Abstract
The hyperthermophilic archaeon Pyrococcus furiosus can utilize different carbohydrates, such as starch, maltose and trehalose. Uptake of α-glucosides is mediated by two different, binding protein-dependent, ATP-binding cassette (ABC)-type transport systems. The maltose transporter also transports trehalose, whereas the maltodextrin transport system mediates the uptake of maltotriose and higher malto-oligosaccharides, but not maltose. Both transport systems are induced during growth on their respective substrates.
Keywords: binding protein, maltodextrin, maltose
Introduction
The hyperthermophilic, anaerobic archaeon Pyrococcus furiosus can grow heterotrophically on some sugars, such as cellobiose, maltose and starch (Fiala and Stetter 1986, Kengen et al. 1993). Maltose and starch metabolism have been studied in some detail (de Vos et al. 1998), but virtually nothing is known about uptake of these carbohydrates into the cell. Pyrococcus furiosus produces a number of starch-hydrolyzing enzymes, such as an extracellular amylopullulanase (Dong et al. 1997) and a-amylase (Jorgensen et al. 1997), and an intracellular α-amylase (Laderman et al. 1993) and α-glucosidase (Costantino et al. 1990). The extracellular enzymes hydrolyze starch into smaller oligosaccharides, which are then transported into the cell via as yet unknown mechanisms, where they are hydrolyzed to glucose. Glucose is further metabolized by the modified Embden-Meyerhof pathway (Schäfer et al. 1994) that involves an ADP-dependent glucokinase and phosphofructokinase (Kengen et al. 1994).
In bacteria, maltose and maltodextrins are usually transported by ATP-binding cassette (ABC) transporters (Ehrmann et al. 1998). In archaea and thermophilic bacteria, ABC transporters appear to play a dominant role in sugar transport. The trehalose/maltose transporter of Thermococcus litoralis has been characterized biochemically, and the trehalose/maltose binding protein (TMBP) and ATP binding subunit MalK have been functionally expressed in Escherichia coli (Horlacher et al. 1998, Greller et al. 1999). This system mediates the uptake of trehalose and maltose but not of maltodextrins. In contrast, a single maltose, trehalose and maltodextrin transporter has been described for the thermophilic bacterium Thermoanaerobacter ethanolicus (Jones et al. 2000). The genome of P. furiosus contains a gene cluster that encodes an ABC-type transporter that is nearly identical to the trehalose/maltose transporter of T. litoralis (DiRuggiero et al. 2000). In addition, it contains an operon that encodes a homolog of the maltose/ maltodextrin transporter of E. coli. Because the second transporter was found to be induced upon growth of P. furiosus on maltose, it was assumed that P. furiosus contains two maltose transport systems, one specific for maltose and trehalose and the other specific for maltose and maltodextrins (DiRuggiero et al. 2000). Here, we show that P. furiosus contains a trehalose/maltose and a maltodextrin transport system. The latter system is specific for maltodextrins only and is not involved in maltose uptake.
Materials and methods
Organisms and growth conditions
Pyrococcus furiosus Vc1 (DSM 3638) was grown routinely at 80 °C in modified Methanococcus medium (Kengen et al. 1993) under anaerobic conditions in the presence of 5 mM of the indicated carbohydrate or with 0.2% (w/v) pyruvate. For growth on peptone, the medium was supplemented with 1% (w/v) elemental sulfur. Escherichia coli DH5a (Hanahan 1983) and BL21-CodonPlus(DE3)-RIL (Stratagene, La Jolla, CA) were grown in LB supplemented with the appropriate antibiotics at 37 °C.
Chemicals
Sugars were from Merck (Darmstadt, Germany). [14C]-Maltose (516 mCi mmol–1) was purchased from Amersham-Radiochemicals (Little Chalfont, Buckinghamshire, U.K.). [14C]-Maltotriose (850 mCi mmol–1) and [14C]-trehalose (850 mCi mmol–1) were kind gifts from Prof. Winfried Boos, Konstanz, Germany.
