Abstract
Attention deficit hyperactivity disorder (ADHD) is a highly heritable disorder affecting some 5-10% of children and 4-5% of adults. The cannabinoid receptor gene (CNR1) is a positional candidate gene due to its location near an identified ADHD linkage peak on chromosome 6, its role in stress and dopamine regulation, its association with other psychiatric disorders that co-occur with ADHD, and its function in learning and memory. We tested SNP variants at the CNR1 gene in two independent samples—an unselected adolescent sample from Northern Finland, and a family-based sample of trios (an ADHD child and their parents). In addition to using the trios for association study, the parents (with and without ADHD) were used as an additional case/control sample of adults for association tests. ADHD and its co-morbid psychiatric disorders were examined. A significant association was detected for a SNP haplotype (C-G) with ADHD (P = 0.008). A sex by genotype interaction was observed as well with this haplotype posing a greater risk in males than females. An association of an alternative SNP haplotype in this gene was found for post-traumatic stress disorder (PTSD) (P = 0.04 for C-A, and P = 0.01 for C-G). These observations require replication, however, they suggest that the CNR1 gene may be a risk factor for ADHD and possibly PTSD, and that this gene warrants further investigation for a role in neuropsychiatric disorders.
Keywords: genes, attention, association, post-traumatic stress disorder
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a common neurobehavioral disorder affecting some 5-10% children and adolescents and 4-5% of adults [Brown et al., 2001; Cuffe et al., 2005; Faraone and Biederman, 2005; Kessler et al., 2006]. ADHD is defined by symptoms of inattention and/or hyperactivity-impulsivity, impairment in at least two settings, and onset in childhood [APA, 1994]. While ADHD has its onset in childhood, the chronic nature of the disorder is evident by the persistence of ADHD into adulthood in 60-70% of cases [McGough et al., 2005]. Neurobiological and genetic research support the hypothesis that ADHD likely represents an extreme on one or more continua of neural processing and that multiple risk genes are responsible for the underlying liability to ADHD, with estimates of heritability at 76% [Faraone et al., 2005]. Despite strong genetic and neurobiological underpinnings, environmental factors are also indicated in both the etiology of ADHD [Hudziak et al., 2005] and its associated impairment [Pressman et al., 2006].
ADHD is a known risk factor for other psychiatric disorders, most notably mood, anxiety, disruptive disorders (oppositional defiant disorder, conduct disorder), and substance abuse or dependence [Biederman et al., 1995; McGough et al., 2005]. However, the mechanism(s) underlying this heightened risk for co-morbid illness is poorly understood. Identification of etiological factors in ADHD and psychiatric co-morbidity are topics of extant research. Recent molecular genetic studies suggest several chromosomal regions may harbor risk genes of moderate effect in ADHD, while candidate gene studies, thus far, support a likely role of dopamine-related systems in ADHD [Maher et al., 2002; Bakker et al., 2003; Arcos-Burgos et al., 2004; Kustanovich et al., 2004; Ogdie et al., 2004; Hebebrand et al., 2006].
The purpose of the present study is to investigate the role of variants of the cannabinoid receptor gene (CNR1) in relation to ADHD and its co-morbid psychiatric disorders. We selected this gene for investigation for several reasons. First, the CNR1 locus (~88.9 Mb or 96-98 cM) falls near one of the regions of interest on chromosome 6 supported by our genome-wide studies (MLS = 3.3 at 89 cM) [Ogdie et al., 2004]. Second, studies of endogenous cannabinoid (EC) regulation in animal models suggest that it plays a role in some aspects of memory, emotional recognition or processing, and reward, each an area of putative dysregulation in ADHD [King et al., 1998; Nigg and Casey, 2005]. Third, individuals with ADHD have increased rates of addiction [Kessler et al., 2005; McGough et al., 2005; Smalley et al., 2007b] which has been suggested to be associated with CNR1 variants.
