Abstract
The study of spontaneous mutations in mice over the last century has been fundamental to our understanding of normal physiology and mechanisms of disease. Here we studied the phenotype and genotype of a novel mouse model we have called the New Zealand Ginger (NZG/Kgm) mouse. NZG/Kgm mice are very large, rapidly growing, ginger-colored mice with pink eyes. Breeding NZG/Kgm mice with CAST/Ei or C57BL/6J mice showed that the ginger coat colour is a recessive trait, while the excessive body weight and large body size exhibit a semidominant pattern of inheritance. Backcrossing F1 (NZG/Kgm × CAST/Ei) to NZG/Kgm mice to produce the N2 generation determined that the NZG/Kgm mouse has two recessive pigmentation variant genes (oca2p and tyrp-1b) and that the tyrp-1b gene locus associates with large body size. Three coat colors appeared in the N2 generation; ginger, brown, and dark. Strikingly, N2 male coat colour associated with body weight; the brown-colored mice weighed the most followed by ginger and then dark. The male brown coat-colored offspring reached adult body weights indistinguishable from NZG/Kgm males. The large NZG/Kgm mouse body size is a result of excessive lean body mass since these mice are not obese or diabetic. NZG/Kgm mice exhibit an unusual pattern of fat distribution; compared with other mouse strains they have disproportionately higher amounts of subcutaneous and gonadal fat. These mice are susceptible to high-fat diet-induced obesity but are resistant to high-fat diet-induced diabetes. We propose NZG/Kgm mice as a novel model to delineate gene(s) that regulate 1) growth and metabolism, 2) resistance to Type 2 diabetes, and 3) preferential fat deposition in the subcutaneous and gonadal areas.
Keywords: mouse model, pigmentation, body weight, body size, body fat distribution
the study of spontaneous mutations in mice over the last century has been fundamental to our understanding of normal physiology and mechanisms of disease (5, 16). Two examples that have contributed significantly to understanding energy homeostasis are the ob/ob and dominant yellow agouti mice. The ob/ob mouse has a mutation in the leptin gene and lacks circulating leptin, a fat cell-derived hormone critical for signaling the body's energy supplies to the brain (28). At the agouti locus, over 25 different alleles have been identified in which phaeomelanin is generally dominant over eumelanin synthesis (22). The two most notable dominant yellow agouti alleles are the lethal yellow (Ay) and the viable yellow (Avy) mutations that develop a dominant pleiotropic syndrome including obesity, insulin resistance, increased linear growth, and yellow coat color. These mice are commonly referred to as “yellow obese mice.”
The mouse agouti gene encodes a secreted protein primarily produced in the hair follicle in wild-type mice (6, 21). Agouti signaling protein (ASP) is an inverse agonist and antagonist at the melanocortin 1 receptor (MC1R) and at the melanocortin 4 receptor (MC4R) (19). The yellow obese mouse phenotype results from dysregulation of the agouti gene leading to ectopic overexpression of ASP. Ectopic ASP prevents α-melanocyte stimulating hormone action at the MC1R resulting in yellow coat color and at the MC4R in the hypothalamus, resulting in obesity and Type 2 diabetes (19). These dominant yellow agouti mice are mildly hyperphagic and show increased lean and fat mass (22). ASP is normally expressed transiently in the skin of 6-day-old mouse pups, giving the yellow color band close to the tip of wild-type mouse fur.
In the 1970s a very large yellow-colored mouse arose spontaneously in Ruakura, New Zealand, during a research program of selection for body weight at different ages and on fat deposition. Four strains of mice, Aw, C57, CBA, and an albino strain called Ruakura White were used for a four-way cross breeding program to select for body size (2), and a yellow mouse that we have named New Zealand Ginger (NZG/Kgm) appeared in the colony. Striking characteristics for the NZG/Kgm mouse are its large body size and ginger coat color. Our investigations, described here, show that the NZG/Kgm mouse is different from the two well-described yellow coat-colored mice, extension (e) recessive yellow and dominant yellow agouti (24). First and foremost, the yellow coat color for NZG/Kgm mice is recessive, and therefore this is not a dominant yellow agouti mutation. Second, a normal coding MC1R is expressed in the skin of NZG/Kgm mice, and therefore it is not an e recessive mouse.
To better understand the phenotype of the NZG/Kgm mouse and the genetics behind the ginger coat color and large body size we bred NZG/Kgm mice with the evolutionarily divergent in-bred mouse strain, CAST/Ei, to generate F1 mice. The F1 mice were backcrossed to NZG/Kgm to produce N2 mice. In addition, we bred NZG/Kgm mice with the common laboratory mouse strain C57BL/6J to generate F1 mice. Here we describe the phenotypes for NZG/Kgm, F1, and N2 mice and compare the NZG/Kgm phenotype with that of C57BL/6J, Avy/a, a/a, and C3HeN mice. Furthermore, we determined whether NZG/Kgm mice were susceptible to high-fat diet-induced obesity. Understanding the genetic loci contributing to NZG/Kgm coat color and large body size may help shed light on novel genes involved in the regulation of lean body mass.
MATERIALS AND METHODS
Mice: Breeding and Diets
The NZG/Kgm mouse is a unique mouse that arose in Ruakura, New Zealand, in the 1970s, and since this time has been maintained as an in-bred colony in the Vernon Jensen Unit, University of Auckland. CAST/Ei mice were imported from the Jackson Laboratory (Bar Harbor, ME). A colony of Avy/a mice (VY/WffC3Hf/Nctr-Avy stock derived from C3H/Di × C57BL/6J F1 hybrids) (26) was a gift from Professor George Wolff (National Center for Toxicological Research/FDA, Jefferson, AR). C3HeN and C57BL/6J mice were purchased from the Vernon Jensen Unit, University of Auckland. The Animal Ethics Committee at the University of Auckland approved all of the animal protocols. Animals were housed up to 10 per cage and provided with normal chow and water ad libitum.
