Abstract
Although cells of flowering plants lack a structurally defined microtubule-organizing center like the centrosome, organization of the spindles and phragmoplasts in mitosis is known to involve the evolutionarily conserved γ-tubulin complex. We have investigated the function of Arabidopsis thaliana NEDD1, a WD40 repeat protein related to the animal NEDD1/GCP-WD protein, which interacts with the γ-tubulin complex. The NEDD1 protein decorates spindle microtubules (MTs) preferentially toward spindle poles and phragmoplast MTs toward their minus ends. A T-DNA insertional allele of the single NEDD1 gene was isolated and maintained in heterozygous sporophytes, and NEDD1's function in cell division was analyzed in haploid microspores produced by the heterozygote. In approximately half of the dividing microspores exhibiting aberrant MT organization, spindles were no longer restricted to the cell periphery and became abnormally elongated. After mitosis, MTs aggregated between reforming nuclei but failed to appear in a bipolar configuration. Consequently, defective microspores did not form a continuous cell plate, and two identical nuclei were produced with no differentiation into generative and vegetative cells. Our results support the notion that the plant NEDD1 homolog plays a critical role in MT organization during mitosis, and its function is likely linked to that of the γ-tubulin complex.
INTRODUCTION
When plant meristematic cells undergo cell division, microtubules (MTs) consisting of α- and β-tubulin heterodimers undergo dynamic reorganization to form arrays of cortical MTs, the preprophase band, spindle, and the phragmoplast. In fungal and animal cells, MT organization is dependent on structurally defined microtubule-organizing centers (MTOCs) of the spindle pole body and the centrosome, respectively. Angiosperm cells are able to organize the MT arrays described above in the absence of an MTOC structure. The spatiotemporal organization of MTs is regulated by many proteins that interact with MTs. Among them, γ-tubulin plays an essential role in MT nucleation, stabilization, and organization by binding specifically to the minus end of MTs (Job et al., 2003; Wiese and Zheng, 2006; Raynaud-Messina and Merdes, 2007). In fungal and animal cells, γ-tubulin predominantly appears at the spindle pole body and the centrosome, respectively. The plant γ-tubulin, however, decorates all MT arrays during mitosis (Liu et al., 1993). In the bipolar arrays of the spindle and phragmoplast MTs, where MT minus ends are readily identified, γ-tubulin is arranged in a gradient, with the highest concentration at spindle poles and phragmoplast distal ends (Liu et al., 1995). More recent data have demonstrated that γ-tubulin, together with its associated proteins, is able to nucleate a new MT from the surface of an extant MT (Murata et al., 2005). In the model cress Arabidopsis thaliana, two γ-tubulin proteins play redundant roles in MT nucleation/organization in both interphase and mitotic cells (Liu et al., 1994; Binarova et al., 2006; Pastuglia et al., 2006).
In eukaryotes, γ-tubulin is part of the γ-tubulin ring complex, or γTuRC, which is >1000 kD in vivo (Zheng et al., 1995). γTuRC contains γ-tubulin and five structurally related accessory subunits, collectively known as γ-tubulin complex proteins (GCPs) (Murphy et al., 2001). Land plants contain the complete set of proteins that constitute a functional γTuRC (Murata et al., 2007). Experimental evidence supports two models for the essential role of γTuRC in MT nucleation (Wiese and Zheng, 2006). In the template model, the γ-tubulin subunits of γ-TuRC make shoulder-to-shoulder contacts, while each interacting with α-tubulin at the MT minus end. The protofilament model, however, suggests that the γ-tubulin subunits are copolymerized into a protofilament. The accessory GCP2-6 protein may function as a scaffold for the interaction between 13 γ-tubulin (GCP1) molecules and the MT minus end. In Arabidopsis, GCP2 and GCP3 are targeted to the nuclear envelope and play a role in MT nucleation (Erhardt et al., 2002; Seltzer et al., 2007). While mechanisms that regulate the interaction between GCP2/3 and the nuclear envelope are unknown, recent findings indicate that GCP2 is involved in organizing cortical MTs by precisely positioning the γ-tubulin–containing complex on preexisting MTs (Nakamura and Hashimoto, 2009). Functions of other GCP subunits have not been studied in plants. In fact, no biochemical evidence of γ-TuRC has been documented in plants to date.
Because γTuRC appears specifically at MTOCs, such as the centrosome in animal cells, an intriguing question is how this large protein complex is localized to the site at which it acts. In the fruitfly Drosophila melanogaster, a centrosome-localized WD40 repeat protein can be copurified with γTuRC (Gunawardane et al., 2003). The fly protein is related to the mouse protein NEDD1, which initially was discovered as a protein primarily expressed in the early stages of central nervous system development but that is developmentally downregulated in the brain (Kumar et al., 1994). NEDD1 (also known as GCP-WD) acts as an anchoring or attachment factor for γTuRC, which facilitates the recruitment of γTuRC to the centrosome in human cells, and is essential for spindle assembly (Haren et al., 2006; Lüders et al., 2006; Manning and Kumar, 2007). In contrast with NEDD1 in human cells, homologous proteins in D. melanogaster and the frog Xenopus laevis interact with γTuRC but are not required for targeting it to the centrosome (Vérollet et al., 2006; Liu and Wiese, 2008). Indeed, the most recent study on this subject shows that the Xenopus NEDD1 protein exists in a complex distinct from γTuRC (Liu and Wiese, 2008). Nevertheless, the aforementioned studies agree that NEDD1/GCP-WD plays a critical role in organizing MTs for MT aster assembly in vitro and in vivo.