There are numerous investigations of CNR1 gene variants in psychiatry, including one study of ADHD among alcoholic individuals where no association was detected [Ponce et al., 2003]. Most studies have utilized a polymorphism (AAT)n in the promoter region of the CNR1 gene, although a few recent studies also include SNP variants within the gene as well [Schmidt et al., 2002; Roeder et al., 2005; Hopfer et al., 2006]. There are positive association studies of CNR1 variants with substance abuse/dependence and schizophrenia [Ujike et al., 2002; Ballon et al., 2006; Martinez-Gras et al., 2006] although results are not consistent, with failure to replicate also reported for each condition analyzed [Tsai et al., 2000; Heller et al., 2001; Herman et al., 2006]. There are also positive and negative reports of CNR1 gene variants with other psychiatric disorders as well, including a positive finding with eating disorder [Siegfried et al., 2004], a negative finding with Tourette syndrome [Gadzicki et al., 2004], a negative finding with bipolar illness [Tsai et al., 2001], and a positive finding with psychiatric sequelae in Parkinson’s disease [Barrero et al., 2005]. Taken together, the findings support a plausible role of the CNR1 gene in a variety of psychiatric disorders but the specific gene variants, strengths of associations, and specificity to particular psychiatric disorders (or underlying liabilities) are unclear.
MATERIALS AND METHODS
Samples
The CNR1 (NM_016083 and NM_033181) gene variants were investigated in two samples: First, a sample of 187 trios (ADHD child and 2 parents) randomly drawn from the affected sibling pair (ASP) sample of the UCLA ADHD Genetic study (the eldest child was selected within an ASP for trio formation) were available to apply family-based association tests (FBATs). There were 130 (69.5%) males and 57 (30.5%) females among 187 children with a mean age of 12.7 ± 3.3 years (range, 5-25). Among the children, 81.8% are Caucasians, 3.2% Hispanic, 4.8% are other ethnic groupings, and 10.2% are mixed-ethnic group, in which parents were from different ethnicity. Of the 187 trios, 374 parents (187 mothers, 187 fathers) were available as a case-control sample using their phenotype status on ADHD and psychiatric diagnoses. However, only Caucasian parents (n = 320) are used for the purpose of the present study to reduced effects due to population stratification. The parental mean age was 43.2 ± 5.2 years (range 31-66 years). The second independent sample consists of adolescent ADHD cases (n = 159) and controls (n = 151) drawn from a Northern Finnish Birth Cohort (NFBC) [Smalley et al., 2007a]. Among that sample, there are 201 (64.8%) males and 109 (35.2%) females with a mean age of 16.1 ± 0.3 years. All subjects signed informed consent prior to data collection as approved by the University of California Institutional Review Board and/or the University of Oulu Institutional Review Board (for the NFBC) as appropriate for the specific sample.
Measures
A similar diagnostic system is in place for samples used in the current analyses. ADHD and other psychiatric diagnoses were determined using a semi-structured psychiatric interview, KSADS-PL for children 5-18 [Kaufman et al., 1997], and SADS-LAR for adults [Fyer et al., 1995] with the Behavioral Disorders section of the KSADS-PL used to determine lifetime and current ADHD, oppositional defiant disorder and conduct disorder. A best estimate procedure [Leckman et al., 1982] is utilized to determine final diagnosis [see Smalley et al., 2000, and Smalley et al., 2007 for a review of assessment procedures]. Lifetime diagnoses are used in the current study.
Genotyping
The CNR1 gene spans 5,469 bp on chromosome 6 [88906306bp-88911775bp; UCSC Human Mar. 2006 (hg18) assembly] and contains a single exon that can be alternatively spliced within the coding sequence. There are two distinct RefSeq transcripts (NM_033181.2 and NM_016083.3) that span the same genomic sequence, and differ solely by alternative splicing. NM_016083.3 contains a single contiguous coding region representing the complete exon1 sequence and encoding a 471 amino acid protein. NM_033181.2 contains a form of exon1 spliced internally that encodes a 411 amino acid protein. SNPs spanning the CNR1 gene were selected from the NCBI dbSNP database and the International HapMap Project (HapMap Rel 19 Oct 05 Build), and physical positions were determined from the NCBI Build 35 human genome assembly. According to the most recent HapMap build (HapMap Rel 19 Jan 07 Build), the SNPs included in this study capture common variation at R2 > 0.8 and MAF > 0.05 for all genotyped SNPs in NM_033181 and NM_016083 with three exceptions (rs12720071, rs16880248, rs4707436) which were not genotyped due to technical difficulties. Genotyping was performed using TaqMan SNP Genotyping Assays and the 7900HT Fast Real-Time PCR System for fluorescent read detection and allelic clustering. All assays were performed in 5 μl volume in 384-well plates according to the manufacturers’ specifications. All SNPs included in the current study were successfully genotyped in >95% of the samples, were in Hardy-Weinberg equilibrium (HWE, P-value cut off = 0.001) in parental samples, and had Mendelian error rates of less than 1%.
