Abstract
The lipid composition of the extremely halophilic archaeon Haloquadratum walsbyi was investigated by thin-layer chromatography and electrospray ionization-mass spectrometry. The analysis of neutral lipids showed the presence of vitamin MK-8, squalene, carotene, bacterioruberin and several retinal isomers. The major polar lipids were phosphatidylglycerophosphate methyl ester, phosphatidylglycerosulfate, phosphatidylglycerol and sulfated diglycosyl diether lipid. Among cardiolipins, the tetra-phytanyl or dimeric phospholipids, only traces of bisphosphatidylglycerol were detected. When the cells were exposed to hypotonic medium, no changes in the membrane lipid composition occurred. Distinguishing it from other extreme halophiles of the Halobacteriaceae family, the osmotic stress did not induce the neo-synthesis of cardiolipins in H. walsbyi. The difference may depend on the three-laminar structure of the cell wall, which differs significantly from that of other Haloarchaea.
Keywords: Archaea, archaeal phospholipids, ether lipids, Halobacteriaceae
Introduction
Among the myriads of halophilic microorganisms inhabiting the brines of salterns or hyper-saline lakes, those having the unusual shape of ultra-thin squares or envelopes have raised much interest (Walsby 1980, Stoeckenius 1981, Javor et al. 1982, DasSarma and Arora 2001, Oren et al. 2006). Phase contrast microscopy analyses of red water samples from salt basins around the world invariably show abundant square cells with sharp corners, measuring 2–5 µm wide, but less than 0.2 µm thick (Anton et al. 1999, Oren et al. 1999, Oren 2002, Walsby 2005). They resemble other extremely halophilic microorganisms in requiring 3–4 M NaCl for growth, but they tolerate 2 M magnesium (Bolhuis et al. 2004).
Halophilic square cells belong to the family Halobacteriaceae (Oren et al. 1996), and are characterized by the presence of highly refractive gas vesicles and poly-hydroxy-butyrate granules (Walsby 1980). Most studies of Haloquadratum walsbyi, the first square-celled archaeon to be discovered (Walsby 1980), have been with cells from natural samples, as the isolation of the organism in axenic culture is difficult. However, two independent groups (Bolhuis et al. 2004, Burns et al. 2004a, 2004b) have recently isolated and cultivated H. walsbyi (Burns et al. 2007). Fresh cultures of H. walsbyi contain, in addition to the cell phenotype abundant in natural environments, large sheets of cells extending to 40 × 40 µm or more, but only 0.1–0.5 µm thick (Bolhuis et al. 2004). The recently described genome of H. walsbyi shows several adaptive traits that allow it to thrive in its extreme ecological niche (Bolhuis et al. 2006). Haloquadratum walsbyi can grow phototrophically (Walsby 2005) due to the presence of the transmembrane protein bacteriorhodopsin (Bolhuis et al. 2006).
We studied the lipids of H. walsbyi, paying particular attention to cardiolipins, a class of phospholipids associated with the membrane protein systems involved in cell bioenergetics (Mileykovskaya et al. 2005). The phospholipids of the two cultured strains of H. walsbyi have previously been described only briefly, and no cardiolipins have been reported until now (Burns et al. 2007). Here we report detailed lipid analyses of cultured H. walsbyiusing a combination of analytical techniques. In particular, we report the first electrospray ionization mass spectrometry (ESI-MS) analyses of the lipids of H. walsbyi (strain HBSQ001). It has been reported that the membrane levels of cardiolipins rise under osmotic stress in many extremely halophilic archaeons (Lobasso et al. 2003, Lopalco et al. 2004), however, we found no evidence for the neo-synthesis of cardiolipins under osmotic stress in H. walsbyi.
Materials and methods
Materials
All organic solvents used were commercially distilled and of the highest available purity (Sigma-Aldrich). Plates for thin-layer chromatography (TLC; silica gel 60A, 20 × 10 cm, layer thickness 0.2 mm, Merck) were washed twice with chloroform/methanol (1:1, v/v) and activated at 120 °C before use.
