Abstract
The Methanocaldococcus jannaschii genome contains putative genes for all four nonoxidative pentose phosphate pathway enzymes. Open reading frame (ORF) MJ0960 is a member of the mipB/talC family of ‘transaldolase-like’ genes, so named because of their similarity to the well-characterized transaldolase B gene family. However, recently, it has been reported that both the mipB and the talC genes from Escherichia coli encode novel enzymes with fructose-6-phosphate aldolase activity, not transaldolase activity (Schürmann and Sprenger 2001). The same study reports that other members of the mipB/talC family appear to encode transaldolases. To confirm the function of MJ0960 and to clarify the presence of a nonoxidative pentose phosphate pathway in M. jannaschii, we have cloned ORF MJ0960 from M. jannaschii genomic DNA and purified the recombinant protein. MJ0960 encodes a transaldolase and displays no fructose-6-phosphate aldolase activity. It retained full activity for 4 h at 80 °C, and for 3 weeks at 25 °C. Methanocaldococcus jannaschii transaldolase has a maximal velocity (Vmax) of 1.0 ± 0.2 µmol min–1 mg–1 at 25 °C, whereas Vmax = 12.0 ± 0.5 µmol min–1 mg–1 at 50 °C. Apparent Michaelis constants at 50 °C were Km = 0.65 ± 0.09 mM for fructose-6-phosphate and Km = 27.8 ± 4.3 µM for erythrose-4-phosphate. When ribose-5-phosphate replaced erythrose-4-phosphate as an aldose acceptor, Vmax decreased twofold, whereas the Km was 150-fold higher. The molecular mass of the active enzyme is 271 ± 27 kDa as estimated by gel filtration, whereas the predicted monomer size is 23.96 kDa, suggesting that the native form of the protein is probably a decamer. A readily available source of thermophilic pentose phosphate pathway enzymes including transaldolase may have direct application in enzymatic biohydrogen production.
Keywords: biohydrogen, fructose-6-phosphate aldolase, fsa, mipB, pentose phosphate pathway, talC
Introduction
The pentose phosphate pathway (PPP) is a key series of enzymatic reactions, which in bacteria and eukaryotes, generates ribose-5-phosphate as a precursor in the biosynthesis of nucleotides. The pathway also generates NADPH for reductive biosynthesis and produces erythrose-4-phosphate, a precursor for the biosynthesis of aromatic amino acids. The PPP is generally divided into oxidative and nonoxidative branches. In the oxidative branch, glucose-6-phosphate is converted to ribulose-5-phosphate in a process that generates two equivalents of NADPH. The nonoxidative branch recycles excess ribulose-5-phosphate back into the glycolytic pathway, producing erythrose-4-phosphate in the process.
Although the genetic and biochemical details of the PPP are fairly well characterized in bacteria and eukaryotes, the pathway in archaea remains enigmatic. With the publication of an increasing number of complete archaeal genome sequences, it has become evident that these genomes are missing many of the recognized PPP genes (Table 1), indicating that either the ‘missing’ genes are of low sequence similarity to known PPP genes, or that alternate pathways exist that make these genes unnecessary (see Cordwell (1999) for a discussion of the implications of ‘missing’ genes in microbial genomes). Putative sequences for the nonoxidative branch enzymes transketolase, transaldolase and ribulose-5-phosphate epimerase have been identified in several archaeal genomes, but are missing from many others. All 16 archaeal genomes in The Institute for Genomic Research (TIGR) database contain a probable gene for the fourth nonoxidative branch enzyme, ribose-5-phosphate isomerase. Evidence from isotopic labeling studies suggests that the oxidative branch is active in some methanogenic species (Choquet et al. 1994), but sequences for these enzymes are missing from all of the known archaeal genomes with the exception of Halobacterium sp. NRC-1, which contains a putative gene for 6-phosphogluconate dehydrogenase. Other reports have suggested that, in methanococci, the oxidative branch is not operative and that the nonoxidative branch may be responsible solely for pentose synthesis (Choquet et al. 1994, Yu et al. 1994, Selkov et al. 1997, Tumbula et al. 1997). To date, only one archaeal PPP enzyme has been purified and characterized: recombinant ribose-5-phosphate isomerase from Pyrococcus horikoshii was recently crystallized and the three-dimensional structure reported (Ishikawa et al. 2002).
Table 1.
