Abstract
Disequilibrium between bone-forming osteoblasts and bone-resorbing osteoclasts is central to many bone diseases. Here, we show that dysregulated expression of translationally controlled isoforms of CCAAT/enhancer-binding protein β (C/EBPβ) differentially affect bone mass. Alternative translation initiation that is controlled by the mammalian target of rapamycin (mTOR) pathway generates long transactivating (LAP*, LAP) and a short repressive (LIP) isoforms from a single C/EBPβ transcript. Rapamycin, an inhibitor of mTOR signalling increases the ratio of LAP over LIP and inhibits osteoclastogenesis in wild type (WT) but not in C/EBPβ null (c/ebpβ−/−) or in LIP knock-in (L/L) osteoclast precursors. C/EBPβ mutant mouse strains exhibit increased bone resorption and attenuated expression of MafB, a negative regulator of osteoclastogenesis. Ectopic expression of LAP and LIP in monocytes differentially affect the MafB promoter activity, MafB gene expression and dramatically affect osteoclastogenesis. These data show that mTOR regulates osteoclast formation by modulating the C/EBPβ isoform ratio, which in turn affects osteoclastogenesis by regulating MafB expression.
Keywords: bone homeostasis, CCAAT/enhancer binding protein β, MafB, mTOR, osteoclast
Introduction
Bone homeostasis is controlled by the interplay between bone-forming osteoblasts and bone-resorbing osteoclasts (Karsenty and Wagner, 2002). The dynamics of bone remodelling is tightly regulated (Boyle et al, 2003; Harada and Rodan, 2003) and is based on intercellular communication between both cell types (Martin and Sims, 2005). Disturbance of the balance between osteoblasts and osteoclasts often results in enhanced bone resorption, usually involving hyperactive osteoclasts and causing focal or generalized bone loss (Helfrich, 2003; Teitelbaum and Ross, 2003; Phan et al, 2004; Ehrlich and Roodman, 2005).
The transcription factor CCAAT/enhancer binding protein β (C/EBPβ) is involved in the differentiation and function of haematopoietic and mesenchymal cell types, including macrophages and adipocytes (Screpanti et al, 1995; Tanaka et al, 1995; Poli, 1998; Rosen and MacDougald, 2006; Uematsu et al, 2007), cell lineages closely related to bone cells. C/EBPβ is a member of the family of C/EBP transcription factors (α, β, γ, δ, ɛ and ζ). C/EBPs have highly conserved basic leucine zipper domains (bZIP) involved in dimerization and DNA binding, and a variable N-terminal region involved in the regulation of gene expression. C/EBPs regulate proliferation and differentiation of many different cell types, including adipocytes, mammary epithelial cells, ovarian luteal cells, keratinocytes, hepatocytes, neuronal cells, intestinal epithelial cells and cells of the haematopoietic lineage (Ramji and Foka, 2002; Johnson, 2005; Nerlov, 2007). The two main members, C/EBPα and β, are required for placentogenesis (Begay et al, 2004), liver functions (Pedersen et al, 2007; Wang et al, 2008), granulopoiesis (Zhang et al, 2002; Hirai et al, 2006) and innate immune functions (Akira and Kishimoto, 1992; Poli, 1998). C/EBPγ is ubiquitously expressed and expression of C/EBPɛ is mostly confined to granulocytes (Ramji and Foka, 2002; Johnson, 2005), whereas C/EBPδ is involved in adipogenesis and neuronal functions (Tanaka et al, 1997; Sterneck and Johnson, 1998).
C/EBPβ is an intronless gene, yet different isoforms are expressed with successively truncated N-termini. The C/EBPβ isoforms were termed LAP*, LAP and LIP (Descombes and Schibler, 1991) and arise by alternative translation initiation at distinct in frame start sites (Calkhoven et al, 2000; Xiong et al, 2001). Alternative translation initiation and thus C/EBPβ isoform production is regulated through the mammalian target of rapamycin (mTOR) pathway: activation of mTOR enhances generation of LIP and inhibition of mTOR enhances LAP production (Calkhoven et al, 2000; Jundt et al, 2005). The truncated and dominant inhibitory isoform (LIP) may also arise by partial proteolysis of long isoforms (Sebastian and Johnson, 2006). All isoforms contain the C-terminal bZIP domain. In addition, the long isoforms LAP* and LAP contain N-terminal transactivation and chromatin remodelling domains. The C/EBPβ isoform ratio is thought to direct cell fate (Kowenz-Leutz and Leutz, 1999; Calkhoven et al, 2002; Nerlov, 2007).
It has been shown that over-expression of full-length C/EBPβ or of its short LIP isoform supports osteoblast differentiation in vitro (Gutierrez et al, 2002; Hata et al, 2005; Villagra et al, 2006). In agreement with this, a recent study showed that absence of C/EBPβ at embryonic and neonatal stages results in delayed bone formation and suppression of osteoblast differentiation (Tominaga et al, 2008). Similarly, transgenic mice expressing only the short LIP isoform in the osteoblast lineage displayed attenuated bone formation (Harrison et al, 2005). Although the action of C/EBPβ in osteoblasts has been addressed in several studies, the general function of C/EBPβ in bone homeostasis, including its potential role in osteoclasts, remains to be examined.
Here, we have used genetically altered mice, either deficient for c/ebpβ (−/−) (Sterneck et al, 1997), or expressing only the truncated LIP isoform from its native genetic locus (LIP knock-in; L/L), to examine C/EBPβ functions in bone tissue. Osteogenesis and osteoclastogenesis were both affected in the absence of C/EBPβ or in the presence of LIP only. Importantly, the translationally controlled ratio between LAP and LIP isoforms strongly affected osteoclastogenesis. Rapamycin, an inhibitor of mTOR, enhanced LAP expression and constrained differentiation of osteoclasts from wild-type (WT, +/+) mice but not from C/EBPβ deficient or L/L mice. Expression of LAP-induced expression of MafB, a negative regulator of osteoclast differentiation (Kim et al, 2007), whereas LIP inhibited expression of MafB and enhanced osteoclastogenesis. Our data suggest that signalling through mTOR affects the C/EBPβ isoform ratio that in turn regulates MafB expression as a major factor controlling osteoclast differentiation and bone homeostasis. Our results raise the possibility that dysregulated translational control of C/EBPβ isoform expression might be involved in bone diseases.
Results
Expression of C/EBPβ in bone cells
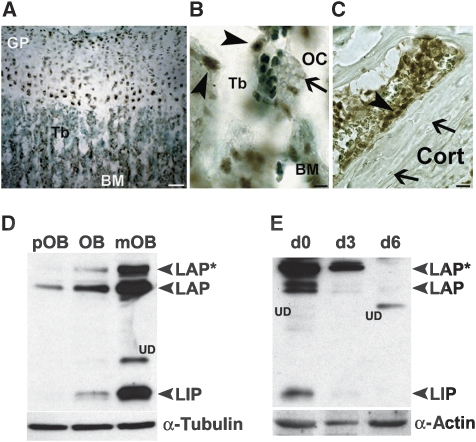
To examine whether C/EBPβ could have a function in bone homeostasis, expression of C/EBPβ was analysed in the different bone cell types. Immunohistochemistry of bone tissue revealed C/EBPβ protein expression in growth plate chondrocytes (Figure 1A) and in osteoblasts (Figure 1B and C, arrowheads), but not in osteoclasts (Figure 1B, arrow) or osteocytes (Figure 1C, arrows). During osteoblast differentiation, expression of all three C/EBPβ protein isoforms increased (Figure 1D). Monocytic precursors derived from the bone marrow expressed all three C/EBPβ protein isoforms and C/EBPβ expression vanished during differentiation into osteoclasts and no C/EBPβ protein was detected in mature osteoclasts (Figure 1E). Thus, C/EBPβ protein expression is reciprocally regulated during differentiation of the two cell lineages controlling bone homeostasis.
Figure 1.