Strains and culture conditions
We studied the square-celled archaeon Haloquadratum walsbyi strain HBSQ001 (provided by F. Rodriguez-Valera), Haloferax volcanii strain WR340 (provided by J. Soppa) and Haloarcula marismortui strain ATCC 43049T (provided by A. Oren). The square cells were grown in liquid growth medium (HAS medium) containing yeast extract (L21, Oxoid) as described by Bolhuis et al. (2004). The medium contained per liter: 195 g NaCl; 50 g MgSO4·7H2O; 35 g MgCl2·6 H2O; 5 g KCl; 0.25 g NaHCO3; 1 g NaNO3; 0.5 g CaCl2·2 H2O; 0.05 g KH2PO4; 0.03 g NH4Cl and 20 ml Tris-HCl (1 M, pH 7.4), supplemented with 0.5 g glycerol, 0.1 g yeast extract and 1 g sodium pyruvate. This strain was grown aerobically in the light in a giratory shaker at 80 rpm and 40 °C. Haloferax volcanii and Haloarcula marismortui were grown in the light at 160 rpm and 37 °C in liquid medium containing neutralized peptone (L34, Oxoid), prepared as previously reported (Lanyi and MacDonald 1979). Cells in the stationary growth phase were collected by centrifugation (10,000 g for 15 min)and, unless otherwise specified, resuspended in 4 M NaCl before lipid extraction.
Lipid extraction and analysis
Total lipids were extracted by the Bligh and Dyer method, as modified for extreme halophiles (Kates 1986); the extracts were carefully dried under N2 before weighing and then dissolved in chloroform. Total lipid extracts were analyzed by one- or two-dimensional TLC on silica gel 60A plates. Polar lipids were separated from neutral lipids by cold acetone precipitation (Kates and Kushwaha 1995). Polar lipids were eluted with Solvent A (chloroform/methanol/90% acetic acid, 65:4:35, v/v) for 1-D TLC, or with Solvent B (chloroform/methanol/water, 65:25:4, v/v) and then with Solvent A for the 2-D TLC; the neutral lipids were separated by TLC in Solvent C (hexane/diethyl ether/acetic acid, 70:30:1, v/v). The following stains were used for the detection of lipids on TLC plates: (1)5% sulfuric acid in water, followed by charring at 120 °C or iodine vapor for all lipids (Kates 1986); (2) molybdenum blue spray reagent (Sigma) specific for phospholipids; (3) 0.5% α-naphtol in methanol/water (1:1, v/v) specific for glycolipids (Siakotos and Rouser 1965); and (4) 2% Azure A in 1 mM sulfuric acid for sulfatides and sulfoglycolipids (Kean 1968). The results were confirmed by comparing the retention factor (Rf) values obtained with those of the reference strains and archaeal lipid standards (Lattanzio et al. 2002, Corcelli and Lobasso 2006).
For negative ion ESI-MS, dried samples of lipid extracts and purified lipids were dissolved in chloroform/methanol (1:1, v/v). The ESI-MS spectra were obtained with an API QSTAR mass spectrometer (Applied Biosystem/MSD Sciex, Concord, ON, Canada) equipped with a Turbo ion spray interface.
Isolation and purification of lipids
The isolation of individual lipid components from the total lipid extracts of archaeal cells was performed by scraping the silica gel in each band from preparative TLC plates and extracting each lipid band from the silica as previously described (Corcelli et al. 2000).
Hypo-osmotic shock of cells
Cells from 5-l liquid cultures were collected by centrifugation, washed and resuspended in 4 M NaCl (about 100 ml). In some experiments, H. walsbyi cells were resuspended in basal salt medium (i.e., HAS medium without yeast extract) or directly in HAS medium, instead of 4 M NaCl. A dialysis bag (molecular weight cut-off 12–14,000 Da) was filled with a concentrated suspension of cells in the presence of DNase I and left in 4-l of distilled water (or in some experiments, 0.1 M NaCl) with stirring at 4 °C for 12 h; the shocked cells were then centrifuged at 28,000 g for 30 min and used for lipid extraction.
Results
Pigments of Haloquadratum walsbyi
The polar lipids of H. walsbyi strain HBSQ001, defined as the acetone insoluble portion of the total extracted lipids, accounted for about 80% by weight, whereas the neutral lipids (including pigments) comprised the remaining lipids. The neutral (acetone-soluble) lipids of H. walsbyi consist mainly of C20–C50 isoprenoids and isoprenoid-derived compounds. These pigments protect the cell (and in particular the DNA) against damage by visible and ultraviolet light and they reinforce the cell membrane and increase its rigidity (Lazrak et al. 1988).