Distribution of putative genes for pentose phosphate enzymes across the completed archaeal genomes in the TIGR database (http:// www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl). Column 2 specifies whether the organism belongs to the Euryarchaeota or Crenarchaeota. Gene designations refer to TIGR locus numbers. The letter X denotes a genome that does not have a putative gene. Abbreviations: TAL = transaldolase; TK(N) = transketolase, N terminal subunit; TK(C) = transketolase, C terminal subunit; Ri5PI = ribose-5-phosphate isomerase; Ru5PE = ribulose-5-phosphate-3-epimerase; G6PDH = glucose-6-phosphate dehydrogenase; and 6GPDH = 6-phosphogluconate dehydrogenase.
| Genome | Eury/Cren | TAL | TK(N) | TK(C) | Ri5PI | Ru5PE | G6PDH | 6GPDH |
| A. fulgidus | E | X | X | X | AF0943 | X | X | X |
| Halobacterium sp. | E | X | X | X | NT01HS1713 | X | X | NT01HS1935 |
| M. acetivorans | E | X | X | X | NT02MA2464 | X | X | X |
| M. jannaschii | E | MJ0960 | MJ0681 | MJ0679 | MJ1603 | MJ0680 | X | X |
| M. kandleri | E | X | X | X | NT01MK1964 | X | X | X |
| M. mazei | E | X | X | X | NT01MM0099 | X | X | X |
| M. thermoautotrophicum | E | X | X | X | NT01MT0629 | X | X | X |
| P. abyssi | E | X | NT01PA0494 | NT01PA0495 | NT01PA0847 | X | X | X |
| P. furiosus | E | X | NT01PF1855 | NT01PF1856 | NT01PF1411 | X | X | X |
| P. horikoshii | E | X | X | X | NT01PH1400 | X | X | X |
| T. acidophilum | E | NT01TA0663 | NT01TA0665 | NT01TA0664 | NT01TA0965 | NT01TA1426 | X | X |
| T. volcanium | E | NT01TV0752 | NT01TV0754 | NT01TV0753 | NT01TV0847 | NT01TV0303 | X | X |
| A. pernix | C | X | NT01AP0460 | NT01AP0459 | NT01AP1510 | X | X | X |
| P. aerophilum | C | X | NT04PA1564 | NT04PA1563 | NT04PA0852 | X | X | X |
| S. solfataricus | C | X | NT02SS0302 | NT02SS0303 | NT02SS1038 | X | X | X |
| S. todokai | C | X | NT02ST2802 | NT02ST2803 | NT20ST1593 | X | X | X |
Only three of the 16 completed archaeal genomes (Methanocaldococcus jannaschii, Thermoplasma acidophilum and Thermoplasma volcanium) contain putative transaldolase genes. These appear to be members of a closely related family of genes encoding ‘transaldolase-like proteins’ that also exist in many bacterial genomes and include the Escherichia coli genes mipB and talC. The assignment of a transaldolase function is based upon the similarity of these sequences to transaldolase B, which has been extensively characterized (Sprenger et al. 1995, Schörken et al. 1998, 2001) and for which crystal structures have been reported for both E. coli (Jia et al. 1996, 1997) and human (Thorell et al. 2000) enzymes. Transaldolase B is approximately 100 amino acids longer than proteins in the ‘transaldolase-like’ family. Recently, the mipB ‘transaldolase-like’ gene was cloned from E. coli, the recombinant protein purified and characterized (Schürmann and Sprenger 2001), and the crystal structure reported (Thorell et al. 2002). The authors were surprised to discover that mipB encoded a fructose-6-phosphate aldolase rather than a transaldolase. They proposed renaming the protein FSA, a name we will use in this report. No aldol cleavage of fructose-6-phosphate had previously been reported from any organism. Like transaldolase, FSA cleaves fructose-6-phosphate into glyceraldehyde-3-phosphate and dihydroxyacetone. Whereas FSA releases both of these molecules as products, transaldolase releases only glyceraldehyde-3-phosphate and transfers the dihydroxyacetone group to an aldose acceptor, typically erythrose-4-phosphate (Figure 1). Thus, FSA does not require an aldose acceptor substrate for turnover to occur, whereas transaldolase does.
Figure 1.
Comparison of the probable reaction mechanisms of fructose-6-phosphate aldolase and transaldolase. Aldolase cleaves the fructose-6-phosphate substrate directly into glyceraldehyde-3-phosphate and dihydroxyacetone, whereas transaldolase requires an aldose substrate (such as erythrose-4-phosphate) to which it transfers the dihydroxyacetone group. Abbreviations: F6P = fructose-6-phosphate; GAP = glyceraldehyde-3-phosphate; E4P = erythrose-4-phosphate; S7P = sedoheptulose-7-phosphate; and DHA = dihydroxyacetone.
The authors reported that E. coli talC encodes a protein with fructose-6-phosphate aldolase activity rather than transaldolase activity. In addition, they reported data from experiments with crude cell extracts, suggesting that the fsa/talC homologs from the bacteria Bacillus subtilis and Thermotoga maritima encode transaldolases, not fructose-6-phosphate aldolases, despite their close homology to fsa from E. coli.
Thus, it appears that the ‘transaldolase-like’ family of proteins is actually two families, the fructose-6-phosphate aldolases and the ‘truncated transaldolases’ that share close sequence similarity to each other. This finding calls into doubt whether the putative transaldolase genes in M. jannaschii, T. acidophilum and T. volcanium actually are transaldolases at all, which further clouds our understanding of the role, if any, of the classical pentose phosphate pathway in archaea.
To determine the function of the ‘transaldolase-like’ gene in archaea, we cloned open reading frame (ORF) MJ0960 from M. jannaschii and expressed, purified and characterized the recombinant protein.