CAST/Ei mice, an evolutionarily divergent in-bred mouse strain used for mapping defective genes, were bred with NZG/Kgm mice to produce F1 mice. Four male CAST/Ei bred with nine NZG/Kgm females to produce a total of 74 F1 and one NZG/Kgm male bred with a female CAST/Ei to produce a total of 13 F1. We were restricted in the availability of CAST/Ei mice for these studies because the CAST/Ei mice were extremely aggressive, and we were unable to establish a breeding colony after two independent shipments of mice from The Jackson Laboratory. Nine F1 progeny were mated to NZG/Kgm mice to produce 72 N2 offspring. C57BL/6J mice were bred with NZG/Kgm mice to produce 29 F1 mice.
Mice were maintained on a 12-h light/dark cycle at 19–23°C and after weaning were fed standard, low-fat, or high-fat diets ad libitum. Standard rodent diet (Teklad Global 18% protein rodent diet) was purchased from Harlan Teklad Gobal Diets (Oxford, UK), and special low- and high-fat rodent diets, D12450B (10 kcal% fat) and D12451 (45 kcal% fat), were purchased from Research Diets (New Brunswick, NJ).
Phenotype Characterization
Growth.
Mice were weaned at 21 days of age, separated by sex, ear notched for identification, and housed up to six per cage. Individual body weights were determined weekly to the nearest 0.1 g from weaning. Body length (nasal-anal) and tail length (anal-tail tip) were measured with calipers to the nearest mm when mice were culled.
Organ weights and blood chemistry.
Mice were fasted overnight (∼16 h) and then euthanized with halothane anesthesia. Blood was collected from the retro-orbital sinus into EDTA-coated vacutainer blood collection tubes (Becton Dickson Vacutainer Systems). The blood was kept on ice until the tubes were centrifuged at 3000 g for 10 min at 4°C. Following cervical dislocation, organs [brain, pituitary, heart, liver, lungs, adrenal glands, spleen, kidneys, stomach, retroperitoneal fat, visceral fat (omental), gonadal fat, subcutaneous (flanking the abdomen) fat, ovaries, testes, and small intestine] were dissected and individually weighed. The stomach and intestine were flushed with saline before being weighed, and the length of the small intestine was determined to the nearest millimeter with a ruler. The ratios of individual organ weights to body weight, expressed as a percentage, were calculated. Plasma glucose was measured using a Perichrom glucose assay (Roche Diagnostics NZ, Auckland, NZ).
Measurement of hormone levels.
Plasma insulin levels were measured using a Mercodia Ultrasensitive Insulin ELISA kit (Australian Laboratory Services, Auckland, NZ). A Quantikine mouse leptin ELISA assay (R&D Systems, Pharmaco, Auckland, NZ) was used to measure plasma leptin levels. Plasma growth hormone and insulin-like growth factor 1 (IGF1) were measured with Quantikine rat growth hormone and mouse/rat IGF1 ELISA kits, respectively, purchased from DSL Australia Pty (NSW, Australia).
High-fat diet-induced obesity.
NZG/Kgm mice were housed in cages of up to six male or female mice per cage and were fed either a control diet (10% calories from fat) or a high-fat diet (45% calories from fat) from weaning. Six mice of each sex were culled at 50, 100, and 150 days of age. Body weights were recorded weekly.
Insulin and glucose tolerance tests.
A glucose tolerance test was performed on NZG/Kgm male mice aged ∼150 days that had been fed either control diet or a high-fat diet from weaning. The mice were fasted overnight (∼16 h) and then at 9:00 AM were injected intraperitoneally (ip) with d-glucose in normal saline (3 g/kg body wt). An insulin tolerance test was performed on mice that were fasted overnight (∼16 h) and then at 9:00 AM were injected ip with human insulin diluted in normal saline (1 IU/kg body wt). Blood was collected from the tail tip at 0 (fasting), 15, 30, 60, 90, 120, 150, and 180 min after either glucose or insulin injection, and the blood glucose was immediately measured by the use of an ACCU-CHEK blood glucose meter [Roche Diagnostics (NZ), Auckland, NZ].
Magnetic Resonance Imaging Method
Magnetic resonance imaging (MRI) was used to compare body fat composition between NZG/Kgm and C57BL/6J mice. Experiments were performed on anaesthetized (ketamine and 2% xylazine) mice using a Varian 4.7T system interfaced with a Unity Inova spectrometer (Varian, Palo Alto, CA). Images were acquired using a birdcage-design radio-frequency coil with inner diameter of 72 mm (m2m Imaging). A gradient-echo pulse sequence (TR = 610 ms, TE = 3.8 ms, flip angle = 70°, matrix = 256 × 128) was used to acquire proton density (PD) weighted images. Fat-saturated images were also acquired using a saturation pulse (flip angle = 90°, duration = 15 ms) followed by a gradient-echo host sequence identical to that used for the PD-weighted images. Both PD and fat-saturated images were obtained for a series of contiguous 1.5 mm thick coronal slices for each mouse. The number of slices varied with animal size and was chosen so that the entire volume of the animal was covered. Body fat composition was quantified by processing of the MR data using ImageJ (23).
Statistics
The growth curves based on body weight were fitted by the logistic model: Y = c/{1 + exp[−b(t − m)] }, where c = the upper asymptote of weight, b = growth rate, and m = time of maximum growth. The procedure NLMIXED in SAS (SAS version 9; SAS Institute, Cary, NC) was used. The animal effect was incorporated by including a normally distributed random variable with mean zero.
Data for body size, organ weights, blood biochemistry, and hormone levels are shown as means ± SE, and statistical analysis was performed by a one-way ANOVA followed by Bonferroni's post hoc test. For multiple comparisons, Kruskal-Wallis and Dunn's nonparametric tests were used. Correlation tests between organ weights, body weights, and hormone levels were performed using the Pearson correlation test after testing for normal distributions. The Spearman correlation test was used for testis weight and insulin levels since these were not normally distributed. GraphPad Prism software (GraphPad Software, San Diego, CA) was used, and a P value <0.05 was considered statistically significant for all statistical methods used.