Although fungi contain GCPs, all appear to lack NEDD1 (Wiese and Zheng, 2006). The land plants examined to date all contain genes encoding proteins that are more similar to NEDD1 than to any other WD40 repeat protein (Murata et al., 2007). While the animal NEDD1 acts at the centrosome in vivo, it is not known if the plant homolog interacts with MTs and if its function is required for MT organization. We have chosen the model plant Arabidopsis to answer these questions because of the availability of rich genetic and genomic resources. We named the Arabidopsis homolog NEDD1, according to the founding member of this family in mouse to avoid it simply being interpreted as being an integral GCP subunit. Published functional in vivo studies in animal systems were largely based on RNA interference–based knockdown or antibody-based protein depletion experiments. Here, we report the isolation of a stable T-DNA insertional mutation at the NEDD1 locus that can be transmitted via sexual reproduction in Arabidopsis. Haploid mutant cells produced by the heterozygous mutant have allowed us to characterize the function of NEDD1 in mitotic cell division.
RESULTS
Arabidopsis NEDD1 Is a Homolog of Animal NEDD1/GCP-WD Proteins
Among the proteins containing WD40 repeats in Arabidopsis, the polypeptide encoded by the locus At5g05970 was found to resemble animal NEDD1/GCP-WD. As determined by the cDNA sequence, this NEDD1 protein of 781 amino acids has a calculated molecular mass of 85 kD and contains six WD40 repeats at the N terminus and coiled coils at the C terminus (Figure 1A). A phylogenetic analysis showed that NEDD1 is closely related to other putative plant orthologs, including NEDD1 proteins from the monocot rice (Oryza sativa), the moss Physcomitrella patens, and the lycophyte Selaginella moellendorffii (Figure 1B; see Supplemental Data Set 1 online). It is more closely related to animal NEDD1/GCP-WD proteins than to other WD40 proteins, like COP1, in Arabidopsis (Figure 1B).
Figure 1.
Arabidopsis NEDD1 Is Related to the NEDD1/GCP-WD Proteins in Animals.
(A) Diagrammatic representation of the At NEDD1 protein. The six WD40 repeats toward the N terminus and the coiled-coil domain toward the C terminus are highlighted.
(B) Phylogenetic relationship among At NEDD1, other plant NEDD1 homologs, and animal NEDD1/GCP-WD proteins. The plant proteins analyzed are from rice (Os NEDD1), the moss P. patens (Pp NEDD1), and the lycophyte S. moellendorffii (Sm NEDD1). The animal proteins include Hs GCP-WD, Mm NEDD1, Xl NEDD1, and Dgp71WD. The Arabidopsis WD40 protein COP1 is used as an outgroup in the phylogenetic analysis. Bootstrap values at the branches represent the percentages obtained in 1000 replications. Only values >50% are presented. The values presented in parentheses reflect identities and similarities, respectively, of the amino acid sequences when compared with At NEDD1. N.A., not applicable.
(C) The native At NEDD1 protein is detected as a band with a molecular mass of 98 kD by immunoblotting. The molecular mass markers (kD) are shown on the right.
Arabidopsis NEDD1 Decorated Mitotic Spindles and the Phragmoplast
Affinity-purified anti-AtNEDD1 antibodies recognized a band of ∼98 kD in immunoblots using protein extracts from 3-d-old etiolated Arabidopsis seedlings (Figure 1C). After the antibodies were preincubated with the glutathione S-transferase (GST)-AtNEDD1 fusion protein used as the antigen for antibody production, the band was no longer detected.
Arabidopsis NEDD1 was localized in root meristematic cells by immunofluorescence using specific antibodies. Distinct NEDD1 localization was first detected in prophase on the nuclear envelope, where it appeared to cap the future spindle poles (Figures 2A to 2D, arrowheads). Later, NEDD1 signals were striking at the poles of prophase spindles (Figures 2E to 2H, arrowheads). NEDD1 was detected along kinetochore MTs of the metaphase spindle, with more prominent signals toward the poles (Figures 2I to 2L). In anaphase, conspicuous NEDD1 signals were detected with the shortening kinetochore fibers (Figures 2M to 2P, arrowheads). At this stage, NEDD1 was not obviously detected in the spindle midzone, where interzonal MTs were bundled. In the developing phragmoplast, NEDD1 appeared more pronounced toward the minus end of MTs (Figures 2Q to 2T). While the dark gap in antitubulin fluorescence in the middle of the phragmoplast was very narrow, that of anti-AtNEDD1 was significantly wider (Figure 2Q, arrows). This wider dark gap persisted as the phragmoplast MT array expanded centrifugally toward the parental plasma membrane.
Figure 2.
Immunolocalization of Arabidopsis NEDD1 in Dividing Meristematic Cells.