Expression of C/EBPβ in bone cells. (A) Immunohistochemistry of the proximal tibia of 4-week-old mice showing expression of C/EBPβ protein (brown precipitate) in the different bone cell types. Lightgreen was used as counterstain. Scale bar, 200 μm. C/EBPβ protein expression in osteoblasts (arrowheads) (B, C), whereas no C/EBPβ protein was detected in differentiated osteoclasts (OC) (arrow), which were identified as large multinucleated cells attached to the bone surface (scale bar, 50 μm) or (C) in osteocytes (arrow, scale bar, 50 μm). Tb, trabeculae; GP, growth plate; BM, bone marrow; Cort, cortical bone (D) Western blot analysis of C/EBPβ isoform expression (LAP*, LAP and LIP) during osteoblast differentiation (pOB, preosteoblasts; OB, osteoblast; mOB, mature osteoblast) and (E) during osteoclast differentiation of bone marrow derived monocytic precursors on the indicated days (d0, d3, d6) after M-CSF and RANK-L addition. Loading was controlled by analysis of α-tubulin and α-actin expression, respectively. UD, undefined bands, which cross-react with the C/EBPβ antibody.
Affected bone mass in c/ebpβ mutant mice
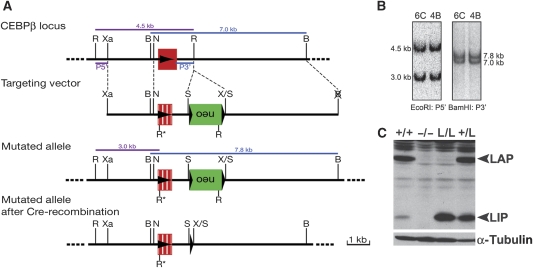
Reciprocal expression of C/EBPβ in osteoblasts and osteoclasts suggested cell-type specific functions of C/EBPβ and its isoforms in bone tissue. To characterize the function of C/EBPβ and its isoforms in bone, mice expressing only the LIP isoform from the endogenous C/EBPβ locus were generated (L/L mice; Figure 2A and B) and compared with WT and c/ebpβ knock-out (−/−) (Sterneck et al, 1997) mice. Analysis of liver protein extracts from 8-week-old mice confirmed expression of the LIP isoform in L/L mice (Figure 2C). L/L mice were born at a slightly reduced Mendelian ratio (18%, n=324). Peripheral blood cell counts, spleen and liver weights and their histological parameters were comparable with WT (+/+) animals (data not shown). Similarly to L/L mice, c/ebpβ−/− mice are born at a reduced Mendelian ratio. However, viable mice developed normally until at least 10 weeks of age (our observation and Tanaka et al, 1995; Sterneck et al, 1997).
Figure 2.
Generation and characterization of LIP knock-in mice. (A) Schematic representation of the targeting strategy used to generate a knock-in (k.i.) of the LIP isoform of C/EBPβ in the endogenous c/ebpβ locus by homologous recombination in embryonic stem (ES) cells. The structure of the genomic c/ebpβ locus, targeting vector and mutated allele are shown. The c/ebpβ gene is intronless and depicted as filled red box and LIP as dashed red box. The arrow indicates the direction of gene transcription. The DNA fragments and their sizes revealed by Southern blot analysis are indicated by thick coloured lines. B, BamHI; N, NotI; R, R*, EcoRI; S, SalI; X, XhoI; Xa, XbaI. Neo, neomycin resistance gene; LoxP sites (black triangles); P5′: 5′ probe; P3′: 3′ probe. (B) Southern blot analysis of ES cell DNA. Genomic DNA of targeted ES cells was isolated from two clones and digested with EcoRI and then hybridized with the 5′ probe (in purple). The WT and mutant allele are detected as 4.5 and 3.0 kb fragments, respectively. BamHI digested DNA was hybridized with the 3′ probe (in blue) that detected a 7.0-kb fragment in the WT and a 7.8-kb fragment in the mutant allele. (C) C/EBPβ protein expression in livers isolated from 8-week-old mice. Loading was controlled by analysis of α-tubulin expression. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice; +/L, heterozygous k.i. mice.
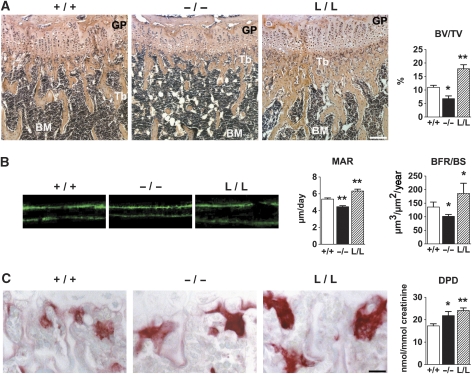
Bone development was analysed by comparing L/L, c/ebpβ knock-out (−/−) (Sterneck et al, 1997) and WT (+/+) mice at the age of 8 weeks. C/ebpβ−/− animals displayed reduced bone length that was restored by replacement of the endogenous c/ebpβ gene with the LIP isoform (data not shown). Histomorphometric analyses of the long bones showed that c/ebpβ−/− mice have a 1.6-fold diminished bone volume, characterized by a reduction of the number and thickness of bone trabeculae (Figure 3A; Supplementary Table 1). In contrast, L/L mice had a 1.7-fold increased bone volume in comparison with WT mice, displaying increased number and thickness of trabeculae (Figure 3A; Supplementary Table 1). However, the number of osteoblasts was not affected in either c/ebpβ−/− or in L/L mice (Supplementary Table 1). These effects on bone tissue were observed at several sites in the skeleton (long bones and vertebrae) (Supplementary Table 1 and data not shown). The observed bone phenotypes were already apparent at birth in both mutants and persisted with age (data not shown). The increased bone volume in L/L mice did not evoke extramedullary haematopoiesis (data not shown). No difference in bone volume compared with WT mice was found in c/ebpβ heterozygous mice, whereas LIP heterozygous mice displayed an intermediate phenotype between WT and homozygous LIP mice (data not shown). Both heterozygotes were therefore not further analysed.
Figure 3.
Affected bone mass in c/ebpβ mutant mice. (A) Histological analyses (haematoxylin-eosin staining) of tibiae of 8-week-old mice, showing an osteopenic phenotype in c/ebpβ-deficient mice (−/−) and an osteosclerotic phenotype in LIP k.i. mice (L/L), compared with WT (+/+) mice. Scale bar, 100 μm. Bar graph displays the histomorphometric quantification of the bone volume (BV/TV, bone volume/total volume). GP, growth plate; Tb, trabeculae; BM, bone marrow. (B) Images of double calcein labelled bones from WT and c/ebpβ mutant mice showing the mineral apposition rate (MAR) and the bone formation rate/bone surface (BFR/BS). (C) Enhanced osteoclastogenesis in c/ebpβ mutant mice. TRACP staining of osteoclasts (red staining) in tibiae of 8-week-old WT and c/ebpβ mutant mice. Lightgreen was used as counterstain. Scale bar, 20 μm. Bar graph shows the urinary excretion of deoxypyridinoline (DPD) cross-links, reflecting osteoclast activity in vivo. For bone histomorphometric measurements, n=8 per group. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice. Data are presented as mean±s.e.m. *P<0.05, **P<0.01 versus WT.
The rate of bone formation was assessed in animals by double calcein labelling. In comparison with WT, c/ebpβ−/− mice displayed decreased mineral apposition and bone formation rate, whereas mineral apposition and bone formation rate was increased in L/L mice (Figure 3B). Bone resorption was enhanced in both c/ebpβ−/− and L/L mice as compared with WT mice. Histomorphometric analyses revealed larger osteoclasts (TRACP-positive cells, red staining) in both c/ebpβ−/− and L/L mice, compared with WT mice (Figure 3C; Supplementary Tables 1 and 2). Although the total number of osteoclasts was not affected, an increase in the number of multinucleated osteoclasts was observed (Supplementary Tables 1 and 2). These enlarged osteoclasts caused enhanced bone resorption, as shown by increased urinary excretion of deoxypyridinoline (DPD) (Figure 3C). Thus, bone formation was decreased in the absence of C/EBPβ and osteoclastogenesis was enhanced, both contributing to the observed osteopenia. Expression of LIP, similarly to the absence of C/EBPβ, also enhanced osteoclastogenesis, that was however, surpassed by enhanced bone formation.
