Abstract
Previously, we showed that the proton permeability of small unilamellar vesicles (SUVs) composed of polar lipid fraction E (PLFE) from the thermoacidophilic archaeon Sulfolobus acidocaldarius was remarkably low and insensitive to temperature (Komatsu and Chong 1998). In this study, we used photon correlation spectroscopy to investigate the time dependence of PLFE SUV size as a function of Ca2+ concentration. In the absence of Ca2+, vesicle diameter changed little over 6 months. Addition of Ca2+, however, immediately induced formation of vesicle aggregates with an irregular shape, as revealed by confocal fluorescence microscopy. Aggregation was reversible upon addition of EDTA; however, the reversibility varied with temperature as well as incubation time with Ca2+. Freeze-fracture electron microscopy showed that, after a long period of incubation (2 weeks) with Ca2+, the PLFE vesicles had not just aggregated, but had fused or coalesced. The initial rate of vesicle aggregation varied sigmoidally with Ca2+ concentration. At pH 6.6, the threshold calcium concentration (Cr) for vesicle aggregation at 25 and 40 °C was 11 and 17 mM, respectively. At pH 3.0, the Cr at 25 °C increased to 25 mM. The temperature dependence of Cr may be attributable to changes in membrane surface potential, which was –22.0 and –13.2 mV at 25 and 40 °C, respectively, at pH 6.6, as determined by 2-(p-toluidinyl)naphthalene-6-sulfonic acid fluorescence. The variation in surface potential with temperature is discussed in terms of changes in lipid conformation and membrane organization.
Keywords: fluorescence, light scattering, membranes, microscopy, pH, surface potential, temperature, vesicle size
Introduction
The major lipid components of the plasma membrane of the thermoacidophilic archaeon Sulfolobus acidocaldarius are tetraether lipids (De Rosa et al. 1986, Langworthy and Pond 1986, Kates 1992), among which the polar lipid fraction E (PLFE) is the main constituent (Lo and Chang 1990). The PLFE contains a mixture of bipolar tetraether lipids with either a glycerol dialkyl calditol tetraether (GDNT; also called calditoglycerocaldarchaeol) or a glycerol dialkyl glycerol tetraether (GDGT; also called caldarchaeol) skeleton (Figure 1) (Lo and Chang 1990, Sugai et al. 1995, Gliozzi et al. 2002). Both GDGT and GDNT have bisubstituted polar head groups and are thus designated bipolar tetraether lipids. Glycerol dialkyl calditol tetraether (~90% of total PLFE) has phospho-myo-inositol on the glycerol end and β-glucose on the calditol end, whereas GDGT (~10% of total PLFE) has phospho-myo-inositol attached to one glycerol and β-D-galactosyl-D-glucose to the other glycerol skeleton. The phospho-myo-inositol groups of PLFE lipids are oriented toward the cytoplasmic side of the cell’s plasma membrane (De Rosa et al. 1983). The nonpolar regions of these lipids consist of a pair of 40-carbon biphytanyl chains, each of which contains up to four cyclopentane rings. The number of cyclopentane rings increases with increasing growth temperature (De Rosa et al. 1980).
Figure 1.
Structures of PLFE lipids: (top) glycerol dialkyl calditol tetraether (GDNT) and (bottom) glycerol dialkyl glycerol tetraether (GDGT). Abbreviations: R1 = inositol; R2 = β-D-glucopyranose; and R3 = β-D-galactosyl-β-D-glucopyranose. The number of cyclopentane rings in each biphytanyl chain can vary from 0 to 4.
In aqueous solution, PLFE lipids form stable multilamellar and unilamellar liposomes (Lo and Chang 1990, Elferink et al. 1992, Bagatolli et al. 2000) in which the lipids span the entire lamellar structure, forming monomolecular membranes (Elferink et al. 1992). Liposomes composed of PLFE lipids exhibit rates of proton permeation and dye leakage that are unusually low and insensitive to temperature (In’t Veld et al. 1992, Chang 1994, Elferink et al. 1994, van de Vossenberg et al. 1995, Komatsu and Chong 1998). These traits have been attributed to the unique chemical structure of bipolar tetraether lipids and their organization within membranes, particularly the network of hydrogen bonds between polar head groups, the rigid and tight packing of lipids within the membrane, and the negative charges on the membrane surface (Elferink et al. 1994, Relini et al. 1994, van de Vossenberg et al. 1995, Komatsu and Chong 1998). The remarkable thermostability of proton permeation across PLFE membranes has provided a partial explanation of how S. acidocaldarius can grow at high temperatures (65–83 °C) (van de Vossenberg et al. 1995) in acidic environments (pH 2–3) and yet maintain the intracellular compartment at pH 6.5 (Brock et al. 1972).
In addition to thermal and acid stability, PLFE and other archaeal bipolar tetraether liposomes exhibit remarkable resistance to mechanical stress and the actions of phospholipases, bile salts and serum proteins (reviewed in Gliozzi and Relini 1996). Because of their extraordinary stability, which permits sterilization (Choquet et al. 1994) and filtration (Bauer et al. 1983), bipolar tetraether liposomes have found wide application in immunoassays (Tomioka et al. 1994) and in vaccine and drug delivery (Ring et al. 1986, Sprott 1992, Elferink et al. 1994, Freisleben et al. 1995, Gliozzi and Relini 1996, Sprott et al. 1997, Krishnan et al. 2000, Patel et al. 2000). Although stability against macrophages and other bioactive species is desirable in liposomes used as drug delivery vehicles, high membrane stability also hinders the release of entrapped drugs. Therefore, a compromise between membrane stability and drug release is required. It is known that drug release from liposomes composed of typical phospholipid bilayers can be facilitated via fusion and lipid mixing, both of which are preceded by vesicle aggregation (Boni et al. 1984, Bentz and Duzgunes 1985). Hence, it is of interest to investigate the mechanisms underlying the aggregation and fusion processes of PLFE liposomes.