Transport and binding studies
Cells grown overnight in 50 ml of medium were harvested under anaerobic conditions, washed once in growth medium without carbon source, resuspended and stored at room temperature until use. Transport assays were performed at 80 °C, under anaerobic conditions maintained by a continuous flow of N2 gas. Cells were used at a protein concentration of 10 µg ml–1, and transport was initiated by addition of the radiolabeled substrate to a final concentration of 10 mM. Samples were taken at intervals, filtered over BA85 nitrocellulose filters (Protran; Schleicher & Schuell, Dassel, Germany), and washed twice with growth medium without carbon source. The radioactivity retained on the filters was determined by liquid scintillation spectrometry. Kinetic constants were estimated from triplicate measurements of the initial uptake rate determined after 10 s.
Binding studies were performed using the method described by Richarme and Kepes (1983). Basically, 1 µM radiolabeled substrate was added to isolated P. furiosus membranes or to purified binding protein (10 µg protein ml–1). Binding assays were performed in 100 µl volume at 60 °C. After 3 min, the binding reactions were terminated by the addition of 2 ml ice-cold 0.1 M LiCl, filtered over BA85 nitrocellulose filters and washed once with 2 ml 0.1 M LiCl. The radioactivity retained on the filters was determined as described above.
Purification of binding proteins
Cells were harvested and resuspended in 50 mM Tris-HCl, pH 7.5, and broken by a single passage through a French Pressure cell at 4.1 MPa. Membranes were collected by centrifugation for 45 min at 100,000 g at 4 °C. The pellet was resuspended in 50 mM Tris-HCl, pH 7.5, washed once and solubilized in 0.5% (v/v) Triton X-100 for 30 min at 37 °C. Non-solubilized material was removed by centrifugation (350,000 g, 15 min, 4 °C), and the supernatant was collected and applied to a concanavalin A (ConA)-Sepharose (Pharmacia, Roosendaal, The Netherlands) column equilibrated with buffer A (25 mM Tris-HCl, pH 7.4, 500 mM NaCl, 0.05% (v/v) Triton X-100). The column was washed thoroughly with buffer A, and bound glycoproteins were eluted with buffer A supplemented with 250 mM a-methyl-mannopyranoside. Fractions were dialyzed overnight against buffer B (25 mM Tris, pH 6.8, 0.05% (v/v) Triton X-100) and measured for substrate binding activity as described above. Fractions containing maltose or maltotriose binding activity were pooled and applied to an HR5/5 MonoQ column (Pharmacia, Uppsala, Sweden) pre-equilibrated with buffer B. Proteins were eluted with a linear gradient of 0 to 500 mM NaCl in buffer B. Fractions were analyzed by SDS-PAGE, assayed for binding activity, pooled and stored at –80 °C.
Cloning and expression of binding proteins
Oligonucleotide primers were designed based on the nucleotide sequence of the complete PF1938 and PF1739 genes as found in the P. furiosus database (http://www.genome.utah.edu/). PF1938 and PF1739 were amplified by PCR (forward 5′-CCCCCGATATCATGAGGAGAGCAACATACGCC-3′,reverse 5′-CCCCCGGATCCTTATCCTTGCATGTTGTTA-
3′; forward 5′-CCCCCGATATCATGAATGTCAAGAAGG TACTGC-3′, reverse 5′-CCCCCTCTAGATTAGCTGTATT GTTTAAC-3′, respectively), and the resulting 1.35-kb fragments were ligated into pBSKS (Stratagene) to yield pSMK10 and pSMK11, respectively. The inserts were transferred to the expression vector pET302 (van der Does et al. 1998) to yield pSMK14 and pSMK16, containing PF1938 and PF1739, respectively, with an amino-terminal hexa-histidine tag.