LIP promotes osteoblast differentiation
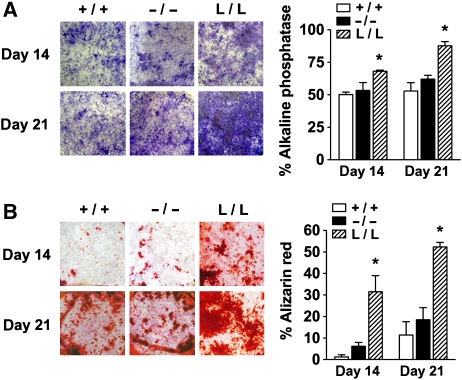
Osteoblasts derived from calvarial bones of L/L mice displayed enhanced differentiation (as also observed for long bones derived osteoblasts, data not shown), as revealed by increased alkaline phosphatase (ALP) activity (Figure 4A), mineralization of bone nodules (alizarin red staining in Figure 4B) and increased expression of early and late osteoblast differentiation markers, such as osteopontin, ALP, bone sialoprotein, collagen type I and osteocalcin (OC) (Supplementary Table 3A). Moreover, expression of the essential osteogenic transcription factors Runx2 and osterix was increased (Supplementary Table 3A), whereas no differences in the expression of several of the Wnt components (Lef1, Tcf1 and Tcf3), or of the AP-1 family members Fra1, Fra2, c-Fos and JunD was observed (Supplementary Table 3B). LIP has been shown earlier to act as a co-activator of Runx2, promoting osteoblast differentiation (Hata et al, 2005), which, together with the enhanced Runx2 and Osterix expression may contribute to enhanced osteogenesis. These data suggest a cell autonomously enhanced differentiation potential of L/L osteoblasts that may underlie the increase in bone formation rate observed in L/L mice. However, differentiation of WT and c/ebpβ−/− osteoblasts in tissue culture, either derived from calvaria or long bones (data not shown) was similar (Figure 4A and B; Supplementary Table 3), suggesting microenvironmental cues might also be involved in the observed osteopenia in c/ebpβ−/− mice (Figure 3A).
Figure 4.
Osteoblast differentiation in c/ebpβ mutants. (A) Primary calvarial osteoblast precursor cells were differentiated and stained for alkaline phosphatase (ALP) activity. The bar graph displays the quantification of the percentage ALP positive area per well area. (B) Bone nodule mineralization of primary calvarial osteoblasts determined by alizarin red staining. The bar graph displays the quantification of alizarin red positive mineralized nodules. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice. Data are presented as mean±s.e.m. *P<0.05, versus WT.
Enhanced osteoclast differentiation of c/ebpβ−/− and L/L cells
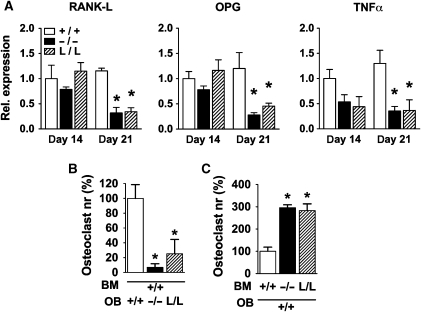
Osteoblasts control the differentiation and activity of osteoclasts by release of signalling molecules such as TNFα, RANK-L and its antagonist OPG (Simonet et al, 1997; Boyle et al, 2003; Teitelbaum and Ross, 2003). Analysis of both c/ebpβ−/− and L/L primary osteoblasts showed diminished expression of the pro-osteoclastogenic factors RANK-L and TNFα and of the antagonist OPG (Figure 5A). Co-culturing C/EBPβ mutant osteoblasts with WT bone marrow cells reduced osteoclast differentiation of WT cells (Figure 5B), in accordance with decreased expression of osteoclastogenic factors by the mutant osteoblasts and co-culturing WT osteoblasts with C/EBPβ mutant bone marrow cells showed enhanced osteoclast differentiation of the C/EBPβ mutant cells (Figure 5C). Thus, paracrine effects do not explain the enhanced osteoclastogenesis observed in both c/ebpβ−/− and L/L mice (Figure 3C) and suggest a cell autonomous mechanism.
Figure 5.
Osteoblast–osteoclast cross-talk in c/ebpβ mutants. (A) Expression of the osteoclastic regulators RANK-L, OPG and TNFα in primary calvarial osteoblast cultures as determined by real-time RT–PCR. Values represent relative expression levels compared with WT on day 14 (set as 1). Data are presented as mean±s.e.m. of three independent experiments. (B) Number of multinucleated osteoclasts formed from WT bone marrow cells (BM) co-cultured with osteoblasts (OB) from the different genotypes, as indicated. (C) Number of multinucleated osteoclasts formed from bone marrow cells of the C/EBPβ mutant mice (as indicated), co-cultured with WT osteoblasts. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice. Data are presented as mean±s.e.m. *P<0.05 versus WT.
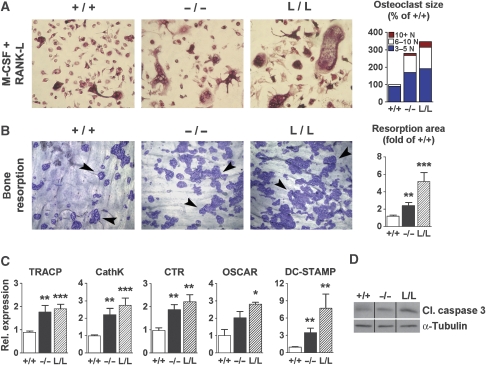
As shown in Figure 6A, bone marrow cell cultures from c/ebpβ−/− and from L/L mice generated more and larger osteoclasts (containing more nuclei/TRACP-positive cell) compared with WT cells after 6 days of treatment with M-CSF and RANK-L. Only 10% of WT osteoclasts contained >5 nuclei/cell, versus 42 and 51% in c/ebpβ−/− and L/L osteoclasts, respectively (P<0.001 compared with WT). The exacerbated osteoclast formation was accompanied by enhanced osteoclast activity, as shown by increased bone resorption on bone slices (Figure 6B), increased expression of the osteoclastic differentiation markers, TRACP, cathepsin K (CathK), calcitonin receptor (CTR), OSCAR (osteoclast-associated receptor) and of the osteoclastic cell fusion marker DC-STAMP (dendritic cell-specific transmembrane protein) (Yagi et al, 2005) (Figure 6C). Expression of RANK (RANK-L receptor) was not affected and expression of c-Fms (M-CSF receptor) was only slightly elevated in osteoclasts of both mutants (data not shown), excluding elevated cytokine receptor levels as a cause of enhanced osteoclastogenesis of mutant cells. These data are consistent with a cell intrinsic defect of osteoclasts in C/EBPβ mutants and are in accordance with the observations made in animals (Figure 3C; Supplementary Table 2).
Figure 6.
c/ebpβ mutations promote osteoclast differentiation. (A) Osteoclast differentiation of bone marrow derived monocytic precursors from WT, c/ebpβ knock-out and LIP k.i. mice and treated with M-CSF and RANK-L. Osteoclasts were stained by TRACP activity (red staining). The bar graph displays the differential quantification of the osteoclasts by number of nuclei per cell (WT cultures set at 100%). (B) Bone resorptive activity of osteoclasts determined by culturing osteoclasts on bovine bone slices and staining resorption pits (arrowheads) with coomassie brilliant blue. The bar graph displays the quantification of the resorption areas expressed as fold of resorbed area in the WT (set at 1). (C) Real-time RT–PCR analysis of expression of the osteoclast markers TRACP, Cathepsin K (CathK), calcitonin receptor (CTR), OSCAR and DC-STAMP in osteoclasts cultured for 6 days in the presence of M-CSF and RANK-L. Values represent relative expression levels compared with WT (set as 1). Data are presented as mean±s.e.m.; n=6 per group. *P<0.05, **P<0.01, ***P<0.001 versus WT. (D) Western blot analysis of cleaved-caspase 3 (cl. caspase 3) expression to determine apoptosis in primary osteoclasts cultured for 6 days in the presence of M-CSF and RANK-L. The samples were run on the same gel, but were noncontiguous, as indicated with the black lines. Loading was controlled by α-tubulin expression. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice.
Cultures of osteoclast precursors exhibited similar numbers of osteoclasts 1 day after seeding (Supplementary Figure 1A; both mononuclear and multi-nucleated TRACP-positive cells), reflecting a comparable pool of osteoclast precursor cells (van der Eerden et al, 2005) in all genotypes tested. However, on day 2 of differentiation, both c/ebpβ−/− and L/L bone marrow cells generated significantly more mononuclear and multinucleated TRACP-positive cells (Supplementary Figure 1A and B), which was accompanied by an increased expression of the osteoclastic differentiation markers, TRACP, CathK, CTR and OSCAR (data not shown). These data indicate that the enhanced osteoclastogenesis of c/ebpβ−/− and L/L cells is initiated at early stages of osteoclast differentiation. In agreement with this, an increase in cleaved-caspase 3 expression was observed in C/EBPβ mutant but not in WT osteoclast cultures (Figure 6D) and likewise, apoptotic cells were present in mutant cultures but not in WT cultures after 6 days (data not shown). These data signify that the presence of enlarged osteoclasts is not caused by a diminished or delayed apoptosis of C/EBPβ mutant cells, but by accelerated differentiation and enhanced cell fusion.