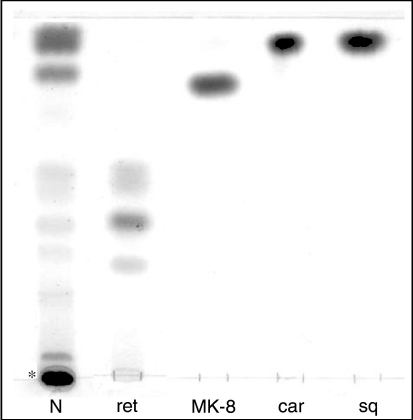
The lipid extract was analyzed by 1-D TLC, and the main neutral lipids were identified by their Rf values relative to standard markers (Figure 1). The results confirmed the presence of the carotenoids carotene and bacterioruberin, the C30-isoprenoid compound squalene and the menaquinone with eight-isoprenoid units vitamin MK-8. Retinal, the prosthetic group of archaeal retinal-containing proteins, was also identified in the lipid extract. As in other Halobacteria having retinal proteins, more than one retinal isomer was found in the lipid extract obtained from cells under room illumination. The exact nature of these isomers is under investigation.
Figure 1.
Pigments of Haloquadratum walsbyi cells. Eighty µg of neutral lipid extract (N) was loaded onto a silica gel 60A plate. Asterisk (*) indicates the carotenoid bacterioruberin. Lipid standards: ret = mixture of retinal isomers; MK-8 = vitamin MK-8; car = β-carotene; and sq = squalene. Lipids were detected by spraying with 5% sulfuric acid and charring at 120 °C.
TLC and ESI-MS polar lipid profiles of H. walsbyi
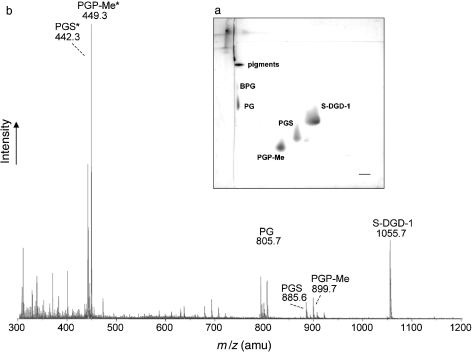
The polar lipids were analyzed by 2-D TLC and ESI-MS (Figure 2). Individual lipid components isolated from the TLC plate were identified by their Rf values relative to those of standard markers and by negative-ion ESI-MS spectra of isolated individual lipids by preparative TLC (not shown). The membrane lipids of many extremely halophilic archaea are diphytanylglycerol diether lipid derivatives (Kates 1993, Corcelli and Lobasso 2006). The main phospholipids present in the lipid extract of H. walsbyi were identified as phosphatidylglycerosulfate (PGS), phosphatidylglycerophosphate methyl ester (PGP-Me) and phosphatidylglycerol (PG). In addition, a very small quantity of archaeal cardiolipin (bisphosphatidylglycerol, BPG) was visible on the TLC (Figure 2a). As regards glycolipids, the H. walsbyi lipid extract contained a glycolipid monosulfated diglycosyl diphytanyl-glycerol, showing the same mobility on the TLC plate as the S-DGD-1 of Haloferax volcanii (see Figure 3).
Figure 2.
Thin layer chromatography (TLC) and electrospray ionization-mass spectrometry (ESI-MS) analyses of the lipid extract of Haloquadratum walsbyi cells. (a) Two-dimensional TLC. Sixty µg of total lipid extract was loaded, and the following pair of solvents was used: Solvent B (chloroform/methanol/water, 65:25:4, v/v) and then Solvent A (chloroform/methanol/ 90% acetic acid, 65:4:35, v/v). (b) Negative ion ESI-MS of total lipid extract of cells. Asterisks (*) indicate bicharged peaks.
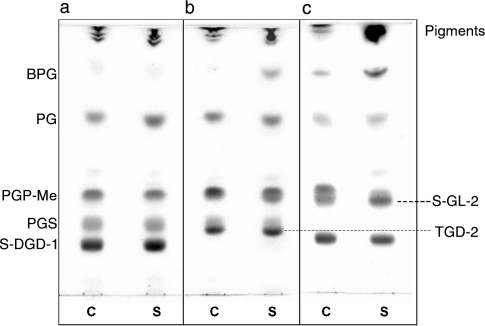
Figure 3.
Effect of osmotic stress on the lipid profiles of (a) Haloquadratum walsbyi, (b) Haloarcula marismortui and (c) Haloferax volcanii. Eighty µg of lipid extracts was loaded on each lane. Eluent: Solvent A. Lipids were detected by spraying with 5% sulfuric acid and charring at 120 °C. Abbreviations: C = whole cells (control); S = cells after hypotonic shock (shocked cells).
