Abstract
A choline-containing phospholipid (PL-4) in Methanopyrus kandleri cells was identified as archaetidylcholine, which has been described by Sprott et al. (1997). The PL-4 consisted of a variety of molecular species differing in hydrocarbon composition. Most of the PL-4 was acid-labile because of its allyl ether bond. The identity of PL-4 was confirmed by thin-layer chromatography (TLC) followed by positive staining with Dragendorff-reagent and fast-atom bombardment–mass spectrometry. A new method of LiAlH4 hydrogenolysis was developed to cleave allyl ether bonds and recover the corresponding hydrocarbons. We confirmed the validity of the LiAlH4 method in a study of the model compound synthetic unsaturated archaetidic acid (2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate). Saturated ether bonds were not cleaved by the LiAlH4 method. The hydrocarbons formed following LiAlH4 hydrogenolysis of PL-4 were identified by gas–liquid chromatography and mass spectrometry. Four kinds of hydrocarbons with one to four double bonds were detected: 47% of the hydrocarbons had four double bonds; 11% had three double bonds; 14% had two double bonds; 7% had one double bond; and 6% were saturated species. The molecular species composition of PL-4 was also estimated based on acid lability: 77% of the molecular species had two acid-labile hydrocarbons; 11% had one acid-labile and one acid-stable hydrocarbon; and 11% had two acid-stable hydrocarbons. To our knowledge, this is the first report of a specific chemical degradation method for the structural analysis of allyl ether phospholipid in archaea.
Keywords: acid-labile phospholipid, LiAlH4 hydrogenolysis, molecular species composition, unsaturated isoprenoid
Introduction
The hyperthermophilic methanoarchaeon, Methanopyrus kandleri, which was isolated from the Guaymas hot vent in California Bay, grows optimally at 97 °C (Kurr et al. 1991). The species is distantly related to all other species of the methanoarchaea (Burggraf et al. 1991). Because polar lipid constituents are a characteristic of the family or genus level of lineage (Koga et al. 1998), we expected to find new polar lipids in this archaeon. The lipid of M. kandleri has been reported to consist of diether-type lipids (Kurr et al. 1991). Hafenbradl et al. (1993, 1996) found 2,3-di-O-geranylgeranyl-sn-glycerol (unsaturated archaeol) in the neutral lipid fraction of M. kandleri, and they reported that the core lipid of the polar lipid fraction comprised only 2,3-di-O-phytanyl-sn-glycerol (saturated archaeol, or simply archaeol). Sprott et al. (1997) detected ion peaks corresponding to saturated archaetidylcholine (2,3-di- O-phytanyl-sn-glycerophosphocholine) and its unsaturated derivatives in fast-atom bombardment–mass spectrometry (FAB–MS) spectra of the total lipid of M. kandleri. Although Sprott et al. (1997) also detected a spot on thin-layer chromatography (TLC) plates that stained positively with Dragendorff reagent, they did not purify this lipid or describe its hydrocarbon composition.
Because Hafenbradl et al. (1996) used acid methanolysis to prepare core lipid, acid-labile allyl ether type core lipids (2,3-di-O-geranylgeranyl-sn-glycerol is an allyl ether type lipid) would be degraded, even if originally present in polar lipids. Therefore, we first looked for an acid-labile polar lipid in the total lipid of M. kandleri. After detecting such a lipid, we developed a new method for preparing hydrocarbon chains linked by allyl ether bonds to a glycerol moiety. In this paper we describe the gross structural determination of archaetidylcholine with unsaturated or saturated isoprenoid hydrocarbon chains (designated PL-4) and the hydrocarbon species composition of the phospholipid. The nomenclature for archaeal ether lipids proposed by Nishihara et al. (1987) is used throughout this paper.
Materials and methods
Chemicals
2,3-Di-O-geranylgeranyl-sn-glycerol (unsaturated archaeol), 2,3-di-O-phytanyl-sn-glycerol-1-phosphate (archaetidic acid) and 2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate (unsaturated archaetidic acid) were prepared as previously described (Morii et al. 2000). 2,3-Di-O-phytanyl-sn-glycerol-1-phosphocholine (saturated archaetidylcholine) was synthesized from archaeol prepared by acid methanolysis from the total lipid of Halobacterium salinarum cells (Nishihara and Koga 1997). Archaeol was first phosphorylated with phosphorus oxychloride, and then choline was bound to the resultant archaetidic acid with choline p-toluene sulfonate according to the method previously described (Brockerhoff and Ayengar 1979, Witzke and Bittman 1986). Geranylgeraniol was kindly supplied by M. Murakami and N. Asakawa (Eisai Co., Tokyo, Japan). Lysophosphatidylcholine was prepared from 1,2-di-O-oleoyl-sn-glycerol-3-phosphocholine by hydrolysis with phospholipase A2 (from bee venom, Sigma) as described by Haverkate and van Deenen (1965).