Previous studies (Relini et al. 1994, 1996) reported that fusogenic agents such as calcium chloride and polyethylene glycol induced fusion between vesicles derived from total lipid extracts of the thermoacidophilic archaeon Sulfolobus solfataricus. However, with vesicles made from the P2 bipolar tetraether lipid fraction of the same archaeon, only vesicle aggregation, not fusion, was observed. The total lipid extracts of S. solfataricus contain both bisubstituted (e.g., GDGT and GDNT) and monosubstituted tetraether lipids, whereas the P2 fraction contains only bisubstituted tetraether lipids (reviewed in Gliozzi et al. 2002). Liposomal membranes made from the P2 fraction have a strict lamellar structure (Gulik et al. 1988), and fusion occurs only when this lamellar structure is disturbed (Relini et al. 1994, 1996). Furthermore, Ca2+-induced aggregation or fusion of bipolar tetraether liposomes occurs on the time scale of tens of minutes (Relini et al. 1994), which is much slower than that of monopolar diester liposomes (Wilschut et al. 1980, Hui et al. 1988). Moreover, unlike most monopolar diester lipid membranes, bipolar tetraether lipid monolayers show no detectable changes in surface tension even when fusion occurs (Relini et al. 1994). As such, bipolar tetraether liposomes possess many unusual properties related to membrane aggregation and fusion that warrant further study.
In this study, we have focused on the calcium-induced aggregation of PLFE liposomes derived from the thermoacidophilic archaeon S. acidocaldarius. The PLFE fraction of S. acidocaldarius is equivalent to the P2 fraction of S. solfataricus (reviewed in Gliozzi et al. 2002). Aggregation was monitored in this study because it is the first step involved in membrane fusion and lipid mixing and because aggregation of bipolar tetraether liposomes has not been studied extensively. In the present study, dynamic light scattering revealed that, in the absence of Ca2+, there was little change in PLFE vesicle diameter over 6 months. Addition of sufficient amounts of Ca2+, however, induced immediate vesicle aggregation, which was largely reversible by EDTA. The initial rate of Ca2+-induced vesicle aggregation and the reversal of this aggregation by EDTA were examined at different temperatures, pH, and Ca2+ concentrations. The morphology of the aggregates was studied by freeze-fracture and confocal fluorescence microscopy. The temperature and pH dependencies of the threshold Ca2+ concentration for vesicle aggregation are discussed in relation to changes in membrane surface potential and lipid organization and conformation.
Materials and methods
Materials
Sulfolobus acidocaldarius cells (strain DSM639, ATCC, Rockville, MD) were grown aerobically and heterotrophically at 69–70 °C and pH 2.5–3.0. Growth was monitored by absorbance at 420 and 540 nm. Cells were harvested just before the stationary phase. Polar lipid fraction E lipids were isolated fromdry cells as previously described (Lo and Chang 1990).
1-Palmitoyl-2-oleoyl-L-α-phosphatidylcholine (POPC) and N-4-nitrobenzo-2-oxa-1,3-diazole phosphatidylethanolamine (N-NBD-PE) were purchased from Avanti Polar Lipids (Alabaster, AL) and 2-(p-toluidinyl)naphthalene-6-sulfonate (TNS) was obtained from Molecular Probes (Eugene, OR). Cholesterol (Sigma, St. Louis, MO) was recrystallized from ethanol.
Liposome preparation
For vesicles without fluorescent probe, PLFE was dispersed in 64:25:4 chloroform:methanol:water (v/v). The solvents were evaporated under nitrogen and the lipid was dried under high vacuum for ~12 h. Vesicles with the fluorescent probe N-NBD-PE were generated in two steps (Khan and Chong 2000). First, PLFE and N-NBD-PE were mixed in 64:25:4 chloroform:methanol:water (v/v), then dried as described above. Second, the dried lipid film was resuspended in 65:25:10 chloroform:methanol:water (v/v) and again dried under nitrogen followed by high vacuum for ~12 h.
To the dried PLFE film (with or without N-NBD-PE), appropriate amounts of either 100 mM citric acid/sodium citrate buffer (pH 3.0) or 50 mM KCl (pH 6.6) containing 0.02% NaN3 were added. The mixture was vigorously vortexed at 65 °C for 12 min to generate multilamellar vesicles (MLVs). Unilamellar vesicles were made from MLVs by the freeze–thaw and extrusion method (Hope et al. 1985). Briefly, MLVs were subjected to at least five cycles of freezing (dry ice/acetone) and thawing (65 °C). Vesicles were then extruded at 65 °C in a lipid extruder (Lipex, Vancouver, BC) through two stacked polycarbonate membranes (1 µm pore size for confocal experiments and 50, 100 or 200 nm pore sizes for the other experiments) under N2 gas pressure (400, 600 and 5000 kPa for pore sizes of 1 µm, 200 nm and < 200 nm, respectively). In N-NBD-PE-labeled PLFE liposomes, the ratio of probe to PLFE was 1:500.