Escherichia coli BL21-ClonePlus(DE3)-RIL, which expresses plasmid-encoded tRNAs for the amino acids leucine, isoleucine and arginine with rare codons, was used for expression. Cells transformed with pSMK14 or pSMK16 were grown to an OD of 0.8 at 660 nm, and induced for 2 h with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Cells were harvested and broken by French Press treatment at 5.5 MPa. The membrane and soluble fraction were collected by centrifugation (350,000 g, 20 min, 4 °C), and analyzed by SDS-PAGE and Western blotting with His-tag antibodies (Dianova, Hamburg, Germany), and by [14C]-maltose or [14C]-maltotriose binding assays at 37 and 60 °C.
Total RNA isolation and Northern analysis
Total RNA was isolated from exponentially growing P. furiosus cells with the TRIZOL reagent (Gibco BRL Life Technologies, Breda, The Netherlands). For Northern blot analysis, 10 µg total RNA was separated on formaldehyde-agarose (1%) gels and transferred to a Zeta-probe membrane (Bio-Rad, Veenendaal, The Netherlands) by capillary blotting. Primers were designed according to the gene sequences present in the P. furiosus (http://www.genome.utah.edu/) database. Probes for PF1938 (forward: 5′-CCCCCGATATCATGAGGAGAG CAACATACGCC-3′; reverse: 5′-TGCCATGTATTCTTCC GC-3′), PF1739 (forward: 5′-CCCCCGATATCATGAATGT CAAGAAGGTACTGC-3′; reverse: 5′-TTAGCTCAACAGT GACCCC-3′) and PF1831 (forward: 5′-ATGGGAGAATTG CCAATTGC-3′; reverse: 5′-TCAGCTCTTAATTGCGAGC- 3′) were labeled with digoxigenin (DIG) by PCR on genomic DNA. Detection was performed with alkaline phosphatase-conjugated DIG antibodies (Boehringer Mannheim, Germany) and CDP-Star (Tropix, Bedford, MA).
Sugar analysis
For the identification of sugar molecules present on the glycosylated binding proteins, 20 µg protein was dialyzed overnight against demineralized water. Sugar moieties were hydrolyzed from the protein by incubation for 5 h in 2 N trifluoroacetic acid (TFA) or 3 h in 4 N HCl at 100 °C. Released saccharides were labeled with 2-aminoacridone (AMAC) and analyzed by gel electrophoresis (Jackson 1994).
Other techniques
Amino-terminal amino acid sequence analysis was performed with purified protein blotted on PVDF membrane by NAPS (Nucleic Acid/Protein Service Unit, Vancouver, Canada). The DNA sequencing was performed by BioMedisch Technologisch Centrum (BMTC, University of Groningen, The Netherlands). Glycoproteins resolved by SDS-PAGE were stained with Periodic Acid Schiff (PAS) stain (Sigma-Aldrich Chemie, Munich, Germany) as described by McGuckin and McKenzie (1958). Protein concentrations were determined with the Bio-Rad DC protein assay.
Results
Identification and heterologous expression of two open reading frames involved in sugar binding
The complete genome sequence of P. furiosus shows two gene clusters that encode binding protein-dependent ABC transporters that are homologous to the maltose/maltodextrin transport operon of E. coli (Table 1, Figure 1). One of the clusters (PF1739–PF1741, PF1744) is identical (99–100% amino acid sequence identity) to the trehalose/maltose transport operon found in the related archaeon Thermococcus litoralis (data not shown). As observed for the possible trehalose/maltose transporter cluster, the gene encoding the ATPase subunit of the second transporter is not located in the operon that contains the genes encoding the binding protein and two permeases (PF1933, PF1936–PF1938). Instead, two open reading frames (ORFs) separate the second permease and the ATPase subunit; one of these ORFs encoded an amylopullulanase (Figure 1). To determine if the two gene clusters encode maltose transporters, the binding proteins encoded by PF1938 and PF1739 were cloned into an E. coli expression vector behind the trc promoter and transformed to E. coli strain BL21-CodonPlus(DE3)-RIL. Although this strain contains an endogenous periplasmic maltose/maltodextrin binding protein, upon expression of the binding proteins from P. furiosus, a significantly elevated level of [14C]-maltose, [14C]-trehalose or [14C]-maltotriose binding was observed at 60 °C (Figure 2). At this temperature, the endogenous E. coli proteins precipitate, leading to low background binding. The expressed binding protein encoded by PF1739 binds [14C]-maltose and [14C]-trehalose, whereas that encoded by PF1938 binds only [14C]-maltotriose. Therefore, PF1739 appears to encode a trehalose/ maltose binding protein (TMBP), whereas PF1938 encodes a maltodextrin binding protein (MDBP).