Haplotypes Construction
Haplotypes were used to test for association in addition to individual SNPs because they are known to often provide more information [Hugot et al., 2001; Rioux et al., 2001]. The Haploview 3.2 software [Barrett et al., 2005] was used to define blocks using the default setting based on Gabriel et al. [2002]. Specifically, criteria included that the upper bound of D′ > 0.98 and the lower bound >0.7, a 0.9 as the upper confidence interval maximum for strong recombination, and a fraction of strong linkage disequilibrium (LD) in information comparison of ≥0.95. The haplotypes were constructed using an EM algorithm, available in Haploview and FBAT. For analyses using logistic regression, the EM algorithm under SAS 9.1 PROC HAPLOTYPE was used to construct haplotypes.
Analyses
Associations of SNPs and haplotypes with the psychiatric diagnostic phenotypes under analysis were assessed using FBAT for LA parent-child trios or a case-control test for the Finnish sample and Caucasian parents in the LA trios. For analysis of the trios, the FBAT [Horvath et al., 2001] was conducted using the FBAT software, which tests for transmission distortion from the null hypothesis of no association: no distortion from the expected 0.5 transmission [Laird et al., 2000]. An offset option (FBAT-O/HBAT-O) was used for adding an offset in the FBAT parametric model in analyzing both affected and unaffected psychiatric diagnostic phenotypes. This option makes the score statistic more efficient [Lunetta et al., 2000]. For all analyses, we reported both a global P-value and the individual P values. The global P-value is found from a distribution of a test of association between a disease status and a gene with “H” haplotypes. The individual P values are computed for a test of one allele versus all others. In addition, permutation P values, based on 10,000 replicates, were also computed to confirm any significant findings as they may achieve a higher precision level.
For any significant associations detected at P < 0.05, logistic regression was used to test for gender by gene interactions and to estimate an odds ratio. In this test, the gene variant (haplotype) is treated as binary (Present or Absent) so that individuals homozygous for the marker allele are assigned twice the weight of heterozygotes in the logistic regression.
The study is considered to be hypothesis generating with the level of significance set at 0.05 in each case. In order to test CNR1 associations with psychiatric disorders co-morbid with ADHD, 5 out of 18 psychiatric disorders were selected because there were ~50 individuals with the diagnosis in at least two of the samples (Finnish, Trios, or Parents). We selected a sample size of 50 to minimize our test comparisons while providing adequate power to detect relatively small odds ratios. Based on power analyses for a case control design in a sample size of 50, we would have 80% power to detect an odds ratio (OR) > 1.8 for haplotype frequencies >0.15 if the level of significance was set at 0.05. For family based analyses and comparable parameters, power is adequate for OR of 2.3 or higher. Although somewhat arbitrary, this criterion kept the number of test comparisons relatively small per group while affording sufficient power to detect a relatively minor gene effect. The power analyses were carried out using the SAS PROC POWER procedure for the case control design and PBAT [Lange and Laird, 2002a,b] for the family based design. We made two exceptions to this sample size selection criterion by including an analysis of substance abuse/dependence (only one sample had the n > 50) and PTSD (maximum sample size of 25) because of previous reports of CNR1 with the former [Ballon et al., 2006] and the specific biological function of ECs (to modify memory of traumatic events) as a compelling argument for a CNR1 role in the latter.
The disorders available for analysis are shown in Table I and include ADHD, “any” anxiety disorder, mood disorder which includes either major depression or dysthymia, disruptive disorder which includes either oppositional defiant disorder or conduct disorder, post-traumatic stress disorder (PTSD) and substance abuse/dependence. “Any anxiety” was defined if any of the following were present: simple phobia, social phobia, PTSD, obsessive compulsive disorder (OCD), generalized anxiety disorder (GAD), separation anxiety, agoraphobia or panic, and adjustment disorder.
TABLE I.
Numbers of ADHD Affected and Non-Affected Having Associated Psychiatric Disorders
| LA child (n=187)b |
LA Caucasian parent (n=320)b |
Finland (n=310)b |
||||
|---|---|---|---|---|---|---|
| Affection statusa | Yes | No | Yes | No | Yes | No |
| ADHD | 187 (70%)c | 0 (0%) | 115 (46%) | 193 (50%) | 159 (71%) | 151 (58%) |
| Any anxiety | 82 (67%) | 104 (72%) | 141 (41%) | 171 (57%) | 71 (54%) | 238 (68%) |
| Mood | 49 (67%) | 137 (70%) | 158 (39%) | 158 (60%) | 57 (47%) | 253 (69%) |
| Disruptive disorder | 104 (74%) | 83 (64%) | 53 (57%) | 262 (48%) | 78 (60%) | 232 (66%) |
| PTSD | 6 (67%) | 181 (70%) | 25 (24%) | 291 (52%) | 17 (29%) | 292 (67%) |
| Substance abuse/dependence | 11 (55%) | 176 (70%) | 110 (65%) | 206 (42%) | 32 (66%) | 278 (65%) |
Lifetime diagnoses.
