Abstract
Statins have been shown to stimulate BMP2 transcription and bone formation. This raises the possibility that they could be useful for enhancing rates of fracture repair. Observational studies in patients treated with oral statins for lipid-lowering have been controversial. The likely reason for their inconsistent effects is that the statin concentration reaching the periphery was too low after oral administration to produce a reproducible biologic effect. Thus, we examined the effects of lovastatin (LV) given transdermally in a well-described preclinical model of fracture repair. Effects on the healing fracture callus were assessed by biomechanical strength, radiographs, and quantitative morphology. LV was administered transdermally (TD) for 5 days after fracture in several doses (0.1–5 mg/kg/d) and compared with vehicle-treated control rats and rats treated with LV by oral gavage (PO) at 5–25 mg/kg/d for 5 days from the day of fracture. Radiological evaluation of bones treated with TD LV showed enhanced fracture repair at 2 and 6 wk. BMD in the callus area at 6 wk was also increased in the TD group compared with vehicle-treated controls (p < 0.05). The force required to break TD-treated bones (0.1 mg/kg/d for 5 days) was 42% greater than vehicle-treated controls (p < 0.02), and there was a 90% increase in stiffness (p < 0.01). PO LV at much higher doses (10 and 25 mg/kg/d) showed increased stiffness but no change in other biomechanical properties. By histological examination, a significant increase was also observed in the size of the callus, surrounding proliferating cell nuclear antigen–positive cells, and osteoblast and osteoclast number in TD-treated rats compared with controls at day 8 after fracture (n = 6). In summary, we found that TD LV in low doses accelerates fracture healing, whereas 10-fold the lipid-lowering dose was required to produce any effect when it was administered orally. These studies provide valuable information on the potential of statins and TD delivery as a new and effective therapeutic modality in fracture repair.
Key words: fracture, statins, transdermal, osteoporosis, orthopedics, fracture repair, HMG-CoA reductase inhibitors
INTRODUCTION
There are ∼6.8 million traumatic fractures in the United States each year. Although the majority eventually heals with restoration of skeletal integrity, the economic costs to the health care system associated with traumatic fractures are estimated to be in the range of $5 billion dollars annually. This is caused in part by hospitalizations, physician visits, and time lost from work while the fractures heal. Any therapeutic approach that could safely and effectively reduce the rates of fracture nonunion or reduce the time to complete healing would be of enormous clinical and financial benefit.
The success of bone morphogenetic protein 2 (BMP2) as a therapeutic agent/biological for fracture repair has shown that enhancement of rates of fracture healing is therapeutically possible. BMP2 has been found to improve fracture healing and to repair bone defects in various animal models and human studies.(1–3) However, there are some problems associated with the therapeutic use of BMP2. It is a peptide growth factor and has to be given by injection. It is expensive to manufacture, and it is not possible to obtain reimbursement in many countries. These costs are passed on to the patient and are prohibitive to the widespread use of BMP2. Moreover, the practical application of this protein is dependent on the carrier system used to deliver it to the site of repair. BMP2 is not simple to deliver, and there have been years of study in attempting to develop a suitable carrier.(4) It must be given locally at the fracture site, because it does not have systemic effects due to its peptide nature and the rapid degradation in the circulation if it is administered systemically.
To circumvent the problems associated with the local administration of BMP2, we have tried a different approach. Statins, natural product compounds that inhibit HMG-CoA reductase and reduce serum cholesterol, increase BMP2 transcription in bone cells.(5) Statins are not effective in reproducibly stimulating bone formation systemically when given in those doses used to lower cholesterol, because they are subject to first-pass metabolism and do not reach sufficient high serum concentrations to produce systemic effects beyond the liver. We have recently shown that transdermal statins stimulate bone formation in rats when given in low doses and are more effective than oral statins.(6)
We found that local application of statins at fracture sites in rats can improve fracture healing,(7) and Skoglund and Aspenberg(8) have shown that local application of simvastatin by continuous injection to mouse femoral fractures improves parameters of biomechanical strength. However, not all fractures are amenable or suitable for local injections at the fracture site, and a safe systemic therapy with easy applicability that enhanced rates of fracture healing would be very attractive.
To determine whether systemic application of lovastatin (LV) enhances rates of fracture healing in a preclinical model, we examined the effects of transdermal (TD) LV in femoral fractures in rats. We analyzed the effect of LV on (1) callus formation, maturation, and fracture healing using radiographic evaluation; (2) callus mechanical strength and integrity using three-point bending; and *3) cell growth activity in the developing callus.
MATERIALS AND METHODS
Fracture model
For these experiments, we used 2-mo-old virgin female Sprague-Dawley intact rats, obtained from Harlan Laboratories, weight-matched and divided into several treatment groups. The study protocol was approved by the Animal Care and Use Committee at the University of Texas Health Science Center, San Antonio, TX, USA.