All cells were stained for NEDD1 (first column), MTs (second column), and DNA (third column). The merged color images have NEDD1 in green, MTs in red, and DNA in blue. Bar = 5 μm.
(A) to (E) At prophase, NEDD1 exhibits a cap-like localization pattern in the future spindle poles on the nuclear envelope (arrowheads).
(E) to (H) At late prophase, NEDD1 is prominently present in the poles of the prophase spindle.
(I) to (L) At metaphase, NEDD1 appears along the kinetochore MT fibers.
(M) to (P) At anaphase, NEDD1 colocalizes with shortening kinetochore fibers (arrowheads), but not obviously with interzonal MTs.
(Q) to (T) NEDD1 is detected in the phragmoplast, with the signal more pronounced toward the minus ends, as indicated by a wider gap of anti-AtNEDD1 fluorescence (arrows) than that of antitubulin.
The NEDD1 localization pattern described above resembled that of γ-tubulin in dividing cells (Liu et al., 1993, 1995). We then tested whether Arabidopsis NEDD1 colocalized with γ-tubulin in cells of a transgenic plant expressing a functional AtNEDD1-c-myc fusion protein (see below). AtNEDD1-c-myc was detected by the mouse anti-c-myc 9E10 antibody and γ-tubulin by a rabbit antibody used in our previous studies (Liu et al., 1993). In a late anaphase cell shown in Figure 3, NEDD1 completely overlapped with γ-tubulin. Thus, we concluded that NEDD1 colocalized with γ-tubulin in dividing cells.
Figure 3.
Colocalization of Arabidopsis NEDD1 with γ-Tubulin.
In a late anaphase cell, the NEDD1 signal (A) appears to overlap with that of γ-tubulin (B). In the merged image (C), the yellow signal indicates that NEDD1 (in green) and γ-tubulin (in red) colocalized. DNA was pseudocolored in blue. Bar = 5 μm.
Distorted Genetic Segregation Caused by the nedd1 Mutation
To elucidate the function of Arabidopsis NEDD1, we sought to isolate a nedd1 mutant. A mutant allele was detected in a collection of T-DNA insertion transformants of Arabidopsis (Samson et al., 2002). The T-DNA was inserted in the third intron and confirmed in a heterozygous plant (Figure 4A). Notably, no homozygous offspring were recovered after genotyping 228 plants derived from self-crosses of this heterozygous NEDD1/nedd1 plant.
Figure 4.
Phenotypic Characterization of the nedd1 Mutant.
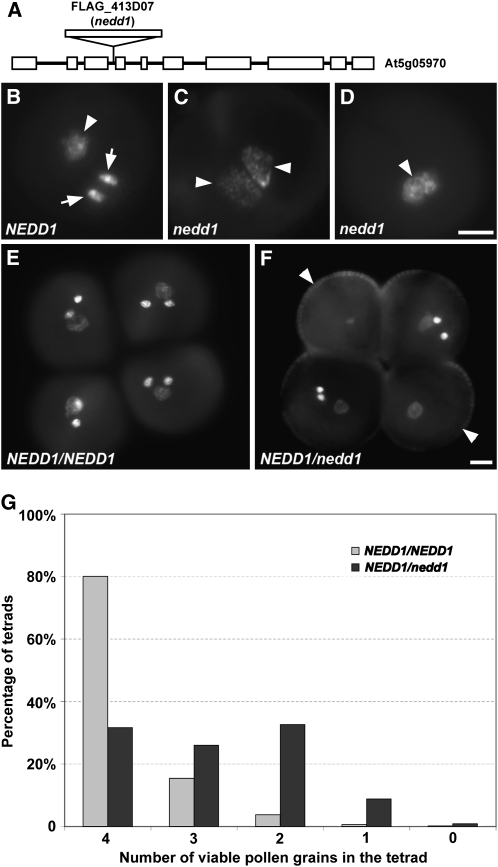
(A) Diagrammatical illustration of the NEDD1 gene structure and the position of the T-DNA insertion rendering the nedd1 mutation. Exons and introns are represented as boxes and lines, respectively.
(B) In wild-type pollen grains, two brighter sperm nuclei (arrows) and a less bright vegetative nucleus (arrowhead) are revealed by DAPI staining.
(C) A defective pollen grain produced by the NEDD1/nedd1 plant contains two identical loosely packed chromatin masses.
(D) A defective pollen grain of NEDD1/nedd1 with overlapping DNA masses.
(E) In the qrt/qrt background, the control plant produced four attached pollen grains, all of which contain the normal configuration of two sperm nuclei plus one vegetative nucleus.
(F) In the qrt/qrt background, the NEDD1/nedd1 plant produces four attached pollen grains: two normal and two (arrowheads) that lack sperm.
(G) Pollen viability assessment in tetrads in the qrt/qrt background. Percentages of tetrads containing 4, 3, 2, 1, or 0 viable pollen grains in the NEDD1/NEDD1 versus the NEDD1/nedd1 plants.
Bars = 5 μm.