Numbers vary due to missing data on genotypes or phenotypes for subjects across conditions.
Number in parentheses reflects the percent that are male.
RESULTS
SNP and Haplotype Description
The CNR1 gene in the current study was investigated using four SNPs. Table II displays their positions and allele information. We note that the minor allele frequencies in our samples were close to those found for Caucasian people in the HapMap project. Using Haploview, two haplotype blocks were detected in both the LA and Finnish samples with the same block boundaries. The first block consists of SNPs 1 and 2, and the second one consists of SNPs 3 and 4. D′ and R2 [Gabriel et al., 2002] are summarized in Table III. We note that the D′ was the same in both samples and the R2 was higher in the second block (≥0.38). The blocks in the LA sample met the default setting [Gabriel et al., 2002] under the Haploview blocking algorithm. The blocks in the Finnish sample did not and were generated based on the solid spine option of LD [Barrett et al., 2005], a method appropriate for cases where D′ > 0.8. We let superscript 1 denote the haplotype that consists of SNPs 1 and 2, and superscript 2 to denote the haplotype consisting of SNPs 3 and 4. Table III also summarizes the haplotype frequencies across samples.
TABLE II.
SNPs Information on CNR1
| Ref SNP ID | Function | Alleles major/minor | Position (bp) | LA children MAF | LA parentsa MAF | LA HWE | Finland MAF | Finland HWE |
|---|---|---|---|---|---|---|---|---|
| rs806368 b | 3′ UTR | C/T | 88906819 | 0.23 | 0.22 | 0.14 | 0.17 | 0.73 |
| rs1049353 | Synonymous | A/G | 88910354 | 0.23 | 0.26 | 0.77 | 0.27 | 1.00 |
| rs806377 c | Non-coding | C/T | 88915442 | 0.47 | 0.48 | 1.00 | 0.47 | 0.87 |
| rs6454674 d | Non-coding | G/T | 88929649 | 0.33 | 0.32 | 0.85 | 0.39 | 0.18 |
MAF, minor allele frequency; HWE, Hardy-Weinberg equilibrium P-value
Frequencies are reported for all parents but the values found in the subset of only Caucasian parents are comparable (data not shown).
TABLE III.
Linkage Disequilibrium Information and Estimated Frequencies of Haplotypes in Two Samples
| LA children | LA parents | Finnish | |
|---|---|---|---|
| Haplotype 1 | |||
| D′ (CI) | NA | 1.0 (0.84, 1.0) | 1.0 (0.80, 1.0) |
| R2 | NA | 0.10 | 0.07 |
| C-A1 a | 0.56 | 0.54 | 0.57 |
| C-G1 | 0.21 | 0.24 | 0.27 |
| T-A1 | 0.21 | 0.20 | 0.16 |
| T-G1 | 0.02 | 0.02 | 0.0 |
| Haplotype 2 | |||
| D′ (CI) | NA | 0.94 (0.87, 0.98) | 0.94 (0.87, 0.97) |
| R2 | NA | 0.38 | 0.49 |
| C-G2 b | 0.22 | 0.23 | 0.15 |
| C-T2 | 0.31 | 0.29 | 0.38 |
| T-G2 | 0.45 | 0.45 | 0.46 |
| T-T2 | 0.02 | 0.03 | 0.01 |
CI denotes 95% confidence interval; NA denotes not available because LD construction was based on founders.
Denotes haplotypes for SNPs 1 and 2.
Denotes haplotypes for SNPs 1 and 2.
Denotes haplotypes for SNPs 3 and 4.
Denotes haplotypes for SNPs 3 and 4.
Results From SNP and Haplotype Associations
We performed an association test of individual SNPs for ADHD and the disorders listed in Table I. At the single SNP level, only PTSD in the LA Caucasian parents showed a significant association, with SNP rs1049353 (allele A, P = 0.011).