LV (Stason Pharmaceuticals) was administered by TD application in the dorsal region of the rat after shaving with an electric clipper. The solution was applied to the same area in each rat using gloves and applying 25 strokes or by oral gavage for 5 days from the same day the fracture was created. The well-described uniform and reproducible fracture defect using a pinned closed transverse rat femoral model was used. This model was chosen because it has been previously characterized by mechanical and histological methods.(9) Animals were anesthetized, and stainless steel Kirschner wires (K wires) (1.1-mm diameter) were inserted in a retrograde fashion through the right femoral shaft exiting the greater trochanter. A standard closed fracture was made in the midshaft using a custom made three-point bending fracture device. Radiographs were taken immediately after the creation of the fracture. Only animals with well-aligned midshaft transverse fractures were included in the study. The procedure was tolerated without complications, and animals were freely mobile after recovery from anesthesia. For histological evaluation, 6 animals/group were killed at day 8 (0 and 2.5 mg/kg/d TD and 25 mg/kg/d orally [PO]).
Subsequently, radiographs were obtained at 2, 4, and 6 wk after fracture. Animals were allowed full weight bearing, free cage activity, and food and water ad libitum. At the completion of the experiment (6 wk), animals were anesthetized with ketamine (10 mg/ml) at a dose of 100 mg/kg body weight and killed by cervical dislocation. Femurs were harvested, stripped of muscle tissue, and stored in 0.145 M NaCl at −70°C for mechanical testing. To determine an effective dose of TD LV, we performed one experiment using doses of 0, 1, and 2.5 mg/kg/d and compared with higher doses of PO LV at 10 and 25 mg/kg/d because we have shown previously(6) that we obtain better efficacy with transdermal delivery (experiment A = five groups, n = 8). We then tried to determine the minimal effective dose of TD LV for fracture healing in a subsequent experiment. Fractured bones were analyzed at 6 wk after receiving 0, 0.1, 1, and 5 mg/kg/d and compared with 5 mg/kg/d of PO LV (experiment B = five groups, n = 10). Some bones were eliminated from the final analysis because of displacement of the pin. Three rats died during anesthesia after taking radiographs.
Radiological analysis
Radiographic analysis was the primary method to assess healing parameters, namely periosteal reaction (callus formation), and bone remodeling. Appearance of the fracture callus was judged by the number of cortices with bridging callus using two radiographic views/animal (anterior–posterior and lateral). Radiographs at 2 and 6 wk after fracture were assessed blindly by four independent investigators using the scoring scale described previously.(7) Basically, a scale (0–5+) was assigned to each animal according to rebridgement (no rebridgement, partial, or complete) of one to four cortices and remodeling of the defect. Once the score was completed, the mean was obtained, and results were expressed as percentage change from vehicle-treated groups (vehicle-treated = 100%).
BMD
BMD of rat femora was evaluated for groups in experiment B using a mouse densitometer, Piximus (GE Medical Systems); BMD was calculated by dividing bone mineral content (g) by the projected bone area (cm2) and assessed in the region of the callus at 6 wk.
Biomechanical testing
To determine mechanical properties, hydrated femurs from experiments A and B were monotonically loaded in three-point bending using an EnduraTEC material testing system (ELF 3300; Bose Corp.). Each rat femur was horizontally positioned on the support rollers (which were 12 mm apart) such that the vertical, rounded impactor loaded the callus. Special care was taken to keep a consistent orientation within each experiment (i.e., bending occurred about the medial–lateral axis). The force-displacement curve was recorded as the impactor loaded the femur at a rate of 3 mm/min into the callus. This data provided the following structural properties: breaking force (maximum load), stiffness (average slope of linear portion of the curve before yielding), and work-to-fracture (area under the force-displacement curve).
Histological and immunohistochemical analysis
After animals were killed, fractured femora from experiment A were removed at 8 (n = 6) and 42 days (n = 7) after the procedure. Extreme care was taken not to traumatize the callus. The bone was cleaned of soft tissue, fixed in 10% formalin for 48 h, demineralized, processed using standard techniques, and sectioned transversally at the fracture site following a previously described method.(10) Briefly, the midposition of the fracture callus was determined from the baseline radiographs, and tissue sections (5 μm in thickness) were generated proximal and distal to the fracture site at fixed increments (500 μm). These sections were stained with H&E or used for TRACP staining and immunohistochemistry.
Immunohistochemistry:
PCNA antibody was obtained from Novus Biologicals. Several consecutive levels of histological sections next to the fracture site, distal and proximal (500 μm), were cut and mounted on poly-l-lysine–coated glass slides. The slides were incubated for 10 min at 30°C in normal horse serum diluted in PBS and triton buffer to block nonspecific antibody staining, and the same procedure was followed as previously described.(11) Histomorphometric analysis was performed using a semiautomated Osteomeasure System (Osteometrics) and digitizing pad and by following standard histomorphometric techniques. Ten regions (0.9 × 0.9 mm in size) containing subperiosteal osteoblastic cells (also including osteoprogenitor cells) were analyzed in each section. The number of PCNA+ cells was counted on day 8 after fracture (experiment A: groups 0 and 2.5 mg/kg/d TD and 25 mg/kg/d PO), and the ratio of PCNA+ cells to total cells was calculated and expressed as a percentage. The average measurement obtained was used as the PCNA score.