Organism
Methanopyrus kandleri cells were grown in a 300-l fermentor as described by Hafenbradl et al. (1996), and the harvested cells were freeze-dried (35 g) and transported from Germany to Japan at ambient temperature and then stored at –20 °C until lipid extraction.
Extraction of lipid
Before lipid extraction, freeze-dried cells (10 g) were ground with a mortar and pestle. Lipid was extracted from the ground cells by the method of Bligh and Dyer (1959) with neutral water as a cell-suspending solvent. Phospholipid-phosphorus (Bartlett 1959) and glycolipid-sugar (Dubois et al. 1956) in the total lipid extract were 2.1 and 69 mg, respectively.
Reductive cleavage of allyl ether bonds with LiAlH4
We successively added 1 ml of diethylether, 40 mg of LiAlH4 powder, and a further 2 ml of diethylether to a chloroform solution of lipid (50 µg/20 µl) in a screw-capped test tube. The mixture was incubated at 100 °C for 90 min in an aluminum block-heating bath with continuous stirring with a magnetic stirrer (Dry-block multi stirrer DM-8, Scinics, Tokyo, Japan) and a small Teflon-coated stirring rod. (The cleavage reaction did not proceed at room temperature.) After the mixture had cooled to room temperature, it was placed in ice and excess hydride was decomposed by the addition of 3 ml of 1 M HCl. The resulting allyl ether-derived hydrocarbons were extracted from the mixture three times with light petroleum.
Chromatography
Core lipids and neutral lipid were subjected to thin-layer chromatography (TLC) on silica gel TLC plates (Merck Art. 5721) with Solvent A (light petroleum:diethyl ether:acetic acid (50:50:1 v/v)) in one dimension. The same kind of TLC plate was used to chromatograph total polar lipid with Solvent B (chloroform:methanol:7 M aqueous ammonia (60:35:8 v/v)) for the first direction, and Solvent C (chloroform:methanol:acetic acid:water (85:30:15:5 v/v)) for the second direction. The choline-containing phospholipid (PL-4) was purified by TLC with Solvent D (chloroform:methanol:acetone:acetic acid:water (6:2:8:2:1 v/v)). Spots were detected by spraying with acid-molybdate reagent (Dittmer and Lester 1964) for phospholipids, Dragendorff reagent (Wagner et al. 1961) for choline-containing lipid, and by charring for all lipids.
Hydrocarbons were analyzed by gas–liquid chromatography (GLC; Shimadzu GC17A, Kyoto, Japan) with a DB-1 capillary column (0.25 mm × 30 m, J & W Scientific, Folson, CA) at a temperature increasing from 180 to 270 °C at a rate of 5 °C min–1. n-Hexacosane or n-tetracosane was used as an internal standard.
Gas chromatography–mass spectrometry (GC–MS)
Hydrocarbons prepared from allyl ether phospholipids with LiAlH4 were analyzed by gas chromatography–mass spectrometry (GC–MS) on a Hewlett-Packard gas chromatograph 5890 with a column of CP-Sil 5CB (0.25 mm × 30 m, GL Science, Tokyo, Japan) connected to a JEOL JMS DX303 mass spectrometer (JEOL, Tokyo, Japan). The temperature of the column in the gas chromatograph was programmed as above. The temperatures of the transfer line and the ion source were 250 and 260 °C, respectively: electron impact (EI) mass spectra were determined at an ionizing voltage of 70 eV.
Mass spectra
Fast-atom bombardment–mass spectrometry (FAB–MS) was performed with a JEOL JMS SX-102A instrument with nitrobenzyl alcohol + NaI matrix in a positive ion mode.
Determination of hydrocarbon composition of PL-4
Because PL-4 was expected to contain various hydrocarbon species differing in numbers of double bonds, hydrocarbon composition was determined by two methods.
Method A: PL-4 was treated with 5% HCl-methanol at 80 °C for 1 h or 100 °C for 3 h. The results of analyses made under the two conditions were similar. The whole reaction mixture was partitioned with a two-phase solvent system of chloroform:methanol:water (10:10:9, v/v). The products in the upper layer (methanol:water phase) were saved as glycerophosphate esters derived from PL-4 with two allyl ether-bonded hydrocarbons (allyl–allyl molecular species). The products in the lower layer (chloroform phase) were separated by TLC (developed twice in the same direction with Solvent D) into unchanged PL-4, lysoarchaetidylcholine derived from PL-4 with one allyl ether-bonded hydrocarbon and one non-allyl ether-bonded hydrocarbon (allyl–non-allyl molecular species), and small amounts of by-products. Silica gel powder corresponding to each spot was scraped off the TLC plate and phosphate was determined by the method of Bartlett (1959). The amount of phosphate in the upper-layer soluble compounds was also determined.
Method B: hydrocarbons derived from allyl ether-bonded lipids were prepared by the LiAlH4 method. Hydrocarbons were quantitatively analyzed by GLC with n-tetracosane (C24) as an internal standard. Saturated hydrocarbons were prepared by HI cleavage (Morii and Koga 1993) and determined by GLC.