Accelerated osteoclast differentiation was accompanied by increased expression of the osteoclastic transcription factor NFATc1 at early stages in differentiation, whereas expression of c-Fos was not affected (Supplementary Figure 1C). In addition, expression of the osteoclastic cell fusion genes ATP6v0d2 and DC-STAMP, as well as of TNFα was increased early in osteoclast differentiation in the C/EBPβ mutant osteoclasts (Supplementary Figure 1C). TNFα has been shown to enhance RANK-L-induced osteoclast differentiation (Lam et al, 2000) and its contribution to the enhanced osteoclastogenesis in c/ebpβ−/− and L/L cells was studied therefore in more detail. Cell cultures from c/ebpβ−/− and L/L mutant mice displayed elevated TNFα protein expression (Supplementary Figure 2A) and neutralization of TNFα by the TNFα antagonist Etanercept (a decoy receptor of TNFα (Childs et al, 2001)) restrained excessive cell fusion in both mutants (Supplementary Figure 2B). No effect of Etanercept on the formation of osteoclasts from WT cultures was observed (Supplementary Figure 2A). Thus, C/EBPβ defects augment TNFα expression, which contributes to the exacerbated osteoclastogenesis.
mTOR regulates osteoclastogenesis by switching C/EBPβ isoforms
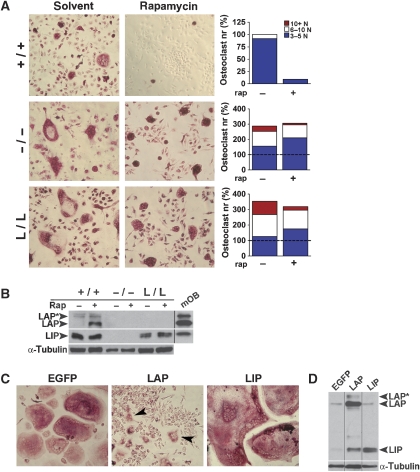
Alternative translation initiation of C/EBPβ isoforms can be shifted towards LAP expression by inhibition of mTOR signalling with rapamycin (Calkhoven et al, 2002; Jundt et al, 2005). Bone marrow derived osteoclasts from WT or C/EBPβ mutant mice were treated with rapamycin and stained for TRACP-activity (Figure 7A). Rapamycin completely blocked formation of multi-nucleated osteoclasts in WT cultures (Figure 7A). In contrast, osteoclast formation was only slightly affected by rapamycin treatment in cells from both c/ebpβ−/− and L/L mice (which do not express or cannot switch to the LAP isoform) (Figure 7A). Protein expression analysis confirmed that rapamycin treatment caused a shift towards LAP expression in WT cultures (approximately five-fold change of the LAP to LIP ratio; Figure 7B). These data show that the inhibition of osteoclastogenesis by rapamycin depends on C/EBPβ LAP, as cells derived from both c/ebpβ−/− and L/L mice are almost unresponsive. We conclude that mTOR adjusts the expression of C/EBPβ isoforms that in turn differentially control osteoclastogenesis.
Figure 7.
The mammalian target of rapamycin (mTOR) regulates osteoclastogenesis by switching C/EBPβ isoforms. (A) Representative pictures of primary bone marrow derived monocytic precursors from indicated genotypes differentiated into osteoclasts in the absence (solvent) or presence of rapamycin. Osteoclasts were stained for TRACP after 6 days (red staining). Bar graphs show quantification of differentiated osteoclasts (by number of nuclei per cell). The values from WT cultures was set at 100% (indicated as dashed line). Note the difference in scale between the results from WT and mutant cultures. A representative experiment is shown. (B) Western blot analysis of C/EBPβ isoform expression (LAP*, LAP and LIP) in primary osteoclasts treated with rapamycin (Rap), as indicated. Positive control consisting of mature osteoblasts (mOB) is shown to indicate the different C/EBPβ isoforms. The positive control was run on the same gel, but was noncontiguous, as indicated with the black line. Loading was controlled by α-tubulin expression. (C) Representative pictures of RANK-L-induced osteoclast differentiation in RAW264.7 cells stably expressing the indicated C/EBPβ isoforms or EGFP control. Osteoclasts were stained for TRACP (red staining). Arrowheads indicate small osteoclasts present in the LAP cultures. (D) Western blot analysis of C/EBPβ isoform expression (LAP*, LAP and LIP) in RAW264.7 cells stably expressing the C/EBPβ isoforms LAP or LIP, or EGFP (as control) and differentiated into osteoclasts. The lanes were run on the same gel, but were noncontiguous as indicated with the black lines. +/+, WT mice; −/−, c/ebpβ−/− mice; L/L, LIP k.i. mice.
The LAP and LIP isoforms were expressed in the RAW264.7 monocytic cell line (Figure 7D) to examine whether C/EBPβ isoforms differentially affect osteoclastogenesis. As shown in Figure 7C, control cells transfected with EGFP differentiated into multi-nucleated osteoclasts in the presence of RANK-L. Ectopic expression of LIP strongly enhanced osteoclastogenesis. Exceptionally large osteoclasts were formed, 40% of which contained >50 nuclei (Figure 7C). In contrast, ectopic expression of LAP almost entirely abolished osteoclast formation (Figure 7C). Most cells remained mononuclear and TRACP-negative and only few, predominantly small TRACP-positive osteoclasts (containing 3–5 nuclei/cell) were present. These data show that the LAP isoform of C/EBPβ restricts osteoclastogenesis and the LIP isoform enhances osteoclastogenesis.
C/EBPβ isoforms regulate osteoclastogenesis through MafB expression
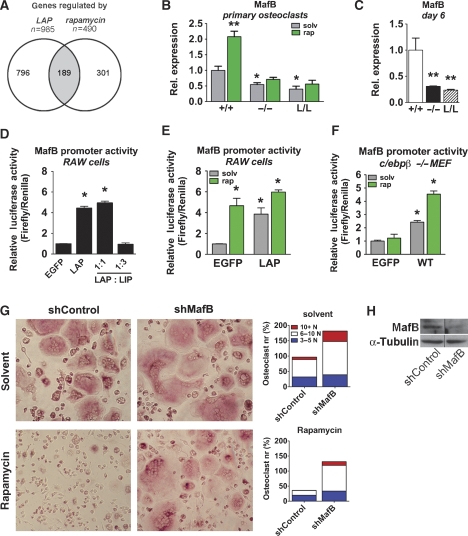
Gene expression profiling was performed to determine possible common genetic targets of C/EBPβ or rapamycin involved in osteoclastogenesis. A total number of 985 genes were differentially expressed upon LAP expression in RAW264.7 cells under osteoclastic differentiation conditions. Similarly, 490 genes were differentially regulated by rapamycin under similar conditions. Interestingly, comparison of LAP and rapamycin-induced gene expression profiles revealed that 39% of the genes were co-regulated (of the 490 differentially expressed genes following rapamycin treatment, 189 genes were shared with the LAP-induced genes) (Figure 8A). The extensive overlap in gene regulation further support the notion of C/EBPβ as an important target of rapamycin in osteoclast differentiation.
Figure 8.