Negative ion ESI-MS of the total cell lipid extract (Figure 2b) identified major signals corresponding to the phospholipids PG at m/z 805.7, PGP-Me at m/z 899.7 and 449.3 (as monocharged and bicharged peaks, respectively) and PGS at m/z 885.6 and 442.3 (as monocharged and bicharged peaks, respectively). A signal of 1055.7 m/z wasalsodetected, as expected, for the glycolipid monosulfated diglycosyl diphytanyl-glycerol. Other minor peaks, such as 731.5 m/z (diagnostic of PA), were detected as fragment ions typically found in negative ion ESI-MS spectra of total polar lipids of extremely halophilic archaea. The diagnostic peak of archaeal cardiolipin (BPG) at m/z 760 (as bicharged peak) was detected as only a small signal. Table 1 summarizes some features of the individual polar lipids isolated and purified from the lipid extract by preparative TLC.Therelative percentages of the individual polar lipids isolated by preparative TLC were: S-DGD-1+PGS, 53%; PGP-Me, 31%; and PG, 16%.
Table 1.
Characterization of the total polar lipids extracted from Haloquadratum walsbyi. The polar lipids were separated by thin layer chromatography (TLC) and stained with molybdenum blue (phosphate), α-naphtol (reducing sugar) and Azure A (sulfate) reagent. M– (m/z) was determined by ESI-MS for each isolated and purified lipid component. Asterisks (*) indicate bicharged molecular ion [M – 2H]2–.
| Lipid | TLC Rf | Molybdenum blue | α-naphtol | Azure A | M– (m/z) |
| S-DGD-1 | 0.36 | – | + | + | 1055.7 |
| PGS | 0.45 | + | – | + | 442.3*, 885.6 |
| PGP-Me | 0.55 | + | – | – | 449.3*, 899.7 |
| PG | 0.80 | + | – | – | 805.7 |
| BPG | 0.92 | + | – | – | 760.0* |
| Neutral lipids + pigments | 0.99–1 | – | – | – | – |
Osmotic shock does not induce cardiolipin neo-synthesis
Besides BPG, substituted cardiolipins or analogues are present in archaea. The structure of three archaeal cardiolipin analogues have been elucidated in extreme halophiles. These dimeric phospholipids consist of a (genus specific) sulfo-glycolipid esterified to phosphatidic acid (PA) and therefore possess two highly acidic groups (one phosphate plus a sulfate) in their polar heads. Among archaeal cardiolipins in the extreme halophiles, we have shown that BPG is ubiquitous (Lattanzio et al. 2002), whereas each glycosyl substituted cardiolipin was present in only one genus of Halobacteriaceae. S-TGD1-PA has been detected only in Halobacterium (Corcelli et al. 2000) and S-DGD-5-PA only in Halorubrum (Lopalco et al. 2004), while the third (S-GL-2) is specific to Haloferax (Sprott et al. 2003).
We have shown that BPG levels are low in H. walsbyi and traces of glycosylated cardiolipins have not been detected. The possibility that the small amounts of cardiolipins in the cell extract are due to incomplete extraction or to a loss of these phospholipids during extraction has been ruled out by performing re-extraction and analysis of lipids possibly still associated with the denaturated proteinaceous material remaining after regular extraction (results not shown).
Recently, it has been demonstrated that exposure of archaeal extreme halophiles to low salt environmental conditions changes the cell membrane lipid composition. Upon exposure to hypotonic solution, the membrane undergoes significant biochemical and structural changes, including an increase in the content of cardiolipin analogues (Lobasso et al. 2003, Lopalco et al. 2004). The neo-synthesis of BPG occurs at the expense of PG (Lopalco et al. 2004), while S-TGD-1 and PGP-Me concur in the neo-formation of the so-called glycocardiolipin S-TGD-1-PA (Lobasso et al. 2003).
Therefore, we investigated the effect of osmotic shock on the lipid pattern of H. walsbyi. Cells in the stationary growth phase were harvested by centrifugation, washed twice and exposed to distilled water for 12 h. Observations by phase contrast microscopy showed that the cells were quickly disrupted by hypo-osmotic shock.
The lipids were extracted and analyzed by TLC and ESI-MS to check for increased cardiolipin content. Figure 3 shows the lipid profiles of control cells (Lane C) and cells after hypo-osmotic shock (Lane S) of H. walsbyi (a), compared with those of two other members of Halobacteriaceae: Haloarcula marismortui (b); and Haloferax volcanii (c). The lipid profile of shocked H. walsbyi cells(Figure 3a, Lane S)was similar to that of control cells (Figure 3a, Lane C), and in particular, no changes in BPG content, or in the presence of other lipid spots were observed. On the contrary, the lipid composition of both Haloarcula marismortui and Haloferax volcanii changed with osmotic stress. The BPG content increased in the lipid extracts of shocked cells. Furthermore lipid bands having similar Rf values to that of PGP-Me were visible on the TLC pattern of shocked cells of Haloarcula marismortui and Haloferax volcanii when staining for glycolipid.