Materials and methods
Cloning of the transaldolase gene from M. jannaschii
Chromosomal DNA from M. jannaschii, obtained from the American Type Culture Collection, was used as a template for polymerase chain reaction (PCR) amplification (Saiki et al. 1988) of the transaldolase gene (TIGR locus MJ0960, GenBank accession no. NP_247955, http://www.ncbi.nlm.nih.gov/). Oligonucleotide primers (Integrated DNA Technologies, Coralville, IA) were 5′-CGGCCCATATGAAATTCT TCTTAGACACTGC-3′ (sense) and 5′-CCGCCGGATCCTT ATTTTCTACTCTTTAAG-3′ (antisense). Underlined bases are engineered restriction sites NdeI and BamHI, respectively, and italicized bases correspond to the start (sense primer) and stop (antisense primer) codons. The PCR reactions (total volume 50 µl) contained 50 ng chromosomal DNA template, 250 µM dNTPs (Promega, Madison, WI), 0.5 µM of each primer, 2.5 units of PfuTurbo DNA polymerase, and the PfuTurbo polymerase buffer (Stratagene, La Jolla, CA). The reaction mixture was incubated in a PTC-100 Peltier Thermocycler (MJ Research, Waltham, MA) at 96 °C for 5 min, cycled 30 times at 96 °C for 1 min, 45 °C for 1 min, and 72 °C for 5 min, held at 72 °C for 10 min, then cooled to 4 °C. The 670 bp PCR product was separated on a low melting point agarose gel, excised, and the DNA extracted with the Wizard PCR Prep Kit following the manufacturer’s protocols. The purified DNA was digested with Nde1 and BamH1 (New England Biolabs, Beverly, MA), then ligated into a pET-11b expression vector (Stratagene) that had been digested with the same two restriction enzymes, dephosphorylated with calf intestinal alkaline phosphatase, and gel purified (Sambrook and Russell 2001). Subcloning efficiency E. coli DH5α competent cells were transformed with the ligation mixture and grown on an LB agar plate containing ampicillin (Sigma-Aldrich, St. Louis, MO). Resulting clones were checked for the presence of inserts by restriction analysis, and inserts were sequenced (Advanced Genetic Analysis Center, University of Minnesota) to confirm the fidelity of the PCR reaction. Plasmid DNA from one of the successful clones was named pTJS-I-24.
Overproduction and purification of recombinant M. jannaschii transaldolase
Competent cells of E. coli expression strain BL21-Codon Plus (DE3) RIL (Stratagene) were transformed with plasmid pTJS-I-24. Isolated colonies from LB agar plates containing ampicillin were used to inoculate 5 ml cultures of LB containing 100 µg ml–1 ampicillin and 30 µg ml–1 chloramphenicol. After approximately 12 h of growth, these starter cultures were used to inoculate 500 ml LB cultures containing 100 µg ml–1 ampicillin and 30 µg ml–1 chloramphenicol. The cultures were grown at 37 °C in 2-liter flasks with rotary shaking at 225 rpm. Cell growth was monitored by measuring optical density at 600 nm (OD600), and expression was induced at approximately OD600 = 0.9 with the addition of isopropyl β-d-thioglucopyranoside (IPTG) to a final concentration of 0.5 mM. The cultures were grown for an additional 4–5 h at 30 °C, then harvested by centrifugation and stored at –20 or –80 °C.
All chromatography was performed at room temperature with a Biologic LP low pressure chromatography system from Bio-Rad (Hercules, CA). Escherichia coli cells were resuspended in a buffer containing 50 mM Tris pH 8.0, 5% glycerol, and 1 mM phenylmethanesulfonyl fluoride, then disrupted by sonication using a Sonifier cell disruptor (3 × 30 s at output level 3) (Heat Systems Ultrasonic, Plainview, NY), and cell debris was cleared by centrifugation. Crude soluble cell extracts were incubated at 70 °C for 20 min and precipitated protein was pelleted by centrifugation. The clarified extract was loaded onto a 2.5 × 20 cm column containing DE52 anion exchange resin (Whatman, Maidstone, Kent, U.K.) equilibrated with a buffer containing 50 mM Tris pH 8.0 and 5% glycerol, and the column was rinsed with the same buffer until the UV absorbance reached baseline. Protein was eluted with a 400 ml linear gradient of 0–600 mM KCl in the same Tris/glycerol buffer at a flow rate of 1 ml min–1. Active fractions were pooled, dialyzed against 50 mM Tris, pH 8.0, loaded onto the DE52 column again, and eluted with the identical KCl gradient. Active fractions were once again pooled, dialyzed against 50 mM Tris, pH 8.0, and loaded onto a 1 × 5 cm DE52 column for concentration. Protein was eluted with a high salt buffer (50 mM Tris, pH 8.0, 1M KCl, 5% glycerol), and fractions containing the concentrated protein were pooled and dialyzed against 50 mM Tris, pH 8.0. The protein solution was brought to 10% glycerol, incubated at 80 °C for 30 minutes, then stored at –20 °C.