RESULTS
NZG/Kgm Mouse is Recessive for Two Old Pigmentation Variant Genes: Pink-Eyed Dilution (oca2p) and Brown (tyrp-1b)
The NZG/Kgm mouse phenotype resembles that of dominant yellow agouti (Avy/a) mice, but NZG/Kgm mice do not have an agouti gene mutation. First, breeding NZG/Kgm mice to CAST/Ei mice produced all brown agouti-colored F1 mice, indicating that the NZG/Kgm ginger coat color is recessively inherited (Fig. 1). Second, a Northern blot showed agouti mRNA is only expressed in skin from 6-day-old NZG/Kgm pups and not in brain, heart, kidney, liver, or testis, ruling out ectopic agouti gene expression (Supplementary Fig. S1),1 and NZG/Kgm mice have a signaling defect downstream of melanocortin peptide signaling (Supplementary Fig. S2). Third, compared with the continuous yellow pigment in Avy/a mice fur, the NZG/Kgm mouse fur has a grayish color close to the skin and a stronger ginger color at the tips of the fur, an indication of a functional ASP (Supplementary Fig. S3).
Fig. 1.
The NZG/Kgm mouse ginger coat colour is recessive and masks a brown (Tyrp1) gene mutation. NZG/Kgm mice were crossed with CAST/Ei mice, and the F1 offspring were backcrossed with NZG/Kgm to obtain the N2 generation. The coat colour for NZG/Kgm mice shows a recessive pattern of inheritance (F1 mice are always brown agouti). N2 mice were either dark, brown, or ginger, suggesting that the NZG/Kgm mouse has 2 pigmentaton gene mutations: pink-eyed dilution (p or Oca2) and brown (Tyrp1) genes. The NZG/Kgm and ginger coat-colored N2 mice have pink eyes.
When NZG/Kgm mice were bred with CAST/Ei mice to produce the F1 generation and then the F1 mice were backcrossed to NZG/Kgm mice to produce the N2 generation, the F1 mice were all brown agouti colored and the N2 offspring exhibited three different coat colors that we have described as dark, brown, and ginger (Fig. 1). The dark and brown mice had black eyes, while the ginger colored mice had pink eyes. The presence of these three N2 coat colors indicated the likelihood of mutations in the NZG/Kgm pink-eyed dilution [now known as oculocutaneous albinism II (Oca2)] and brown [now known as tyrosinase-related protein 1 (Tyrp-1)] genes.
The NZG/Kgm ginger coat color mapped to the Oca2 locus, and the Oca2 gene was the only gene at this locus that was not expressed as measured by RT-PCR or Northern blotting in tissues derived from the NZG/Kgm mouse (Supplementary Figs. S4–S6). PCR analysis of a fragment of the NZG/Kgm Oca2 gene determined that it is characteristic of the original p allele (oca2p) (Supplementary Fig. S7) (4). The mRNA for the coding NZG/Kgm tyrosinase-related protein 1 gene was sequenced and found to contain the same four nucleotide mutations (two silent and two that alter the coding) previously described for the allele that confers the brown coloration for mice (11, 27) (tyrp-1b) (Supplementary Fig. S8).
NZG/Kgm Mice Grow Very Rapidly, and Both Male And Female Adult NZG/Kgm Mouse Body Weights Exceed Those of Dominant Yellow Agouti (Avy/a) Mice
A striking characteristic of NZG/Kgm mice and one that is not shared with Avy/a mice is the very rapid growth rate observed for both males and females, with growth curves reaching a plateau at a younger age (∼60 days for NZG/Kgm) than those for Avy/a mice (∼100 days for Avy/a) (Figs. 2 and 3, A and B). Adult NZG/Kgm male mice are heavier than age-matched Avy/a male mice (Figs. 2 and 3A and Supplementary Fig. S9), while NZG/Kgm and Avy/a female mice obtain similar adult body weights (Figs. 2 and 3B). The increased growth and body size of the NZG/Kgm mouse are not a result of any defect in expression or function of the neural melanocortin receptors that are known to influence mouse body weight. NZG/Kgm mice express both Mc3r and Mc4r mRNA in the brain and the coding sequences of both of these genes in the NZG/Kgm mouse are normal. (Data not shown; see supplementary methods and Supplementary Table S1).
Fig. 2.
NZG/Kgm mice grow more rapidly and reach a greater body weight compared with Avy/a mice. NZG/Kgm male (35), NZG/Kgm female (36), Avy/a yellow male (2), Avy/a yellow female (2), Avy/a pseudoagouti male (1), Avy/a pseudoagouti female (1), a/a black male (3), and a/a black female (3) were weighed weekly from weaning. Body weight (BW) is plotted against age in days (Day).
Fig. 3.
NZG/Kgm mice are not obese, nor are they diabetic in contrast to Avy/a mice, which are obese and diabetic. Male (A) and female (B) NZG/Kgm, Avy/a, and a/a mice were fed normal chow and tap water ad libitum from weaning. At ∼50, 100, and 150 days of age the mice were assigned to either the “Free Fed” group or the “16h starved group.” The 16 h starved group were starved for 16 h prior to anesthetic, when all mice had their body weight recorded and then plasma collected for glucose, insulin, and leptin measurements. All mice were euthanized following blood collection. Data shown represent means ± SE of groups. Significant differences compared with age-matched NZG/Kgm mice: *P < 0.05; **P < 0.01; ***P < 0.001; n = number of mice per group.