Analysis of osteoclast and osteoblast numbers in areas of cancellous bone in the callus:
The number of osteoclasts (Oc.Surf/BSurf) and osteoblasts (Ob.Surf/BSurf) at day 8 after fracture from vehicle-treated, TD (2.5 mg/kg/d), and PO LV (25 mg/kg/d) were assessed in demineralized histological sections of the fracture site after H&E staining or staining for the TRACP enzyme. Ten regions (0.9 × 0.9 mm in size) containing cancellous bone were analyzed in each section. The osteoclast and osteoblast surface index were calculated, and the average measurement was determined and expressed using nomenclature described previously by Parfitt et al.(12)
Callus dimensions:
Callus diameter (CDm) was measured in two orthogonal planes at the fracture line using a sliding caliper at 8 days and 6 wk after fracture.
Statistical analysis
Data are expressed as the mean ± SE. Statistical differences between groups were evaluated with one-way ANOVA. When the ANOVA performed over all groups was significantly different among the groups, statistical differences between two groups were subsequently analyzed using Tukey's multiple comparison test. p < 0.05 was considered significant.
RESULTS
Radiological analysis
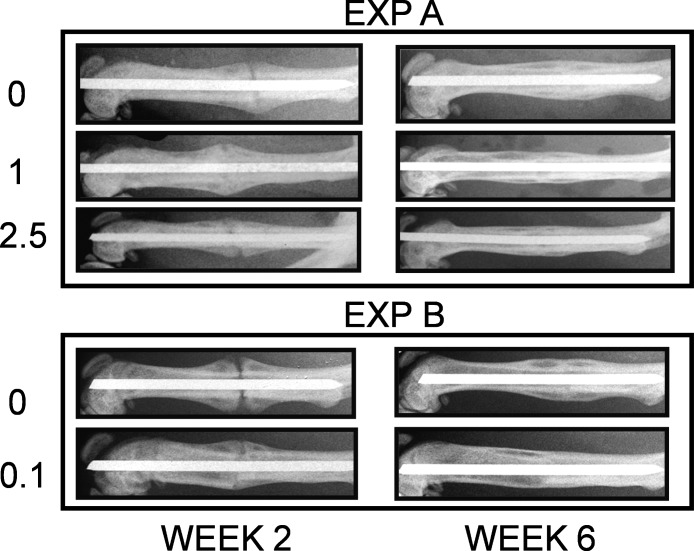
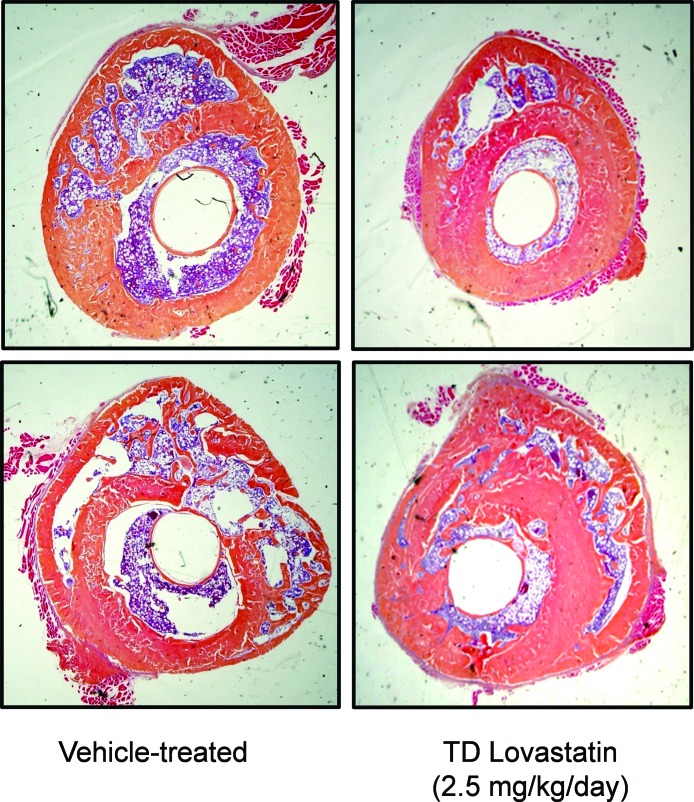
Callus formation was observed on radiographic examination by 2 wk in all animals. In experiment A, in groups treated with TD LV, fractured femurs showed enhanced repair at 2 wk compared with vehicle-treated animals. By week 6, almost complete healing of the TD-treated femurs was observed, with very small callus present, whereas large callus and incomplete bridging of cortical bone could clearly be seen radiographically in the vehicle-treated animals. Radiographs from experiment B also showed a significant acceleration of healing even at the lowest dose tested, 0.1 mg/kg/d (Fig. 1). In this experiment, TD LV at 1 and 5 mg/kg/d showed similar results as with experiment A (data not shown).
FIG. 1.
Radiographic images of fractured femurs (experiments A and B) at 2 and 6 wk after procedure was performed. Two images representative of each group (vehicle and TD LV) are shown (n = 8, experiment A; n = 10, experiment B).