Results
Neutral lipids
Total lipid of M. kandleri was chromatographed on a TLC plate with Solvent A. Three spots corresponding to neutral lipids that co-migrated with archaeol, unsaturated archaeol (2,3-di-O-geranylgeranyl-sn-glycerol) and geranylgeraniol were detected. Detection of unsaturated archaeol confirmed the result of Hafenbradl et al. (1996). The lipids in the three spots were not further characterized.
An acid-labile choline-containing phospholipid, PL-4
Two-dimensional TLC of total lipid of M. kandleri with Solvents B and C revealed 20 or more spots by acid charring, of which about 11 spots were acid molybdate reagent-positive (phospholipid). The two-dimensional TLC chromatogram was essentially identical with that previously reported (Hafenbradl et al. 1996). Two of the phospholipids were positive to Dragendorff reagent, suggesting that they contained tertiary ammonium salt (e.g., choline). Because choline-containing lipid from M. kandleri has been reported by Sprott et al. (1997) based on FAB–MS and TLC of total lipid, we attempted to demonstrate the allyl ether structure of this lipid by specific chemical cleavage of purified lipid. One of these choline-containing phospholipids (PL-4) co-migrated with synthetic archaetidylcholine (Rf = 0.20 when developed with Solvent D). The PL-4 was partially acid labile, that is, when total lipid was treated with 5% HCl-methanol at 100 °C for 3 h, the amount of PL-4 greatly decreased but a small amount of it remained intact on TLC. The other choline-containing phospholipid had an Rf = 0.14, and was not further characterized. We purified PL-4 by one-dimensional TLC with Solvent D as a developing solvent.
Fast-atom bombardment–mass spectrometry of PL-4
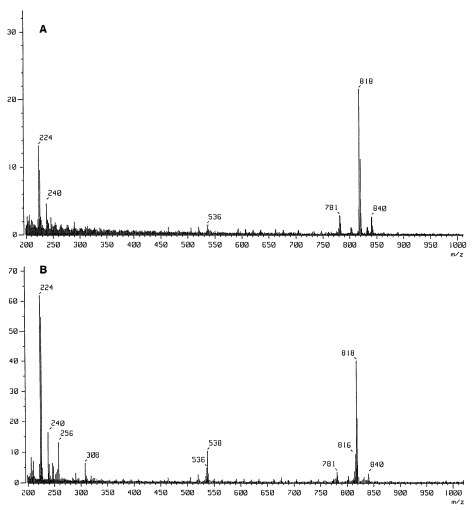
We compared FAB–MS spectrum of PL-4 with that of standard archaetidylcholine with saturated phytanyl chains (2,3-di-O-phytanyl-sn-glycerol-1-phosphocholine, MW = 817) that had been chemically synthesized. Figure 1 shows the positive ion FAB–MS spectra of synthetic saturated archaetidylcholine and PL-4. Synthetic saturated archaetidylcholine (Figure 1A) had a major peak at m/z 818 ([M + H]+). This lipid may correspond to the lipid shown by Sprott et al. (1997) to have signals at m/z 802.7 and 818.9 on negative and positive ion mass spectra, respectively. Fragment peaks at m/z 781 ([M + Na – N+(CH3)3]+), 536 ([M – C20H41]+), 240 ([M + H – C20H41 – OC20H41]+) and 224 ([M + H – 2(OC20H41)]+) were also observed. The observed peak at m/z 840 was assigned to [M + Na]+.
Figure 1.
Positive ion FAB–MS spectra of synthetic 2,3-di-O-phytanyl-sn-glycero-phosphocholine (saturated archaetidylcholine, MW = 817, A) and PL-4, the mixture of saturated and unsaturated analogs of archaetidylcholine (B).
The FAB–MS spectrum of PL-4 (Figure 1B) also had peaks at m/z 818, 781, 536, 240, 224 and 840 (cf. Figure 1A), indicating that PL-4 contained saturated archaetidylcholine. Furthermore, peaks at m/z 816, 538, 308 and 256 were observed in the spectrum of PL-4. It is reasonable to consider that the peak at m/z 816 was derived from the protonated molecular ion ([M¢ + H]+) of an unsaturated analog of archaetidylcholine, where M¢ (MW = 815) was archaetidylcholine with one hydrocarbon chain with one double bond (-C20H39) and one saturated hydrocarbon chain (-C20H41). Unfortunately, the peaks corresponding to the molecular ions or the protonated molecular ions of other unsaturated analogs were not observed. The peaks at m/z 536 ([M – C20H41]+ or [M¢ – C20H39]+), 256 ([X + H – 2R]+), 240 ([X + H – R – OR]+) and 224 ([X + H – 2OR]+) represented fragment ions, where X represents archaetidylcholine analogs and R a hydrocarbon moiety. It is not known if the peaks at m/z 538 and 308 (Figure 1B) originated from the unsaturated analog of archaetidylcholine.