C/EBPβ isoforms regulate osteoclastogenesis through MafB expression (A) Venn diagram and number of differentially regulated genes in RAW264.7 cells induced to differentiate into osteoclasts for 2 days as identified by gene array analysis. Control cells (empty vector) and cells stably expressing LAP were compared with cells treated with rapamycin. (B) Real-time RT–PCR analysis of MafB expression in c/ebpβ−/− and L/L osteoclasts cultured for 2 days with M-CSF and RANK-L as well as in the absence (grey bars) or presence of rapamycin (green bars), as indicated. Values represent relative expression levels compared with WT (set as 1). (C) Real-time RT–PCR analysis of MafB expression in osteoclasts of WT, c/ebpβ−/− and L/L mice cultured for 6 days under osteoclastogenic conditions. Values represent relative expression levels compared with WT (set as 1). Data are presented as mean±s.e.m.; n=6 per group. *P<0.05, **P<0.01 versus WT. (D) Luciferase reporter assay using a mouse MafB promoter reporter. RAW264.7 cells were transfected with empty control (EGFP) or with C/EBPβ isoforms. Values were normalized to CMV-promoter driven Renilla luciferase activity. (E) Luciferase reporter assay using a mouse MafB promoter reporter. RAW264.7 cells were transfected with empty control (EGFP) or LAP expression vector, treated with RANK-L and with rapamycin (green bars), as indicated. (F) C/EBPβ-deficient MEFs were transfected with the mouse MafB promoter reporter and control (empty vector, EGFP) or C/EBPβ (WT) and treated with rapamycin (green bars), as indicated. Data are presented as mean±s.e.m, *P<0.05 versus control. (G) Representative pictures of RANK-L-induced osteoclast differentiation of RAW264.7 cells with stable MafB short hairpin interfering RNA (shMafB) or control, in the absence (solvent) or presence of rapamycin, as indicated. Osteoclasts were stained for TRACP (red staining). Bar graphs show quantification of differentiated osteoclasts (number of nuclei per cell), in the absence (solvent) or presence of rapamycin. The values from control cultures in the presence of solvent are set at 100%. A representative experiment is shown. (H) Knock-down of MafB using a shRNA in RAW264.7 cells as determined by western blot analysis. The lanes were run on the same gel, but were noncontiguous. Loading was controlled by analysis of α-tubulin expression.
Among the LAP and rapamycin activated genes, MafB stands out (66-fold and 7.4-fold upregulated by LAP and rapamycin treatment, respectively). MafB is a bZIP transcription factor, recently suggested to be a negative regulator of osteoclast differentiation (Kim et al, 2007). Rapamycin treatment of WT osteoclasts increased expression of MafB (Figure 8B) and decreased expression of various osteoclastic markers (data not shown), confirming the profiling data. In the C/EBPβ mutant osteoclasts that are unresponsive to rapamycin treatment and pursue unrestricted osteoclastogenesis, MafB expression remains low and unaffected after 2 days of culture (Figure 8B). In agreement with this, rapamycin did not affect increased expression of NFATc1, ATP6v0d2 or DC-STAMP in C/EBPβ mutant osteoclasts (data not shown). In the absence of LAP (c/ebpβ−/− and L/L osteoclasts), MafB expression even further decreases during osteoclast differentiation compared with WT on day 6 of culture (Figure 8C).
The MafB promoter contains a potential C/EBP binding site (Huang et al, 2000) and LAP expression resulted in increased MafB promoter activity, whereas LIP repressed MafB promoter driven transactivation (Figure 8D). Treatment of RAW264.7 cells (under osteoclastic differentiation conditions) with rapamycin, similarly to LAP expression, resulted in transactivation of the MafB promoter (Figure 8E). These data suggest that the LAP isoform of C/EBPβ inhibits osteoclastogenesis by increasing MafB expression and that absence of the long C/EBPβ isoforms or presence of LIP opposes MafB expression, resulting in enhanced osteoclast differentiation. To examine these possibilities, gene reporter studies were carried out in C/EBPβ-deficient mouse embryonic fibroblasts (MEFs). In the absence of C/EBPβ, the MafB promoter could not be activated by rapamycin (Figure 8F); however, complementation with full-length C/EBPβ (WT, a construct that contains the upstream open reading frame (uORF)) activated the MafB promoter, which was further enhanced by rapamycin (Figure 8F).
We conclude that rapamycin mediated mTOR inhibition that switches C/EBPβ expression towards the LAP isoform, upregulates MafB which then in turn inhibits osteoclastogenesis. In support of this idea, down-regulation of MafB expression by interferring RNA overruled rapamycin treatment whereas control small hairpin RNA (shRNA) constructs did not affect differentiation (Figure 8G and H).
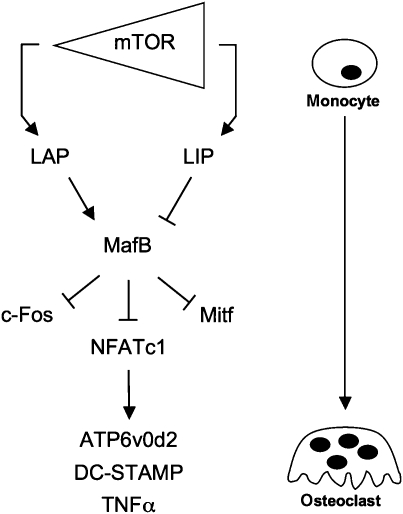
Altogether these data show that C/EBPβ mediates the inhibitory effect of rapamycin on osteoclast differentiation, by regulating MafB. Rapamycin induces the production of the LAP isoform of C/EBPβ and LAP induces the expression of MafB, which subsequently inhibits osteoclast differentiation by negatively regulating osteoclastic transcription factors, including NFATc1 (Kim et al, 2007) and its downstream targets DC-STAMP, ATP6v0d2 and TNFα (Kim et al, 2008) (Figure 9).
Figure 9.
C/EBPβ as a switch in osteoclastogenesis. Schematic representation of how differences in mTOR activity alters the C/EBPβ isoform ratio to regulate osteoclastogenesis. Rapamycin inhibits mTOR, which causes enhanced expression of LAP. LAP induces expression of MafB. MafB inhibits NFATc1 and other osteoclastic transcriptional regulators (c-Fos and Mitf), which results in the down-regulation of osteoclastic genes including TNFα and the cell fusion genes ATP6v0d2 and DC-STAMP (partially derived from Kim et al, 2007, 2008). Enhanced expression of LAP therefore inhibits osteoclast differentiation, whereas LIP (produced at high mTOR activity) forces osteoclast differentiation.
Discussion
Here, we show that isoforms of the transcription factor CCAAT/enhancer binding protein (C/EBP) β and its alternative translational initiation are critical in bone formation and resorption. We describe two complex novel bone phenotypes in animals that either lack all C/EBPβ isoforms or that express only the truncated LIP isoform. C/EBPβ-deficient mice and mice that express the LIP isoform only (L/L) display enhanced bone resorption. However, only C/EBPβ-deficient mice suffer from osteopenia due to combined decrease in bone formation and enhanced resorption, whereas osteogenesis in LIP k.i. mutants exceeds enhanced bone resorption and results in increased bone mass. Although resolving the complexity of the described bone phenotypes requires further analysis, including constructing conditional and cell-type specific k.i. mutants, important conclusions can be drawn from our observations.
C/EBPβ and bone homeostasis
C/EBPβ deficiency resulted in reduced postnatal osteogenesis, as suggested by recent findings obtained in prenatal and newborn mice (Tominaga et al, 2008), as well as in adult mice (Zanotti et al, 2009). C/EBPβ therefore displays non-redundant functions as other C/EBP family members are co-expressed in osteogenesis (C/EBPδ and the more distantly related CHOP (C/EBPζ)) (Umayahara et al, 1999; Gutierrez et al, 2002; Pereira et al, 2006, 2007; Shirakawa et al, 2006), but do not rescue the phenotype of C/EBPβ-deficient mice. Tominaga and colleagues showed osteoblast differentiation defects in c/ebpβ−/− osteoblasts (Tominaga et al, 2008), whereas in our cell culture experiments, the differentiation potential of C/EBPβ-deficient osteoblasts was not affected, as also observed when C/EBPβ was downregulated in primary osteoblasts (Zanotti et al, 2009). This discrepancy might be related to differences in the genetic background that apparently causes a more severe phenotype in C57Bl/6 as illustrated by the strongly reduced mendelian ratio (data not shown) (Tominaga et al, 2008), in comparison with our results obtained from a mixed genetic background. Thus, the observed reduced osteogenesis in c/ebpβ−/− mice in vivo may involve micro-environmental effects that impinge on osteoblast function.