The absence of a homozygous mutant in the progeny of the heterozygote prompted us to investigate how the mutation segregated during sexual reproduction. Because the T-DNA fragment was linked to both the BAR gene and the kanamycin-resistant gene, we examined the ratio of BASTA-resistant and -sensitive seedlings among the offspring of a self-crossed heterozygous plant (Table 1). Mendel's Law of Independent Assortment predicts a 3:1 ratio of resistant to sensitive seedlings; however, a ratio of 0.641:1 (n = 461) was obtained, indicating that the segregation was seriously distorted. To test if the segregation distortion was caused by the T-DNA insertion at the NEDD1 locus, a genomic NEDD1 fragment containing a 2-kb promoter region was introduced by transformation into the heterozygous plant. When the offspring of a transformant with the otherwise NEDD1/nedd1 background were tested by exposure to the herbicide BASTA, a 2.79:1 (n = 197) ratio of resistant to sensitive plants was detected (Table 1). Transformation with the control pGWB16 vector rendered a ratio of 0.668:1 (n = 402), similar to that derived from the untransformed parent. This critical genetic evidence allowed us to conclude that the segregation distortion was caused by the T-DNA insertion at the NEDD1 locus, and this insertion-caused inactivation was rescued by our At NEDD1 construct.
Table 1.
Genetic Segregation of the NEDD1/nedd1 Plant and Transformed Plants
| Parent | BASTAr | BASTAs | r/s Ratio | P Valuea |
|---|---|---|---|---|
| NEDD1/nedd1 | 180 | 281 | 0.641 | P < 0.0001 |
| NEDD1/nedd1 pGWB16 | 161 | 241 | 0.668 | P < 0.0001 |
| NEDD1/nedd1 pGWB-NEDD1 | 145 | 52 | 2.790 | P = 0.6509 |
P values were calculated by the χ2 test based on the expected resistant (BASTAr):sensitive (BASTAs) ratio of 3 (or 3:1) among progeny.
Gametophytic Defects Caused by the nedd1 Mutation
This segregation distortion phenotype of the NEDD1/nedd1 plant could be caused either by embryo lethality or defects in gametophyte development. Using Nomarski differential interference contrast optics, developing embryos in NEDD1/nedd1 ovules were indistinguishable from those of control plants. Therefore, we investigated the transmission efficiency (TE) of the mutation through gametophytes. Reciprocal crosses between the wild-type plant and the NEDD1/nedd1 heterozygote were performed. Because the T-DNA construct used for generating the insertion mutation also carried the kanamycin-resistant gene in addition to the BAR gene, the inheritance of the mutation could be tested by kanamycin resistance of the progeny. When all gametophytes were functional, the TE of the mutant allele through the male or female gamete would be 100%. When the NEDD1/nedd1 pollen grains were used to pollinate the stigma of a wild-type plant, however, transmission of the nedd1 mutation (the male TE) was as low as 21.8% (Table 2). Conversely, wild-type pollen grains were used to pollinate a NEDD1/nedd1 plant. The resulting TE through the female gamete was 48.3%, which was higher than the male TE but still significantly lower than the perfect value of 100%. Taken together, the segregation distortion exhibited by the NEDD1/nedd1 plant was largely caused by defective male gametophytes and, to a lesser extent, by defects in female gametophytes.
Table 2.
TE of the nedd1 Mutation in Reciprocal Crosses between NEDD1/nedd1 and Wild-Type (NEDD1/NEDD1) Plants
| ♀ Parent | ♂ Parent | kanr Progeny | kans Progeny | TEa |
|---|---|---|---|---|
| NEDD1/NEDD1 | NEDD1/nedd1 | 96 | 440 | ♂ TE = 21.8% |
| NEDD1/nedd1 | NEDD1/NEDD1 | 87 | 180 | ♀ TE = 48.3% |
TE of the nedd1 mutation through mutant gametes was the ratio of NEDD1/nedd1 to NEDD1/NEDD1 progeny, expressed as kanr and kans for kanamycin resistant and sensitive, respectively. TEs of the nedd1 mutation through the male and female gametes were expressed as ♂ TE and ♀ TE, respectively.
The seriously reduced TE of the T-DNA allele through the male gamete prompted us to examine pollen grains harvested from the NEDD1/nedd1 plant. Using 4',6-diamidino-2-phenylindole (DAPI) staining, most if not all of the pollen grains (n = 131) collected from mature anthers of the wild-type plant appeared to contain three nuclei. Among them, two were the brightly stained sperm nuclei, and the faintly stained nucleus was the vegetative nucleus (Figure 4B). Comparatively, only 45.8% (n = 1003) of pollen grains collected from a NEDD1/nedd1 plant exhibited the trinucleate configuration present in wild-type pollen grains. We established that 21.9% of pollen grains contained one or two loosely packed DNA masses, which each resembled the DNA mass of the vegetative nucleus in wild-type pollen grains (Figures 4C and 4D). The remaining pollen grains (32.3%) were shrunken, with no discernible nuclei.