The fragment peaks at m/z 536, 240 and 224 are seen in Figures 1A and 1B. However, compared with the molecular ion peak in Figure 1B, the relative intensities of the fragment peaks, especially the peak at m/z 224, were higher than those of the saturated standard. Moreover, the peak of m/z 256 was only detected in Figure 1B. These results suggest that the unsaturated analog molecular species were easily fragmented at the position of allyl ether bond under the FAB–MS conditions employed.
LiAlH4 reductive cleavage of allyl ether bonds of synthetic unsaturated archaetidic acid and identification of allyl ether-bonded hydrocarbons
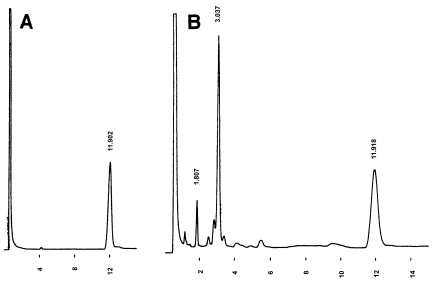
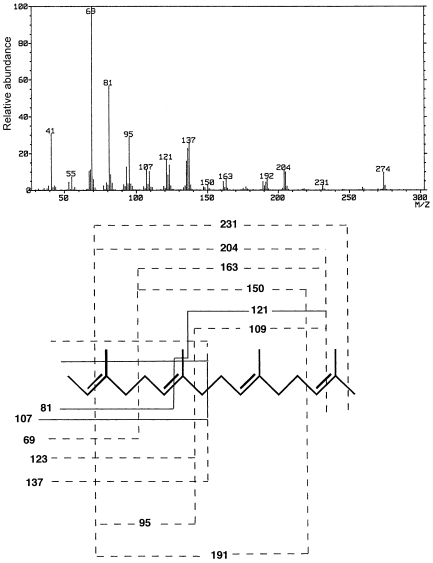
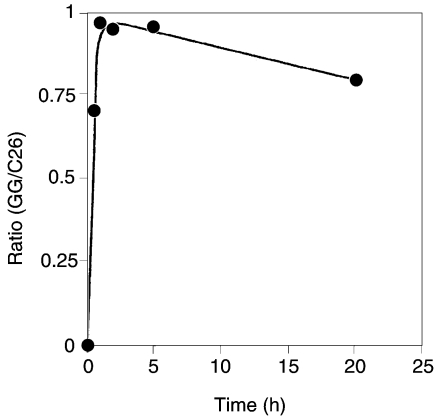
Because the allyl ether bonds of PL-4 are acid-labile, the hydrocarbons or core lipid of the lipid could not be prepared by traditional methods, such as HI cleavage, BCl3 treatment, HCl-methanolysis, HF hydrolysis, or acetolysis. Several attempts at reductive cleavage of ether bonds with H2/PtO2 or NaBH4 were also unsuccessful. Because LiAlH4 has been used for reductive cleavage of acyl ester bonds and phosphodiester bonds of alkenyl-acyl phospholipids and alkyl-acyl phospholipids (Wood and Snyder 1968), we used the LiAlH4 method to cleave allylic ether bonds, initially, for the model compound of synthetic 2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate and then for PL-4. The GLC of the final hydrocarbon product of the model compound showed one major peak (Figure 2B), which when subjected to GC–MS had a molecular ion peak (M+ = 274) and a spectrum that showed a fragmentation pattern consistent with that expected for 2,6,10,14-tetramethylhexadec-2,6,10,14-tetraene (MW = 274, Figure 3). When saturated archaetidic acid or archaeol was subjected to the same treatment, no peak appeared in the GLC profile, suggesting that LiAlH4 cleavage is specific to the allyl ether bond. The time course of the reaction at 100 °C is shown in Figure 4. We adopted an incubation time of 90 min for further studies.
Figure 2.
Gas-liquid chromatogram of hydrocarbons prepared by LiAlH4 from a model ether lipid, 2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate. Panels A and B are the chromatograms of samples before and after reductive cleavage, respectively. The peaks eluted at 11.9 min and 3.0 min were the internal standard, n-hexacosane and geranylgeranyl chain-derived hydrocarbon (2,6,10,14-tetramethylhexadec-2,6,10,14-tetraene), respectively.
Figure 3.
A positive ion EI-MS spectrum of the hydrocarbon product prepared by LiAlH4 reductive cleavage of di-O-geranylgeranyl-sn-glycerol-1-phosphate (unsaturated archaetidic acid). The hydrocarbon product was separated by GLC and the 3.0 min peak on the chromatogram shown in Figure 2 was subjected to MS. The lower part of the figure shows a possible fragmentation pattern of the hydrocarbon, which coincides well with the mass spectrum.
Figure 4.