Gel filtration chromatography
A 1 ml sample of purified protein at a concentration of 0.5 mg ml–1 in a buffer of 50 mM Tris, pH 8.0 and 10% glycerol was loaded onto two 1 × 45 cm gel filtration columns (Sephacryl 300-HR, Sigma-Aldrich) connected in series. The column was eluted at 1 ml min–1 with a buffer containing 50 mM Tris, pH 8.0 plus 100 mM KCl. Protein molecular mass standards were carbonic anhydrase (29 kDa), bovine serum albumin (66 kDa), alcohol dehydrogenase (150 kDa), β-amylase (200 kDa), apoferritin (443 kDa) and thyroglobulin (669 kDa) (Sigma-Aldrich). The void volume of the column was defined as the elution volume of blue dextran.
Transaldolase assays
The standard transaldolase coupled assay (Tsolas and Joris 1964) was carried out, in which glyceraldehyde-3-phosphate (produced by transaldolase in a reaction with fructose-6-phosphate and an aldose acceptor, generally erythrose-4-phosphate) is converted first to dihydroxyacetone phosphate by triosephosphate isomerase and then reduced to glycerol phosphate by α-glycerophosphate dehydrogenase. This occurs with the concomitant oxidation of NADH to NAD+, which can be followed spectrophotometrically by observing the decrease in the characteristic absorbance of NADH at 340 nm (ε = 6290 M–1 cm–1).
In general, transaldolase activity assays were carried out at 25 or 50 °C in 50 mM imidazole, pH 7.5, with 5 mM fructose-6-phosphate, 100 µM erythrose-4-phosphate, 100 µM NADH, and 5 units each of the auxiliary enzymes triosephosphate isomerase and glycerol phosphate dehydrogenase (Sigma-Aldrich). The 1-ml reactions were each run in a standard 1 cm path length quartz cuvette, and absorbance at 340 nm (A340) was monitored by a Beckman DU-50 spectrophotometer (Beckman Coulter, Fullerton, CA). The reactions were initiated by addition of transaldolase, generally in a quantity sufficient to observe a ΔA340 of approximately –0.010 to –0.020 min–1. Reactions were allowed to run for 3–5 min. Control reactions were run in an identical manner except that the enzyme storage buffer was substituted for enzyme solution. To ensure that initial rates were being measured, absorbance was monitored every 30 s and the values plotted to check for linearity. In addition, the quantity of transaldolase was adjusted to ensure that less than 15% of the limiting substrate was consumed.
To confirm that auxiliary enzymes were being used at saturating levels, reactions were run with half and double the amount of auxiliary enzymes. In addition, the activity of the auxiliary enzyme mixture was measured directly in 1-ml reactions containing 50 mM imidazole, pH 7.5, 1.5 mM dihydroxyacetone phosphate and 0.1 mM NADH. At 25 °C, the rate of NADH oxidation over a 5 min period was approximately 100 times higher than the rate observed in the transaldolase assay, whereas at 50 °C, the rate was about 400 times higher.
The requirement for acceptor substrate was tested by combining all reaction components at 25 °C, except erythrose-4-phosphate and transaldolase. Transaldolase (1.8 µg) was added and the A340 value recorded every 30 s for 5 min. At this point, erythrose-4-phosphate was added (10 µl of a 10 mM stock solution) and absorbance readings were recorded for another 7 min.
Enzyme stability tests were performed by incubating 100 µl aliquots of the enzyme in enzyme storage buffer (50 mM Tris, pH 8.0, 10% glycerol) in 0.67 ml microcentrifuge tubes at the desired temperature for varying lengths of time, after which the samples were stored at –20 °C until they could be assayed together for activity.
The pH activity profile was determined at 25 °C using five different buffers, each at 50 mM: sodium acetate (pH 5.0, 5.8), sodium PIPES (pH 5.8, 6.2), imidazole chloride (pH 6.2, 6.5, 7.0, 7.5), sodium HEPES (pH 7.5, 8.0), Tris chloride (pH 8.0, 8.5, 9.0) and sodium CHES (pH 9.0, 9.5, 10.0) (Sigma-Aldrich).
The apparent Michaelis constant for fructose-6-phosphate was determined by measuring initial rates with 150 μM erythrose-4-phosphate and concentrations of fructose-6-phosphate from 100 µM to 10 mM. The apparent Michaelis constants for erythrose-4-phosphate and ribose-5-phosphate were determined by measuring initial rates with 5 mM fructose-6-phosphate and varying concentrations of erythrose-4-phosphate (10–200 µM) or ribose-5-phosphate (0.5–30 mM). The experiments were performed in triplicate, and kinetic constants were determined by nonlinear regression fit to the Michaelis-Menten equation using KaleidaGraph (Synergy Software, Reading, PA).
Results
Cloning and expression of the M. jannaschii transaldolase gene
The transaldolase gene (TIGR locus MJ0960) in M. jannaschii was amplified by PCR (Saiki et al. 1988) with PfuTurbo DNA polymerase and short oligonucleotide primers in which unique restriction sites were engineered. The 670 bp PCR fragment was ligated as an Nde1–BamH1 cassette into the pET-11b expression vector. The pET-11b plasmid containing the MJ0960 insert (pTJS-I-24) was isolated and the insert region was found to have the same sequence as that of the target gene in the TIGR and NCBI databases.