Although the above result revealed an abnormality in dividing microspores, it was unclear whether the defect was caused by microsporogenesis or postmeiotic microgametogenesis. The quartet (qrt) mutation causes the four microspores formed during meiosis to remain attached (Figure 4E) (Preuss et al., 1993). We observed that the nedd1 allele did not affect the formation of the tetrad (Figure 4F). The control qrt/qrt NEDD1/NEDD1 plant produced attached pollen grains in units of four (pollen tetrads) with the trinucleate configuration, as shown by DNA staining with DAPI (Figure 4E). The qrt/qrt NEDD1/nedd1 plant, however, produced tetrads with two pollen grains in the typical trinucleate configuration and the other two having either one or two loosely packed chromatin masses (Figure 4F). Therefore, the gametophytic defect observed earlier in Figures 4C and 4D was caused by abnormal postmeiotic events.
The defect in male gametogenesis was assessed quantitatively by examining pollen grains produced by the qrt/qrt NEDD1/nedd1 plant in units of tetrads by DAPI staining, using the qrt/qrt NEDD1/NEDD1 plant as a control. In the control plant, 75.5% of the tetrads (n = 143) have all four pollen grains containing three nuclei of two brightly stained sperm nuclei and one less bright vegetative nucleus, as shown in Figure 4E. In the qrt/qrt NEDD1/nedd1 plant, however, no tetrads had four normal pollen grains. Instead, 86.2% of the tetrads contained two normal pollen grains plus two abnormal ones that had one or two nuclei or that appeared to be shrunken. The viability of pollen grains was also assayed using Alexander's stain (Alexander, 1969). Most pollen tetrads produced by the control qrt/qrt NEDD1/NEDD1 plant had four viable pollen grains, as shown by purple staining (Figure 4G; see Supplemental Figure 1 online). By contrast, the majority of qrt/qrt NEDD1/nedd1 plants produced pollen tetrads with at least one, often two, and sometimes three nonviable pollen grains (Figure 4G; see Supplemental Figure 1 online).
Because of the reduced TE of the mutant allele through the female gamete, we also examined female gametophytes produced by the NEDD1/nedd1 plant. Wild-type plants produced the female gametophyte of the embryo sac containing one egg cell, two synergid cells, three antipodals, and one central cell with a fused polar nucleus (see Supplemental Figures 2A and 2B online). Using the whole-mount protocol, the antipodals were less obvious than other cells. Among chemically cleared ovules produced by the NEDD1/nedd1 plant (n = 194), 7% had two nuclei (see Supplemental Figures 2C and 2D online) and 15% of the ovules only contained one nucleus (see Supplemental Figures 2E and 2F online). This finding was consistent with the TE of the mutant allele through the female gametes determined earlier. As 50% of the ovules of the NEDD1/nedd1 plant would contain embryo sacs with the nedd1 genotype, a 48.3% TE would reflect ∼25.9% defective embryo sacs.
Arabidopsis NEDD1 Plays a Critical Role in MT Organization during Mitotic Cell Division in the Microspore
Because no homozygous nedd1 mutants could be recovered, the NEDD1 gene is most likely essential. Fortunately, in Arabidopsis, meiotic cytokinesis takes place after both karyokinesis processes are completed, so that haploid microspores carrying mutations in essential genes like NEDD1 are produced by heterozygous parents. Once the nedd1 microspores underwent cell division, the resulting gametophytic materials allowed us to analyze how mitosis might have been affected by the mutation. We analyzed MT organization during mitosis in dividing microspores. During the development from the microspore to pollen (male gametophyte), two mitoses take place. The microspore undergoes a unique asymmetrical cell division. With a short spindle having one pole at the periphery of the microspore, the aligned chromosomes were placed very close to the cell periphery (Figures 5A and 5B). The peripheral spindle pole remained at the cortex throughout mitosis as if it were tethered to the plasma membrane. At late anaphase, as indicated by the approach of sister chromatids toward the spindle poles, interzonal MTs were polymerized (Figures 5C and 5D). At telophase, the interzonal MTs were organized as parallel bundles (Figures 5E and 5F). These MTs were later rearranged into a bipolar phragmoplast array, concomitant with the formation of two nuclei (Figures 5G and 5H). Consequently, the cell plate was formed while phragmoplast MTs gradually depolymerized in the central region and polymerized at the periphery (Figures 5I and 5J). The completion of this mitotic cell division resulted in the formation of a small lense-shaped generative cell and a large vegetative cell (Figures 5K and 5L; Lee et al., 2007). The chromatin of the generative nucleus was much more condensed than that of the vegetative nucleus, as highlighted by arrowheads in Figures 5J and 5L. The vegetative nucleus displayed MTs radiating from the surface of the nuclear envelope (Figures 5K and 5L).
Figure 5.
Defects in MT Organization Caused by the nedd1 Mutation during the First Mitosis in Developing Pollen.
Rows represent stages of the division as labeled for metaphase (Met), anaphase (Ana), telophase (Telo), telophase/cytokinesis transition (Telo/Cyto), cytokinesis (Cyto), and two-cell stage (2-Cell). MTs are shown in the first and third columns and DNA in the second and fourth columns. Bar = 5 μm.
(A) to (L) Control NEDD1 microspores undergoing mitosis.
(A) and (B) A peripherally positioned metaphase spindle.
(C) and (D) Interzonal MTs appear upon the arrival of chromotids near the spindle poles at anaphase.