Measurement of surface potential
To determine the surface potential of PLFE liposomes, the steady-state fluorescence intensity of the membrane probe TNS was measured. This probe is an anionic amphipathic molecule that binds strongly to lipid membranes (Huang and Charlton 1972, Easter et al. 1978, Lakowicz and Hogen 1981), mainly via van der Waals interactions (Seelig and Ganz 1991). In aqueous medium, TNS has a low quantum yield; however, upon binding to lipid vesicles, the fluorescence intensity of TNS (IL; dimensionless) is enhanced. Both IL and the concentration of TNS in the bulk solution ([TNS]b; µM) are related to membrane surface potential (Ψo; mV) according to Equation 1 (Eisenberg et al. 1979, Cafiso et al. 1989):
where β is a proportionality constant, F is the Faraday constant (9.65 × 104 C mol–1), R is the gas constant (8.3145 J mol–1 K–1), T is absolute temperature and [L] is lipid concentration (µM).
We measured IL as a function of [TNS]b at a fixed lipid concentration (16 µM). At constant [L], there is a linear relationship between IL and [TNS]b, and the slope of the plot of IL versus [TNS]b gives Ψo. Appropriate amounts of TNS were incubated with lipid vesicles at the desired temperature for 4–6 h prior to measurement of IL; thereafter, no further enhancement of fluorescence was observed. This experiment was performed with two lipid systems: PLFE unilamellar vesicles and POPC unilamellar vesicles containing 19 mol% cholesterol (prepared as described in Komatsu and Chong 1998). At neutral pH, the Ψo of the zwitterionic lipid POPC is zero. Then,
where IPFLE and IPOPC are the fluorescence intensities of TNS with PLFE and POPC liposomes, respectively, Ψo,PLFE is the surface potential of PLFE liposomes and [TNS]b,lipid is the bulk concentration of TNS when the designated lipid is used. The liposomes with probe were stirred while fluorescence intensity was measured with an SLM 8000C fluorometer (SLM Instruments, Urbana, IL). The excitation wavelength was 320 nm (2-nm band-pass) and the emission was observed between 380 and 560 nm (8-nm band-pass). Sample temperatures were maintained with a circulating bath. Blank readings (vesicles or TNS in buffer) were subtracted from the sample IL readings.
Photon correlation spectroscopy
The hydrodynamic diameters of the extruded PLFE vesicles were measured by photon correlation spectroscopy using a Malvern Zetasizer 1000HAS spectrometer (Malvern Instruments, Worcestershire, U.K.). The light source was a 10 mW He-Ne laser (633 nm) and the scattered light was measured at a right angle using an avalanche photodiode detector. The instrument was calibrated with 200-nm latex beads in 10 mM NaCl. The solution was filtered through a 0.22-µm syringe filter and degassed prior to measurements. Because very dilute solutions were used, refractive index and viscosity values for water were used for calculation of hydrodynamic diameters. Specifically, the refractive index was 1.33 at all temperatures examined, and the viscosity was 0.891, 0.653 and 0.488 cP (1 cP = 0.001 Pa s) at 25, 40 and 54 °C, respectively (Weast 1987). Data were analyzed with the Contin algorithm (Malvern Instruments), which calculates the Z-average size and polydispersity. The former is the mean vesicle hydrodynamic diameter and the latter is a measure of the width of the vesicle size distribution. To induce aggregation, various volumes of 204 mM CaCl2 were added to a solution of PLFE vesicles. To reverse aggregation, an appropriate amount of 100 mM EDTA was added.
Freeze-fracture electron microscopy
Samples without cryoprotectant were placed between copper specimen carriers (Balzers, Liechtenstein) and rapidly plunged into liquid propane. Fracturing and replicating was performed at –115 °C in a Balzers BAF 400T Freeze-Etch unit. Replicas were cleaned with NaHClO3 solution and viewed on a Philips 300 transmission electron microscope at a typical magnification of 27,000×.
Confocal fluorescence microscopy
Liposomes labeled with N-NBD-PE were incubated with or without Ca2+ and then adsorbed to a glass coverslip. Images were captured with a Model TCS-SP confocal laser scanning microscope (Leica Microsystems, Exton, PA) using an argon/krypton laser for excitation at 488 nm. Fluorescence (around ~534 nm) was detected in the photon counting mode as a single channel through a 100× oil-immersion lens.
Results and discussion
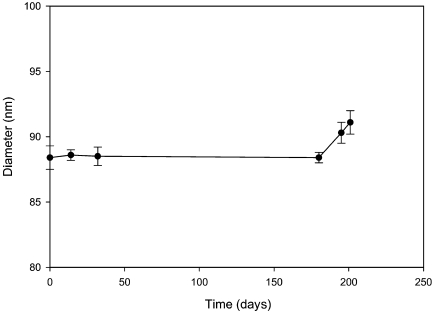
In this study, we investigated the kinetics of the change in size of PLFE SUVs as a function of Ca2+ concentration. The photon correlation spectroscopy data show that, in the absence of Ca2+, the mean diameter of PLFE SUVs in 50 mM KCl is stable for 6 months at room temperature (~24 °C) (Figure 2). Furthermore, the mean size of PLFE SUVs remained constant at temperatures ranging from 25 to 55 °C (data not shown).
Figure 2.
Vesicle size of PLFE small unilamellar vesicles as a function of storage time at room temperature in 50 mM KCl. The PLFE concentration was 0.05 mM.