Table 1.
Homology between Pyrococcus furiosus and Escherichia coli maltose/maltodextrin transporter clusters.
| Pyrococcus furiosus | Escherichia coli | |||
| MalE | MalF | MalG | MalK | |
| PF1938 | 281 (46)2 | |||
| PF1937 | 35 (56) | |||
| PF1936 | 33 (55) | |||
| PF1933 | 47 (63) | |||
| PF1739 | 28 (44) | |||
| PF1740 | 36 (54) | |||
| PF1741 | 41 (63) | |||
| PF1744 | 46 (64) | |||
1 Identity. 2 Similarity.
Figure 1.
Organization of genes encoding the (A) maltodextrin (indicated in grey) and (B) trehalose/maltose (indicated in black) transporters.
Figure 2.
Functional expression of PF1938 and PF1739 in Escherichia coli BL21-CodonPlus(DE3)-RIL. (A) [14C]-Maltose, (B) [14C]-maltotriose and (C) [14C]-trehalose binding activities were measured before (–) and after (+) induction of the promotor.
Induction of binding proteins
To study the functional expression of the maltose and maltotriose binding proteins in P. furiosus, binding studies with [14C]-maltose (Figure 3B), [14C]-maltotriose (Figure 3C) and [14C]-trehalose (Figure 3D) were performed using membranes isolated from P. furiosus cells grown on different substrates. Membranes from cells grown on pyruvate showed no binding of the tested substrates. On the other hand, membranes derived from maltotriose-, maltose- or starch-grown cells exhibited binding of all three tested substrates, whereas membranes of trehalose-grown cells showed binding of maltose and trehalose only.
Figure 3.
(A) Northern blot analysis of total RNA extracted from Pyrococcus furiosus cells grown on maltose, maltotriose, pyruvate, starch or trehalose, with the gene encoding MDBP (PF1938) or TMBP (PF1739) as probe. A P. furiosus histone (PF1831) was used as an internal control for the total amount of RNA. (B) [14C]-Maltose, [14C]-maltotriose (C) and [14C]-trehalose (D) binding to membranes derived from P. furiosus cells grown on different substrates. The binding studies were performed in triplicate.
To correlate the carbohydrate binding activities to the expression of the respective binding proteins, northern blotting was performed to probe for the genes encoding TMBP (PF1739) and MDBP (PF1938) (Figure 3A). The expression of a P. furiosus histone (PF1831) was used to control for equal loading with mRNA. When cells were grown on trehalose, a low level of MDBP expression was observed, but [14C]-maltotriose binding activity was not detectable in the membrane fraction. Maltodextrin binding protein was highly expressed when cells were grown on maltotriose and starch. Although membranes derived from maltose-grown cells exhibited high maltotriose binding (Figure 3C), MDBP was only poorly expressed under these conditions. Trehalose/maltose binding protein was highly expressed when cells were grown on trehalose, maltose, maltotriose and starch (Figure 3A). The mRNA levels and the substrate binding activities of the respective binding proteins did not match completely in a reproducible way. Taken together, the results suggest that both TMBP and MDBP are functionally expressed when cells are grown on maltose and maltotriose, whereas in starch and trehalose-grown cells, TMBP is most prominently present.