NZG/Kgm Mice are Larger and Have Increased Lean Mass Compared With C3H/HeN, a/a, Avy/a, and C57BL/6J Mouse Strains
The NZG/Kgm male mouse phenotype was compared with the phenotypes of male C3H/HeN, a/a and Avy/a mouse strains (Figs. 2; 3, A and B; 8B; and Supplementary Figs. S9–S17). Of these mouse strains, the NZG/Kgm mouse was the heaviest and had the longest body and tail length (Supplementary Fig. S9). The large body size of the NZG/Kgm mouse appears to be attributable to increased lean mass rather than increased fat mass (Supplementary Figs. S13, S15, S16, and S17). When the sum of gonadal, retroperitoneal, subcutaneous, and visceral fats was expressed as a percentage of body weight, the male NZG/Kgm mouse has the least fat compared with C3H/HeN, a/a, Avy/a mice. The nonobese NZG/Kgm mouse phenotype is also reflected in the NZG/Kgm mouse plasma leptin levels that are similar to those for a/a and C3H/HeN mice and ∼3.5-fold lower than those for obese age-matched Avy/a mice (Fig. 3A and Supplementary Fig. S10).
Fig. 8.
The large body weight of NZG/Kgm mice shows a semi-dominant pattern of inheritance. A: growth curves for NZG/Kgm and F1 from CAST/Ei × NZG/Kgm crosses are shown. No growth curves are available for CAST/Ei mice. The observed growth curves (mean at each age ± SE, n ≥ 35) for each strain are shown. The logistic model showed statistically significant differences between all growth curves. B: growth curves for C57BL/6J × NZG/Kgm crosses are shown. The observed growth curves (mean at each age ± SE, n ≥ 10) for each strain are shown. The logistic model showed statistically significant differences between all growth curves.
NZG/Kgm Mice have Disproportionately Higher Amounts of Retroperitoneal, Gonadal, and Inguinal Fat Compared With C57BL/6J, C3H/HeN, a/a, and Avy/a Mouse Strains
Although the NZG/Kgm mouse is not obese, it does have an unusual distribution of body fat. The fat mass in both male and female NZG/Kgm mice had disproportionately higher amounts of retroperitoneal fat compared with the other mouse strains (Supplementary Figs. S13 and S18), and female NZG/Kgm mice also had a disproportionately higher amount of gonadal fat compared with C57BL/6J mice (Supplementary Fig. S18).
Sequential MRI images obtained from anaesthetized male NZG/Kgm and C57BL/6J mice showed distinctly different fat distributions for male NZG/Kgm and C57BL/6J mice (Fig. 4, Supplementary Figs. S24 and S25). Male NZG/Kgm mice had very little visceral fat, and most of their fat mass comprised subcutaneous and gonadal fat. In contrast, the C57BL/6J mice had most of their fat mass in the visceral area.
Fig. 4.
HF-Magnetic resonance imaging analysis of NZG/Kgm and C57BL/6J mouse body composition shows NZG/Kgm mice have increased subcutaneous and gonadal fat mass compared with C57BL/6J mice. Sequential MRI images (each 2 mm thick) were obtained from anaesthetized male NZG/Kgm and C57BL/6J mice. ImageJ was used to merge together the anatomical image with the fat suppressed image for each 2 mm slice. The red represents the fat in the mouse, and the green represents the anatomical area that is not fat. The percentage body fat quantitated using ImageJ was 6.0% for the NZG/Kgm mouse and 7.1% for the C57BL/6J mouse. The NZG/Kgm mouse was 96 days of age and weighed 54 g, and the C57BL/6J mouse was 128 days of age and weighed 31 g.
NZG/Kgm Mice were not Obese or Diabetic
NZG/Kgm mice did not develop obesity or Type 2 diabetes in contrast to Avy/a mice. NZG/Kgm mouse plasma levels of glucose and insulin measured in free-feeding and overnight-fasted animals were similar to those measured in lean, nondiabetic a/a mice, but significantly less than the plasma glucose and insulin levels measured in obese and diabetic Avy/a mice (Fig. 3, A and B). The severity of diabetes in Avy/a mice increased between 50 and 150 days of age.
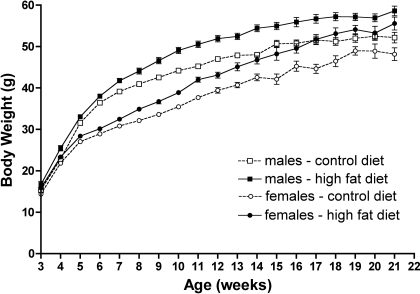
NZG/Kgm Mice were Susceptible to High-Fat Diet-induced Obesity but Resistant to High-Fat Diet-induced Diabetes or Insulin Resistance
The body weights of both male and female NZG/Kgm mice were increased when the mice were fed a moderately high fat (45% calories from fat) diet from weaning. The logistic model was fitted to the growth curves, and it showed statistically significant differences between all growth curves (Fig. 5). Significant increases in retroperitoneal, gonadal, and visceral fat contributed to the high-fat diet-induced increased body weight (data not shown). The high-fat diet-induced obesity in the NZG/Kgm mice did not, however, induce diabetes or insulin resistance (Fig. 6). Fasting blood glucose levels for mice on the high-fat diet were no different from fasting blood glucose levels for mice on the control diet, and the only significant difference for the insulin tolerance test was between the control and high-fat diet mice at 180 min following the insulin injection. The plasma glucose levels in the mice on the high-fat diet were significantly elevated compared with control animals at 30, 60, 90, and 120 min following glucose injection but were not significantly different at 150 and 180 min.
Fig. 5.
NZG/Kgm mice develop high-fat diet-induced obesity. NZG/Kgm mice were housed in cages of up to 6 male or female mice per cage and were fed either a control diet (11% calories from fat) or a high fat diet (45% calories from fat) from weaning until 150 days of age. Body weights were recorded weekly. The observed growth curves (mean at each age ± SE, n ≥ 10) for each strain are shown. The logistic model showed statistically significant differences between all growth curves (P < 0.05).
Fig. 6.