An SDS-PAGE gel of crude cell extract from E. coli BL21 cells transformed with pTJS-I-24 and induced with IPTG showed a heavy band at approximately 24 kDa (Figure 2).
Figure 2.
SDS-PAGE analysis of purified M. jannaschii transaldolase. Lane 1: 10 kDa protein molecular mass marker. Lane 2: Crude extract. Lane 3: Crude extract after heat treatment. Lane 4: DE52-purified transaldolase.
Data on the purification of M. jannaschii transaldolase is compiled in Table 2. Incubation of clarified cell extract at 70 °C for 20 min followed by centrifugation resulted in the formation of a pellet of heat-precipitated protein and a significant reduction in total soluble protein. Chromatography on DE52 anion exchange resin with a KCl gradient of 0–600 mM resulted in a peak containing transaldolase activity centered at approximately 250 mM KCl. However, SDS-PAGE analysis of this fraction revealed that the sample was contaminated with protein from overlapping peaks (data not shown). Therefore, the sample was chromatographed again on the same DE52 column, and the resulting protein was found to be pure by SDS-PAGE analysis (Figure 2). The yield from a 1-liter culture of cells (5.5 g wet cells) was approximately 7.8 mg of pure protein, with a total activity of 8.6 units at 25 °C and 103 units at 50 °C (one unit is defined as the amount of protein that will convert 1 µmol of fructose-6-phosphate to glyceraldehyde-3-phosphate in 1 min).
Table 2.
Purification data for M. jannaschii transaldolase. Activity was assayed at 25 °C. Abbreviation: ND = not determined (activity of crude extract before heat treatment was not determined due to significant amounts of E. coli transaldolase activity).
| Sample | Total protein (mg) | Total activity (µmol min–1) | Specific activity (µmol min–1 mg–1) | Yield (%) |
| Crude | 106 | ND | ND | ND |
| Crude-heat treated | 35 | 16.8 | 0.48 | 100 |
| DE52 (1) | 15.3 | 13.3 | 0.87 | 67 |
| DE52 (2) | 7.8 | 8.6 | 1.1 | 51 |
Identification of enzyme activity
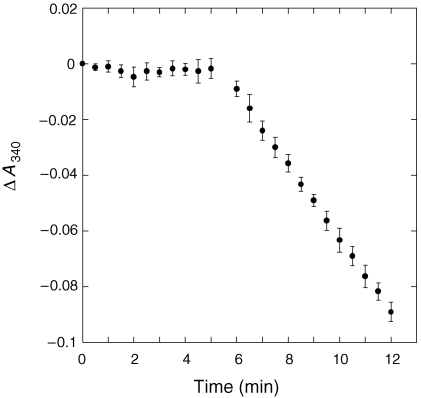
One of the key questions of this study was whether the ORF at TIGR locus MJ0960 encodes transaldolase, as annotated in the genomic databases, or fructose-6-phosphate aldolase, as suggested by its close homology to the recently characterized fsa gene from E. coli. Although the two reactions are similar, fructose-6-phosphate aldolase does not require an aldose acceptor substrate for turnover to occur, whereas transaldolase does (Figure 1). The protein encoded by MJ0960 was shown to have transaldolase activity and did not act as a fructose-6-phosphate aldolase (Figure 3). Without an acceptor substrate (erythrose-4-phosphate or ribose-5-phosphate in this study), enzyme turnover was not observed. Even when 18 µg enzyme was used (50 times more than in the typical transaldolase assay) in a test for aldolase activity at 50 °C, the resulting ΔA340 was at background level, meaning aldolase activity was not observed above a detection limit of approximately 0.01 µmol min–1 mg–1. Turnover began immediately upon addition of acceptor substrate.
Figure 3.
Requirement for an acceptor substrate for enzyme turnover. Each value represents the mean of triplicate assays. Erythrose-4-phosphate was added at the 5 min mark. See Materials and methods for details.
It was necessary to ensure that any enzyme activity being observed was that of the recombinant archaeal protein rather than that of the E. coli host. Crude cell extracts from a control culture transformed with the pET-11b plasmid (no insert) did contain measurable transaldolase activity. However this activity was irreversibly inactivated (to a detection limit of approximately 0.01 µmol min–1 mg–1) by incubating the extract at 80 °C for 20 min. In contrast, crude extract from the MJ0960-containing strain retained transaldolase activity after prolonged heat treatment, and the purified recombinant enzyme was shown to be stable at 80 °C (see below).
Properties of M. jannaschii transaldolase
Because M. jannaschii cultures grow optimally at 85 °C (Jones et al. 1983), it was not surprising to observe that the recombinant transaldolase was stable at high temperatures. No activity was lost after incubation at 80 °C for 4 h, and after 24 h, the enzyme still retained approximately 50% of its original activity. The enzyme was also extremely stable at 25 °C, retaining all of its activity after 3 weeks. The protein was less stable at higher temperatures, losing approximately 20% of its activity after 15 min at 90 °C and approximately 50% after 15 min at 100 °C.