(E) and (F) At telophase, interzonal MTs are bundled and begin to be organized in an antiparallel fashion.
(G) and (H) A mature phragmoplast is formed.
(I) and (J) During cytokinesis, MT signals are prominent at the periphery of an expanding phragmoplast array. At this stage, the DNA mass of the generative cell nucleus (arrowhead) is much more compact than that of the vegetative nucleus.
(K) and (L) Upon completion of the microspore mitosis, the generative chromatin is brightly labeled (arrowhead). MTs are nucleated from the surface of the vegetative nuclear envelope.
(M) to (X) Mutant (nedd1) microspores undergoing mitosis.
(M) and (N) A metaphase spindle has its peripheral pole anchored near the plasma membrane and the other pole beyond the center of the cytoplasm.
(O) and (P) The anaphase spindle has elongated interzonal MTs.
(Q) and (R) The interzonal MTs become bundled at telophase.
(S) to (V) The interzonal MTs remain highly bundled and fail to be organized into a functional phragmoplast array during telophase and cytokinesis.
(W) and (X) MT bundles are randomly arranged between two reformed nuclei.
Abnormal MT organization was detected in approximately half of the dividing microspores produced by the NEDD1/nedd1 heterozygous plant. Among these abnormal cells, long mitotic spindles were formed almost completely spanning the cytoplasm (Figures 5M and 5N). The spindle had one pole positioned at the cortex as in the control cells, but the other pole extended across the cytoplasm. During anaphase and telophase, such long spindles persisted in these abnormal cells, and MTs appeared as conspicuous bundles between the reforming nuclei (Figures 5O to 5R). We quantitatively scored the length of metaphase and anaphase spindles (Figure 6). In dividing microspores collected from the wild type, metaphase spindles had lengths of 4.63 ± 0.58 μm (n = 24). Dividing microspores collected from the NEDD1/nedd1 heterozygous plant were separated into two groups. For those having spindles positioned peripherally, their metaphase spindles had an average length of 4.74 ± 1.07 μm (n = 30), similar to that of the wild type. The elongated spindles in a subset of the dividing NEDD1/nedd1 microspores had an average length of 6.78 ± 0.88 μm (n = 39) at metaphase. Among cells undergoing anaphase, the wild type had spindle lengths of 5.44 ± 0.88 μm (n = 24). Normal anaphase spindles in dividing microspores produced by NEDD1/nedd1 had lengths of 5.26 ± 0.26 μm (n = 9); and abnormal ones had lengths of 8.29 ± 1.55 μm (n = 27). Hence, it is likely that the spindles in the nedd1 microspores were significantly longer than those of the control.
Figure 6.
Spindle Lengths of Wild-Type and Mutant Microspore Cells.
The length (in micrometers) was measured for both metaphase and anaphase spindles of wild-type, normal short spindles produced by the heterozygous NEDD1/nedd1 plant (Het-S) and abnormal long ones by NEDD1/nedd1 (Het-L).
We also detected serious defects in phragmoplast MT organization in dividing microspores produced by the NEDD1/nedd1 heterozygous plant. MT bundles formed between two reforming nuclei failed to reveal a bipolar configuration (Figures 5S to 5V). Instead, thick MT bundles persisted from telophase to cytokinesis (Figures 5S and 5U). The abnormal MT bundles between two reforming nuclei did not undergo depolymerization in a centrifugal pattern as seen in wild-type cells (cf. Figures 5U to 5I). Eventually, these MT bundles became disorganized in the original site, and MTs did not polymerize on the nuclear envelope of the vegetative nucleus (Figures 5W and 5X). We randomly surveyed 100 postanaphase cells produced by the NEDD1/nedd1 heterozygous plant and found that 47 had normal phragmoplast MT configurations, and the remaining 53 microspores showed disorganized phragmoplast MTs. Thus, the dividing microspores with normal phragmoplast MT arrays likely had the NEDD1 genotype, and those with abnormal MTs had the nedd1 mutant allele.
A striking phenomenon was that the two nuclei formed in defective pollen grains had similar intensity when stained by DAPI (Figures 5V and 5X). This suggests that neither nucleus had acquired the fate of the generative nucleus. The two nuclei were positioned symmetrically in the cytoplasm. Such a phenomenon could be due to the two nuclei being suspended in the shared cytoplasm, as shown in other mutants (Lee et al., 2007).
Aborted Cell Plate Formation Caused by the nedd1 Mutation
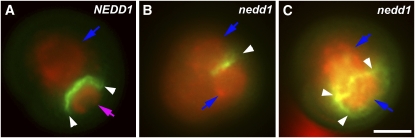
Because neither nucleus acquired the appearance of the generative cell, failed cytokinesis was suspected in dividing microspores. We tested whether the abnormal MT organization during cytokinesis in mutant cells would lead to defects in cell plate formation and consequently to the loss of identity of the generative nucleus. The cell plate was revealed by aniline blue staining, which detects callose in the cell plate, while DNA was stained with DAPI to reveal the positions of the nuclei. A hemispherical arched cell plate was detected between the two nuclei in wild-type pollen grains (Figure 7A). Approximately half of the dividing microspores collected from the NEDD1/nedd1 heterozygous plant had normal cell plate formation, while the other half were aberrant (n = 131) (Figures 7B and 7C). Incomplete cell plates were found in some cells (Figure 7B), and branched or scattered callose-rich cell plates were observed in others (Figure 7C). This result suggested that mutant cells failed to form a complete cell plate, resulting in two nuclei suspended in the same cytoplasm. Consequently, gametogenesis failed because the generative cell was not produced.