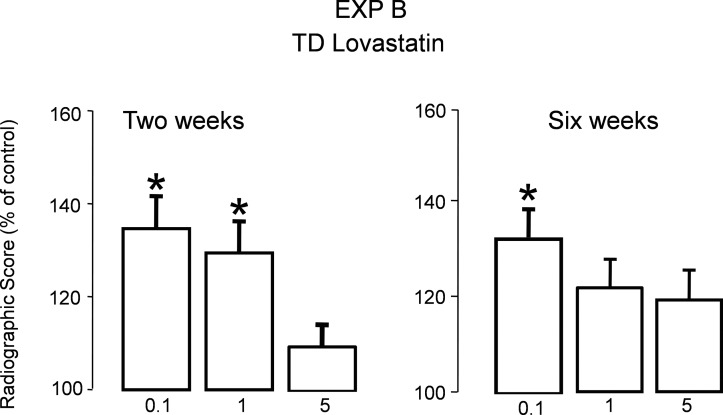
Using a grading scale based on rebridgement of the cortices and acceleration of healing as described in the Materials and Methods section, radiological evaluation of animals treated with TD LV at 2 and 6 wk indicated a significant increase in the healing rate (close to 40% compared with vehicle-treated) as shown in Fig. 2 (p < 0.001).
FIG. 2.
Radiological score obtained at 2 and 6 wk for experiment B (TD LV groups) using the grading scale presented in the Materials and Methods section (n = 10). Bars represent percentage increase compared with vehicle treated group ± SE.
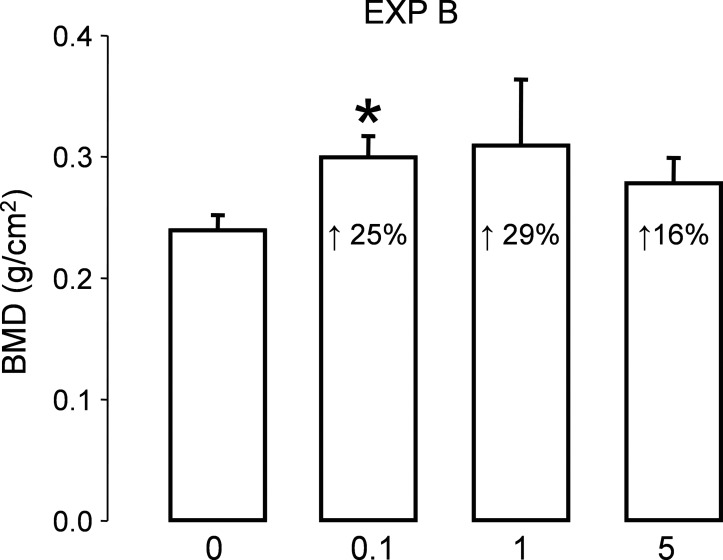
BMD
All the femurs from experiment B were analyzed at the callus region, and BMD is presented in Fig. 3. There was an increase in BMD in the groups treated with TD LV, being significant at 0.1 mg/kg/d. No changes were seen in the group treated with PO LV (data not shown).
FIG. 3.
BMD of the total callus at the fracture site was obtained for experiment B at 6 wk (n = 6). Bars represent mean ± SE and percentage increase compared with vehicle-treated is shown inside each bar. p < 0.05.
Histomorphometric analysis
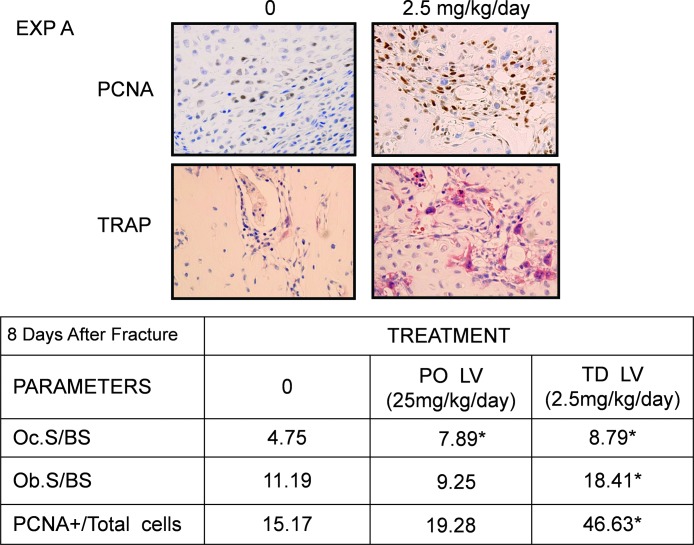
Histomorphometry was performed in one of the experiments (experiment A). The fractured bones at 8 days after fracture in TD LV-treated rats (1 and 2.5 mg/kg/d) showed significant increase in the size of the callus (Table 1: 22% and 13%, respectively), with newly formed cartilage and trabecular bone within the callus (data not shown). The presence of cell proliferation in the fracture calluses was studied using immunohistochemistry with anti-PCNA monoclonal antibodies as described in the Materials and Methods section. Eight days after the fracture, many PCNA+ cells could be found in the entire callus, more abundantly in the fibrous layers the periosteum. The number of PCNA+ cells increased significantly with TD LV (Fig. 4, top). We also observed an increase in the osteoclast number in groups treated with PO (25 mg/kg/d) and TD LV (2.5 mg/kg/d; Fig. 4, bottom) compared vehicle-treated animals, but only TD LV showed a significant increase in osteoblast number (see table for histomorphometric analysis). In the TD group, areas of endochondral ossification could be detected in the callus.