NZG/Kgm mice do not develop Type 2 diabetes when fed a high-fat diet from weaning. NZG/Kgm mice (males) were housed in cages of up to 6 mice per cage and were fed either a control diet (10% calories from fat) or a high-fat diet (45% calories from fat) from weaning until 150 days of age. Glucose tolerance (GTT) and insulin tolerance tests were performed at 139 and 146 days old, respectively. Blood glucose levels were measured immediately before and at the times indicated on the x-axis, after glucose or insulin injection. Blood glucose levels were measured from a drop of blood collected from the tail vein using Roche Accu-Check Glucometer test strips. Each data point represents the mean ± SE of measurements from 3 animals. Significant differences between control and high-fat diet data points at each time point were found (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
Male N2 Brown Mice were Heavier Than N2 Ginger and Dark Mice
The body weights of the male, but not female, N2 generation associated with their coat colors (Fig. 7). Significant differences between growth curves for male dark N2, brown N2, and ginger N2 were determined using the logistic model with the brown N2 being the heaviest, followed by the ginger N2 and the dark N2 were the lightest. The body weights of male brown N2 were similar to the body weights for male NZG/Kgm mice from ∼90 days of age. In contrast with the male N2, there were no significant differences between the growth curves for female dark N2, ginger N2, and brown N2 mice. All female N2 mice weighed significantly less than female NZG/Kgm mice.
Fig. 7.
Male, but not female, NZG/Kgm mouse coat color associates with body weight. NZG/Kgm mice were crossed with CAST/Ei mice and the F1 offspring were backcrossed to NZG/Kgm mice to obtain the N2 generation. The observed growth curves (mean at each age ± SE, n ≥ 11) for each strain are shown. The logistic model showed statistically significant differences between all growth curves. This demonstrates that male body weight associates with coat colour in these offspring and the male brown colored offspring are heavier than the ginger colored offspring. The number of male mice with each coat colour were 12 brown, 16 ginger, and 17 dark. The number of female mice with each coat colour were 13 brown, 11 ginger, and 15 dark.
Inheritance of NZG/Kgm Excessive Body Weight and Body Size was Semidominant and Influenced by Other Genes When NZG/Kgm Mice were Crossed With CAST/Ei Mice
The excessive body weight and body length of NZG/Kgm mice showed a semidominant pattern of inheritance when NZG/Kgm mice were bred with CAST/Ei mice. The logistic model was fitted to NZG/Kgm and F1 mouse growth curves, and it showed statistically significant differences between all growth curves (Fig. 8A). The body weight and body length of 150-day-old NZG/Kgm male and female mice were also significantly different from 150-day-old F1 (NZG/Kgm × CAST/Ei cross) mice (Fig. 9). The excessive body weight for NZG/Kgm mice also showed a semidominant pattern of inheritance when NZG/Kgm mice were bred with C57BL/6J mice (Fig. 8B). The logistic model was fitted to NZG/Kgm, C57BL/6J, and F1 (NZG/Kgm × C57BL/6J cross) mouse growth curves, and it showed statistically significant differences between all growth curves.
Fig. 9.
Phenotypic inheritance in the NZG/Kgm × CAST/Ei cross. The inheritance of large NZG/Kgm mouse body size is observed in male (open bars) but not female (solid bars) N2 generation mice. NZG/Kgm mice were bred with CAST/Ei mice to obtain the F1 generation. F1 mice were then backcrossed with NZG/Kgm mice to obtain the N2 generation. Mice were housed up to 6 mice per cage and were fed normal chow and water ad libitum from weaning. At 150 days old, the mice were anaesthetized, and plasma was collected for insulin and glucose measurements. Mice were subsequently culled and body weight, body length, and tail length were recorded. Organs were collected and weighed. Data shown represent means ± SE of groups of at least 8 animals (all ∼150 days old) (significant difference compared with NZG/Kgm mice: *P < 0.05, **P < 0.01, ***P < 0.001).
The inheritance of NZG/Kgm mouse body size and organ weights are shown in Fig. 9. The inheritance of the excessive NZG/Kgm body weight and body length was observed in male but not female, N2 mice generated from backcrossing F1 from the NZG/Kgm × CAST/Ei cross to NZG/Kgm mice. Neither F1 nor N2 generations inherited the long NZG/Kgm tail length.
Male and female F1 mice had significantly less (P < 0.01) retroperitoneal fat and female F1 significantly less (P < 0.01) gonadal fat compared with age-matched NZG/Kgm mice. Only female N2 mice had significantly less retroperitoneal and gonadal fat compared with NZG/Kgm mice. The male N2 brown mice had significantly more (P < 0.05) gonadal fat compared with NZG/Kgm mice.
Testis weight was significantly reduced for F1, N2 ginger, and N2 dark mice but not for N2 brown mice compared with NZG/Kgm. Female F1 and N2 dark mice had significantly smaller hearts compared with NZG/Kgm mice. Male and female NZG/Kgm mice had significantly larger spleens compared with F1 and N2 mice. All three coat colored N2 males, F1, N2 brown, and N2 dark females had significantly smaller kidneys compared with NZG/Kgm mice. Compared with NZG/Kgm mice, pituitary weights were reduced for all F1 and N2 mice except the male N2 ginger mice. Male and female F1 and N2 dark mice had significantly reduced lung weights compared with NZG/Kgm mice. Female F1, N2 ginger, N2 brown, and N2 dark all had significantly reduced liver weights compared with NZG/Kgm mice. The male liver weights for NZG/Kgm, F1 and all N2 mice were not significantly different. No differences in brain or adrenal weights were observed for F1 or N2 mice compared with NZG/Kgm mice.
Hyperglycemia and Hyperinsulinemia Arose in F1 and N2 Generations From the NZG/Kgm × CAST/Ei Cross
F1 (NZG/Kgm × CAST/Ei) mice were backcrossed with NZG/Kgm mice to obtain the N2 generation. Mice at ∼150 days of age were bled after an overnight fast and their plasma glucose and insulin levels are compared in Fig. 10. Male and female F1 mice were hyperglycemic and hyperinsulinemic compared with NZG/Kgm mice. Plasma glucose levels for all N2 were not significantly different from NZG/Kgm mice. Male, but not female, N2 mice were hyperinsulinemic compared with NZG/Kgm mice.