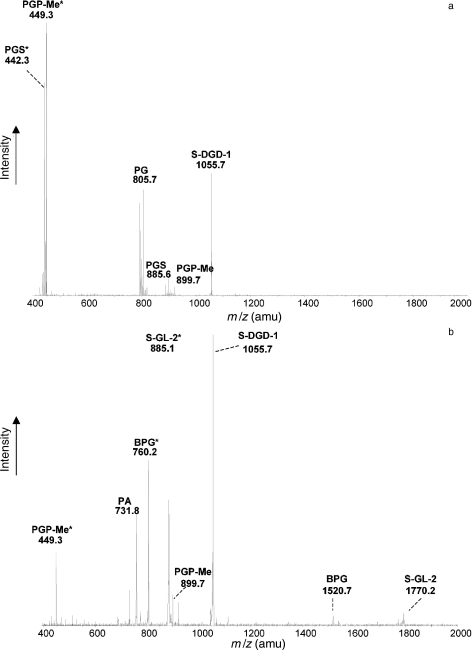
To examine thoroughly the lipid profile of H. walsbyi cells exposed to osmotic shock, the lipid extract was analyzed by ESI-MS. Figure 4a shows the negative ion ESI-MS spectrum of shocked cells of H. walsbyi. The lipid pattern is similar to that previously shown for whole cells (Figure 2b). In particular, no peak for BPG (at m/z 760, as bicharged peak) or other bicharged peaks were detected.
Figure 4.
Electrospray ionization-mass spectrometry (ESI-MS) analyses of total lipids of Halobacteria exposed to hypotonic stress. Total lipids were extracted from shocked cells of (a) Haloquadratum walsbyi and (b) Haloferax volcanii upon hypotonic stress. Experimental conditions were the same as in Figure 3. Asterisk (*) indicates the bicharged peaks.
The ESI-MS lipid profile of Haloferax volcanii upon osmotic stress shows strong signals corresponding to both BPG (m/z 760.2) and S-GL-2 (m/z 885.1, as bicharged peak) (Figure 4b), which were barely present in the control cells (see Sprott et al. 2003).
To check whether the lack of response in H. walsbyi to osmotic stress was due to the use of a resuspension medium lacking nutrients and important divalent cations, osmotic shock experiments were performed on cells resuspended in basal salt medium (i.e., HAS medium without yeast extract) or directly on cells in growth medium, but no differences in the cardiolipin content of the lipid extracts of shocked H. walsbyi cellswere found (results not shown).
Our results show that under osmotic stress, Haloferax volcanii and Haloarcula marismortui increase the cardiolipin content of their membranes, as observed in Halobacterium salinarum (Lobasso et al. 2003) and Halorubrum sp. (Lopalco et al. 2004), whereas Haloquadratum walsbyi does not modify its lipid composition (Table 2).
Table 2.
Cardiolipins as osmo-regulated lipids in Halobacteriaceae. The Haloarcula experiments are in progress to confirm the exact structure of this novel osmo-regulated glycosyl-cardiolipin.
| Genus | Osmoregulated cardiolipins | Reference |
| Haloquadratum | – | Present work |
| Haloarcula | BPG, TGD-2-PA | Present work |
| Haloferax | BPG, S-GL-2 | Sprott et al. 2003 |
| Halobacterium | BPG, S-TGD-1-PA | Lobasso et al. 2003 |
| Halorubrum | BPG, S-DGD-5-PA | Lopalco et al. 2004 |
Discussion
Although the main polar lipids of H. walsbyi have been described (Burns et al. 2007), there have been no past studies showing whether cells of these halophilic archaea possess glycosyl-cardiolipin analogues. The main finding of this study is that, unlike other extremely halophilic archaeal microorganisms, no glycosyl-cardiolipin analogue was detected in the lipid extract of H. walsbyi and that the archaeal cardiolipin BPG is found only in trace amounts, even in cells exposed to severe hypo-osmotic shock.
Lipids of H. walsbyi cells, in both high (i.e., control) and low (i.e., under hypotonic stress) salt media, were analyzed by TLC and ESI-MS, in parallel with those of two other members of Halobacteriaceae, Haloarcula marismortui and Haloferax volcanii.