Table 1.
Callus Diameter (CDm) at 8 Days and 6 wk after fracture
| Experiment A | 8 days (mm) (n = 4) | 6 wk (mm) (n = 8) |
| Vehicle | 6.82 ± 0.07 | 5.84 ± 0.35 |
| PO 10 | ND | 4.85 ± 0.22 |
| PO25 | 8.32 ± 0.73 | 4.91 ± 0.22 |
| TD 1 | 9.61 ± 0.8† | 4.56 ± 0.19† |
| TD 2.5 | 9.47 ± 1.05* | 4.92 ± 0.14† |
CDm (mm) was measured at 8 days and 6 wk after fracture for experiment A. Number of animals for each time point is indicated in the table. After 8 days of fracture, TD LV (1 and 2.5 mg/kg/d) had a larger callus (>40% than control), but after 6 wk, the callus size decreased substantially and was <22% than the control group.
* Significantly different from vehicle, p < 0.001.
† Significantly different from vehicle, p < 0.05.
FIG. 4.
Representative histological sections comparing vehicle-treated vs. TD LV (2.5 mg/kg/d for 5 days) after 8 days of fracture. Several sections 500 μm proximal and distal to the fracture site were stained for the analysis. Ten regions as described in the Materials and Methods section were analyzed in each section. The table under the images shows the results of the histomorphometric analysis. Osteoclast and osteoblast surface as well as the number of PCNA+ cells were counted, and the ratio of PCNA+ cells to total cells was calculated and expressed as a percentage.
Cross-sectional images comparing controls with groups treated with TD LV showed more active endochondral ossification that appeared subperiosteally as new and more mature bone at the end of the 6 wk (Fig. 5).
FIG. 5.
Two representative images of transverse sections (at fracture site) 6 wk after fracture. Sections were stained with H&E (n = 6). Fracture can still be observed in the control sections. In the TD LV-treated group, the primary cortex and the new layer of bone have been remodeled and replaced with compact bone underlying the new external periosteal layer.
Biomechanical testing: three-point bending
After 6 wk of healing, femurs of rats treated with TD LV for 5 days at 1 and 2.5 mg/kg/d in experiment A and at 0.1 mg/kg/d in experiment B were significantly stronger than the controls (Table 2). The force required to break the bone was 42% greater than vehicle-treated controls. Oral LV showed some increase in structural strength at 5, 10, and 25 mg/kg/d, but this effect was not significant. The structural stiffness of the fractured femur was also significantly increased when rats were treated with TD LV even at 0.1 mg/kg/d. In comparison, PO LV only significantly increased stiffness at high doses (10 and 25 mg/kg/d).
Table 2.
Biomechanical Testing
| Parameter |
Experiment A
|
Experiment B
|
||||||||
| PO | PO | TD | TD | PO | TD | TD | TD | |||
| Dose (mg/kg/d) | 0 | 10 | 25 | 1 | 2.5 | 0 | 5 | 0.1 | 1 | 5 |
| N | 5 | 6 | 6 | 5 | 7 | 9 | 8 | 8 | 8 | 9 |
| 125.5 ± 8.2 | 135.0 ± 11 | 138.0 ± 1.1 | 178.1 ± 12* | 149 ± 7.6* | 135.4 ± 17.2 | 161.5 ± 13.7 | 192.6 ± 17* | 175 ± 15.8* | 154.2 ± 9.8 | |
| Maximum force (N) | ↑ 7.6% | ↑ 10% | ↑ 42% | ↑ 18.5% | ↑ 19% | ↑ 42% | ↑ 29% | ↑ 14% | ||
| 321 ± 20.2 | 559 ± 68.7* | 596.2 ± 102* | 566 ± 89* | 594 ± 26* | 252 ± 55 | 275.4 ± 38 | 477 ± 58* | 382 ± 49* | 419 ± 60* | |
| Stiffness (N/mm) | ↑ 74% | ↑ 85.7% | ↑ 76% | ↑ 85% | ↑ 9% | ↑ 90% | ↑ 52% | ↑ 67% | ||
| 35.4 ± 15 | 19.2 ± 1.6 | 26.6 ± 5.8 | 57.7 ± 5 | 27.1 ± 2.5 | 70.8 ± 16 | 62.6 ± 13.7 | 54 ± 8.9 | 54 ± 13.2 | 61 ± 8.7 | |
| Work to fracture (N-mm) | ↑ 63% | |||||||||
Biomechanical testing evaluated at 6 wk by three-point bending for experiments A and B in groups treated with PO and TD lovastatin. Number of femora tested for each treatment groups is indicated in the table. Results are expressed as mean ± SE. Numbers after arrows represent the percentage increase compared with vehicle-treated controls.
* p < 0.02, compared with vehicle control.