Figure 7.
Defects in Cell Plate Formation Caused by the nedd1 Mutation.
Callose in the cell plate (green; white arrowheads) and DNA/nuclei (red) are labeled. In the control (NEDD1), the first mitotic cell division of the microspore results in a hemispherical cell plate separating the generative cell and vegetative cell (A). In the mutant (nedd1), an incomplete cell plate (B) or a highly branched one (C) is observed. The blue arrows point at the vegetative nucleus in the control or identical nuclei in the mutant and the purple arrow at the generative nucleus. Scale bar, 5 μm.
DISCUSSION
Our results show that in Arabidopsis, the WD40 repeat protein NEDD1 acts on the minus ends of spindle and phragmoplast MTs, as previously shown for γ-tubulin (Liu et al., 1993). A T-DNA insertional mutation at the NEDD1 locus can only be kept in the heterozygous status, suggesting that NEDD1 is an essential gene. The mutation caused frequent abortion of gametogenesis, especially for the male gametophyte (Figure 4). The asymmetric mitotic division in dividing microspores is compromised by the mutation (Figure 5). This mutation alters the organization of phragmoplast MTs, which leads to failure of cytokinesis and results in pollen grains that lack sperm cells (Figure 7). These results support the notion that At NEDD1 plays a critical role in MT organization.
NEDD1 Is an Essential Protein in Arabidopsis
In animal cells, NEDD1/GCP-WD interacts with multiple subunits of γ-TuRC and MTs directly (Gunawardane et al., 2003; Liu and Wiese, 2008). Several lines of evidence indicate that it plays a role in targeting γ-TuRC to the centrosome and/or mediating the interaction between γ-TuRC and MTs (Haren et al., 2006; Lüders et al., 2006; Liu and Wiese, 2008). Ultimately, the function of this WD40 protein is indispensible for both centrosome-dependent and -independent MT organization and consequently for spindle formation. To date, yeasts and other advanced fungi do not have an obvious NEDD1 homolog based on sequence comparison. The fact that plants produce NEDD1 homologs suggests that the ancestral protein must have evolved in ancient times, before the evolution of the kingdoms of multicellular plants and animals. Elucidating the essential function of Arabidopsis NEDD1 has allowed us to conclude that the plant homolog likely exercises similar activities as its animal counterparts. It is noteworthy that, unlike metazoans, the fission yeast homologs of GCP4-6 of γ-TuRC are not essential for MT nucleation at the spindle pole body and for cell cycle progression (Fujita et al., 2002; Venkatram et al., 2004; Anders et al., 2006). This may be true for other fungi, as the budding yeast Saccharomyces cerevisiae lacks GCP4-6 (Vinh et al., 2002). Thus, the presence of NEDD1 in plants and animals implies that these organisms have evolved sophisticated mechanisms that regulate the activity of γ-TuRC for MT organization.
The presence of NEDD1 homologs in earlier land plants like mosses and lycophytes points at a conserved role of NEDD1 in plants. The numbers of the NEDD1 genes in P. patens (four Pp NEDD1 genes) and the lycophyte S. moellendorffii (two Sm NEDD1 genes) are surprising. Whether those isoforms express different means of regulating γ-TuRC awaits functional characterization of the proteins.
The essential role of Arabidopsis NEDD1 also provides direct evidence that it regulates MT organization in a centrosome-independent manner. Plants have developed noncentrosomal MTOC sites, like the nuclear envelope and plastid surface, which are decorated by γ-tubulin and other GCPs (Schmit, 2002; Brown and Lemmon, 2007). It would be interesting to establish whether NEDD1 proteins play a role in nucleating MTs on these noncentrosomal MTOCs. Recently, a NEDD1–red fluorescent protein fusion was observed as fluorescent dots in the interphase cytoplasm when expressed using the 35S promoter (Motose et al., 2008). It was unclear, however, whether those dots represented MT nucleation sites and whether NEDD1 plays a role in MT organization during interphase. Our results showed that upon nuclear reformation during cell division, MTs failed to be organized on the surface of the vegetative nucleus in mutant pollen grains (Figure 5W). This supports the notion that NEDD1 is probably required for organizing MTs into the radial array in Arabidopsis. It has been demonstrated that a truncated version of Arabidopsis homologs of GCP2 and GCP3 is targeted to the nuclear envelope when expressed in tobacco (Nicotiana tabacum) cells (Seltzer et al., 2007). An obvious question lies in whether NEDD1 functions in mediating the localization of GCP2/3 to the nuclear envelope. We feel that it is likely that NEDD1 is an essential component of MTOCs in plant cells.