Upon cell osmotic downshift, the cardiolipin content increased in the plasma membranes of both Haloarcula marismortui and Haloferax volcanii. BPG-enrichment due to osmotic shock was observed in both strains. Two glycosyl-cardiolipin analogues were present only in shocked cells.The shocked cells of Haloferax volcanii contained the previously described glycosyl-cardiolipin S-GL-2 (Sprott et al. 2003), and a new lipid, likely a glycosyl-cardiolipin analogue, was detected in the lipid extract of Haloarcula marismortui by TLC.
We showed that, unlike other members of Halobacteriaceae, osmotic shock did not influence the phospholipid composition of H. walsbyi. In fact, the lipid profile of hypo-osmotically shocked cells was both qualitatively and quantitatively identical to that of control cells. In particular, no BPG-enrichment was observed, and no glycosyl-cardiolipins were found in the shocked cell extract.
The difference in response to osmotic shock between H. walsbyi and other members of Halobacteriaceae may be related to the difference in the structures of the cell membrane and wall and, in particular, to the structural–functional inter-relationships between the cytoplasmic membrane and wall.
Cell envelopes of archaea differ from those of bacteria and show remarkable structural and chemical diversity. Murein, the typical sacculus-forming polymer of bacteria, and lipopolysaccharide-containing outer membranes, characteristic of gram-negative bacteria, are absent in archaea. Crystalline surface layers (S-layers) are common in both prokaryotic domains, and they consist of protein or glycoprotein subunits. However, S-layers in archaea have a form-stabilizing function, especially when they are the only layer enveloping the cytoplasmic membrane (Kates 1993).
Many members of Halobacteriaceae have an S-layer, which consists of subunits of a large glycoprotein, having negatively charged amino acids to counteract high Na+ concentrations. These glycoproteins require high NaCl concentrations and relatively large amounts of magnesium or other divalent cations for structural stability; when suspended in low salt solutions, the wall protein denatures, leading to lysis and cell death.
Halobacterium salinarum, Halorubrum trapanicum, Haloferax volcanii and Haloarcula marismortui, in which cardiolipin content increases in response to osmotic shock, allpossess similar cell surface glycoproteins (Nakamura et al. 1992).
Haloquadratum walsbyi strain HBSQ001 has a thick rigid cell wall that does not depend on high salt for structural stability. Electron cryomicroscopy showed that the cell wall of HBSQ001 displayed a three-layered structure (Burns et al. 2007).
Haloquadratum walsbyi expresses a water-enriched capsule byencoding halomucin (Bolhuis et al. 2006), a large protein homologous to mammalian mucins, which play an important role in protecting tissues against desiccation (Hollingsworth and Swanson 2004). Animal mucins may contain sialic acids, which cap the end of polysaccharide side-chains of these proteins. Although sialic acids have not been detected in archaea, two essential sialic acid biosynthesis genes have been found in the genome of H. walsbyi (Bolhuis et al. 2006).
Haloquadratum walsbyi could synthesize a poly-gamma-glutamate capsule (Bolhuis et al. 2006) by means of a poly-gamma-glutamate biosynthesis protein complex CapBCA (Ashiuchi and Misono 2002). A cross-linked matrix of poly-gamma-glutamate may contribute to the cell wall rigidity and maintenance of the unique square morphology. We suggest that osmotic shock does not modify the phospholipid composition of H. walsbyi membranes, particularly its cardiolipin content, due to its cell wall architecture.
The neo-synthesis of cardiolipin in response to osmotic stress is characteristic not only of halophilic archaea, but also of many other prokaryotes with a rigid three-layered cell wall. Cardiolipin neo-synthesis under osmotic stress has been documented for Rhodobacter sphaeroides (Catucci et al. 2004) and Staphylococcus aureus (Okabe et al. 1980), and its induction in the cytoplasmatic membrane is strictly related to selective disruption or impairment of the cell wall. The loss of cell wall can be induced either by treatment with exogenous lytic enzyme (resulting in protoplast) or by activation of endogenous autolytic enzyme by high sucrose concentrations (resulting in autoplast). Sphaeroplastes of Rhodobacter and protoplastes of Staphylococcus obtained after lysozyme treatment of bacteria are indeed enriched in cardiolipin. As lysozyme is inactive on the cell well of H. walsbyi cells, experiments are in progress to find agents able to selectively hydrolyze or induce autolytic degradation of the cell wall of H. walsbyi.
Acknowledgments
This work was supported by the Ministero Italiano dell’Università e della Ricerca (Fondi d’Ateneo), the Ministero Italiano della Difesa (Contract no. 1999/13.12.2005) and the Istituto per i Processi Chimico-Fisici del Consiglio Nazionale delle Ricerche, Bari, Italy (CNR-IPCF). We thank S.O. Cuadros for suggestions in the preparation of the culture medium.