DISCUSSION
In this study, we showed that LV administered transdermally stimulates rates of fracture healing in a well-described model of fracture repair in rats, as has been previously shown for BMP2.(1,13) The effects were seen at much lower doses of TD LV than those required by the oral route to cause any effect on bone. Rats were treated with TD LV for (once daily for 5 days only) after fracture, and results showed accelerating healing of fractures as assessed by radiological evaluation, biomechanical testing, and histological examination. LV administered transdermally accelerated the maturation process that resulted in faster healing and improved structural strength of the fractured femur. LV, however, did not improve work-to-fracture, likely because of the nature of the healing callus. Intact bones exhibit a certain degree of displacement after yielding, and this postyield displacement contributes to the work-to-fracture. Healing calluses, on the other hand, typically break near the yield point, and so their work-to-fracture is a product of maximum force and stiffness. Because treatment by LV tended to stiffen the callus, the displacement at fracture was less pronounced. Given that work-to-fracture is a function of both force and displacement, treatment did not increase this property. At a later time point of healing in which the callus has undergone significant remodeling, the postyield behavior would be expected to return increasing the work-to-fracture.
The callus size at the time when biomechanical testing was performed (6 wk) was significantly smaller with TD LV than the control, indicating an improvement in material properties. In contrast, the callus size 1 wk after fracture was significantly bigger in femurs treated with TD LV with increase in PCNA+ cells and osteoclast and osteoblast number, suggesting acceleration of tissue regeneration starting the first week after fracture. Similar to our studies in preclinical models of osteoporosis,(6) LV did not give a classical sigmoidal dose–response curve. This has been found by others with simvastatin(8) and with biological effects of other growth factors, as well as BMP2 itself.(14) The reasons are not entirely clear but may be caused by counter-regulatory mechanisms common in growth factor cascades when higher doses are used. An alternative possibility is that LV uptake may be saturated at low drug concentrations at sites of drug uptake. Flat dose–response effects have been reported with other commonly used effective drugs, including β-blockers (hypotensive effect) and benzodiazepines (duration of apnea).(15,16)
We have previously shown that LV administered transdermally increased trabecular bone volume in intact and ovariectomized rats.(6) Now we showed its effects on fracture healing in a well-established rat model. Young rats were used in this experiment because we wanted to evaluate the statin effect in a model where the early period of fracture healing is not compromised. Future experiments will include older animals and ovariectomized rats.
Normal fracture healing is a complex, multistep process involving cellular events influenced and regulated by local and systemic factors. The most common biological failure in fracture healing involves an improperly formed callus during the first weeks after fracture. Despite statistics from the American Academy of Orthopaedic Surgeons indicating that ∼6.8 million fractures occur in the United States each year, the treatment of fractures has remained essentially unchanged for centuries. In addition, 5–10% of these fractures will have some difficulty healing. Frost(17,18) proposed that treatments that reliably induce callus formation will resolve the problem of biological failures and improve rates of fracture healing. Whereas progress has been made in the treatment of more complex fractures with the use of techniques like intramedullary fixation, ultrasound, electricity, etc., most cases are routinely treated with rest and cast immobilization. However, recent advances in understanding the regulatory factors controlling fracture healing have suggested that a number of compounds may be used to stimulate bone growth and initiate and enhance the cascade of events involved in callus formation and maturation.
It is known now that the expression of members of the TGFβ superfamily, especially BMPs, may be crucial in fracture healing. In several animal models, BMP2 has been shown to accelerate skeletal defect repair and fracture healing, as indicated by radiographic assessment and increased biomechanical strength.(1–3) BMP receptors and signaling proteins have also been identified within bone cells of the fracture callus.(19,20) As a result, the BMP family has been suggested to be actively involved in fracture healing.(21,22)
BMPs, particularly BMP2, which is well known for its osteogenic activity, are highly expressed during fracture repair. Bostrom,(23) using monoclonal antibodies against BMP2 and BMP4, showed increased expression of these BMPs in the healing fracture callus. Immediately after the fracture occurs, very few cells express these factors, but as the process of endochondral ossification continues, there is substantial increase in their expression by primitive mesenchymal and chondrocytic cells. In the callus undergoing intramembranous ossification, there is also increased expression of BMPs by periosteal cells and osteoblasts, suggesting a critical role of these proteins in fracture repair. In recent years, clinical trials using a highly concentrated human extract of BMP have shown promising results for the treatment of nonunions and spine fusion, and BMPs have been approved for clinical applications in nonunions of bone fractures resistant to conventional therapy. However, the delivery of BMPs has proven to be difficult, particularly in the selection of optimal carriers. Ideal conditions for their use as osteoinductive factors in fracture repair still need to be clarified before they can be widely used for this indication.
