg. For example, in vitro IgM, anti-HBc production requires three signals: BCR, cognate Th, and TLR-7; and LPS signaling through TLR-4 can substitute for the TLR-7 signal. However, the amount of LPS present even in a highly contaminated HBcAg preparation had no significant effect on anti-HBc antibody production in vivo (Fig. 9). Similarly, we observed non-specific activation of DC/MØ in the presence of full length HBcAg183 in vitro at relatively high concentrations (≥ 1.0 μg/ml), which may be mediated by HBcAg C-terminal binding to cell membranes (9, 52) (data not shown). However, the in vivo relevance is not apparent because DC/MØ do not efficiently present exogenous HBcAg183 to resting or primed CD4+ T cells; and, in the absence of TLR-7 signaling, full length HBcAg183 and truncated HBcAg149 are equally immunogenic. Therefore, the particulate structure of the HBcAg characterized by the optimal spacing of the antigenic loops at the tip of the capsid spikes, which are capable of crosslinking BCRs, together with efficient CD4+ T cell recognition explain the efficient adaptive humoral response to HBcAg, which can certainly be enhanced by the innate immune mechanisms described in this report.
Acknowledgments
We would like to gratefully acknowledge Francis V Chisari (The Scripps Research Institute, La Jolla, CA) and members of his laboratory, especially Alana Althage, Masanori Isogawa, and Holly Maier for scientific discussions, excellent help and technical assistance. We also thank Melissa Grace for preparation of the manuscript.
Footnotes
The study was supported by NIH grants R01 AI049730 and R01 AI20720 and grants from the Swedish Cancer Foundation. Lars Frelin was supported by grants from Erik and Edith Fernstroms Foundation and Gålö Foundation/Gemzeús Foundation.
Abbreviations: HBcAg, hepatitis B core antigen; APC, antigen presenting cell; TLR, toll-like receptor; BMDC, bone marrow derived dendritic cell; CFA, complete Freund’s adjuvant; TCR-Tg, T cell receptor-transgenic; DC/MØ, dendritic/macrophage cells; LPS, lipopolysaccharide; BCR, B cell receptor
References
- 1.Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986;234:1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- 2.Milich DR, McLachlan A, Stahl S, Wingfield P, Thornton GB, Hughes JL, Jones JE. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988;141:3617–3624. [PubMed] [Google Scholar]
- 3.Milich DR, Peterson DL, Schodel F, Jones JE, Hughes JL. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 1995;69:2776–2785. doi: 10.1128/jvi.69.5.2776-2785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milich DR, McLachlan A, Thornton GB, Hughes JL. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987;329:547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- 5.Schodel F, Moriarty AM, Peterson DL, Zheng JA, Hughes JL, Will H, Leturcq DJ, McGee JS, Milich DR. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992;66:106–114. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milich DR, Chen M, Schodel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci U S A. 1997;94:14648–14653. doi: 10.1073/pnas.94.26.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belnap DM, Watts NR, Conway JF, Cheng N, Stahl SJ, Wingfield PT, Steven AC. Diversity of core antigen epitopes of hepatitis B virus. Proc Natl Acad Sci U S A. 2003;100:10884–10889. doi: 10.1073/pnas.1834404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. doi: 10.1038/386088a0. [DOI] [PubMed] [Google Scholar]
- 9.Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–3176. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 10.Vanlandschoot P, Van Houtte F, Serruys B, Leroux-Roels G. The arginine-rich carboxy-terminal domain of the hepatitis B virus core protein mediates attachment of nucleocapsids to cell-surface-expressed heparan sulfate. J Gen Virol. 2005;86:75–84. doi: 10.1099/vir.0.80580-0. [DOI] [PubMed] [Google Scholar]
- 11.Vanlandschoot P, Van Houtte F, Serruys B, Leroux-Roels G. Contamination of a recombinant hepatitis B virus nucleocapsid preparation with a human B-cell activator. J Virol. 2007;81:2535–2536. doi: 10.1128/JVI.02507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanlandschoot P, Van Houtte F, Ulrichts P, Tavernier J, Leroux-Roels G. Immunostimulatory potential of hepatitis B nucleocapsid preparations: lipopolysaccharide contamination should not be overlooked. J Gen Virol. 2005;86:323–331. doi: 10.1099/vir.0.80605-0. [DOI] [PubMed] [Google Scholar]
- 13.Riedl P, Stober D, Oehninger C, Melber K, Reimann J, Schirmbeck R. Priming Th1 immunity to viral core particles is facilitated by trace amounts of RNA bound to its arginine-rich domain. J Immunol. 2002;168:4951–4959. doi: 10.4049/jimmunol.168.10.4951. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 15.Schodel F, Peterson D, Zheng J, Jones JE, Hughes JL, Milich DR. Structure of hepatitis B virus core and e-antigen. A single precore amino acid prevents nucleocapsid assembly. J Biol Chem. 1993;268:1332–1337. [PubMed] [Google Scholar]
- 16.Billaud JN, Peterson D, Barr M, Chen A, Sallberg M, Garduno F, Goldstein P, McDowell W, Hughes J, Jones J, Milich D. Combinatorial approach to hepadnavirus-like particle vaccine design. J Virol. 2005;79:13656–13666. doi: 10.1128/JVI.79.21.13656-13666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 18.Sallberg M, Ruden U, Magnius LO, Norrby E, Wahren B. Rapid “tea-bag” peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol Lett. 1991;30:59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 19.Lazdina U, Alheim M, Nystrom J, Hultgren C, Borisova G, Sominskaya I, Pumpens P, Peterson DL, Milich DR, Sallberg M. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol. 2003;84:139–146. doi: 10.1099/vir.0.18678-0. [DOI] [PubMed] [Google Scholar]