Interestingly, expression of the LIP isoform from the murine C/EBPβ locus prevents the osteopenic phenotype, enhances osteoblast differentiation and even leads to an osteosclerotic phenotype in the animal. Our results therefore suggest that C/EBPβ, even without its transactivation domain, carries out essential functions in osteogenesis. It has been suggested that the bZIP domain of C/EBPβ might serve as a scaffold for the assembly of a transcriptionally functional complex consisting of C/EBPβ, Runx2 and ATF4, which may enhance activation of osteogenic genes (Hata et al, 2005; Tominaga et al, 2008). Although LIP is thought to act as a transdominant inhibitor, it has also been suggested that long C/EBPβ isoforms could act as repressors (Kowenz-Leutz et al, 1994; Williams et al, 1995; Lamb et al, 2003; Mo et al, 2005) and that LIP might abrogate repression by removal of long C/EBPβ isoforms (repression of a repressor) (Lamb et al, 2003). The fact that we see both, up- and downregulated genes in C/EBPβ-deficient osteoblasts, supports the notion that C/EBPβ may function as both, activator and repressor in the same cell type and in a gene context specific manner. However, how exactly removal of the transactivation function of C/EBPβ contributes to the enhanced gene expression in osteoblasts will require additional experiments.
Collagen promoter based transgenic mice, which overexpress LIP in the osteoblast lineage develop osteopenia (Harrison et al, 2005), whereas the L/L k.i. mice described here display increased bone mass. This discrepancy could be due to dissociation of the coupling process between LIP expression in osteoblasts and osteoclasts, or to gene dosage effects. The LIP-transgene might be temporally misexpressed (Harrison et al, 2005) and the observed osteopenia has recently been suggested to be secondary to decreased OC expression in the bone (Tominaga et al, 2008). Moreover, in the LIP transgenic mice, all isoforms of the endogenous C/EBPβ messenger RNA are still present and could contribute to the observed phenotype. In our study, LIP replaces the endogenous C/EBPβ gene, resulting in the sole expression of LIP when C/EBPβ becomes expressed. Cell type and/or conditional knock-in animals will have to be constructed to solve any remaining discrepancies and to unravel the molecular mechanism of LIP function in osteogenesis. Nevertheless, all data show that C/EBPβ is part of the transcription factor network regulating bone cell functions.
C/EBPβ controls osteoclastogenesis through mTOR mediated isoform switching and MafB gene regulation
A major result of this study is the identification of C/EBPβ as regulator of osteoclastogenesis. Lack of C/EBPβ, or lack of the transactivating isoforms, resulted in the formation of enlarged, hyperactive osteoclasts in animals and in tissue culture. Alternative translation initiation from a single mRNA may generate different C/EBPβ isoforms. The C/EBPβ isoform ratio is adjusted by an uORF that senses the activity status of the translation machinery, in particular the eukaryotic translation initiation factor eIF4E, which is controlled by the mTOR pathway. Rapamycin treatment has been shown to inhibit osteoclastogenesis in bone marrow cell cultures (Glantschnig et al, 2003; Kneissel et al, 2004) and to prevent ovariectomy-induced bone loss in rats (Kneissel et al, 2004), yet the mechanism downstream of mTOR remained unknown (Boyce et al, 2006). Our data provide the genetic link and a rational explanation for the rapamycin effect by showing that it depends on switching between C/EBPβ isoforms.
The phenotypic coincidence of the c/ebpβ knock-out and the L/L genotypes on one hand and the surprisingly large overlap between genes that are activated by LAP and by rapamycin on the other hand suggested that full-length C/EBPβ may induce expression of an osteolytic inhibitor. Loss of an inhibitor of osteoclastogenesis as the mechanism of C/EBPβ activation is consistent with the gain-of-function phenotype observed in both c/ebpβ−/− and in L/L cells.
We found that MafB was strongly upregulated by LAP and by rapamycin and suppressed by LIP and by c/ebpβ deficiency. MafB is a bZIP transcription factor, important for podocyte differentiation (Sadl et al, 2002; Moriguchi et al, 2006), rhombomere specification in the early hindbrain (Cordes and Barsh, 1994) and for respiratory control (Blanchi et al, 2003). Moreover, in the haematopoietic system, MafB regulates myeloid differentiation and promotes macrophage differentiation (Sieweke et al, 1996; Kelly et al, 2000). MafB deficiency in macrophages results in an altered actin organization (Aziz et al, 2006) and reduced F4/80 expression (Moriguchi et al, 2006). Recently, MafB has been associated with osteoclast differentiation. MafB negatively regulates RANK-L-induced osteoclastogenesis by attenuating DNA binding of the key regulators NFATc1, c-Fos and Mitf (Kim et al, 2007).
Here, we show that inhibition of osteoclastogenesis by either expression of LAP or by rapamycin depends on MafB. Furthermore, and as observed in c/ebpβ−/− and L/L osteoclasts, decreased levels of MafB result in increased levels of the key regulator of osteoclastogenesis NFATc1 and its downstream target OSCAR (Kim et al, 2007). NFATc1 is not only a key regulator of osteoclast differentiation (Takayanagi et al, 2002; Asagiri et al, 2005; Aliprantis et al, 2008), but also regulates the cell fusion genes ATP6v0d2, DC-STAMP (Kim et al, 2008) and TNFα expression (Peng et al, 2001; Kaminuma et al, 2008). We found that in c/ebpβ−/− and L/L osteoclasts the cell fusion genes ATP6v0d2, DC-STAMP and TNFα, were upregulated. Hyperactive osteoclasts and increased TNFα production are often involved in bone diseases, including osteoporosis (Rodan and Martin, 2000; Helfrich, 2003; Teitelbaum and Ross, 2003; Ehrlich and Roodman, 2005). Autocrine TNFα production in osteoclasts derived from c/ebpβ−/− or L/L mice indeed contributed to augmented osteoclastogenesis, consistent with earlier reports (Lam et al, 2000; Fuller et al, 2002; Kim et al, 2008).
Therefore, our data provide strong genetic and mechanistical evidence that adjustment of the C/EBPβ isoform ratio by alternative translation of C/EBPβ determines the MafB expresssion status and osteoclastogenesis (Figure 9). We speculate that changes in C/EBPβ isoform ratio could be involved in human bone diseases and drugs targeting translational control and thus altering the C/EBPβ isoform ratio may possibly enable novel therapeutic strategies in the management and treatment of osteolytic bone diseases, such as osteoporosis and multiple myeloma.
Materials and methods
Generation of c/ebpβ mutant mice
The c/ebpβ knock-out mouse strain has been described earlier (Sterneck et al, 1997) and the LIP knock-in mice have been generated as described in the Supplementary data. The genetic background of both c/ebpβ knock-out and LIP knock-in mice was 129 × C57Bl/6. Mice were kept under pathogen-free conditions. Mice were bred from heterozygous breeders and littermates were compared with each other. The WT littermates of both c/ebpβ knock-out and LIP knock-in mice displayed similar bone parameters (bone histomorphometry and in cell culture experiments). Therefore, the WT control group is displayed as a single group. Animals were analysed at the age of 8 weeks. Both female and male mice showed the same phenotype and were analysed as one group. Mice were provided with standard mouse diet and water ad libitum on a 12-h light–dark cycle. All procedures and animal experiments were conducted in compliance with protocols approved by the institutional Animal Care and Use Committee.
Osteoclast cultures
Primary bone marrow cells from 8-week-old mice were cultured as described earlier (de Vries et al, 2005), in the presence of 30 ng/ml recombinant M-CSF (R&D Systems) and with or without 20 ng/ml recombinant murine RANK-L-TEC (R&D Systems) for 6 days on plastic or for 7 days on bovine cortical bone slices. The TNFα antagonist Etanercept (Enbrel) was used at 10 μg/ml. Rapamycin (0.5 μM) and Etanercept treatment were started at day 0. TRACP staining was performed with the leukocyte acid phosphatase kit (Sigma). For osteoclast cultures of 2 days, BMMs were prepared as described before (Takayanagi et al, 2002) and osteoclast differentiation was induced as described above for 2 days. To assess the resorptive activity of osteoclasts, bone slices were stained with coomassie brilliant blue to visualize resorption pits as described earlier (de Vries et al, 2005). RAW264.7 cells (ATCC) were cultured in the presence of 20 ng/ml recombinant murine RANK-L-TEC for 6 days to induce osteoclast differentiation.
Osteoblast cultures
Primary osteoblast precursors were cultured from neonatal mice (1–6 days old) as described earlier (Jochum et al, 2000). After 14 and 21 days of culture, cells were fixed stained for ALP and mineralized bone nodules were identified by staining with 2% Alizarin Red S (Sigma). Co-cultures of osteoblasts and osteoclasts were performed as described earlier (van't Hof, 2003).