Statins stimulate bone formation in vitro by increasing expression and production of BMP2, which acts as an autocrine-paracrine factor in osteoblast differentiation.(24) The effects of the statins on bone formation in vivo are impaired in transgenic mice, which are unresponsive to BMP2.(25) Importantly, however, even transient exposure to the drug leads to prolonged effects. In vitro, a mere 6-h exposure to a statin leads to a prolonged effect on bone formation in bone organ cultures, apparent even after 14 days of continuous culture (IR Garrett, GE Gutierrez, and GR Mundy, unpublished data, 2001). Similar results have been found in vivo in rats, where statin administration to the skin for 5 days is associated with a 150% increase in bone formation rates 35 days later.(6)
The effect of statins on bone is related to their capacity to inhibit HMG-CoA reductase, because other nonstatin compounds that reduce concentrations of this enzyme also stimulate BMP2 production and bone formation. In addition, downstream metabolites of mevalonic acid block the effects of statins on bone formation and on BMP2 expression.(25) Statins enhance the generation of NO by bone cells, and this seems to be a key intermediate in their effects on the BMP2 promoter. These effects are blocked by pharmacologic inhibitors of endothelial nitric oxide synthase (eNOS) and are abrogated in eNOS-null mutant mice.(26,27)
Statins are a group of HMG-CoA reductase inhibitors that are widely prescribed as potent cholesterol-lowering agents. Also, statins have been shown to dramatically increase bone formation rates in rodents(5,26) and BMD in postmenopausal women.(28) Statin-treated osteoblastic cells show increased levels of osteoblast differentiation markers including type 1 collagen, alkaline phosphatase, and osteocalcin.(29) This effect is a result of increased transcription and prolonged stimulation of the BMP2 gene in osteoblasts,(5,30,31) chondrocytes,(32) and smooth muscle cells.(33)
Recently, fractures in mice infused with simvastatin have shown increased callus transverse areas and mechanical strength, resulting in improved fracture healing.(8) These results correlate with our own observations when histological sections were analyzed 8 and 42 days after fracture. Statins have also been shown to exert a protective effect against nonpathological fracture among older women(34–36) and have been associated with a significant reduction in fracture risk in elderly men.(37,38)
Others have also shown acceleration in fracture healing in an ovariectomized rat model when simvastatin was applied locally by multiple daily injections into the adjacent subcutaneous tissue.(39) However, the DMSO formulation described would not be suitable for human use, multiple local injections would be problematic for patients, and the effective dose was 100-fold higher than the effective transdermal dose described here.
In conclusion, statins may represent a novel therapeutic approach for the treatment of skeletal fractures, with substantial clinical advantages over existing treatment modalities. Relatively large oral doses of statins are required to induce bone formation in humans, and high statin concentrations have been associated with side effects such as myotoxicity.(40–42) However, this study showed the efficacy of low doses of TD- compared with PO-delivered LV to enhance callus formation and progression in a preclinical rat mid-diaphyseal fracture model.
ACKNOWLEDGMENTS
This work was supported in part by NIH Grants SBIR IR43AR050288-01A1 and R01 AR 048801 and a Veterans Affairs Merit Review to GRM.
Footnotes
Drs Gutierrez, Garrett, McCluskey, Rossini, Flores, and Mundy have stock in OsteoScreen and OsteoGenix, which own issued patents on the effects of statins on bone. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Bax BE, Wozney JM, Ashhurst DE. Bone morphogenetic protein-2 increases the rate of callus formation after fracture of the rabbit tibia. Calcif Tissue Int. 1999;65:83–89. doi: 10.1007/s002239900662. [DOI] [PubMed] [Google Scholar]
- 2.Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: A biomechanical and histological study in rats. Bone. 2002;30:816–822. doi: 10.1016/s8756-3282(02)00740-8. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85:1425–1435. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hollinger JO, Leong K. Poly(alpha-hydroxy acids): Carriers for bone morphogenetic proteins. Biomaterials. 1996;17:187–194. doi: 10.1016/0142-9612(96)85763-2. [DOI] [PubMed] [Google Scholar]
- 5.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez G, Lalka D, Garrett I, Rossini G, Mundy G. Transdermal application of lovastatin to rats causes profound increases in bone formation and plasma concentrations. Osteoporos Int. 2006;17:1033–1042. doi: 10.1007/s00198-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 7.Garrett IR, Gutierrez GE, Rossini G, Nyman J, McCluskey B, Flores A, Mundy GR. Locally delivered lovastatin nanoparticles enhance fracture healing in rats. J Orthop Res. 2007;25:1351–1357. doi: 10.1002/jor.20391. [DOI] [PubMed] [Google Scholar]
- 8.Skoglund B, Aspenberg P. Locally applied simvastatin improves fracture healing in mice. BMC Musculoskelet Disord. 2007;8:98–104. doi: 10.1186/1471-2474-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Wronski TJ, Hollinger JO, Einhorn TA. Application of histomorphometric methods to the study of bone repair. J Bone Miner Res. 2005;20:1715–1722. doi: 10.1359/JBMR.050702. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki A, Jingushi S, Oda Y, Izumi T, Shida JI, Tsuneyoshi M, Sugioka Y. Localization and quantification of proliferating cells during rat fracture repair: Detection of proliferating cell nuclear antigen by immunohistochemistry. J Bone Miner Res. 1997;12:96–102. doi: 10.1359/jbmr.1997.12.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt H, Christensen KS, Lind M, Hansen ES, Hall DWR, Hvid I. Recombinant human bone morphogenetic protein 2 enhances bone healing in an experimental model of fractures at risk of non-union. Injury. 2005;36:489–494. doi: 10.1016/j.injury.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Radomsky ML, Aufdemorte TB, Swain LD, Fox WC, Spiro RC, Poser JW. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J Orthop Res. 1999;17:607–614. doi: 10.1002/jor.1100170422. [DOI] [PubMed] [Google Scholar]