The addition of Ca2+, however, had a pronounced effect on particle size. Figure 3 shows the effect of Ca2+ concentration on mean PLFE particle diameter over time in 50 mM KCl at 40 °C. At low Ca2+ concentrations (e.g., < 10.2 mM), PLFE unilamellar vesicles showed no appreciable change in size over time. However, at higher Ca2+ concentrations (e.g., 15.0– 25.1 mM), mean particle diameter increased over time (Figure 3). The vesicle size distribution also grew broader over time. The increase in particle size is attributable either to vesicle aggregation or to fusion or coalescence (lipid mixing). In either case, the change in size is presumably triggered by binding of Ca2+ to the negatively charged phospho-myo-inositol groups, followed by charge neutralization, complex formation and membrane structural changes (Wilschut et al. 1985). Figure 3 also shows that the rate of Ca2+-induced aggregation or fusion of PLFE liposomes is low (on the order of tens of minutes) compared with the rate of aggregation of negatively charged monopolar diester liposomes at comparable Ca2+ and lipid concentrations (on the order of seconds) (Sundler and Papahadjopoulos 1981).
Figure 3.
Effect of Ca2+ concentration (10.2–25.1 mM) on the time course of mean PLFE particle diameter in 50 mM KCl at 40 °C.
Confocal fluorescence microscopy also showed that, in the absence of Ca2+, the diameter of the extruded vesicles was relatively uniform (initially about 500 nm; Figure 4A). However, at high Ca2+ concentrations, vesicles formed aggregates with irregular shapes and heterogeneous sizes (Figure 4B). These observations are supported by the photon correlation spectroscopy data, which revealed an increase in size as well as a broadening of the size distribution. Note that the two studies used different vesicle preparations with different initial vesicle sizes.
Figure 4.
Confocal fluorescence images of N-NBD-PE-labeled PLFE large unilamellar vesicles in 50 mM KCl (A) without Ca2+ (mean vesicle diameter = 499.4 nm) and (B) with 35.3 mM Ca2+.
As shown in Table 1, the increase in PLFE particle size following incubation with 12.5 mM Ca2+ for 4 h at 25 °C was reversed (~98%) by addition of a twofold excess of EDTA (Wilschut et al. 1980, 1981), suggesting that little vesicle fusion had occurred. In conjunction with the decrease in size, the polydispersity also decreased (data not shown). Furthermore, the data in Table 1 demonstrate that the reversal of particle size change by EDTA is dependent on both temperature and the incubation time of the vesicles with Ca2+. At higher temperatures, and when the incubation time with Ca2+ is increased, the degree of reversal engendered by EDTA is decreased. These results strongly suggest that Ca2+ causes not only PLFE vesicle aggregation, but also some vesicle fusion or coalescence. However, if the Ca2+-induced size increase had been a result of fusion or coalescence only, the size increase would have been completely nonreversible by a twofold excess of EDTA (Leventis et al. 1986). These results are in agreement with previous findings that, at room temperature, Ca2+ induces aggregation, but not fusion, of vesicles made from the P2 lipid fraction from S. solfataricus (Relini et al. 1994, 1996). The PLFE fraction from S. acidocaldarius and the P2 fraction from S. solfataricus both consist primarily of bisubstituted tetraether lipids carrying negative charges at neutral pH.
Table 1.
Effect of EDTA on Ca2+-induced aggregation of PLFE vesicles.
| Temperature (°C) | Vesicle aggregation | Reversal of aggregation | |||||
| Initial particle | [Ca2+] (mM) | Incubation | Resulting particle | [EDTA] (mM) | Incubation | Final particle | |
| diameter (nm) | time (h) | diameter (nm) | time (h) | diameter (nm) | |||
| 25 | 117 | 12.5 | 4 | 1245 | 25 | 12 | 140 |
| 25 | 90 | 10.0 | 154 | 2545 | 20 | 10 | 180 |
| 40 | 89 | 19.6 | 120 | 2374 | 40 | 12 | 352 |
A possible explanation for the limited fusion of PLFE liposomes at 25 °C after a brief (4-h) incubation with Ca2+ is the rigid and tight membrane packing in these liposomes (Kao et al. 1992, Chang 1994, Komatsu and Chong 1998, Bagatolli et al. 2000, Gabriel and Chong 2000, Khan and Chong 2000). Vesicle fusion would require a membrane defect or destabilization (Papahadjopoulos et al. 1977, 1990, Hui et al. 1981). Another possibility is that the bulky hydrated inositol group sterically inhibits fusion by preventing the formation of dehydrated complexes between the apposed PLFE vesicles, as is the case with phosphatidylinositol membranes, which do not undergo fusion following Ca2+-induced aggregation (Sundler and Papahadjopoulos 1981). In addition, a greater amount of energy would be expected to be required for the molecular rearrangements necessary for fusion of liposomes composed of bipolar lipids, because one head group would be expected to completely traverse the lipid core. Coalescence of liposomes composed of bipolar lipids would not occur in the manner typically observed for fusion of liposomes composed of monopolar lipids, where single monolayers interact to form hemifusion intermediates (Ellens et al. 1989). Note that, in many monopolar diester liposomes, divalent cation-induced vesicle aggregation is followed by immediate membrane fusion, which cannot be reversed by addition of EDTA.
Fusion or coalescence may occur as a result of impurities in the PLFE (Chang and Lo 1991). An alternative explanation is that calcium ions induce lateral segregation of the bulky phospho-myo-inositol groups in the plane of the membrane. As a result, the less bulky polar head groups (i.e., those with nonitol and glucose) may become more readily susceptible to dehydration, leading to hydrophobic interactions between the apposed vesicles and eventually to fusion or lipid mixing. Lipid lateral reorganization could be a slow process, on the time scale of minutes to days; this may explain why incubation of PLFE vesicles with Ca2+ for a longer period of time lowers the reversibility of vesicle size change by EDTA (Table 1).