As shown in Table IV(A), haplotype 2 (C-G2) was associated with ADHD in the Finnish sample (, P = 0.008 and global P = 0.05). The proportion of C-G2 was higher in cases (19%) than in controls (11%). No significant association in either the LA Caucasian parent sample (, P = 0.16 and global P = 0.29) or the LA trios (Z = -0.57, P = 0.57, global P = 0.91) was observed.
TABLE IV.
(A) ADHD Association with Haplotype 2 (SNPs 3 and 4) in the Finnish Sample (n=310), (B) PTSD Association With Haplotype 1 (SNPs 1 and 2) in LA Caucasian Parent Sample (n=320)*
| Estimate haplotype frequencies |
||||
|---|---|---|---|---|
| (A) Phenotype | C-G2 | C-T2 | T-G2 | T-T2 |
| ADHD (n=159) | 0.19 | 0.37 | 0.43 | 0.01 |
| Not ADHD (n=151) | 0.11 | 0.39 | 0.49 | 0.01 |
| 7.10 | 0.15 | 1.95 | 0.33 | |
| P-value | 0.008 | 0.50 | 0.16 | 0.56 |
| Estimate haplotype frequencies |
|||
|---|---|---|---|
| (B) Phenotype | C-A1 | C-G1 | T-A1 |
| PTSD (n=22) | 0.65 | 0.12 | 0.23 |
| Not PTSD (n=280) | 0.50 | 0.29 | 0.21 |
| 4.21 | 6.53 | 0.08 | |
| P-value | 0.04 | 0.01 | 0.78 |
, P-value=0.05 global analysis of association
Variation in sample sizes reflects cases that were missing either genotype data or specific diagnoses were uknown
(B) , P-value=0.03 global analysis of association.
Two variants (C-A1, C-G1) within haplotype 1 were associated with PTSD in the LA Caucasian parents (global P = 0.03) as shown in Table IV(B). No difference was observed in LA trios (global P = 0.37), although very few children and comorbid PTSD (n = 6). Although no difference was observed in the Finnish sample (global P = 0.20) either, a similar trend in C-A1 and C-G1 distributions was observed. C-A1 was more common in PTSD cases (71%) compared to non-PTSD controls (55%), while C-G1 was less common among the PTSD (20%) compared to non-PTSD (28%) in that sample as well. Using the permutation method, the permutation P values for C-G2 and ADHD was 0.02 while the permutation P values for C-A1, C-G1, and PTSD were 0.11 and 0.04, respectively. A depiction of the SNPs, haplotypes and significant associations is shown in Figure 1.
Fig. 1.
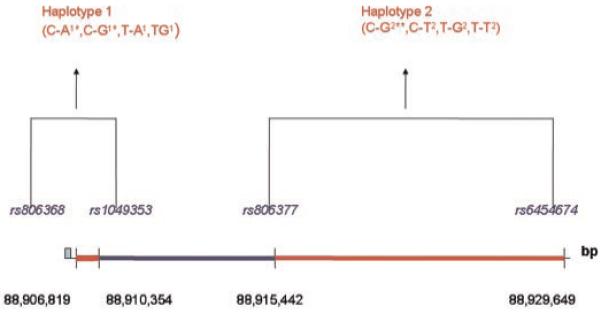
Haplotypes and associations with attention deficit hyperactivity disorder (ADHD) and PTSD at CNR1. *Haplotypes associated with post-traumatic stress disorders (PTSD), **haplotype associated with ADHD. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.].
Testing for haplotype by gender interactions, we found a significant interaction between the C-G2 haplotype and gender for ADHD (P = 0.03) in the Finnish sample. The interaction reflects the approximate threefold increased risk of ADHD in males carrying the haplotype compared to females (carrying it or not) or males without the haplotype.
DISCUSSION
In the current study we find support that variants at CNR1 are associated with ADHD and PTSD. The findings are similar to those of other investigations of this gene, in that they are of relatively small effect size, and do not replicate necessarily across samples. In the current study, we see support for an association of CNR1 and PTSD but findings require replication in larger samples. The failure to replicate across samples may reflect shared underlying constructs embedded in diagnostic classifications (e.g., emotional regulation difficulties) and/or inadequate power to detect small effect sizes. There may be an important role of gender in mediating the association of CNR1 and psychiatric illness, however, and future research needs to include a possible role of genotype by gender interactions.