Fig. 10.
Phenotypic inheritance in the NZG/Kgm × CAST/Ei cross. Male (open bars) and female (solid bars) F1 mice were hyperglycemic and hyperinsulinemic compared with NZG/Kgm mice. Male, but not female, N2 mice were hyperinsulinemic compared with NZG/Kgm mice. NZG/Kgm mice were bred with CAST/Ei mice to obtain the F1 generation. F1 mice were then backcrossed with NZG/Kgm mice to obtain the N2 generation. Mice were housed up to 6 mice per cage and were fed normal chow and water ad libitum from weaning. At 150 days of age, the mice were anaesthetized, and plasma was collected after an overnight fast for insulin and glucose measurements. Data shown represents means ± SE of groups of at least 8 animals (significant difference compared with NZG/Kgm mice: *P < 0.05, **P < 0.01).
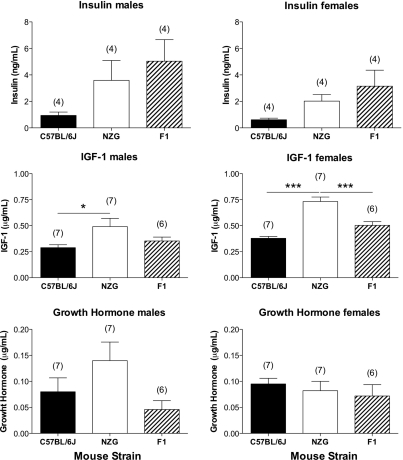
NZG/Kgm Mice had Higher Circulating Levels of IGF1 Compared With C57BL/6J mice
Fasting insulin, insulin like growth factor 1 (IGF1) and growth hormone (GH) were measured by ELISA assays on frozen plasma collected from 18 wk old C57BL/6J, NZG/Kgm, and F1 (C57BL/6J X NZG/Kgm) (see Fig. 8B) male and female mice. Male and female NZG/Kgm mice had higher circulating levels of IGF1 compared with C57BL/6J mice (Fig. 11). Female NZG/Kgm mice also had significantly higher IGF1 levels compared with female F1 mice. Neither the IGF1 gene nor the IGF1 receptor gene is encoded on mouse chromosome 4, where the Tyrp1 gene is located. No significant differences were observed for either gender for plasma insulin or GH between NZG/Kgm, C57BL/6J and F1 mice.
Fig. 11.
NZG/Kgm mice have higher circulating levels of IGF1 compared with C57BL/6J mice. Insulin, IGF1, and growth hormone (GH) were measured by ELISA assays on frozen plasma collected from 18 wk old C57BL/6J, NZG/Kgm, and F1 from the C57BL/6J × NZG/Kgm cross (see Fig. 8B) male and female mice. Data shown represent means ± SE (n = number of mice; *P ≤ 0.05; ***P ≤ 0.001).
DISCUSSION
We have characterized a novel mouse model, the NZG/Kgm mouse, and commenced studying the genotypic contributions to its phenotype. Our investigations into the inheritance of coat color and large body size inherent of the NZG/Kgm mouse confirm a discovery made by Mendelian mouse geneticists in the early 1900s who observed that several pigmentation gene loci associate with body weight in mice.
The NZG/Kgm mouse is a very large, rapidly growing, ginger-colored mouse with pink eyes. Importantly, we have determined from breeding NZG/Kgm mice with CAST/Ei mice that the NZG/Kgm mouse has at least two pigmentation variant genes (oca2p and tyrp-1b) and that the tyrp1b gene locus associates with large body size and heavy body weight. The NZG/Kgm oca2p and tyrp1b alleles are likely to have been inherited from Aw or the albino strain of mice. The albino phenotype is a result of a mutation in the tyrosinase gene, and this mutation can mask an oca2p and/or tyrp1b mutant phenotype. We have determined that the ginger coat color is a recessive trait, while the excessive body weight and large body size exhibits a semidominant pattern of inheritance. The large NZG/Kgm mouse body size is a result of excessive lean body mass since these mice are not obese or diabetic. NZG/Kgm mice exhibit an unusual pattern of fat distribution; compared with other mouse strains they have disproportionately higher amounts of subcutaneous and gonadal fat. These mice are susceptible to high-fat diet-induced obesity but are resistant to high-fat diet-induced diabetes.
The NZG/Kgm mouse shares phenotypic characteristics with the dominant yellow agouti mouse (Avy/a). Both are large mice with phaeomelanic coat colors, and both have excessive lean mass and body length. Both strains are susceptible to high-fat diet-induced obesity. This is where the similarities end. NZG/Kgm mice have pink eyes and Avy/a mice have black eyes. NZG/Kgm mice have fur with a light gray base and ginger tips while Avy/a have uniformly yellow fur from base to tip. Compared with Avy/a mice, NZG/Kgm mice grow very rapidly from weaning; adult NZG/Kgm mouse body weight exceeds Avy/a mouse body weight. Avy/a mice are both obese and diabetic, while NZG/Kgm mice are not obese or diabetic.