Time course of LiAlH4 cleavage reaction of an allyl ether. 2,3-Di-O-geranylgeranyl-sn-glycerol-1-phosphate was reacted with LiAlH4 as described in Materials and methods. Samples were taken at the times indicated and analyzed by GC. The ratio of peak areas of 2,6,10,14-tetramethylhexadec-2,6,10,14-tetraene to the internal standard was recorded.
Reductive cleavage of PL-4
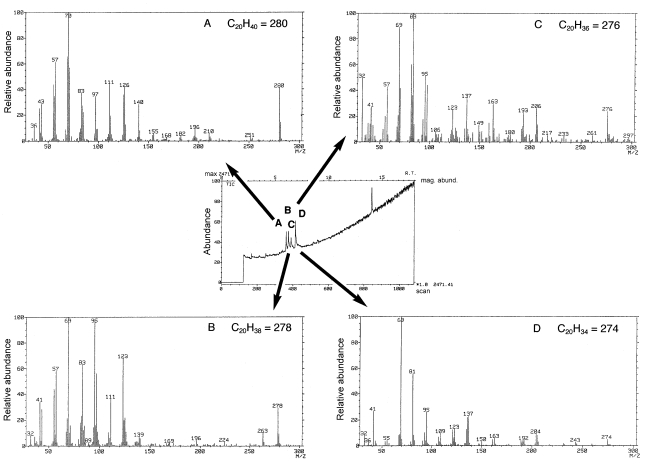
Reductive cleavage with LiAlH4 was applied to the analysis of the PL-4 hydrocarbons. Total ion chromatography (TIC) of hydrocarbons showed four peaks and GC–MS showed molecular ion signals at m/z 280 (C20H40), 278 (C20H38), 276 (C20H36) and 274 (C20H34) (Figure 5), corresponding to C20 hydrocarbons with one, two, three and four double bonds, respectively. Because the MS spectrum shown in Figure 5D was essentially identical to the mass spectrum of geranylgeranyl hydrocarbon shown in Figure 3, the PL-4 hydrocarbon chain with four double bonds was most probably an isoprenoid chain with double bonds at the 2, 6, 10 and 14 positions (2,6,10,14-tetramethylhexadec-2,6,10,14-tetraene). Although signals at m/z 123 and 109 were flagged in Figure 5D instead of m/z 121 and 107 as in Figure 3, the latter signals were also seen in Figure 5D. They were not flagged because they were not the highest peaks among the relevant peak groups. We were unable to determine the positions of double bonds in hydrocarbons giving signals shown in Figures 5A–5C.
Figure 5.
Mass spectra of the LiAlH4 reductive cleavage products of PL-4. Center panel, TIC chromatogram. Panels A, B, C, and D are mass spectra of peaks A, B, C, and D of the TIC chromatogram.
Composition of molecular species of PL-4
The acid-labile molecular species composition of PL-4 was determined by Method A (see Materials and methods). About 75% of PL-4 phosphate was changed to water-soluble products. Seven spots were detected following TLC of the chloroform-soluble fraction obtained after HCl-degradation. Unchanged archaetidylcholine, archaetidic acid, lysoarchaetidic acid and lysoarchaetidylcholine were identified by comparing their mobility with standard lipids, including lysophosphatidylcholine. Archaetidic acid and lysoarchaetidic acid were by-products formed from the non-allyl ether molecular species and allyl–non-allyl molecular species of PL-4, respectively, by removing choline by acid. Lysoarchaetidylcholine was also derived from allyl–non-allyl molecular species of PL-4. The unknown spots were less than 2%. Phosphate contents of archaetidic acid and unchanged archaetidylcholine were combined as non-allyl–non-allyl molecular species. The composition of the hydrocarbon species obtained by two independent analyses is summarized in Table 1. The largest component of PL-4 was the allyl ether-bonded species. Half of the remainder had one allyl ether hydrocarbon and one non-allyl ether hydrocarbon. Only 11% of PL-4 was non-allyl molecular species.
Table 1.
Composition of acid-labile allyl ether-bonded hydrocarbons of PL-4. Allyl ether bonds of PL-4 were degraded with 5% HCl-methanol at 80 °C for 1 h or 100 °C for 3 h. The products were partitioned with a Bligh-Dyer solvent. The chloroform-soluble products were separated by TLC into the unchanged archaetidylcholine (AC), lysoarchaetidylcholine (lyso AC), lysoarchaetidic acid (lyso AA), archaetidic acid (AA), and small amounts of by-products. Phosphate contents of the water-soluble product and of each spot on the TLC plates were determined.