References
- R1.Anton J., Llobet-Brossa E., Rodriguez-Valera F., Amann R. Fluorescence in situ hybridization analysis of the prokaryotic community inhabiting crystallizer ponds. Environ. Microbiol. 1999;1:517–523. doi: 10.1046/j.1462-2920.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- R2.Ashiuchi M., Misono H. Biochemistry and molecular genetics of poly-gamma-glutamate synthesis. Appl. Microbiol. Biotechnol. 2002;59:9–14. doi: 10.1007/s00253-002-0984-x. [DOI] [PubMed] [Google Scholar]
- R3.Bolhuis H., Te Poele E.M., Rodriguez-Valera F. Isolation and cultivation of Walsby’s square archaeon. Environ. Microbiol. . 2004;6:1287–1291. doi: 10.1111/j.1462-2920.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- R4.Bolhuis H., Palm P., Wende A., Falb M., Rampp M., Rodriguez-Valera F., Pfeiffer F., Oesterhelt D. The genome of the square archaeon Haloquadratum walsbyi: life at the limits of water activity. BMC Genomics. 2006;7:169–180. doi: 10.1186/1471-2164-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R5.Burns D.G., Camakaris H.M., Janssen P.H., Dyall-Smith M.L. Cultivation of Walsby’s square haloarchaeon. FEMS Microbiol. Lett. 2004;238:469–473. doi: 10.1016/j.femsle.2004.08.016. [DOI] [PubMed] [Google Scholar]
- R6.Burns D.G., Camakaris H.M., Janssen P.H., Dyall-Smith M.L. Combined use of cultivation-dependent and cultivation-independent methods indicates that members of most Haloarchaeal groups in an Australian crystallizer pond are cultivable. Appl. Environ. Microbiol. 2004;70:5258–5265. doi: 10.1128/AEM.70.9.5258-5265.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R7.Burns D.G., Janssen P.H., Itoh T., Kamekura M., Li Z., Jensen G., Rodriguez-Valera F., Bolhuis H., Dyall-Smith M.L. Haloquadratum walsbyi gen. nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain. Int. J. Syst. Evol. Microbiol. 2007;57:387–392. doi: 10.1099/ijs.0.64690-0. [DOI] [PubMed] [Google Scholar]
- R8.Catucci L., Depalo N., Lattanzio V.M., Agostano A., Corcelli A. Neo-synthesis of cardiolipin in Rhodobacter sphaeroides under osmotic stress. Biochemistry. 2004;43:15066–15072. doi: 10.1021/bi048802k. [DOI] [PubMed] [Google Scholar]
- R9.Corcelli A., Lobasso S. Characterization of lipids of halophilic Archaea. In: Rainey A.F., Oren A., editors. Methods in Microbiology—Extremophiles. Amsterdam: Elsevier; 2006. pp. 585–613. [Google Scholar]
- R10.Corcelli A., Colella M., Mascolo G., Fanizzi F.P., Kates M. A novel glycolipid and phospholipid in the purple membrane. Biochemistry. 2000;39:3318–3326. doi: 10.1021/bi992462z. [DOI] [PubMed] [Google Scholar]
- R11.DasSarma S., Arora P. Encyclopedia of Life Sciences. London: Nature Publishing Group; 2001. Halophiles; pp. 1–9. [Google Scholar]
- R12.Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- R13.Javor B., Requadt C., Stoeckenius W. Box-shaped halophilic bacteria. J. Bacteriol. 1982;151:1532–1542. doi: 10.1128/jb.151.3.1532-1542.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R14.Kates M. Techniques of lipidology. In: Burdon R.H., Van knippenberg P.H., editors. Vol. 3. Elsevier, Amsterdam: 1986. pp 100–110, 114–115, 153–154, 163–164, 251–253. [Google Scholar]
- R15.Kates M. Membrane lipids of Archaea. In: Kates M., Kushner D.J., Matheson A.T., editors. The Biochemistry of Archaea (Archaebacteria) Amsterdam: Elsevier; 1993. pp. 261–295. [Google Scholar]
- R16.Kates M., Kushwaha S.C. Isoprenoids and polar lipids of extreme halophiles. In: Dassarma S., Fleischman E.M., editors. Archaea: A Laboratory Manual, Halophiles. New York: Cold Spring Harbor Laboratory Press; 1995. pp. 35–54. [Google Scholar]
- R17.Kean E.L. Rapid, sensitive spectrophotometric method for quantitative determination of sulphatides. J. Lipid Res. . 1968;9:319–327. [PubMed] [Google Scholar]