ECs play an important role in neural plasticity, stress response, and learning and memory. CNR1 receptors are found in particularly high density in the hippocampus and amygdala, regions known to play a role in emotional regulation and memory, and regions suggested to play a role in ADHD [Plessen et al., 2006], emotional regulation [Urry et al., 2006], and other psychiatric disorders (e.g., bipolar, mood, anxiety disorders). They are found on axonal terminals of GABAergic inhibitory neurons containing neuropeptide cholecystokinin (CCK), a neuropeptide known to bind to CCKB receptors and play a role in anxiety. ECs are known to increase CCK release and also have interactions with other neurotransmitter systems including dopamine [Price et al., 2007] and serotonergic systems [Braida et al., 2007]. Genes involved in dopamine and serotonin regulation have already been implicated in both ADHD [Kim et al., 2005], anxiety and mood disorders [Hariri et al., 2006] and are also associated with variation in hypothalamic-pituitary-axis (HPA) stress response [King et al., 1998].
In rodents, endogenous cannabinoids have numerous influences on cognition and behavior as evident by the use of antagonists and agonists of cannabinoids. Agonists impair memory in rats, increase slow wave sleep and rapid eye movement sleep at the expense of wakefulness, impair prepulse inhibition, and recognition memory [Castellano et al., 2003]. The specific mechanism of ECs and CNR1 activity in the brain are currently being delineated but it is well known that many psychiatric disorders, including ADHD, schizophrenia, PTSD, anxiety, and mood disorders have various memory and inhibition impairments. The potential involvement of reward systems in multiple psychiatric disorders, including ADHD, again suggests the plausible association of CNR1 and a range of conditions.
These data provide support for a putative role of endogenous cannabinoids in ADHD, and PTSD. The CNR1 gene may contribute to shared underlying risk continua, such as emotional dysregulation in response to stress, across these diverse diagnostic groups. Increased amygdala activity, poor stress reactivity as reflected by HPA response, and poor prefrontal cortical modulation is a plausible underlying mechanism of liability that may be shared across disorders. Recent imaging studies support amygdala and hippocampal differences in ADHD [Plessen et al., 2006], studies of siblings of ADHD individuals support prefrontal-limbic circuitry differences as a putative endophenotypes [Durston et al., 2006], and studies of stress response in ADHD support abnormalities in HPA axis responsivity [King et al., 1998]. Taken together with the current findings, we suggest that this gene may be an important risk variant in the emotional regulation difficulties underlying ADHD, PTSD, and possibly other co-morbid conditions (such as mood disorder); however, the role of CNR1 is likely small, particularly at the level of psychiatric diagnosis, so future work using more refined phenotypes or endophenotypes of affect regulation are necessary.
The current findings require additional work and replication. Further investigation of affect related traits associated with ADHD may improve our understanding of the role the CNR1 gene may have in this condition and co-morbid disorders. Further refinement of putative DNA variants in CNR1 with functional outcomes is needed to delineate the causal variants contributing to psychiatric illness.
ACKNOWLEDGMENTS
This work was inspired through discussions with TEF and supported by an NIMH grant 063706 (Smalley). Thanks to Jennifer Kitil for administrative support and all the families that participated in this research.
Grant sponsor: NIMH; Grant number: 063706; Grant sponsor: The Juselius Foundation, Finland; the Academy of Finland.
Footnotes
ELECTRONIC DATABASE INFORMATION
The accession number and URLs for data presented herein are as follows:
National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene&cmd=retrieve&dopt=graphics&list_uids=1268; NCBI dbSNP database, http://www.ncbi.nlm.nih.gov/snp/; International Hap-Map Project, http://hapmap.org/; TaqMan SNP Genotyping Assays, http://www.appliedbiosystems.com/; FBAT software, http://www.biostat.harvard.edu/~fbat/default.html; Haploview software, http://www.broad.mit.edu/mpg/haploview/; PBAT, http://www.biostat.harvard.edu/~clange/default.htm; SAS, http://www.sas.com/.