| Fraction | Allyl ether bonded hydrocarbon in PL-4 | Allyl ether bonded hydrocarbon (%)1 | Non-allyl ether-bonded hydrocarbon (%)2 | |
| No. | % | |||
| Water-soluble (a) | 2 | 77 | ||
| Lyso AC (b) | 1 | 5 | 83 | |
| Lyso AA (c) | 1 | 6 | ||
| Unchanged AC (d) | 0 | 9 | ||
| AA + unknown (e) | 0 | 2 | 17 | |
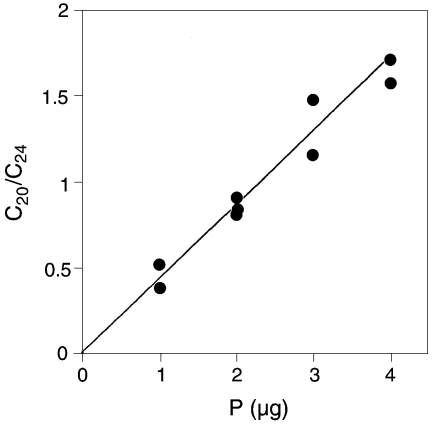
The hydrocarbon composition was analyzed more directly by Method B (see Materials and methods). Although the overall recovery of C20 hydrocarbons by this method was incomplete (25%, which was constant throughout several analyses), a linear relationship was obtained between the amount of phosphate of the model compound (unsaturated archaetidic acid) and the ratio of GLC integrated areas of C20 hydrocarbon to C24 hydrocarbon (Figure 6). Saturated hydrocarbon (S) was prepared by HI cleavage, allyl ether-bonded hydrocarbons with one, two, three or four double bonds (A) were analyzed after LiAlH4 cleavage, and the remainder (U = 100 – S – A) was assumed to be non-allyl unsaturated hydrocarbons (Table 2). The total percentage of the four kinds of allyl ether species was 78%, which coincided well with 83% allyl ether type in PL-4 based on acid lability (calculated from the data shown in Table 1 as: (2a + b + c)/2%). The most abundant hydrocarbon was the four-double-bond type (geranylgeranyl type), but the exact position of unsaturation of hydrocarbons with three or less double bonds was not determined, except at position 2.
Figure 6.
Standard curve for the quantification of allyl ether-bonded hydrocarbons by GLC after LiAlH4 hydrogenolysis. The ratio C20/ C24 represents the area ratio of the GLC profile integrated between the sample of geranylgeranyl hydrocarbon (C20) to the known amount of internal standard (C24). Abbreviation: P represents the amount of phosphate in the sample (2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate) that was subjected to analysis.
Table 2.
Composition of hydrocarbon chains of PL-4. Allyl ether-bonded hydrocarbons were prepared with LiAlH4 by Method B as described in Materials and methods. Non-allyl ether-bonded hydrocarbons were prepared by HI cleavage. Hydrocarbons were quantitatively analyzed by GLC with n-tetracosane (C24) as an internal standard.
| Ether-bonded hydrocarbon | % |
| Saturated | 6 |
| Non-allylic unsaturated | 16 |
| Allylic unsaturated | |
| Total | 78 |
| With 4 double bonds | 47 |
| With 3 double bonds | 11 |
| With 2 double bonds | 14 |
| With 1 double bond | 7 |
Discussion
Our data confirmed the presence, in M. kandleri, of archaetidylcholine with zero to four double bonds on its two hydrocarbon chains. This is the first example of the isolation of allyl ether-bonded hydrocarbons from purified archaeal phospholipid by a specific chemical cleavage. Allyl ether type lipids containing a phytol (monounsaturated allyl alcohol) side chain on the glycerol moiety have been reported for Halobacterium lacusprofundi (Franzmann et al. 1988) and Methanococcoides burtonii (Nichols and Franzmann 1992). Halobacterium lacusprofundi seems to have more unsaturated hydrocarbons, but their structures have not been identified. Unsaturated hydrocarbons with two or more double bonds from H. lacusprofundi were not recovered after HI cleavage of ether bonds, suggesting that they are acid-labile allyl ether-bonded chains. Qiu et al. (1998) presented evidence for the presence of archaeal phospholipids containing one, two and three double bonds in their isoprenoid chains in the haloalkaliphilic archaeon, Natronobacterium magadii. Because H. lacusprofundi and M. burtonii,which contain unsaturated ether lipids, were isolated from low-temperature salt lakes in Antarctica (Franzmann et al. 1988, 1992), Nichols and Franzmann (1992) speculated that the presence of unsaturated ether lipids in both archaea is a result of adaptation to low temperature. Our finding of a higher degree of unsaturation and more abundant unsaturated ether lipids in the hyperthermophilic methanoarchaeon M. kandleri than in H. lacusprofundi and M. burtonii provides strong evidence against their speculation. Other cases in which archaea growing optimally at high and low temperatures possess similar types of lipids have been described by Morii et al. (1988) and Nishihara et al. (1989).
Hydroxyarchaeols are another type of acid-labile ether core lipid that are easily degraded by acid treatment (Sprott et al. 1990). It is expected that a hydroxyl group at position 3 of the phytanyl chain can be easily converted to an allylic double bond.