To study the pattern of inheritance of the NZG/Kgm mouse phenotype we bred NZG/Kgm with CAST/Ei mice to obtain F1 mice and then backcrossed the F1 with NZG/Kgm mice to obtain the N2 generation. Inheritance of the NZG/Kgm mouse excessive body weight and body length in male, but not female, F1 and N2 mice suggests a sexually dimorphic pattern of inheritance for these traits and/or multiple factors influence the NZG/Kgm tyrp1b or linked gene mutation phenotype, including nongenetic factors. A sexually dimorphic pattern of inheritance of fat and liver mass, with contribution from CAST/Ei mouse dominant genes is suggested by 1) the overall decreased retroperitoneal fat mass in male and female F1, but only female N2, generations and 2) the overall decreased gonadal fat mass and liver weight in female, but not male, F1 and N2 generations compared with NZG/Kgm mice. The decreased spleen and pituitary weights observed for both male and female F1 and N2 generations is also suggestive of a dominant pattern of inheritance for these traits from the CAST/Ei mice. The decreased lung weight in male and female F1 and N2 dark, but not N2 ginger or N2 brown mice, suggests complex interactions between NZG/Kgm and CAST/Ei mouse genes affecting lung size. Similarly, the decreased kidney weight observed for female F1 and male and female N2 dark suggests complex interactions between NZG/Kgm and CAST/Ei mouse genes affecting kidney size. The decreased heart weight observed for female, but not male F1 and N2 dark mice, is suggestive of a dominant, sexually dimorphic pattern of inheritance for NZG/Kgm genes affecting heart size.
Future studies, in which NZG/Kgm mice will be bred with C57BL/6J and other mouse strains, will ultimately determine whether NZG/Kgm mouse or CAST/Ei genes are dominant or recessive for each of these traits and whether there are any maternal effects. Thirteen of the seventy-two F1 (CAST/Ei × NZG/Kgm) mice were derived from a CAST/Ei dam, and it appeared that these F1 did not exhibit excessive body weight (data not shown), which is suggestive of a maternal effect on body size. We have already crossed male and female NZG/Kgm with C57BL/6J mice, and the F1 phenotype confirmed a semidominant pattern of inheritance of the NZG/Kgm excessive body weight (Fig. 8B). This semidominant pattern of excessive body weight inheritance was observed for both male and female mice in contrast with the inheritance for males only for the NZG/Kgm × CAST/Ei cross.
A striking and unusual characteristic for male and female NZG/Kgm mice is the very rapid growth rate after weaning. Growth may even be rapid from birth, but birth weights and neonatal growth rates were not measured. There appears to be only one other mouse described in the literature that has a similar rapid neonatal growth rate, and this is known as the high growth (hg/hg) mouse (7–10). The hg/hg mouse has a spontaneous partially recessive autosomal mutation that increases growth rate and body size, but the mouse is not obese. The hg/hg phenotype results from a lack of expression of the suppressor of cytokine signaling 2 (Socs2) gene (17). NZG/Kgm mice do not have the same defect since they do express the Socs2 gene (Supplementary Fig. S19). Despite hg/hg and NZG/Kgm mice having different gene defects contributing to their phenotypes, they have similar plasma GH, IGF1, and insulin profiles. Both NZG/Kgm and hg/hg mice have significantly higher plasma IGF1 levels compared with C57BL/6J mice, while both strains have GH and insulin levels that are either similar or lower than those for C57BL/6J mice (Fig. 11) (8, 20). Increased plasma IGF1 levels could contribute to the rapid neonatal growth and large adult body size phenotype observed for hg/hg and NZG/Kgm mice. Interestingly, the source of the circulating IGF1 to promote growth in the hg/hg mouse appears to be nonhepatic tissues and GH independent (8).
NZG/Kgm mice, similar to hg/hg mice, are generally proportional in the size of most body components (Supplementary Fig. S20) (10). Two exceptions are female brain and male spleen weights that did not significantly correlate with body weight. The reason for this lack of correlation is not clear. Female, but not male adrenal weights, and pituitary weights of either sex also did not correlate with body weight. Variations in the dissection and weighing of the small adrenal and pituitary tissues may have contributed to this lack of correlation or there may be real NZG/Kgm mouse sex differences in pituitary and adrenal mass. Interestingly, retroperitoneal and gonadal fat mass positively correlated with body weight (Supplementary Fig. S20), but fasting plasma leptin levels did not correlate with body weight for either male or female mice (Supplementary Fig. S21). Leptin is a hormone produced from fat that circulates to the brain to inform about fat mass, and circulating leptin levels are usually directly proportional to body fat mass and body mass index (1, 18). Fasting plasma leptin levels correlated with gonadal and retroperitoneal fat mass in female but not male NZG/Kgm mice (Supplementary Fig. S22). This difference may be due to the smaller sample size for male (n = 12) compared with females (n = 21), or alternatively, the various fat pads contributed differently to the plasma pool of leptin. We found male and female NZG/Kgm mice had disproportionately higher amounts of subcutaneous and gonadal fat mass compared with other mouse strains, and this may have affected the correlation of male fat mass with circulating leptin levels. Fat distribution is under genetic control (3) and is considered a better predictor of diabetes than obesity, with the amount of abdominal fat being associated with higher risk of disease (13). Subcutaneous inguinal fat is intrinsically different from visceral fat and produces substances that systemically improve glucose homeostasis (25). It is possible that the unusual NZG/Kgm mouse fat distribution protects NZG/Kgm mice from high fat diet-induced diabetes. Plasma insulin levels did not correlate with fasting gonadal or retroperitoneal fat mass for either male or female NZG/Kgm mice.
Our study is not the first time that pigmentation gene loci have been associated with body weight in mice. The p allele of the pink-eyed dilution gene (Oca2) locus was reported to slightly decrease body weight, while the b allele of the brown or Tyrp-1 gene locus was associated with an increase in body weight (16). Maltese (“blue”) dilution is another coat color gene locus that was associated with increased body size, while leaden and pallid coat color gene loci were reported to decrease body size (16). An association between pigmentation gene loci and body weight is not restricted to mice. Three different types of sexual skin color exist in primates, and the darkest scrotal coloration in adult male vervet monkeys associates with increased body weight (12). In Soay sheep, there is a strong association between dark coat color and large body size that is present from the time of birth (15). Soay sheep are either dark or light tawny, and the variation in coat color is controlled by a single autosomal locus at which the dark allele is dominant to the light allele. The light phenotype is determined by a Tyrp-1 linked gene at a locus encoding for homozygosity of a single recessive amino acid transversion at coding position 869 in the tyrosinase-related protein 1 gene (14, 15). Interestingly, the brown mouse coat color that associates with increased body weight is also determined by a locus encoding for homozygosity of a Tyrp-1 gene mutation. The mutation, however, is not at the same coding position of Tyrp-1 as that found in Soay sheep (Supplementary Fig. S8) (27).