Maltose uptake
Pyrococcus furiosus cells grown on maltose readily accumulated [14C]-maltose under anaerobic conditions at 80 °C (Figure 4). The transient uptake rates indicate rapid metabolism of the labeled substrate in the cell. Maltose uptake showed a steep temperature dependence, and uptake was hardly detectable at temperatures below 40 °C (results not shown). Initial rates of maltose uptake were used to determine the affinity of the transport system. The Km for maltose uptake is 30–40 nM at 80 °C. This transporter is therefore a high affinity transport system.
Figure 4.
Maltose transport in Pyrococcus furiosus cells. Accumulation of 10 mM [14C]-maltose was assayed under anaerobic conditions at 80 °C (filled circles). Inhibition of maltose accumulation in maltose-grown cells was studied by adding a 100-fold excess of unlabeled maltose (open circles), maltotriose (filled squares) or trehalose (oen squares). Transport studies were performed in triplicate.
The effect of a 100-fold excess of unlabeled maltotriose and trehalose on maltose uptake was studied (Figure 4). [14C]-Maltose uptake was not inhibited by the unlabeled maltotriose, but was effectively reduced by a 100-fold excess of trehalose. This observation indicates that maltose uptake is mediated by a system that also accepts trehalose as a substrate.
Purification of the binding proteins
A high level of maltose binding was observed in membranes derived from maltose-grown cells. These membranes were solubilized with Triton X-100, and the binding protein purified to homogeneity by concanavalin A (ConA) affinity chromatography and subsequent MonoQ anion exchange chromatography, using [14C]-maltose binding at 60 °C to monitor the purification (Figure 5). The maltose binding activity corresponded with a 45-kDa protein. To characterize the substrate specificity of this protein, an inhibition assay was used: addition of excess unlabeled substrate led to a decrease in binding of [14C]-labeled substrate, whereas addition of unlabeled non-substrate did not lead to a decrease of [14C]-labeled substrate binding. Addition of 10-fold excess unlabeled trehalose or maltose completely abolished [14C]-maltose binding, whereas an excess of glucose, maltotriose or maltotetraose had no effect (Figure 6A). The 45-kDa binding protein was therefore identified as TMBP, which has only maltose and trehalose as substrates.
Figure 5.
Purification of trehalose/maltose binding protein (TMBP): Coomassie stained gel. The TMBP was purified from Triton X-100 solubilized membranes by ConA affinity chromatography and MonoQ anion exchange chromatography.
Figure 6.
Substrate specificity of the ConA fraction derived from cells grown on maltose. The binding of (A) 1 mM [14C]-maltose or (B) 1 mM [14C]-maltotriose to the ConA fraction was inhibited by addition of a 10-fold excess of unlabeled substrate.
The maltodextrin binding protein was partially purified from Triton X-100 solubilized membranes, derived from cells grown on maltose by ConA chromatography. The binding of [14C]-maltotriose was used to monitor the purification. The substrate specificity of MDBP was studied using the inhibition assay as explained above. The [14C]-maltotriose binding activity of the active fraction was effectively inhibited by maltotriose and maltotetraose, but not by glucose or maltose (Figure 6B). Therefore, this binding protein is a maltodextrin binding protein.
Glycosylation of the binding proteins
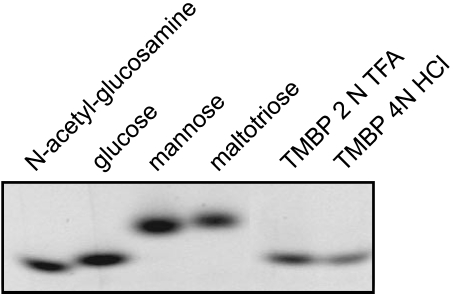
Both TMBP and MDBP appear to be glycosylated proteins because they bind to the ConA affinity column, which specifically binds terminally mannosylated and glucosylated glycoproteins. The purified TMBP could be stained with the glycoprotein-specific Periodic Acid Schiff (PAS) stain. To identify the carbohydrate moieties, purified TMBP was hydrolyzed and the released sugars were labeled with the fluorophore 2-aminoacridone (AMAC). After polyacrylamide gel electrophoresis, only glucose molecules were observed, suggesting that TMBP is glycosylated with glucose moieties (Figure 7).