- 20.Frelin L, Alheim M, Chen A, Soderholm J, Rozell B, Barnfield C, Liljestrom P, Sallberg M. Low dose and gene gun immunization with a hepatitis C virus nonstructural (NS) 3 DNA-based vaccine containing NS4A inhibit NS3/4A-expressing tumors in vivo. Gene Ther. 2003;10:686–699. doi: 10.1038/sj.gt.3301933. [DOI] [PubMed] [Google Scholar]
- 21.Kuhober A, Pudollek HP, Reifenberg K, Chisari FV, Schlicht HJ, Reimann J, Schirmbeck R. DNA immunization induces antibody and cytotoxic T cell responses to hepatitis B core antigen in H-2b mice. J Immunol. 1996;156:3687–3695. [PubMed] [Google Scholar]
- 22.Kuhrober A, Wild J, Pudollek HP, Chisari FV, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–1212. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 23.Frelin L, Wahlstrom T, Tucker AE, Jones J, Hughes J, Lee BO, Billaud J, Peters C, Whitacre D, Peterson D, Milich DR. A mechanism to explain the selection of the HBeAg-negative mutant during chronic HBV infection. J Virol (In Press) 2008 doi: 10.1128/JVI.01902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watts TH, Brian AA, Kappler JW, Marrack P, McConnell HM. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984;81:7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt-Jones EA, Liano D, HayGlass KA, Benacerraf B, Sy MS, Abbas AK. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988;140:3773–3778. [PubMed] [Google Scholar]
- 26.Liu Y, Wu Y, Ramarathinam L, Guo Y, Huszar D, Trounstine M, Zhao M. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–1362. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 27.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848–2856. [PubMed] [Google Scholar]
- 28.Epstein MM, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naïve T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 31.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5- B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa K, Hardy RR. Development and function of B-1 cells. Curr Opin Immunol. 2000;12:346–353. doi: 10.1016/s0952-7915(00)00098-4. [DOI] [PubMed] [Google Scholar]
- 33.Lazdina U, Cao T, Steinbergs J, Alheim M, Pumpens P, Peterson DL, Milich DR, Leroux-Roels G, Sallberg M. Molecular basis for the interaction of the hepatitis B virus core antigen with the surface immunoglobulin receptor on naïve B cells. J Virol. 2001;75:6367–6374. doi: 10.1128/JVI.75.14.6367-6374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watts NR, Cardone G, Vethanayagam JG, Cheng N, Hultgren C, Stahl SJ, Steven AC, Sallberg M, Wingfield PT. Non-canonical binding of an antibody resembling a naïve B cell receptor immunoglobulin to hepatitis B virus capsids. J Mol Biol. 2008;379:1119–1129. doi: 10.1016/j.jmb.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan C, Morel L, Yang P, Wakeland EK. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998;41:1652–1662. doi: 10.1002/1529-0131(199809)41:9<1652::AID-ART17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 36.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 37.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 39.Billaud JN, Peterson D, Lee BO, Maruyama T, Chen A, Sallberg M, Garduno F, Goldstein P, Hughes J, Jones J, Milich D. Advantages to the use of rodent hepadnavirus core proteins as vaccine platforms. Vaccine. 2007;25:1593–1606. doi: 10.1016/j.vaccine.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills DM, Cambier JC. B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: molecular dynamics and immunologic consequences. Semin Immunol. 2003;15:325–329. doi: 10.1016/j.smim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 42.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 43.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naïve B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 44.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanlandschoot P, Leroux-Roels G, Cooper A, Tal G, Shaul Y. The role of heparan sulfate and TLR2 in cytokine induction by hepatitis B virus capsids. J Immunol. 2005;175:6253–6255. doi: 10.4049/jimmunol.175.10.6253. [DOI] [PubMed] [Google Scholar]
- 46.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J Immunol. 2004;172:1777–1785. doi: 10.4049/jimmunol.172.3.1777. [DOI] [PubMed] [Google Scholar]
- 47.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisicaro Paola VC, Carolina Boni, Marco Massari, Cristina Mori, Alessandro Zerbini, Alessandra Orlandini, Luca Sacchelli, Gabriele Missale, Carlo Ferrari. Early Kinetics of Innate and Adaptive Immune Responses During Hepatitis B Virus Infection. Gut. 2009 doi: 10.1136/gut.2008.163600. BMJ Publishing Group Ltd & British Society of Gastroenterology, Published Online First. [DOI] [PubMed] [Google Scholar]
- 51.Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, Dusheiko GM, Bertoletti A. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 52.Cooper A, Shaul Y. Clathrin-mediated endocytosis and lysosomal cleavage of hepatitis B virus capsid-like core particles. J Biol Chem. 2006;281:16563–16569. doi: 10.1074/jbc.M601418200. [DOI] [PubMed] [Google Scholar]