The conclusions drawn from the EDTA results are supported by freeze-fracture electron microscopy data (Figure 5). Figure 5A shows the freeze-fracture micrograph of PLFE unilamellar vesicles in the absence of Ca2+. The mean diameter of these vesicles was 84 nm. The liposomes were not aggregated and lacked a preferential membrane fracture plane, in agreement with previous results obtained with the P2 fraction from S. solfataricus (De Rosa et al. 1986, Gliozzi and Relini 1996). However, after incubation with 15.1 mM CaCl2 for 2 weeks, PLFE vesicles in 50 mM KCl had not just aggregated, they had fused or coalesced, as indicated by cross-fractured structures visible in the micrographs (Figure 5B). Cross-fractures would be anticipated where there are no bilayers, and thus no true fracture planes. Furthermore, the vesicles formed after incubation with CaCl2 did not consist of tight concentric layers. Our electron microscopy data are in agreement with observations made by Lo and Chang (1990), who looked at PLFE multilamellar vesicles. We initially observed unilamellar vesicles (Figure 5A), which coalesced to form multilamellar vesicles (multiple rings) after incubation with Ca2+ for about 2 weeks (Figure 5B). If the initial unilamellar PLFE vesicles had merely aggregated, not fused or coalesced, then we would have expected the vesicles in Figure 5B to look like those shown in Figure 5A, but at a higher density.
Figure 5.
Electron micrographs of freeze-fracture replicas of 500 µl of 0.4 mg ml–1 PLFE unilamellar vesicles in 50 mM KCl. (A) Vesicles without Ca2+; mean vesicle diameter ~84 nm. (B) Vesicles incubated with 15.1 mM Ca2+ for 2 weeks. Magnification = 46,000×.
The threshold Ca2+ concentration for PLFE vesicle aggregation can be determined from the photon correlation spectroscopy data. The first 15 min of data from the plot of mean vesicle diameter versus aggregation time (e.g., Figure 3) were fitted to a linear line, the slope of which gives the initial rate of vesicle aggregation. The initial rate of PLFE vesicle aggregation varied sigmoidally with Ca2+ concentration (Figure 6). The Ca2+ concentration corresponding to the midpoint of the abrupt change (50% change) in vesicle size is the threshold calcium concentration (Cr) for vesicle aggregation. We found that the Cr increased with increasing temperature, but decreased with increasing pH (Table 2).
Figure 6.
Plots of the initial rate of Ca2+-induced PLFE vesicle aggregation versus Ca2+ concentration ([Ca2+]) at 25 °C (filled circles), 40 °C (open circles) and 54 °C (inverted triangles) in 50 mM KCl (pH 6.6).
Table 2.
Effects of pH and temperature on the threshold Ca2+ concentration (Cr) for PLFE vesicle aggregation and on the surface potential (Ψo) of PLFE vesicles (117 nm in diameter) prior to aggregation.
| pH | Temperature (°C) | Cr (mM) | Ψo (mV) |
| 6.6 | 25 | 11 | –22.00 ± 0.003 |
| 6.6 | 40 | 17 | –13.20 ± 5.30 |
| 6.6 | 54 | 18 | –10.28 ± 3.49 |
| 3.0 | 25 | 25 | ND1 |
| 3.0 | 40 | 60 | ND |
1ND = not determined, because the TNS method works well only at neutral pH.
Because membrane surface charge may affect Ca2+ binding and thus change the value of Cr, we determined the temperature dependence of the membrane surface potential of PLFE vesicles using TNS fluorescence. The surface potential of PLFE SUVs at pH 6.6 and 25 °C was determined to be –22 mV (Table 2), which is comparable with the published zeta potential (–34.3 mV) for PLFE SUVs of similar sizes at pH 7.6 and 20 °C (Komatsu and Chong 1998). The surface potential of PLFE liposomes decreased (i.e., became less negative) with increasing temperature (Table 2). When the surface potential is less negative, more calcium ions are required to form stable complexes with PLFE vesicles. This explains why Cr increases with increasing temperature (Table 2). The same rationale explains why Cr increases with decreasing pH (Table 2). The negative charge on the phospho-myo-inositol group in PLFE lipids should be significantly lower at pH 3.0 than at pH 6.6, leading to a lower surface potential on PLFE liposomes and a higher Cr at pH 3.0 than at pH 6.6. This interpretation is consistent with the observed decrease in the threshold calcium concentration for lipid mixing with increasing phosphatidic acid (PA) content in PA–phosphatidylcholine mixtures (Leventis et al. 1986), as PA is negatively charged at neutral pH.
Temperature may induce changes in polar head group orientation, leading to changes in dipole potential. However, Cafiso et al. (1989) found that the absorption of TNS to monopolar lipid membranes is independent of the dipole potential of the lipid head group, presumably because the charge of TNS is located such that it is unaffected by a change in dipole potential. Therefore, it is unlikely that a change in lipid dipole potential is responsible for the change in surface potential with temperature (Table 2), unless the location of TNS in PLFE liposomes is different from its location in non-archaeal monopolar lipid membranes.