The molecular mass of the recombinant enzyme under nondenaturing conditions was estimated to be 271 ± 27 kDa by gel filtration with standards of known molecular mass. This is 10–12 times the calculated molecular mass of the transaldolase monomer (23.96 kDa). Given its close similarity to the decameric FSA from E. coli (Thorell et al. 2002), the active form of the enzyme is most likely a decamer.
The enzyme was assayed with various buffers over a wide pH range. A plateau was observed at pH 7.0–8.5, with activity dropping to 83 ± 2, 44 ± 3 and 11 ± 2% at pH = 9.0, 9.5 and 10.0, respectively. At lower pH ranges, activity dropped to 60 ± 2, 50 ± 8 and 7 ± 1% at pH = 6.5, 6.2 and 5.8, respectively. The enzyme showed similar activity in its optimal pH range with imidazole, HEPES and Tris buffers.
The enzyme displayed classical saturating kinetic behavior with its substrates fructose-6-phosphate and erythrose-4-phosphate. The maximum velocity (Vmax) at 25 °C was 1.0 ± 0.2 µmol min–1 mg–1, corresponding to kcat = 24 min–1 per monomer, whereas at 50 °C, Vmax = 12.0 ± 0.5 µmol min–1 mg–1. Apparent Michaelis constants at 25 °C were Km = 0.64 ± 0.04 mM for fructose-6-phosphate and Km = 15.6 ± 2.8 µM for erythrose-4-phosphate. The results were similar at 50 °C: Km = 0.65 ± 0.09 mM for fructose-6-phosphate and Km = 27.8 ± 4.3 µM for erythrose-4-phosphate. Ribose-5-phosphate was also tested as an aldose acceptor in place of erythrose-4-phosphate. For the reaction with fructose-6-phosphate and ribose-5-phosphate at 25 °C, Vmax = 0.52 ± 0.03 µmol min–1 mg–1 and Km for ribose-5-phosphate was 2.2 ± 0.2mM. This value is based upon nonlinear regression fit to the Michaelis-Menten equation with ribose-5-phosphate concentrations ranging from 0.5 to 15 mM. At concentrations higher than 15 mM, the reaction rate decreased.
Amino acid sequence analysis
A BLASTP search with the M. jannaschii transaldolase sequence against the NCBI protein database yielded a set of 40 sequences with expectation values ranging from 10–79 to 10–49, followed by a sharp transition to sequences with expectation values of 10–37 and higher. The first set included ORF TM0295 from Thermotoga maritima and ORF NT01BS4693 from Bacillus subtilis, both of which are reported to encode transaldolase based on experiments with crude cell extracts (Schürmann and Sprenger 2001). Compared with the MJ0960 protein, the E. coli FSA and TalC sequences have expectation values of 10–32 and 10–30, respectively. A BLASTP search with the E. coli FSA sequence yielded five sequences, including E. coli TalC, with expectation values ranging from 10–72 to 10–49, followed by a sharp transition to sequences with expectation values of 10–32 and above. Compared with the longer TalB protein from E. coli, MJ0960 has an expectation value of 10–17, whereas FSA and TalC from E. coli have expectation values of 10–5 and 10–7, respectively. These data confirm the suggestion made by Schürmann and Sprenger (2001) that, in bacteria and archaea, there is a family of ‘truncated transaldolases,’ of which the MJ0960 protein is a member, and a separate family of fructose-6-phosphate aldolases, characterized by FSA and TalC from E. coli. None of the genomes in the NCBI database contained genes for members of both families.
A comparison of multiple sequence alignments (ClustalW Version 1.8, Thompson et al. 1994) (Figure 4) revealed several amino acids that are uniquely conserved in the transaldolase group and that are likely to be located in or near the enzyme’s active site, based on the similarity of the transaldolases to E. coli FSA, for which the three-dimensional structure is known (Thorell et al. 2002). The results are the same as those reported by Thorell and coworkers (2002) in a similar analysis using a smaller sequence set, with the addition of Gly131 and His170 of the M. jannaschii protein. These residues will be interesting targets for site-directed mutagenesis analysis of the determinants of FSA versus transaldolase activity.
Figure 4.
Alignment of M. jannaschii transaldolase (MJ0960) and E. coli FSA (Ec FSA) sequences. Conserved residues among 40 transaldolase and six FSA sequences are in bold, with conservative substitutions in parentheses above or below the sequence. The regions corresponding to the eight β sheets of the FSA α/β barrel structure (Thorell et al. 2002) are underlined. Arrows indicate conserved residues that are unique to the transaldolase sequences and that are in or near the probable active site of the enzyme.