Figure 7.
Glycosylation of TMBP. Glycosyl moieties were visualized on acrylamide gels with the fluorescent probe AMAC after hydrolysis of the purified protein with 2 N TFA or 4 N HCl as described in Materials and methods.
Discussion
Pyrococcus furiosus contains two operons involved in α-glucoside transport, a trehalose/maltose and a maltodextrin transport system. Both systems are members of the ATP-binding cassette family of transporters. The maltodextrin transport operon shows homology to the maltose/maltodextrin transporter of E. coli. Surprisingly, however, this system is not involved in the uptake of maltose but binds higher malto-oligosaccharides only. The binding protein was heterologously expressed in E. coli to confirm the substrate binding specificity. Recently, the crystallization of the P. furiosus maltodextrin binding protein has been reported in the presence of maltose (Evdokimov et al. 2001). Surprisingly, maltotriose was found in the binding pocket. This result is now confirmed by our biochemical data. Apparently, the maltodextrin binding protein exhibits such an extremely high binding affinity for maltotriose that it binds the minute amount of contaminants present in the maltose solution. This is consistent with the hypothesis that hyperthermophilic organisms utilize highly efficient ABC transporters to survive when substrate concentrations are extremely low (Elferink et al. 2001).
The trehalose/maltose transporter is identical to the system described in the related archaeon T. litoralis (DiRuggiero et al. 2000). Also, the binding protein of this transporter has been crystallized and its structure has been solved (Diez et al. 2001). The trehalose/maltose transport operon in both organisms is flanked by inverted repeats. In T. litoralis, a hypothetical transposon is located upstream of this fragment. It was hypothesized that this fragment was acquired recently by one of the organisms by lateral gene transfer (DiRuggiero et al. 2000). Because the maltotriose transporter does not transport maltose, it is possible that T. litoralis acquired the trehalose/ maltose transporter from P. furiosus, because maltose and trehalose are the only sugars so far known to be used for growth by T. litoralis (Neuner et al. 1990). On the other hand, genes encoding the trehalose/maltose transporter are absent in both P. horikoshii and P. abyssi. Although the maltodextrin transporter is present in P. abyssi, this organism is unable to grow on starch or other sugars (Erauso et al. 1993). It is unknown if P. glycovorans (Barbier et al. 1999), which can grow on sugars, contains the trehalose/maltose transporter. It is therefore possible that both P. furiosus and T. litoralis acquired the genes encoding the trehalose/maltose transporter from a third organism.
Both MDBP and TMBP are induced by growth on α-glucosides. Surprisingly, MDBP was also found to be induced after growth on maltose. This induction could be caused by the presence of small amounts of maltotriose in the maltose solution. Maltotriose induces not only MDBP but also TMBP. This most likely relates to extracellular α-glucosidases that cleave the maltotriose into glucose and maltose. The latter induces the trehalose/maltose transport system. When P. furiosus is grown on starch, the substrate is first cleaved extracellularly by an amylopullulanase, which cleaves starch into maltodextrins (Dong et al. 1997) (See Figure 8). The maltodextrins are subsequently hydrolyzed to smaller subunits (G2, G3–G7) by an extracellular a-amylase (Jorgensen et al. 1997). Therefore, when utilizing starch as a carbon source, both maltose and small malto-oligosaccharides are formed. Once transported inside, the malto-oligosaccharides are most likely further hydrolyzed to maltose by the intracellular α-amylase. In this respect, maltose metabolism in P. furiosus and T. litoralis differ. In the latter organism, maltose is hydrolyzed by MalP and 4-α-glucanotransferase, similar to the system found in E. coli (Xavier et al. 1999). In P. furiosus, however, maltose is hydrolyzed by α-glucosidase to glucose, which then enters a modified Embden-Meyerhof pathway. The concerted action of the two transport systems for α-glucosides permits P. furiosus to utilize these components efficiently.