Another possible explanation for the decrease in surface potential with increasing temperature is that higher temperatures induce more phospho-myo-inositol groups to turn toward the intravesicular side of the membrane. This explanation would be consistent with the previous finding that, in vivo, the phospho-myo-inositol moieties of bipolar tetraether lipids mainly face the intracellular compartment of thermoacidophilic archaea at the high growth temperatures characteristic of these microorganisms (De Rosa et al. 1983). However, from an energetic point of view, flip-flop of PLFE lipids in tightly packed membranes would be difficult to achieve. Nevertheless, a change in lipid transmembrane asymmetry has previously been proposed to explain why the rate and temperature sensitivity of proton permeation across PLFE liposomal membranes are reduced as vesicle diameter decreases (Komatsu and Chong 1998). We cannot exclude the possibility that a temperature-induced change in lipid lateral organization (Bagatolli et al. 2000) (rather than transmembrane asymmetry) in the PLFE membrane affects TNS binding and fluorescence, thus changing the surface potential.
Archaeal bipolar tetraether liposomes have wide technological applications (Ring et al. 1986, Tomioka et al. 1994, Freisleben et al. 1995, Krishnan et al. 2000, Patel et al. 2000) because they are thermo- and acid-stable and resistant to mechanical stress and the actions of phospholipases, bile salts and serum proteins (reviewed in Gliozzi and Relini 1996). In a previous study (Komatsu and Chong 1998), we showed that small PLFE liposomes (diameter ~60 nm) exhibited lower proton permeability than large PLFE liposomes (diameter ~240 nm). Furthermore, the proton permeability of small PLFE liposomes is less sensitive to temperature than that of PLFE large unilamellar vesicles, changing by less than 2 × 10–10 cm s–1 from 25 to 82 °C (Komatsu and Chong 1998). The photon correlation spectroscopic data in this study (Figure 1) provide evidence that the size of PLFE SUVs (~87–90 nm in diameter) is extraordinarily stable for at least 6 months. All of these properties suggest that PLFE SUVs could be developed for applications such as drug delivery and bioassays. The high stability of PLFE SUVs should result in a long circulation time in vivo when these liposomes are used as drug or vaccine delivery vehicles. However, if the vesicles are extraordinarily stable, the entrapped materials cannot easily be released. According to our study, elevation of the local Ca2+ concentration in the target tissue could circumvent this problem by causing PLFE liposomes to undergo slow and limited vesicle fusion or coalescence to release the entrapped materials. The high Ca2+ concentration and long incubation time used in this study may be unrealistic for medical applications; however, mixtures of PLFE with phosphatidylethanolamines, which are known to facilitate membrane fusion and leakage, may enable controlled release of entrapped materials under physiological conditions. Whether aggregation, fusion or coalescence of PLFE liposomes occurs in the presence of other divalent cations or fusogenic agents has yet to be determined.
Acknowledgments
This work was supported in part by a grant from the NSF (MCB-9513669) and a grant from the U.S. Army Research Office (DAAD19-02-1-0077).
References
- R1.Bagatolli L., Gratton E., Khan T.P., Chong P.L.-G. Two-photon fluorescence microscopy studies of bipolar tetraether giant liposomes from thermoacidophilic archaebacteria Sulfolobus acidocaldarius . Biophys. J. 2000;79:416–425. doi: 10.1016/S0006-3495(00)76303-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R2.Bauer S., Heckmann K., Six L., Strobl C. Hyperfiltration through crosslinked monolayers. II. Desalination. 1983;46:369–378. [Google Scholar]
- R3.Bentz J., Duzgunes N. Fusogenic capacities of divalent cations and effect of liposome size. Biochemistry. 1985;24:5436–5443. doi: 10.1021/bi00341a023. [DOI] [PubMed] [Google Scholar]
- R4.Boni L.T., Hah J.S., Hui S.W., Mukherjee P., Ho J.T., Jung C.Y. Aggregation and fusion of unilamellar vesicles by poly(ethylene glycol) Biochim. Biophys. Acta. 1984;775:409–418. doi: 10.1016/0005-2736(84)90198-6. [DOI] [PubMed] [Google Scholar]
- R5.Brock T.D., Brock K.M., Belly R.T., Weiss R.L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- R6.Cafiso D., Mclaughlin A., Mclaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- R7.Chang E.L. Unusual thermal stability of liposomes made from bipolar tetraether lipids. Biochem. Biophys. Res. Commun. 1994;202:673–679. doi: 10.1006/bbrc.1994.1983. [DOI] [PubMed] [Google Scholar]
- R8.Chang E.L., Lo S.-L. Extraction and purification of tetraether lipids from Sulfolobus acidocaldarius . In: Fleischmann E.M., Place A.R., Robb R.T., Schreier H.J., editors. Maryland Biotechnology Institute, Univ. Maryland System, Baltimore, MD. Univ. Maryland System, Baltimore, MD: Maryland Biotechnology Institute; 1991. pp. 2.3.1–2.3.14. [Google Scholar]