Discussion
In this study, we cloned ORF MJ0960 from M. jannaschii, the function of which had been placed in doubt by the recent report that a close homolog in E. coli encodes an enzyme with novel aldolase activity (Schürmann and Sprenger 2001). We show here that the MJ0960 gene product is in fact a transaldolase and not a fructose-6-phosphate aldolase like the FSA protein in E. coli. This supports the hypothesis that a complete nonoxidative pentose phosphate pathway is operative in M. jannaschii and at least two other archaeal species, T. acidophilum and T. volcanium.
In this study, the standard coupled assay for transaldolase activity described by Tsolas and Joris (1964) was used, at reaction temperatures of 25 and 50 °C, well below the probable optimum of approximately 80–85 °C for an M. jannaschii enzyme. Unfortunately, this assay is not suitable for higher temperatures because of the use of mesophilic auxiliary enzymes and the highly heat-labile substrate, glyceraldehyde-3-phosphate. Nevertheless, transaldolase activity specific to the recombinant MJ0960 gene product was conclusively demonstrated, and kinetic constants were measured at 25 and 50 °C with reproducible results. Efforts are underway to develop a continuous high temperature assay using the recombinant enzyme encoded by MJ1411, the M. jannaschii homolog of a Thermoproteus tenax glyceraldehyde-3-phosphate dehydrogenase recently characterized by Brunner et al. (1998).
Heat-stable transaldolase activity was shown to be present in the crude extract of an E. coli strain engineered to express the MJ0960 gene, and the enzyme was purified by heat treatment and anion exchange chromatography. The purified enzyme displayed an absolute requirement for the presence of an aldose acceptor substrate in the reaction mixture in order for turnover to occur (Figure 3), confirming its function as a transaldolase rather than a fructose-6-phosphate aldolase.
Recombinant transaldolase from M. jannaschii displayed robust heat stability, retaining full activity for at least 4 h at 80 °C and for at least 3 weeks at room temperature. A thermostable carbon–carbon bond-forming enzyme such as transaldolase may have practical applications. In a general sense, enzymes from extremophilic organisms are attracting continued interest for their inherent stability in harsh conditions (Niehaus et al. 1999, Bruins et al. 2001, Haki and Rakshit 2003). More specifically, thermostable pentose phosphate enzymes could greatly increase the efficiency of a hydrogen bioproduction procedure that employs a novel archaeal hydrogenase (Woodward et al. 2000).
The Michaelis constants measured for the archaeal enzyme were somewhat lower than those reported for E. coli transaldolase B (Sprenger et al. 1995): Km for erythrose-4-phosphate was 16% and Km for fructose-6-phosphate was 53% of the respective values for the bacterial enzyme. The lower Km values may be an indication of lower intracellular substrate concentrations, as well as a reflection of a primarily anabolic function for the enzyme in M. jannaschii.
Gel filtration experiments with molecular mass standards indicated that the native protein is probably a decamer, correlating well with the known decameric structure of E. coli FSA (Thorell et al. 2002). This, along with the finding that many highly conserved amino acids from the “truncated transaldolase” subfamily can be mapped to the active site pocket of the FSA crystal structure (Thorell et al. 2002), strongly suggests that the two proteins are closely related not only in catalytic strategy and primary structure, but in three-dimensional structure as well. This enzyme pair has already been recognized (Thorell et al. 2002) as an interesting example of how small structural changes can determine different catalytic outcomes.
The positive identification of the MJ0960 gene product as a transaldolase also adds credence to the hypothesis that at least three of the 16 archaeal species for which complete genomes are currently available have operative nonoxidative pentose phosphate pathways. Other archaeal genomes, however, are missing recognizable genes for the nonoxidative branch, and oxidative branch genes are virtually nonexistent in archaea (Table 1). It remains to be seen whether analogous enzymes with novel structures exist in these species, or whether the classical roles of the pentose phosphate pathway, generation of NADH and the biosynthetic building blocks for nucleotides and aromatic amino acids, are filled by other biochemical players.
Acknowledgments
This work was supported by a grant from the Cottrell College Science Awards program of the Research Corporation.