Figure 8.
Scheme of α-glucoside metabolism in Pyrococcus furiosus.
Acknowledgments
This work was supported by the Earth and Life Sciences Foundation (ALW), which is subsidized by the Netherlands Organization for Scientific Research (NWO). The authors thank Sonja-V. Albers and Marieke Elferink for helpful discussions.
References
- R1.Barbier G., Godfroy A., Meunier J.R., Querellou J., Cambon M.A., Lesongeur F., Grimont P.A., Raguenes G. Pyrococcus glycovorans sp. nov., a hyperthermophilic archaeon isolated from the East Pacific Rise. Int. J. Syst. Bacteriol. 1999;49:1829–1837. doi: 10.1099/00207713-49-4-1829. [DOI] [PubMed] [Google Scholar]
- R2.Costantino H.R., Brown S.H., Kelly R.M. Purification and characterization of an alpha-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115 degrees C. J. Bacteriol. 1990;172:3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R3.de Vos W.M., Kengen S.W.M., Voorhorst W.G., Van der Oost J. Sugar utilization and its control in hyperthermophiles. Extremophiles. 1998;2:201–205. doi: 10.1007/s007920050061. [DOI] [PubMed] [Google Scholar]
- R4.Diez J., Diederichs K., Greller G., Horlacher R., Boos W., Welte W. The crystal structure of a liganded trehalose/maltose-binding protein from the hyperthermophilic archaeon Thermococcus litoralis at 1.85 Å. J. Mol. Biol. 2001;305:905–915. doi: 10.1006/jmbi.2000.4203. [DOI] [PubMed] [Google Scholar]
- R5.DiRuggiero J., Dunn D., Maeder D.L., Holley-Shanks R., Chatard J., Horlacher R., Robb F.T., Boos W., Weiss R.B. Evidence of recent lateral gene transfer among hyperthermophilic archaea. Mol. Microbiol. 2000;38:684–693. doi: 10.1046/j.1365-2958.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- R6.Dong G., Vieille C., Zeikus J.G. Cloning, sequencing, and expression of the gene encoding amylopullulanase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl. Environ. Microbiol. 1997;63:3577–3584. doi: 10.1128/aem.63.9.3577-3584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R7.Ehrmann M., Ehrle R., Hofmann E., Boos W., Schlosser A. The ABC maltose transporter. Mol. Microbiol. 1998;29:685–694. doi: 10.1046/j.1365-2958.1998.00915.x. [DOI] [PubMed] [Google Scholar]
- R8.Elferink M.G., Albers S.V., Konings W.N., Driessen A.J. Sugar transport in Sulfolobus solfataricus is mediated by two families of binding protein-dependent ABC transporters. Mol. Microbiol. 2001;39:1494–1503. doi: 10.1046/j.1365-2958.2001.02336.x. [DOI] [PubMed] [Google Scholar]
- R9.Erauso G., Reysenbach A.-L., Godfroy A., Meunier J.-R., Crump B., Partensky F., Baross J.A., Marteinsson V., Barbier G., Pace N.R., Prieur D. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch. Microbiol. 1993;160:338–349. [Google Scholar]
- R10.Evdokimov A.G., Anderson D.E., Routzahn K.M., Waugh D.S. Structural basis for oligosaccharide recognition by Pyrococcus furiosus maltodextrin-binding protein. J. Mol. Biol. 2001;305:891–904. doi: 10.1006/jmbi.2000.4202. [DOI] [PubMed] [Google Scholar]
- R11.Fiala G., Stetter K.O. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100 °C. Arch. Microbiol. 1986;145:56–61. [Google Scholar]
- R12.Greller G., Horlacher R., Diruggiero J., Boos W. Molecular and biochemical analysis of MalK, the ATP-hydrolyzing subunit of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis . J. Biol. Chem. 1999;274:20259–20264. doi: 10.1074/jbc.274.29.20259. [DOI] [PubMed] [Google Scholar]