- R9.Choquet C.G., Patel G.B., Beveridge T.J., Sprott G.D. Stability of pressure-extruded liposomes made from archaebacterial ether lipids. Appl. Microbiol. Biotechnol. 1994;42:375–384. doi: 10.1007/BF00902745. [DOI] [PubMed] [Google Scholar]
- R10.De Rosa M., Esposito E., Gambacorta A., Nicholaus B. Effects of temperature on ether lipid composition of Caldariella acidophila . Phytochemistry. 1980;19:827–831. [Google Scholar]
- R11.De Rosa M., Gambacorta A., Nicolaus B. A new type of cell membrane in thermophilic archaebacteria, based on bipolar ether lipids. J. Membrane Sci. 1983;16:287–294. [Google Scholar]
- R12.De Rosa M., Gambacorta A., Gliozzi A. Structure, biosynthesis and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R13.Easter J.H., Detoma R.P., Brand L. Fluorescence measurements of environmental relaxation at the lipid–water interface region of bilayer membranes. Biochim. Biophys. Acta. 1978;508:27–38. doi: 10.1016/0005-2736(78)90186-4. [DOI] [PubMed] [Google Scholar]
- R14.Eisenberg M., Gresalfi T., Riccio T., Mclaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979;18:5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- R15.Elferink M.G.L., De Wit J.G., Demel R., Driessen A.J.M., Konings W.N. Functional reconstitution of membrane proteins in monolayer liposomes from bipolar lipids of Sulfolobus acidocaldarius. J. Biol. Chem. 1992;267:1375–1381. [PubMed] [Google Scholar]
- R16.Elferink M.G.L., De Wit J.G., Driessen A.J.M., Konings W.N. Stability and proton-permeability of liposomes composed of archaeal tetraether lipids. Biochim. Biophys. Acta. 1994;1193:247–254. doi: 10.1016/0005-2736(94)90160-0. [DOI] [PubMed] [Google Scholar]
- R17.Ellens H., Siegel D.P., Alford D., Yeagle P.L., Boni L., Lis L.J., Quinn P.J., Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989;28:3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- R18.Freisleben H.-J., Bormann J., Litzinger D.C., Lehr F., Rudolph P., Schatton M., Huang L. Toxicity and biodistribution of liposomes of the main phospholipid from the archaebacterium Thermoplasma acidophilum . J. Liposome Res. 1995;5:215–223. [Google Scholar]
- R19.Gabriel J.L., Chong P.L.-G. Molecular modeling of archaebacterial bipolar tetraether liposomes by perylene fluorescence. Chem. Phys. Lipids. 2000;105:193–200. doi: 10.1016/s0009-3084(00)00126-2. [DOI] [PubMed] [Google Scholar]
- R20.Gliozzi A., Relini A. Lipid vesicles as model systems for archaea membranes. In: Barenholz Y., Lasic D.D., editors. Handbook of Nonmedical Applications of Liposomes. Boca Raton: Vol. II. CRC Press; 1996. pp. 329–348. [Google Scholar]
- R21.Gliozzi A., Relini A., Chong P.L.-G. Structure and permeability properties of biomimetic membranes of bolaform archaeal tetraether lipids. J. Membrane Sci. 2002;206:131–147. [Google Scholar]
- R22.Gulik A., Luzzati V., De Rosa M., Gambacorta A. Tetraether lipid components from a thermoacidophilic archaebacterium. J. Mol. Biol. 1988;201:429–435. doi: 10.1016/0022-2836(88)90149-0. [DOI] [PubMed] [Google Scholar]
- R23.Hope M.J., Bally M.B., Webb G., Cullis P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- R24.Huang C.H., Charlton J.P. Interactions of phosphatidylcholine vesicles with 2-p-toluidinylnaphthalene-6-sulfonate. Biochemistry. 1972;11:735–740. doi: 10.1021/bi00755a010. [DOI] [PubMed] [Google Scholar]
- R25.Hui S.W., Stewart T.P., Boni L.T., Yeagle P.L. Membrane fusion through point defects in bilayers. Science. 1981;212:921–923. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- R26.Hui S.W., Nir S., Stewart T.P., Boni L.T., Haung S.K. Kinetic measurements of fusion of phosphatidylserine-containing vesicles by electron microscopy and fluorometry. Biochim. Biophys. Acta. 1988;941:130–140. doi: 10.1016/0005-2736(88)90173-3. [DOI] [PubMed] [Google Scholar]
- R27.In’t Veld G., Driessen A.J.M., Konings W.N., Elferink M.G.L. Reconstitution of the leucine transport system of Lactococcus lactis into liposomes composed of membrane-spanning lipids from Sulfolobus acidocaldarius . Biochemistry. 1992;31:12493–12499. doi: 10.1021/bi00164a028. [DOI] [PubMed] [Google Scholar]
- R28.Kao Y.L., Chang E.L., Chong P.L.-G. Unusual pressure dependence of the lateral motions of pyrene-labeled phosphatidylcholine in bipolar lipid vesicles. Biochem. Biophys. Res. Commun. 1992;188:1241–1246. doi: 10.1016/0006-291x(92)91364-v. [DOI] [PubMed] [Google Scholar]
- R29.Kates M. Archaebacterial lipids: structure, biosynthesis and function. In: Danson M.J., Hough D.W., Lunt G.G., editors. The Archaebacteria: Biochemistry and Biotechnology. London: Portland Press; 1992. pp. 51–72. [PubMed] [Google Scholar]
- R30.Khan T.K., Chong P.L.-G. Studies of archaebacterial bipolar tetraether liposomes by perylene fluorescence. Biophys. J. 2000;78:1390–1399. doi: 10.1016/S0006-3495(00)76692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R31.Komatsu H., Chong P.L.-G. Low permeability of liposomal membranes composed of bipolar tetraether lipids from thermoacidophilic archaebacterium Sulfolobus acidocaldarius . Biochemistry. 1998;37:107–115. doi: 10.1021/bi972163e. [DOI] [PubMed] [Google Scholar]