REFERENCES
- APA . Diagnostic and statistical manual of mental disorders, 4th edition (DMS-IV) Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Pineda D, Lopera F, Palacio JD, Palacio LG, Rapoport JL, Berg K, Bailey-Wilson JE, Muenke M. Attention-deficit/hyperactivity disorder in a population isolate: Linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am J Hum Genet. 2004;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker SC, van der Meulen EM, Buitelaar JK, Sandkuijl LA, Pauls DL, Monsuur AJ, van ’t Slot R, Minderaa RB, Gunning WB, Pearson PL, et al. A whole-genome scan in 164 Dutch sib pairs with attention-deficit/hyperactivity disorder: Suggestive evidence for linkage on chromosomes 7p and 15q. Am J Hum Genet. 2003;72(5):1251–1260. doi: 10.1086/375143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon N, Leroy S, Roy C, Bourdel MC, Charles-Nicolas A, Krebs MO, Poirier MF. (AAT)n repeat in the cannabinoid receptor gene (CNR1): Association with cocaine addiction in an African-Caribbean population. Pharmacogenomics J. 2006;6(2):126–130. doi: 10.1038/sj.tpj.6500352. [DOI] [PubMed] [Google Scholar]
- Barrero FJ, Ampuero I, Morales B, Vives F, de Dios Luna Del Castillo J, Hoenicka J, Garcia Yebenes J. Depression in Parkinson’s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1) Pharmacogenomics J. 2005;5(2):135–141. doi: 10.1038/sj.tpj.6500301. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152(11):1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404,the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol. 2007;555(2-3):156–163. doi: 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, Feldman HM, Pierce K, Wolraich ML. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107(3):E43. doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: Animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2(6):389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- Cuffe SP, Moore CG, McKeown RE. Prevalence and correlates of ADHD symptoms in the national health interview survey. J Atten Disord. 2005;9(2):392–401. doi: 10.1177/1087054705280413. [DOI] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60(10):1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. What is the prevalence of adult ADHD? Results of a population screen of 966 adults. J Atten Disord. 2005;9(2):384–391. doi: 10.1177/1087054705281478. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Mannuzza S, Chapman TF, Martin LY, Klein DF. Specificity in familial aggregation of phobic disorders. Arch Gen Psychiatry. 1995;52(7):564–573. doi: 10.1001/archpsyc.1995.03950190046007. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gadzicki D, Muller-Vahl KR, Heller D, Ossege S, Nothen MM, Hebebrand J, Stuhrmann M. Tourette syndrome is not caused by mutations in the central cannabinoid receptor (CNR1) gene. Am J Med Genet Part B. 2004;127B(1):97–103. doi: 10.1002/ajmg.b.20159. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: Perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Dempfle A, Saar K, Thiele H, Herpertz-Dahlmann B, Linder M, Kiefl H, Remschmidt H, Hemminger U, Warnke A, et al. A genome-wide scan for attention-deficit/hyperactivity disorder in 155 German sib-pairs. Mol Psychiatry. 2006;11(2):196–205. doi: 10.1038/sj.mp.4001761. [DOI] [PubMed] [Google Scholar]
- Heller D, Schneider U, Seifert J, Cimander KF, Stuhrmann M. The cannabinoid receptor gene (CNR1) is not affected in German i.v. drug users. Addict Biol. 2001;6(2):183–187. doi: 10.1080/13556210020040271. [DOI] [PubMed] [Google Scholar]
- Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet Part B. 2006;141B(5):499–503. doi: 10.1002/ajmg.b.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, Rhee SH, Smolen A, Krauter K, Hewitt JK, et al. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet Part B. 2006;141(8):895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype-phenotype associations. Eur J Hum Genet. 2001;9(4):301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Derks EM, Althoff RR, Rettew DC, Boomsma DI. The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ Rating Scales-Revised. Am J Psychiatry. 2005;162(9):1614–1620. doi: 10.1176/appi.ajp.162.9.1614. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, Greenhill LL, Jaeger S, Secnik K, Spencer T, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: Results from the national comorbidity survey replication. Biol Psychiatry. 2005;57(11):1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Badner J, Cheon KA, Kim BN, Yoo HJ, Kim SJ, Cook E, Jr, Leventhal BL, Kim YS. Family-based association study of the serotonin transporter gene polymorphisms in Korean ADHD trios. Am J Med Genet Part B. 2005;139B(1):14–18. doi: 10.1002/ajmg.b.30214. [DOI] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biol Psychiatry. 1998;44(1):72–74. doi: 10.1016/s0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Kustanovich V, Ishii J, Crawford L, Yang M, McGough JJ, McCracken JT, Smalley SL, Nelson SF. Transmission disequilibrium testing of dopamine-related candidate gene polymorphisms in ADHD: Confirmation of association of ADHD with DRD4 and D RD5. Mol Psychiatry. 2004;9(7):711–717. doi: 10.1038/sj.mp.4001466. [DOI] [PubMed] [Google Scholar]
- Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(Suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Lange C, Laird NM. On a general class of conditional tests for family-based association studies in genetics: The asymptotic distribution, the conditional power, and optimality considerations. Genet Epidemiol. 2002a;23(2):165–180. doi: 10.1002/gepi.209. [DOI] [PubMed] [Google Scholar]
- Lange C, Laird NM. Power calculations for a general class of family-based association tests: Dichotomous traits. Am J Hum Genet. 2002b;71(3):575–584. doi: 10.1086/342406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: A methodological study. Arch Gen Psychiatry. 1982;39(8):879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Lunetta KL, Faraone SV, Biederman J, Laird NM. Family-based tests of association and linkage that use unaffected sibs, covariates, and interactions. Am J Hum Genet. 2000;66(2):605–614. doi: 10.1086/302782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Ferrell RE, Vanyukov MM. Dopamine system genes and attention deficit hyperactivity disorder: A meta-analysis. Psychiatr Genet. 2002;12(4):207–215. doi: 10.1097/00041444-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Hoenicka J, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Perez-Hernandez E, Ampuero I, Ramos-Atance JA, Palomo T, Rubio G. (AAT)n repeat in the cannabinoid receptor gene, CNV R1: Association with schizophrenia in a Spanish population. Eur Arch Psychiatry Clin Neurosci. 2006;256(7):437–441. doi: 10.1007/s00406-006-0665-3. [DOI] [PubMed] [Google Scholar]
- McGough JJ, Smalley SL, McCracken JT, Yang M, Del’Homme M, Lynn DE, Loo S. Psychiatric comorbidity in adult attention deficit hyperactivity disorder: Findings from multiplex families. Am J Psychiatry. 2005;162(9):1621–1627. doi: 10.1176/appi.ajp.162.9.1621. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Ogdie MN, Fisher SE, Yang M, Ishii J, Francks C, Loo SK, Cantor RM, McCracken JT, McGough JJ, Smalley SL, et al. Attention deficit hyperactivity disorder: Fine mapping supports linkage to 5p13, 6q12, 16p13, and 17p11. Am J Hum Genet. 2004;75(4):661–668. doi: 10.1086/424387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(7):795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G, Hoenicka J, Rubio G, Ampuero I, Jimenez-Arriero MA, Rodriguez-Jimenez R, Palomo T, Ramos JA. Association between cannabinoid receptor gene (CNR1) and childhood attention deficit/hyperactivity disorder in Spanish male alcoholic patients. Mol Psychiatry. 2003;8(5):466–467. doi: 10.1038/sj.mp.4001278. [DOI] [PubMed] [Google Scholar]
- Pressman LJ, Loo SK, Carpenter EM, Asarnow JR, Lynn D, McCracken JT, McGough JJ, Lubke GH, Yang MH, Smalley SL. Relationship of family environment and parental psychiatric diagnosis to impairment in ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45(3):346–354. doi: 10.1097/01.chi.0000192248.61271.c8. [DOI] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC, Giuffrida A. CB(1)-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem. 2007;101(2):389–396. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Rioux JD, Daly MJ, Silverberg MS, Lindblad K, Steinhart H, Cohen Z, Delmonte T, Kocher K, Miller K, Guschwan S, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat Genet. 2001;29(2):223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- Roeder K, Bacanu SA, Sonpar V, Zhang X, Devlin B. Analysis of single-locus tests to detect gene/disease associations. Genet Epidemiol. 2005;28(3):207–219. doi: 10.1002/gepi.20050. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65(3):221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, Berry EM. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: Differences between restricting and binging/purging subtypes. Am J Med Genet Part B. 2004;125B(1):126–130. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Del’Homme M, NewDelman J, Gordon E, Kim T, Liu A, McCracken JT. Familial clustering of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1135–1143. doi: 10.1097/00004583-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, Ebeling H, Hurtig T, Humphrey LA, McCracken JT, Varilo T, et al. Prevalence and psychiatric Comorbidity of attention deficit hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1575–1583. doi: 10.1097/chi.0b013e3181573137. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wang YC, Hong CJ. Association study of a cannabinoid receptor gene (CNR1) polymorphism and schizophrenia. Psychiatr Genet. 2000;10(3):149–151. doi: 10.1097/00041444-200010030-00008. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wang YC, Hong CJ. Association study between cannabinoid receptor gene (CNR1) and pathogenesis and psychotic symptoms of mood disorders. Am J Med Genet. 2001;105(3):219–221. doi: 10.1002/ajmg.1259. [DOI] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Nakata K, Tanaka Y, Takeda T, Kodama M, Fujiwara Y, Sakai A, Kuroda S. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7(5):515–518. doi: 10.1038/sj.mp.4001029. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