- R13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- R14.Horlacher R., Xavier K.B., Santos H., Diruggiero J., Kossmann M., Boos W. Archaeal binding protein-dependent ABC transporter: molecular and biochemical analysis of the trehalose/ maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis . J. Bacteriol. 1998;180:680–689. doi: 10.1128/jb.180.3.680-689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R15.Jackson P. High-resolution polyacrylamide gel electrophoresis of fluorophore-labeled reducing saccharides. Methods Enzymol. 1994;230:250–265. doi: 10.1016/0076-6879(94)30017-8. [DOI] [PubMed] [Google Scholar]
- R16.Jones C.R., Ray M., Dawson K.A., Strobel H.J. High-affinity maltose binding and transport by the thermophilic anaerobe Thermoanaerobacter ethanolicus 39E. Appl. Environ. Microbiol. 2000;66:995–1000. doi: 10.1128/aem.66.3.995-1000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R17.Jorgensen S., Vorgias C.E., Antranikian G. Cloning, sequencing, characterization, and expression of an extracellular alpha-amylase from the hyperthermophilic archaeon Pyrococcus furiosus in Escherichia coli and Bacillus subtilis . J. Biol. Chem. 1997;272:16335–16342. doi: 10.1074/jbc.272.26.16335. [DOI] [PubMed] [Google Scholar]
- R18.Kengen S.W.M., Luesink E.J., Stams A.J., Zehnder A.J. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus . Eur. J. Biochem. 1993;213:305–312. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- R19.Kengen S.W.M., De Bok F.A., Van Loo N.D., Dijkema C., Stams A.J., De Vos W.M. Evidence for the operation of a novel Embden-Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus . J. Biol. Chem. 1994;269:17537–17541. [PubMed] [Google Scholar]
- R20.Laderman K.A., Davis B.R., Krutzsch H.C., Lewis M.S., Griko Y.V., Privalov P.L., Anfinsen C.B. The purification and characterization of an extremely thermostable alpha-amylase from the hyperthermophilic archaebacterium Pyrococcus furiosus . J. Biol. Chem. 1993;268:24394–24401. [PubMed] [Google Scholar]
- R21.McGuckin W.F., McKenzie B.F. An improved periodic acid fuchsin sulfite staining method for evaluation of glycoproteins. Clin. Chem. 1958;4:476–483. [PubMed] [Google Scholar]
- R22.Neuner A., Jannasch H.W., Belkin S., Stetter K.O. Thermococcus litoralis sp. nov.: A new species of extremely thermophilic marine archaebacteria. Arch. Microbiol. 1990;153:205–207. [Google Scholar]
- R23.Richarme G., Kepes A. Study of binding protein-ligand interaction by ammonium sulfate-assisted adsorption on cellulose esters filters. Biochim. Biophys. Acta. 1983;742:16–24. doi: 10.1016/0167-4838(83)90353-9. [DOI] [PubMed] [Google Scholar]
- R24.Schäfer T., Xavier K.B., Santos H., Schönheit P. Glucose fermentation to acetate and alanine in resting cell suspensions of Pyrococcus furiosus: Proposal of a novel glycolytic pathway based on 13C labelling data and enzyme activities. FEMS Microbiol. Lett. 1994;121:107–114. [Google Scholar]
- R25.van der Does C., Manting E.H., Kaufmann A., Lutz M., Driessen A.J.M. Interaction between SecA and SecYEG in micellar solution and formation of the membrane-inserted state. Biochemistry. 1998;37:201–210. doi: 10.1021/bi972105t. [DOI] [PubMed] [Google Scholar]
- R26.Xavier K.B., Peist R., Kossmann M., Boos W., Santos H. Maltose metabolism in the hyperthermophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes. J. Bacteriol. 1999;181:3358–3367. doi: 10.1128/jb.181.11.3358-3367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]