- R32.Krishnan L., Dicaire C.J., Patel G.B., Sprott G.D. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect. Immun. 2000;68:54–63. doi: 10.1128/iai.68.1.54-63.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R33.Lakowicz J.R., Hogen D. Dynamic properties of the lipid–water interface of model membranes as revealed by lifetime-resolved fluorescence emission spectra. Biochemistry. 1981;20:1366–1373. doi: 10.1021/bi00508a051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R34.Langworthy T.A., Pond J.L. Membranes and lipids of thermophiles. In: Brock T.D., editor. Thermophiles: General, Molecular, and Applied Microbiology. New York: John Wiley & Sons; 1986. pp. 107–134. [Google Scholar]
- R35.Leventis R., Gagne J., Fuller N., Rand R.P., Silvius J.R. Divalent cation induced fusion and lipid lateral segregation in phosphatidylcholine-phosphatidic acid vesicles. Biochemistry. 1986;25:6978–6987. doi: 10.1021/bi00370a600. [DOI] [PubMed] [Google Scholar]
- R36.Lo S.-L., Chang E.L. Purification and characterization of a liposomal-forming tetraether lipid fraction. Biochem. Biophys. Res. Commun. 1990;167:238–243. doi: 10.1016/0006-291x(90)91756-i. [DOI] [PubMed] [Google Scholar]
- R37.Papahadjopoulos D., Vail W.J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim. Biophys. Acta. 1977;465:579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- R38.Papahadjopoulos D., Nir S., Duzgunes N. Molecular mechanisms of calcium-induced membrane fusion. J. Bioenerg. Biomembr. 1990;22:157–179. doi: 10.1007/BF00762944. [DOI] [PubMed] [Google Scholar]
- R39.Patel G.B., Agnew B.J., Deschatelets L., Fleming L.P., Sprott G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000;194:39–49. doi: 10.1016/s0378-5173(99)00331-2. [DOI] [PubMed] [Google Scholar]
- R40.Relini A., Cassinadri D., Mirghani Z., Brandt O., Gambacorta A., Trincone A., De Rosa M., Gliozzi A. Calcium-induced interaction and fusion of archaeobacterial lipid vesicles: a fluorescence study. Biochim. Biophys. Acta. 1994;1194:17–24. doi: 10.1016/0005-2736(94)90198-8. [DOI] [PubMed] [Google Scholar]
- R41.Relini A., Cassinadri D., Fan Q., Gulik A., Mirghani Z., De Rosa M., Gliozzi A. Effect of physical constraints on the mechanisms of membrane fusion: bolaform lipid vesicles as model systems. Biophys. J. 1996;71:1789–1795. doi: 10.1016/S0006-3495(96)79379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R42.Ring K., Henkel B., Valenteijn A., Gutermann R. Studies on the permeability and stability of liposomes derived from a membrane-spanning bipolar archaebacterial tetraether lipid. In: Schmidt K.H., editor. Liposomes as Drug Carriers. Stuttgart: Georg Thieme Verlag; 1986. pp. 100–123. [Google Scholar]
- R43.Seelig J., Ganz P. Nonclassical hydrophobic effect in membrane binding equilibria. Biochemistry. 1991;30:9354–9359. doi: 10.1021/bi00102a031. [DOI] [PubMed] [Google Scholar]
- R44.Sprott G.D. Structures of archaebacterial membrane lipids. J. Bioenerg. Biomembr. 1992;24:555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- R45.Sprott G.D., Tolson D.L., Patel G.B. Archaeosomes as novel antigen delivery systems. FEMS Microbiol. Lett. 1997;154:17–22. doi: 10.1111/j.1574-6968.1997.tb12618.x. [DOI] [PubMed] [Google Scholar]
- R46.Sugai A., Sakuma R., Fukuda I., Kurosawa N., Itoh Y.H., Kon K., Ando S., Itoh T. The structure of the core polyol of the ether lipids from Sulfolobus acidocaldarius . Lipids. 1995;30:339–344. doi: 10.1007/BF02536042. [DOI] [PubMed] [Google Scholar]
- R47.Sundler R., Papahadjopoulos D. Control of membrane fusion by phospholipid headgroups. I. Phosphatidate/phosphatidylinositol specificity. Biochim. Biophys. Acta. 1981;649:743–750. doi: 10.1016/0005-2736(81)90179-6. [DOI] [PubMed] [Google Scholar]
- R48.Tomioka K., Kii F., Fukuda H., Katoh S. Homogeneous immunoassay of antibody by use of liposomes made of a model lipid of archaebacteria. J. Immunol. Methods. 1994;176:1–7. doi: 10.1016/0022-1759(94)90345-x. [DOI] [PubMed] [Google Scholar]
- R49.van de Vossenberg J.L.C.M., Ubbink-Kok T., Elferink M.G.L., Driessen A.J.M., Konings W.N. Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol. Microbiol. 1995;18:925–932. doi: 10.1111/j.1365-2958.1995.18050925.x. [DOI] [PubMed] [Google Scholar]
- R50.Weast R.C. 68th Edn. . Boca Raton, FL: CRC Press; 1987. Handbook of chemistry and physics; pp. E-374–F-39. [Google Scholar]
- R51.Wilschut J., Duzgunes N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- R52.Wilschut J., Duzgunes N., Papahadjopoulos D. Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry. 1981;20:3126–3133. doi: 10.1021/bi00514a022. [DOI] [PubMed] [Google Scholar]
- R53.Wilschut J., Nir S., Scholma J., Hoekstra D. Kinetics of Ca2+-induced fusion of cardiolipin-phosphatidylcholine vesicles: correlation between vesicle aggregation, bilayer destabilization and fusion. Biochemistry. 1985;24:4630–4636. doi: 10.1021/bi00338a023. [DOI] [PubMed] [Google Scholar]