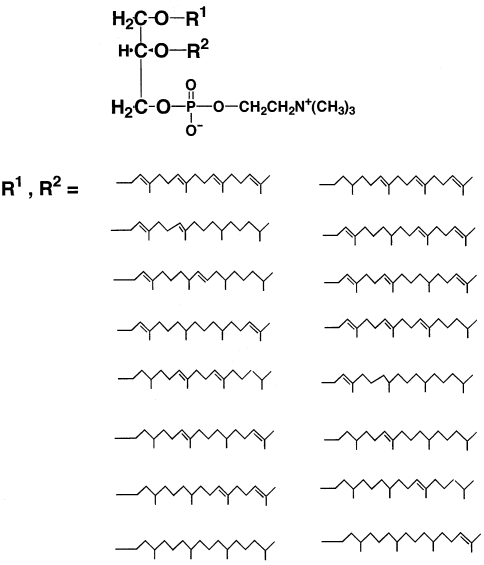
We showed that unsaturated PL-4 is a mixture of archaetidylcholine with one, two, three or four double bonds in one hydrocarbon chain, but the exact positions of the double bonds were not determined. In the traditional isoprenoid biosynthetic pathway, a geranylgeraniol derivative is synthesized as the first C20 product and is used as the only isoprenoid precursor of ether bond formation. Formation of archaetidic acid and CDP-archaeol intermediates of archaeal ether polar lipid biosynthesis are specific to geranylgeranyl chains (Zhang and Poulter 1993, Morii et al. 2000). Therefore, the hydrocarbon chain of PL-4 with four double bonds is most likely a geranylgeranyl chain. Assuming that the double bonds are located at positions 2, 6, 10 and 14 as in a geranylgeranyl chain, it is possible that there are 16 molecular species that have zero to four double bonds randomly distributed at the four positions. Because archaetidylcholine has two hydrocarbon chains in one molecule, 256 molecular species are possible. Among the 256 molecular species, 64 are allyl–allyl ether types. Figure 7 shows the possible structures of archaetidylcholine.
Figure 7.
Possible structures of archaetidylcholine. Although the positions of double bonds on the hydrocarbon chains are assumed to be at 2, 6, 10 and 14, the exact positions were not determined.
Although the exact composition of the molecular species could not be determined, an approximation is given in Table 2. To our knowledge, this is the first estimation of the molecular species composition of archaeal polar lipid. Lysoarchaetidic acid was presumed to be a by-product formed by removal of choline with acid (simple removal of choline is estimated to be less than 2% as shown by the amount of archaetidic acid formed (the bottom line of Table 1)). However, we also offer an alternative explanation for the presence of lysoarchaetidylcholine. Among the acid hydrolysis products of PL-4, lysoarchaetidylcholine might be derived from sn-3-allyl-sn-2-non-allyl molecular species and lysoarchaetidic acid from sn-2-allyl-sn-3-non-allyl molecular species. We speculate that the choline moiety is removed through cyclic phosphate formation on a free hydroxyl group adjacent to the phosphate group that is formed during acid hydrolysis of the sn-2-allyl ether bond of the latter phospholipid. Because the former molecular species could not form a free hydroxyl group adjacent to the phosphate group, the choline moiety would be intact. If true, the positional isomers of PL-4 with one allyl ether hydrocarbon could be separately determined (5% of sn-3-allyl and 6% of sn-2-allyl molecular species).
Acknowledgments
The authors thank Drs. M. Murakami and N. Asakawa (Eisai Co.) for supplying geranylgeraniol.
References
- R1.Bartlett G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- R2.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- R3.Brockerhoff H., Ayengar N.K.N. Improved synthesis of choline phospholipids. Lipids. 1979;14:88–89. [Google Scholar]
- R4.Burggraf S., Stetter K.O., Rouviere P., Woese C.R. Methanopyrus kandleri—an archaeal methanogen unrelated to all other known methanogens. Syst. Appl. Microbiol. 1991;14:346–351. doi: 10.1016/s0723-2020(11)80308-5. [DOI] [PubMed] [Google Scholar]
- R5.Dittmer J.C., Lester R.L. A simple, specific spray for the detection of phospholipids on thin-layer chromatograms. J. Lipid Res. 1964;5:126–127. [PubMed] [Google Scholar]
- R6.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- R7.Franzmann P.D., Stackebrandt E., Sanderson K., Volkman J.K., Cameron D.E., Stevenson P.L., Mcmeekin T.A., Burton H.R. Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep lake, Antarctica. Syst. Appl. Microbiol. 1988;11:20–27. [Google Scholar]
- R8.Franzmann P.D., Springer N., Ludwig W., Conway De Macario E., Rohde M. A methanogenic archaeon from Ace lake, Antarctica: Methanococcoides burtonii sp. nov. Syst. Appl. Microbiol. 1992;15:573–581. [Google Scholar]
- R9.Hafenbradl D., Keller M., Thiericke R., Stetter K.O. A novel unsaturated archaeal ether core lipid from the hyperthermophile Methanopyrus kandleri . Syst. Appl. Microbiol. 1993;16:165–169. [Google Scholar]