- 15.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–324. [PubMed] [Google Scholar]
- 16.Love JN. Beta-blocker toxicity: A clinical diagnosis. Am J Emerg Med. 1994;12:356–357. doi: 10.1016/0735-6757(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 17.Frost HM. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop. 1989;248:294–309. [PubMed] [Google Scholar]
- 18.Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop. 1989;248:283–293. [PubMed] [Google Scholar]
- 19.Kloen P, Doty SB, Gordon E, Rubel IF, Goumans MJ, Helfet DL. Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am. 2002;84:1909–1918. doi: 10.2106/00004623-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ. BMP signaling components are expressed in human fracture callus. Bone. 2003;33:362–371. doi: 10.1016/s8756-3282(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 21.Spector JA, Luchs JS, Mehrara BJ, Greenwald JA, Smith LP, Longaker MT. Expression of bone morphogenetic proteins during membranous bone healing. Plast Reconstr Surg. 2001;107:124–134. doi: 10.1097/00006534-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom MP. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop. 1998;355(Suppl):S116–S123. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- 24.Harris SEFJ, Harris MA, Ghosh-Choudhury N, Dallas MR, Wozney J, Mundy GR. Recombinant bone morphogenetic protein 2 accelerates bone cell differentiation and stimulates BMP 2 mRNA expression and BMP 2 promoter activity in primary fetal rat calvarial osteoblast cultures. Mol Cell Differ. 1995;3:137–155. [Google Scholar]
- 25.Zhao M, Harris SE, Horn D, Geng Z, Nishimura R, Mundy GR, Chen D. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol. 2002;157:1049–1060. doi: 10.1083/jcb.200109012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrett IR, Gutierrez G, Mundy GR. Statins and bone formation. Curr Pharm Des. 2001;7:715–736. doi: 10.2174/1381612013397762. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, de Fernanz Marticorena I, Hukkanen M, Huang P, MacIntyre I, Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001;158:247–257. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355:2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 29.Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem. 2004;92:458–471. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000;271:688–692. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 31.Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, Dang G. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun. 2003;308:458–462. doi: 10.1016/s0006-291x(03)01408-6. [DOI] [PubMed] [Google Scholar]
- 32.Hatano H, Maruo A, Bolander ME, Sarkar G. Statin stimulates bone morphogenetic protein-2, aggrecan, and type 2 collagen gene expression and proteoglycan synthesis in rat chondrocytes. J Orthop Sci. 2003;8:842–848. doi: 10.1007/s00776-003-0724-9. [DOI] [PubMed] [Google Scholar]
- 33.Emmanuele L, Ortmann J, Doerflinger T, Traupe T, Barton M. Lovastatin stimulates human vascular smooth muscle cell expression of bone morphogenetic protein-2, a potent inhibitor of low-density lipoprotein-stimulated cell growth. Biochem Biophys Res Commun. 2003;302:67–72. doi: 10.1016/s0006-291x(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 34.Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, Goodman MJ, Gurwitz JH, LaCroix AZ, Platt R. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet. 2000;355:2185–2188. doi: 10.1016/S0140-6736(00)02400-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang PS, Solomon DH, Mogun H, Avorn J. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA. 2000;283:3211–3216. doi: 10.1001/jama.283.24.3211. [DOI] [PubMed] [Google Scholar]
- 36.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283:3205–3210. doi: 10.1001/jama.283.24.3205. [DOI] [PubMed] [Google Scholar]
- 37.Scranton RE, Young M, Lawler E, Solomon D, Gagnon D, Gaziano JM. Statin use and fracture risk: Study of a US veterans population. Arch Intern Med. 2005;165:2007–2012. doi: 10.1001/archinte.165.17.2007. [DOI] [PubMed] [Google Scholar]
- 38.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HAP. Use of statins and fracture: Results of 4 prospective studies and cumulative meta-analysis of observational studies and controlled trials. Arch Intern Med. 2004;164:146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 39.Wang JW, Xu SW, Yang DS, Lv RK. Locally applied simvastatin promotes fracture healing in ovariectomized rat. Osteoporos Int. 2007;18:1641–1650. doi: 10.1007/s00198-007-0412-2. [DOI] [PubMed] [Google Scholar]
- 40.Fuentes I, Aguilera C. Myopathy secondary to the treatment with inhibitors of HMG-CoA reductase. Med Clin (Barc) 1998;111:700–704. [PubMed] [Google Scholar]
- 41.Duell PB, Connor WE, Illingworth DR. Rhabdomyolysis after taking atorvastatin with gemfibrozil. Am J Cardiol. 1998;81:368–369. doi: 10.1016/s0002-9149(97)00907-7. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson RH, Wang P, Glueck CJ. Myositis and rhabdomyolysis associated with concurrent use of simvastatin and nefazodone. JAMA. 1997;277:296–297. doi: 10.1001/jama.277.4.296. [DOI] [PubMed] [Google Scholar]