- R18.Lanyi J.K., MacDonald R.E. Light-induced transport in Halobacterium halobium . Methods Enzymol. 1979;56:398–407. doi: 10.1016/0076-6879(79)56038-8. [DOI] [PubMed] [Google Scholar]
- R19.Lattanzio V.M.T., Corcelli A., Mascolo G., Oren A. Presence of two novel cardiolipins in the halophilic archaeal community in the crystallizer brines from the salterns of Margherita di Savoia (Italy) and Eilat (Israel) Extremophiles. 2002;6:437–444. doi: 10.1007/s00792-002-0279-2. [DOI] [PubMed] [Google Scholar]
- R20.Lazrak T., Wolff G., Albrecht A.M., Nakatani Y., Ourisson G., Kates M. Bacterioruberins reinforce reconstituted Halobacterium lipid membranes. Biochim. Biophys. Acta. 1988;939:160–162. [Google Scholar]
- R21.Lobasso S., Lopalco P., Lattanzio V.M.T., Corcelli A. Osmotic shock induces the presence of glycocardiolipin in the purple membrane of Halobacterium salinarum . J. Lipid Res. 2003;44:2120–2126. doi: 10.1194/jlr.M300212-JLR200. [DOI] [PubMed] [Google Scholar]
- R22.Lopalco P., Lobasso S., Babudri F., Corcelli A. Osmotic shock stimulates de novo synthesis of two cardiolipins in an extreme halophilic archaeon. J. Lipid Res. 2004;45:194–201. doi: 10.1194/jlr.M300329-JLR200. [DOI] [PubMed] [Google Scholar]
- R23.Mileykovskaya E., Zhang M., Dowhan W. Cardiolipin in energy transducing membranes. Biochemistry (Moscow) 2005;70:154–158. doi: 10.1007/s10541-005-0095-2. [DOI] [PubMed] [Google Scholar]
- R24.Nakamura S., Aono R., Mizutani S., Takashina T., Grant W.D., Horikoshi K. The cell surface glycoprotein of Haloarcula japonica TR-1. Biosci. Biotechnol. Biochem. 1992;56:996–998. doi: 10.1271/bbb.56.996. [DOI] [PubMed] [Google Scholar]
- R25.Okabe A., Hirai Y., Hayashi H., Kanemasa Y. Alteration in phospholipid composition of Staphylococcus aureus during formation of autoplast. Biochim. Biophys. Acta. 1980;617:28–35. doi: 10.1016/0005-2760(80)90221-0. [DOI] [PubMed] [Google Scholar]
- R26.Oren A. Halophilic Microorganisms and Their Environments. Dordrecht: Kluwer Academic Publishers; 2002. Pigments of halophilic microorganisms; pp. 173–206. [Google Scholar]
- R27.Oren A., Duker S., Ritter S. The polar lipid composition of Walsby’s square bacterium. FEMS Microbiol. Lett. 1996;138:135–140. [Google Scholar]
- R28.Oren A., Ventosa A., Gutiérrez M.C., Kamekura M. Haloarcula quadrata sp. nov., a square, motile archaeon isolated from a brine pool in Sinai (Egypt). Int. J. Syst. Bacteriol. 1999;49:1149–1155. doi: 10.1099/00207713-49-3-1149. [DOI] [PubMed] [Google Scholar]
- R29.Oren A., Pri-El N., Shapiro O., Siboni N. Buoyancy studies in natural communities of square gas-vacuolate archaea in saltern crystallizer ponds. Saline Systems. 2006;2:4–10. doi: 10.1186/1746-1448-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R30.Siakotos A.N., Rouser G. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J. Am. Oil Chem. Soc. 1965;42:913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- R31.Sprott G.D., Larocque S., Cadotte N., Dicaire C.J., Mcgee M., Brisson J.R. Novel polar lipids of halophilic eubacterium Planococcus H8 and archaeon Haloferax volcanii . Biochim. Biophys. Acta . 2003;1633:179–188. doi: 10.1016/j.bbalip.2003.08.001. [DOI] [PubMed] [Google Scholar]
- R32.Stoeckenius W. Walsby’s square bacterium: fine structure of an orthogonal procaryote. J. Bacteriol. 1981;148:352–360. doi: 10.1128/jb.148.1.352-360.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R33.Walsby A.E. A square bacterium. Nature. 1980;283:69–71. [Google Scholar]
- R34.Walsby A.E. Archaea with square cells. Trends Microbiol. 2005;13:193–195. doi: 10.1016/j.tim.2005.03.002. [DOI] [PubMed] [Google Scholar]