- R10.Hafenbradl D., Keller M., Stetter K.O. Lipid analysis of Methanopyrus kandleri . FEMS Microbiol. Lett. 1996;136:199–202. [Google Scholar]
- R11.Haverkate F., van Deenen L.L.M. Isolation and chemical characterization of phosphatidylglycerol from spinach leaves. Biochim. Biophys. Acta. 1965;106:78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- R12.Koga Y., Morii H., Akagawa-Matsushita M., Ohga M. Correlation of polar lipid composition with 16S rRNA phylogeny in methanogens. Further analysis of lipid component parts. Biosci. Biotechnol. Biochem. 1998;62:230–236. doi: 10.1271/bbb.62.230. [DOI] [PubMed] [Google Scholar]
- R13.Kurr M., Huber R., König H., Jannasch H.W., Fricke H., Trincone A., Kristjansson J.K., Stetter K.O. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110 °C. Arch. Microbiol. 1991;156:239–247. [Google Scholar]
- R14.Morii H., Koga Y. Tetraether type polar lipids increase after logarithmic growth phase of Methanobacterium thermoautotrophicum in compensation for the decrease of diether lipids. FEMS Microbiol. Lett. 1993;109:283–288. [Google Scholar]
- R15.Morii H., Nishihara M., Koga Y. Composition of polar lipids of Methanobrevibacter arboriphilicus and structure determination of the signature phosphoglycolipid of Methanobacteriaceae . Agric. Biol. Chem. 1988;52:3149–3156. [Google Scholar]
- R16.Morii H., Nishihara M., Koga Y. CTP: 2,3-di-O-geranylgeranyl-sn-glycerol-1-phosphate cytidyltransferase in the methanogenic archaeon Methanothermobacter thermoautotrophicus . J. Biol. Chem. 2000;275:36568–36574. doi: 10.1074/jbc.M005925200. [DOI] [PubMed] [Google Scholar]
- R17.Nichols P.D., Franzmann P.D. Unsaturated diether phospholipids in the Antarctic methanogen Methanococcoides burtonii . FEMS Microbiol. Lett. 1992;98:205–208. [Google Scholar]
- R18.Nishihara M., Koga Y. Purification and properties of sn-glycerol-1-phosphate dehydrogenase from Methanobacterium thermoautotrophicum: Characterization of the biosynthetic enzyme for the enantiomeric glycerophosphate backbone of ether polar lipids of archaea. J. Biochem. 1997;122:572–576. doi: 10.1093/oxfordjournals.jbchem.a021791. [DOI] [PubMed] [Google Scholar]
- R19.Nishihara M., Morii H., Koga Y. Structure determination of a quartet of novel tetraether lipids from Methanobacterium thermoautotrophicum . J. Biochem. 1987;101:1007–1015. doi: 10.1093/oxfordjournals.jbchem.a121942. [DOI] [PubMed] [Google Scholar]
- R20.Nishihara M., Morii H., Koga Y. Heptads of polar ether lipids of an archaebacterium, Methanobacterium thermoautotrophicum: Structure and biosynthetic relationship. Biochemistry. 1989;28:95–102. [Google Scholar]
- R21.Qiu D.-F., Games M.P.L., Xiao X.-Y., Games D.E., Walton T.J. Application of high-performance liquid chromatography/ electrospray mass spectrometry for the characterization of membrane lipids in the haloalkaliphilic archaebacterium Natronobacterium magadii . Rapid Commun. Mass Spectrom. 1998;12:939–946. [Google Scholar]
- R22.Sprott G.D., Ekiel I., Dicaire C. Novel, acid-labile, hydroxydiether lipid cores in methanogenic bacteria. J. Biol. Chem. 1990;265:13735–13740. [PubMed] [Google Scholar]
- R23.Sprott G.D., Agnew B.J., Patel G.B. Structural features of ether lipids in the archaeobacterial thermophiles Pyrococcus furiosus, Methanopyrus kandleri, Methanothermus fervidus, and Sulfolobus acidocaldarius . Can. J. Microbiol. 1997;43:467–476. [Google Scholar]
- R24.Wagner H., Hörhammer L., Wolff P. Dünnschichtchromatographie von Phosphatiden und Glykolipiden. Biochem. Z. 1961;334:175–184. [PubMed] [Google Scholar]
- R25.Witzke N.M., Bittman R. Convenient synthesis of racemic mixed-chain ether glycerophosphocholines from fatty alkyl allyl ethers: Useful analogs for biophysical studies. J. Lipid Res. 1986;27:344–351. [PubMed] [Google Scholar]
- R26.Wood R., Snyder F. Quantitative determination of alk-1-enyl- and alkyl-glyceryl ethers in neutral lipids and phospholipids. Lipids. 1968;3:129–135. doi: 10.1007/BF02531729. [DOI] [PubMed] [Google Scholar]
- R27.Zhang D., Poulter C.D. Biosynthesis of archaebacterial ether lipids—formation of ether linkages by prenyltransferases. J. Am. Chem. Soc. 1993;115:1270–1277. [Google Scholar]