References
- R1.Bruins M.E., Janssen A.E.M., Boom R.M. Thermozymes and their applications: a review of recent literature and patents. Appl. Biochem. Biotechnol. 2001;90:155–186. doi: 10.1385/abab:90:2:155. [DOI] [PubMed] [Google Scholar]
- R2.Brunner N.A., Brinkmann H., Siebers B., Hensel R. NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase from Thermoproteustenax . J. Biol. Chem. 1998;273:6149–6156. doi: 10.1074/jbc.273.11.6149. [DOI] [PubMed] [Google Scholar]
- R3.Choquet C.G., Richards J.C., Patel G.B., Sprott G.D. Ribose biosynthesis in methanogenic bacteria. Arch. Microbiol. 1994;161:481–488. [Google Scholar]
- R4.Cordwell S.J. Microbial genomes and “missing” enzymes: redefining biochemical pathways. Arch. Microbiol. 1999;172:269–279. doi: 10.1007/s002030050780. [DOI] [PubMed] [Google Scholar]
- R5.Haki G.D., Rakshit S.K. Developments in industrially important thermostable enzymes: a review. Bioresour. Technol. 2003;89:17–34. doi: 10.1016/s0960-8524(03)00033-6. [DOI] [PubMed] [Google Scholar]
- R6.Ishikawa K., Matsui I., Payan F., Cambillau C., Ishida H., Kawarabayasi Y., Kikuchi H., Roussel A. A hyperthermostable D-ribose-5-phosphate isomerase from Pyrococcus horikoshii: characterization and three-dimensional structure. Structure. 2002;10:877–886. doi: 10.1016/s0969-2126(02)00779-7. [DOI] [PubMed] [Google Scholar]
- R7.Jia J., Huang W., Schörken U., Sahm H., Sprenger G.A., Lindqvist Y., Schneider G. Crystal structure of transaldolase B from Escherichia coli suggests a circular permutation of the alpha/beta barrel within the class I aldolase family. Structure. 1996;4:715–724. doi: 10.1016/s0969-2126(96)00077-9. [DOI] [PubMed] [Google Scholar]
- R8.Jia J., Schörken U., Lindqvist Y., Sprenger G.A., Schneider G. Crystal structure of the reduced Schiff-base intermediate complex of transaldolase B from Escherichia coli: mechanistic implications for class I aldolases. Protein Sci. 1997;6:119–124. doi: 10.1002/pro.5560060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R9.Jones W.J., Leigh J.A., Mayer F., Woese C.R., Wolfe R.S. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 1983;136:254–261. [Google Scholar]
- R10.Niehaus F., Bertoldo C., Kahler M., Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999;51:711–729. doi: 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- R11.Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T., Mullis K.B., Erlich H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- R12.Sambrook J., Russell D.W. 3rd Edn. . Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning: a laboratory manual. [Google Scholar]
- R13.Schörken U., Jia J., Sahm H., Sprenger G.A., Schneider G. Disruption of Escherichia coli transaldolase into catalytically active monomers: evidence against half-of-the-sites mechanism. FEBS Lett. 1998;441:247–250. doi: 10.1016/s0014-5793(98)01521-x. [DOI] [PubMed] [Google Scholar]
- R14.Schörken U., Thorell S., Schürmann M., Jia J., Sprenger G.A., Schneider G. Identification of catalytically important residues in the active site of Escherichia coli transaldolase. Eur. J. Biochem. 2001;268:2408–2415. doi: 10.1046/j.1432-1327.2001.02128.x. [DOI] [PubMed] [Google Scholar]
- R15.Schürmann M., Sprenger G.A. Fructose-6-phosphate aldolase is a novel Class I aldolase from Escherichia coli and is related to a novel group of bacterial transaldolases. J. Biol. Chem. 2001;276:11055–11061. doi: 10.1074/jbc.M008061200. [DOI] [PubMed] [Google Scholar]
- R16.Selkov E., Maltsev N., Olsen G.J., Overbeek R., Whitman W.B. A reconstruction of the metabolism of Methanococcus jannaschii from sequence data. Gene. 1997;197:GC11–26. doi: 10.1016/s0378-1119(97)00307-7. [DOI] [PubMed] [Google Scholar]
- R17.Sprenger G.A., Schörken U., Sprenger G., Sahm H. Transaldolase B of Escherichia coli K-12: cloning of its gene, talB, and characterization of the enzyme from recombinant strains. J. Bacteriol. 1995;177:5930–5936. doi: 10.1128/jb.177.20.5930-5936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R18.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R19.Thorell S., Gergely P., Banki K., Perl A., Schneider G. The three-dimensional structure of human transaldolase. FEBS Lett. 2000;475:205–208. doi: 10.1016/s0014-5793(00)01658-6. [DOI] [PubMed] [Google Scholar]
- R20.Thorell S., Schürmann M., Sprenger G.A., Schneider G. Crystal structure of decameric fructose-6-phosphate aldolase from Escherichia coli reveals inter-subunit helix swapping as a structural basis for assembly differences in the transaldolase family. J. Mol. Biol. 2002;319:161–171. doi: 10.1016/S0022-2836(02)00258-9. [DOI] [PubMed] [Google Scholar]
- R21.Tsolas O., Joris L. Transaldolase. In: Boyer P.D., editor. The Enzymes. New York: Vol. 7. 3rd Edn. Academic Press; 1964. pp. 259–280. [Google Scholar]
- R22.Tumbula D.L., Teng Q., Bartlett M.G., Whitman W.B. Ribose biosynthesis and evidence for an alternative first step in the common aromatic amino acid pathway in Methanococcus maripaludis . J. Bacteriol. 1997;179:6010–6013. doi: 10.1128/jb.179.19.6010-6013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R23.Woodward J., Orr M., Cordray K., Greenbaum E. Enzymatic production of biohydrogen. Nature. 2000;405:1014–1015. doi: 10.1038/35016633. [DOI] [PubMed] [Google Scholar]
- R24.Yu J.P., Lapado J., Whitman W.B. Pathway of glycogen metabolism in Methanococcus maripaludis . J. Bacteriol. 1994;176:325–332. doi: 10.1128/jb.176.2.325-332.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]