Protein analysis
All cell types were lyzed with RIPA buffer. For osteoblasts, proteins were isolated before adding osteoblastogenic agents (considered as preosteoblasts), 7 days after adding osteogenic agents (considered as osteoblasts) and at the appearance of bone nodules (considered as mature osteoblasts). For osteoclasts, proteins were isolated from bone marrow derived monocytic precursors at day 0, 3 and 6 after addition of M-CSF and RANK-L. For the liver protein extracts, 8-week-old animals were used. Proteins were separated on 15% SDS–polyacrylamide gels, transferred onto nitrocellulose membranes and probed with anti-C/EBPβ (C-19), anti-α-tubulin or anti-α-actin (all from Santa Cruz Biotechnology Inc.), anti-MafB (Abcam) and anti-cleaved caspase-3 (Cell Signaling) using standard procedures.
Biochemical assays
In vivo osteoclast activity was determined by measuring total DPD cross-links in urine samples, which was expressed relative to urinary creatinine levels (Metra Biosystems, Inc.). Eight animals of each genotype were analysed.
Media samples of osteoclast cultures were collected on day 6 of cell culture and used for quantifying TNFα protein levels by ELISA (BD Biosciences).
RNA isolation and real-time RT–PCR
Total RNA of primary osteoclasts was isolated on day 6 of culture using the RNeasy mini kit (Qiagen) and cDNA was synthesized using the SuperScript II reverse transcriptase, as described by the manufacturer (Invitrogen). Real-time RT–PCR was performed on an ABI PRISM 7000 (Applied Biosystems) using the SYBR Green PCR Master Mix (Applied Biosystems) as described earlier (de Vries et al, 2006). RNA of osteoblasts was isolated using TriPure isolation reagent (Roche) and cDNA was synthesized as described for the osteoclasts. Real-time RT–PCR analysis was performed on a LightCycler type II (Roche) using the SYBR Green PCR Master Mix (Roche). Expression of GAPDH was used to normalize individual RNA expression levels. The data are expressed as relative RNA expression levels and calculated using the comparative CT method. The WT expression level was set at 1. Sequences of primer pairs used can be obtained upon request.
Expression of C/EBPβ isoforms in RAW264.7 cells
The cDNAs of rat c/ebpβ isoforms LAP and LIP were cloned into a pIRES-EGFP vector. RAW264.7 cells were transfected with FuGENE (Roche) and after 48 h, the cells were washed once and selected for 2–3 weeks in the presence of neomycin. EGFP-positive cells were sorted by FACS and were induced to differentiate into osteoclasts as described above. Three independent experiments were performed.
Gene array analyses and shRNA experiments
For gene array analyses, total RNA was isolated from RAW264.7 control cells (empty vector) treated with rapamycin or cells expressing LAP, using the RNeasy mini kit (Qiagen). The cells were induced to differentiate into osteoclasts by treatment with 20 ng/ml RANK-L for 48 h. RNA (1 μg) expression analysis was performed using the Agilent 4x44K whole genome mouse array (Agilent) and analysed per manufacturer's instructions at ImaGenes (Berlin, Germany). Each gene array experiment was performed in triplicate.
For short hairpin interfering RNA experiments, shRNAs were expressed in psiRNA (Invivogen). shRNA oligos against mouse MafB were designed using the ‘InvivoGen's siRNA Wizard' program (http://www.sirnawizard.com/design.php). As control, a non-specific shRNA was used. Sequences targeted by shRNAs: Control (5′-GTCCATCGAACTCAGTAGCT-3′) and mouse MafB (5′-GGCAACTAACGCTGCAACTCT-3′). Cells were transfected with FuGENE (Roche) and after 48 h, the cells were washed once and selected for 2–3 weeks in the presence of zeocin. EGFP-positive cells were sorted by FACS and were induced to differentiate into osteoclasts as described above.
Luciferase gene reporter assay
The MafB luciferase reporter plasmid containing the –609 bp MafB promoter inserted in the promoter-less luciferase vector pGVB2 was a gift from M Sakai (Hokkaido University School of Medicine, Sapporo, Japan) (Huang et al, 2000). RAW264.7 cells or spontaneously immortalized C/EBPβ−/− MEFs were transfected with TransIT-LT1 (Mirus) with the MafB reporter plasmid, the indicated C/EBPβ expression constructs and a CMV-promoter driven Renilla luciferase vector as an internal control at a ratio of 5:1. RANK-L or rapamycin were added to the RAW264.7 cells and rapamycin to the MEFs 7 h after transfection. Firefly and Renilla luciferase activity was assayed 48 h after transfection. Firefly luciferase activity was normalized to Renilla luciferase to control for transfection efficiency. The data are representative of three independent experiments, duplicates are plotted as the mean±s.e.m.
Statistical analysis
In all experiments, data are expressed as means±s.e.m. and c/ebpβ−/− and LIP k.i. mice were compared with WT mice. Statistical differences between groups were determined by one-way ANOVA with Dunnett post-test. A P-value of <0.05 was considered to be statistically significant.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figures Legends
Supplementary Table 1
Supplementary Data
Acknowledgments
We are indebted to Drs C Birchmeier and T Müller (MDC, Berlin, Germany) for providing reagents, advice and help with targeted mouse genetics. We are grateful to Dr J-P David (German Rheumatism Research Center Berlin, Germany) and Dr GR Burmester (Charité, University Medicine Berlin, Germany) for providing Etanercept, Dr M Sakai (Department of Biochemistry, Hokkaido University School of Medicine, Sapporo Japan) for providing the MafB luciferase reporter construct. Furthermore, we thank Dr H-P Rahn (MDC, Berlin, Germany) for FACS sorting, R Zarmstorff and P Gossen-Heinrich for technical assistance. We also thank Dr BCJ van der Eerden (Erasmus Medical Center, Rotterdam, The Netherlands) for helpful discussions and advice as well as Dr K Zaragoza, Dr K Wethmar and the other members of the Leutz lab for discussions. This work was supported by an individual Marie Curie fellowship from the European Community to JJS (MEIF-CT-2005-009611) and the Berliner Krebsgesellschaft (LEFF200708).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akira S, Kishimoto T (1992) IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev 127: 25–50 [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH (2008) NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest 118: 3775–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202: 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz A, Vanhille L, Mohideen P, Kelly LM, Otto C, Bakri Y, Mossadegh N, Sarrazin S, Sieweke MH (2006) Development of macrophages with altered actin organization in the absence of MafB. Mol Cell Biol 26: 6808–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begay V, Smink J, Leutz A (2004) Essential requirement of CCAAT/enhancer binding proteins in embryogenesis. Mol Cell Biol 24: 9744–9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH (2003) MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci 6: 1091–1100 [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L, Yao Z, Shakespeare WC, Wang Y, Metcalf CA III, Sundaramoorthi R, Dalgarno DC, Iuliucci JD, Sawyer TK (2006) Future anti-catabolic therapeutic targets in bone disease. Ann N Y Acad Sci 1068: 447–457 [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337–342 [DOI] [PubMed] [Google Scholar]
- Calkhoven CF, Muller C, Leutz A (2000) Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev 14: 1920–1932 [PMC free article] [PubMed] [Google Scholar]
- Calkhoven CF, Muller C, Leutz A (2002) Translational control of gene expression and disease. Trends Mol Med 8: 577–583 [DOI] [PubMed] [Google Scholar]
- Childs LM, Goater JJ, O′Keefe RJ, Schwarz EM (2001) Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res 16: 338–347 [DOI] [PubMed] [Google Scholar]
- Cordes SP, Barsh GS (1994) The mouse segmentation gene kr encodes a novel basic domain-leucine zipper transcription factor. Cell 79: 1025–1034 [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Schoenmaker T, Beertsen W, van der Neut R, Everts V (2005) Effect of CD44 deficiency on in vitro and in vivo osteoclast formation. J Cell Biochem 94: 954–966 [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Schoenmaker T, Wattanaroonwong N, van den Hoonaard M, Nieuwenhuijse A, Beertsen W, Everts V (2006) Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts. J Cell Biochem 98: 370–382 [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U (1991) A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67: 569–579 [DOI] [PubMed] [Google Scholar]
- Ehrlich LA, Roodman GD (2005) The role of immune cells and inflammatory cytokines in Paget's disease and multiple myeloma. Immunol Rev 208: 252–266 [DOI] [PubMed] [Google Scholar]
- Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ (2002) TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 143: 1108–1118 [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA (2003) M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 10: 1165–1177 [DOI] [PubMed] [Google Scholar]
- Gutierrez S, Javed A, Tennant DK, van Rees M, Montecino M, Stein GS, Stein JL, Lian JB (2002) CCAAT/enhancer-binding proteins (C/EBP) beta and delta activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem 277: 1316–1323 [DOI] [PubMed] [Google Scholar]