A mouse with orange-colored fur resembling the coat and eye color of the NZG/Kgm mouse has previously been described: “Genotypes B/B; A+/A+; p/p and b/b; A+/A+; p/p are scarcely distinguishable phenotypically, both having orange-colored fur and pink eyes. Although the coat color superficially resembles that of dominant yellow agouti mice, their hairs have a light gray base while dominant yellow agouti mice have hairs that are uniformly yellow from base to tip.” (16). Other than coat and eye color there appears to be no further characterization of these mice in the literature. We have recently identified recessive oca2p and tryp1b mutations for the NZG/Kgm mouse and the tyrp1b mutant allele associated with increased body size and weight in male mice. Whether the Tyrp1 gene or closely linked genes at the Tyrp1 gene locus regulate body size and lean body mass, and the multifactorial regulation of this gene locus phenotype, will only be determined through future studies on congenic mice and/or knockout or knock-in mice with tryp1b mutant gene. Finally, we propose NZG/Kgm mice as a model to delineate gene(s) that regulate 1) growth and metabolism, 2) resistance to Type 2 diabetes, and 3) preferential fat deposition in the subcutaneous and gonadal areas.
GRANTS
Funding for this project was provided from The Health Research Council of NZ, Auckland Medical Research Foundation, Auckland Uniservices, Lottery Health NZ, and Diatranz NZ. J. K. Naggert was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-46977.
DISCLAIMER
The views expressed in this paper do not necessarily represent those of the U. S. Food and Drug Administration.
Acknowledgments
We acknowledge the intellectual and technical help of Dr. Laurence Dumont, Dr. Chia-Shan Wu, Dr. Scott Graham, Dr. Philip Daniel, Carol Wang, Olga Shevstova, and Margaret McGregor.
Address for reprint requests and other correspondence: K. G. Mountjoy, Dept. of Physiology, Faculty of Medical and Health Sciences, Univ. of Auckland, Private Bag 92019, Auckland 1023, New Zealand (e-mail: kmountjoy@auckland.ac.nz).
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Arora S, Anubhuti. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides 40: 375–401, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baker RL, Carter AH, Cox EH. The effect of selection for body weight at different ages on fat deposition in mice. Proc NZ Soc Anim Prod 39: 118–128, 1979. [Google Scholar]
- 3.Bradford GE, Famula TR. Evidence for a major gene for rapid postweaning growth in mice. Genet Res 44: 293–308, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Brilliant MH, Ching A, Nakatsu Y, Eicher EM. The original pink-eyed dilution mutation (p) arose in Asiatic mice: implications for the H4 minor histocompatibility antigen, Myod1 regulation and the origin of inbred strains. Genetics 138: 203–211, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockmann GA, Bevova MR. Using mouse models to dissect the genetics of obesity. Trends Genet 18: 367–376, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell 71: 1195–1204, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Corva PM, Horvat S, Medrano JF. Quantitative trait loci affecting growth in high growth (hg) mice. Mamm Genome 12: 284–290, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Corva PM, Medrano JF. Diet effects on weight gain and body composition in high growth (hg/hg) mice. Physiol Genomics 3: 17–23, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Corva PM, Medrano JF. Quantitative trait loci (QTLs) mapping for growth traits in the mouse: a review. Genet Select Evol 33: 105–132, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Famula TR, Calvert CC, Luna E, Bradford GE. Organ and skeletal growth in mice with a major gene for rapid postweaning growth. Growth Dev Aging 52: 145–150, 1988. [PubMed] [Google Scholar]
- 11.Feklman HW The brown variation and growth of the house mouse. Am Nat 69: 370–374, 1935. [Google Scholar]
- 12.Gerald MS, McGuire MT. Secondary sexual coloration and CSF 5-HIAA are correlated in vervet monkeys (Cercopithecus aethiops sabaeus). J Med Primatol 36: 348–354, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 26: 372–379, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Gratten J, Beraldi D, Lowder BV, McRae AF, Visscher PM, Pemberton JM, Slate J. Compelling evidence that a single nucleotide substitution in TYRP1 is responsible for coat-colour polymorphism in a free-living population of Soay sheep. Proc Royal Soc B: Biol Sci 274: 619–626, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratten J, Wilson AJ, McRae AF, Beraldi D, Visscher PM, Pemberton JM, Slate J. A localized negative genetic correlation constrains microevolution of coat color in wild sheep. Science 319: 318–320, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gruneberg H The genetics of the mouse. London: Bentley House, New York, Toronto, Bombay, Calcutta, Madras: Macmillan: Cambridge at the University Press, 1943, p. 1–334.
- 17.Horvat S, Medrano JF. Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics 72: 209–212, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab 278: E1–E14, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371: 799–802, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Medrano JF, Pomp D, Sharrow L, Bradford GE, Downs TR, Frohman LA. Growth hormone and insulin-like growth factor-I measurements in high growth (hg) mice. Genet Res 58: 67–74, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev 7: 454–467, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Miltenberger RJ, Mynatt RL, Wilkinson JE, Woychik RP. The role of the agouti gene in the yellow obese syndrome. J Nutr 127: 1902S-1907S, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Rasband WS ImageJ. 1997. –2008.
- 24.Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72: 827–834, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metabolism 7: 410–420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff GL Body composition and coat color correlation in different phenotypes of “viable yellow” mice. Science 147: 1145–1147, 1965. [DOI] [PubMed] [Google Scholar]
- 27.Zdarsky E, Favor J, Jackson IJ. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics 126: 443–449, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]