- Harada S, Rodan GA (2003) Control of osteoblast function and regulation of bone mass. Nature 423: 349–355 [DOI] [PubMed] [Google Scholar]
- Harrison JR, Huang YF, Wilson KA, Kelly PL, Adams DJ, Gronowicz GA, Clark SH (2005) Col1a1 promoter-targeted expression of p20 CCAAT enhancer-binding protein beta (C/EBPbeta), a truncated C/EBPbeta isoform, causes osteopenia in transgenic mice. J Biol Chem 280: 8117–8124 [DOI] [PubMed] [Google Scholar]
- Hata K, Nishimura R, Ueda M, Ikeda F, Matsubara T, Ichida F, Hisada K, Nokubi T, Yamaguchi A, Yoneda T (2005) A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol Cell Biol 25: 1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich MH (2003) Osteoclast diseases. Microsc Res Tech 61: 514–532 [DOI] [PubMed] [Google Scholar]
- Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG (2006) C/EBPbeta is required for ‘emergency' granulopoiesis. Nat Immunol 7: 732–739 [DOI] [PubMed] [Google Scholar]
- Huang K, Serria MS, Nakabayashi H, Nishi S, Sakai M (2000) Molecular cloning and functional characterization of the mouse MafB gene. Gene 242: 419–426 [PubMed] [Google Scholar]
- Jochum W, David JP, Elliott C, Wutz A, Plenk H Jr, Matsuo K, Wagner EF (2000) Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med 6: 980–984 [DOI] [PubMed] [Google Scholar]
- Johnson PF (2005) Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118: 2545–2555 [DOI] [PubMed] [Google Scholar]
- Jundt F, Raetzel N, Muller C, Calkhoven CF, Kley K, Mathas S, Lietz A, Leutz A, Dorken B (2005) A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein beta and NF-kappaB activity in Hodgkin and anaplastic large cell lymphomas. Blood 106: 1801–1807 [DOI] [PubMed] [Google Scholar]
- Kaminuma O, Kitamura F, Kitamura N, Hiroi T, Miyoshi H, Miyawaki A, Miyatake S (2008) Differential contribution of NFATc2 and NFATc1 to TNF-alpha gene expression in T cells. J Immunol 180: 319–326 [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2: 389–406 [DOI] [PubMed] [Google Scholar]
- Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T (2000) MafB is an inducer of monocytic differentiation. EMBO J 19: 1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N (2007) MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 109: 3253–3259 [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SH, Ha Kim J, Choi Y, Kim N (2008) NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol 22: 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneissel M, Luong-Nguyen NH, Baptist M, Cortesi R, Zumstein-Mecker S, Kossida S, O′Reilly T, Lane H, Susa M (2004) Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone 35: 1144–1156 [DOI] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Leutz A (1999) A C/EBP beta isoform recruits the SWI/SNF complex to activate myeloid genes. Mol Cell 4: 735–743 [DOI] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Twamley G, Ansieau S, Leutz A (1994) Novel mechanism of C/EBP beta (NF-M) transcriptional control: activation through derepression. Genes Dev 8: 2781–2791 [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106: 1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME (2003) A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114: 323–334 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA (2005) Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 11: 76–81 [DOI] [PubMed] [Google Scholar]
- Mo X, Kowenz-Leutz E, Laumonnier Y, Xu H, Leutz A (2005) Histone H3 tail positioning and acetylation by the c-Myb but not the v-Myb DNA-binding SANT domain. Genes Dev 19: 2447–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S (2006) MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol 26: 5715–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C (2007) The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol 17: 318–324 [DOI] [PubMed] [Google Scholar]
- Pedersen TA, Bereshchenko O, Garcia-Silva S, Ermakova O, Kurz E, Mandrup S, Porse BT, Nerlov C (2007) Distinct C/EBPalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J 26: 1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL, Gerth AJ, Ranger AM, Glimcher LH (2001) NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14: 13–20 [DOI] [PubMed] [Google Scholar]
- Pereira RC, Stadmeyer L, Marciniak SJ, Ron D, Canalis E (2006) C/EBP homologous protein is necessary for normal osteoblastic function. J Cell Biochem 97: 633–640 [DOI] [PubMed] [Google Scholar]
- Pereira RC, Stadmeyer LE, Smith DL, Rydziel S, Canalis E (2007) CCAAT/Enhancer-binding protein homologous protein (CHOP) decreases bone formation and causes osteopenia. Bone 40: 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TC, Xu J, Zheng MH (2004) Interaction between osteoblast and osteoclast: impact in bone disease. Histol Histopathol 19: 1325–1344 [DOI] [PubMed] [Google Scholar]
- Poli V (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 273: 29279–29282 [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P (2002) CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289: 1508–1514 [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896 [DOI] [PubMed] [Google Scholar]
- Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S (2002) The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol 249: 16–29 [DOI] [PubMed] [Google Scholar]
- Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, Bistoni F, Frati L, Cortese R, Gulino A, Ciliberto G, Constantini F, Poli V (1995) Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J 14: 1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian T, Johnson PF (2006) Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle 5: 953–957 [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Maeda S, Gotoh T, Hayashi M, Shinomiya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K, Imamura T (2006) CCAAT/enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol Cell Biol 26: 6105–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Frampton J, Graf T (1996) MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell 85: 49–60 [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319 [DOI] [PubMed] [Google Scholar]
- Sterneck E, Johnson PF (1998) CCAAT/enhancer binding protein beta is a neuronal transcriptional regulator activated by nerve growth factor receptor signaling. J Neurochem 70: 2424–2433 [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF (1997) An essential role for C/EBPbeta in female reproduction. Genes Dev 11: 2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T (1995) Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 80: 353–361 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S (1997) Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J 16: 7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4: 638–649 [DOI] [PubMed] [Google Scholar]
- Tominaga H, Maeda S, Hayashi M, Takeda S, Akira S, Komiya S, Nakamura T, Akiyama H, Imamura T (2008) CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell 19: 5373–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S, Kaisho T, Tanaka T, Matsumoto M, Yamakami M, Omori H, Yamamoto M, Yoshimori T, Akira S (2007) The C/EBPbeta isoform 34-kDa LAP is responsible for NF-IL-6-mediated gene induction in activated macrophages, but is not essential for intracellular bacteria killing. J Immunol 179: 5378–5386 [DOI] [PubMed] [Google Scholar]
- Umayahara Y, Billiard J, Ji C, Centrella M, McCarthy TL, Rotwein P (1999) CCAAT/enhancer-binding protein delta is a critical regulator of insulin-like growth factor-I gene transcription in osteoblasts. J Biol Chem 274: 10609–10617 [DOI] [PubMed] [Google Scholar]
- van't Hof RJ (2003) Osteoclast formation in the mouse coculture assay. Methods Mol Med 80: 145–152 [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP (2005) The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102: 17507–17512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagra A, Cruzat F, Carvallo L, Paredes R, Olate J, van Wijnen AJ, Stein GS, Lian JB, Stein JL, Imbalzano AN, Montecino M (2006) Chromatin remodeling and transcriptional activity of the bone-specific osteocalcin gene require CCAAT/enhancer-binding protein beta-dependent recruitment of SWI/SNF activity. J Biol Chem 281: 22695–22706 [DOI] [PubMed] [Google Scholar]
- Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA (2008) HDAC1 promotes liver proliferation in young mice via interactions with C/EBPbeta. J Biol Chem 283: 26179–26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Baer M, Dillner AJ, Johnson PF (1995) CRP2 (C/EBP beta) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J 14: 3170–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Hsieh CC, Kurtz AJ, Rabek JP, Papaconstantinou J (2001) Regulation of CCAAT/enhancer-binding protein-beta isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucleic Acids Res 29: 3087–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202: 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti S, Stadmeyer L, Smerdel-Ramoya A, Durant D, Canalis E (2009) Misexpression of CCAAT/enhancer binding protein beta (C/EBPβ) causes osteopenia. J Endocrinol 201: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Nelson E, Radomska HS, Iwasaki-Arai J, Akashi K, Friedman AD, Tenen DG (2002) Induction of granulocytic differentiation by 2 pathways. Blood 99: 4406–4412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figures Legends
Supplementary Table 1
Supplementary Data