The nedd1 Mutation Alters MT Organization during Mitotic Cell Division
Two major problems were encountered by the dividing nedd1 microspores. In the wild type, the first postmeiotic mitosis is an asymmetrical division, which ensures the differentiation of the small lens-shaped generative cell and the large vegetative (tube) cell. Currently, the mechanism that regulates the asymmetrical spindle positioning is unclear. It seemed that the nedd1 pollen grains had eliminated the restriction for the spindle to be placed at the peripheral zone. While one spindle pole was probably still anchored to a peripheral position, the other pole is positioned near the center of the cytoplasm and was unlikely fixed in a particular position. NEDD1 may play a role in mediating the interaction between the γ-TuRC and unknown cytoplasmic components to allow MT minus ends at the spindle pole to be correctly fixed and positioned. Uncovering the mechanisms underlying the apparent differences between the two wild-type spindle poles during microspore mitosis may provide clues to understanding the general rules of asymmetrical cell division in plants.
γ-Tubulin is indispensible for the assembly of a bipolar spindle (Binarova et al., 2006; Pastuglia et al., 2006), but the nedd1 mutation did not seem to affect karyokinesis itself (Figures 5M to 5R). This suggests that Arabidopsis NEDD1 may not be required for γ-tubulin's role in spindle assembly, but is required for the spindle to be positioned correctly. One possible scenario is that there might be enough NEDD1 proteins left from meiosis to satisfy the minimal requirement for spindle assembly.
The other serious consequence of mutating the NEDD1 gene was the disorganization of interzonal MTs, which prevented the assembly of a functional bipolar phragmoplast array. At late anaphase, interzonal MTs coalesced between the two reforming nuclei, but they were not reorganized into a bipolar configuration. These MTs eventually became randomized (Figure 5W). The bipolar configuration of MTs is essential for deposition of carbohydrates and membranous materials and leads to the formation of the cell plate (Lee et al., 2007). In nedd1 pollen grains, the defect in organizing MTs into a phragmoplast array might be attributed to the lack of MT nucleation activity at the minus end. Such a collapse of phragmoplast MTs has also been observed in dividing cells lacking γ-tubulin, which also causes the production of defective pollen grains with two identical nuclei (Pastuglia et al., 2006). Thus, our results suggest that Arabidopsis NEDD1 is likely required for the function of γ-tubulin in organizing phragmoplast MTs.
NEDD1 and Unequal Pollen Mitosis
In various species of the orchid Phalaenopsis, the unequal first pollen mitosis is preceded by the formation of a generative pole MT system (GPMS) that guides the migration of the microspore nucleus (Brown and Lemmon, 1991a). The appearance of elongated spindles in the nedd1 microspore suggests that the nucleus most likely failed to migrate to the cell periphery. Thus, NEDD1 may play a critical role in GPMS formation if indeed such a GPMS-based nuclear migration mechanism is conserved among flowering plants.
During cytokinesis, the hemispherical cell plate is brought about by the hemispherical phragmoplast formed between the vegetative and generative nuclei. In orchids, again, MTs radiate from the surface of the generative nucleus, while MTs of the vegetative half-phragmoplast terminate in the endoplasmic reticulum (Brown and Lemmon, 1991b). Because the hemispherical phragmoplast curves around the generative nucleus, it has been proposed that the generative nucleus defines a cytoplasmic domain by forming a radiating MT system from its surface (Brown and Lemmon, 1992). Our results also support the notion that NEDD1 likely plays a critical role in establishing the hemispherical phragmoplast MT array because the nedd1 mutant fails to form such an array (Figures 5U and 5W).
Given these nedd1 phenotypes, the results suggest that the later cytokinetic failure might be caused by the earlier inaccurate positioning of the spindle. In the nedd1 mutant microspore, the phragmoplast elongated instead of expanding toward the periphery. Therefore, the position of the spindle might dictate the expansion of the phragmoplast during cytokinesis. Alternatively, the expansion of the phragmoplast might be directly dependent on NEDD1 for generating new MTs from the generative nuclear envelope. The lack of NEDD1 might have prevented the formation of the MT array in the hemispherical phragmoplast; consequently, no hemispherical cell plate was formed. The final collapse of MTs between the two nuclei would support a role of NEDD1 in MT nucleation and organization at this stage.
Another striking phenotype of nedd1 is that no nucleus formed during microspore mitosis differentiated into the generative nucleus, suggesting that peripheral attachment of the nucleus might be critical for the differentiation. We hypothesize that NEDD1 is required for establishing a GPMS-like structure, which may be required for anchoring the nucleus to the cell periphery. This scenario would allow the peripheral nucleus to differentiate into the generative nucleus.
Cell Cycle–Dependent Activity of NEDD1
The human NEDD1/GCP-WD protein has a Ser-411 residue of a SPIR motif, which is phosphorylated by Cdk1 during mitosis. This phosphorylation event is critical for its function in recruiting the γ-TuRC complex to spindle MTs but not to the centrosome; consequently, it is crucial for the assembly of functional spindles (Haren et al., 2006; Lüders et al., 2006). A similar phosphorylation event was detected in mitotic extracts in Xenopus (Liu and Wiese, 2008). By contrast, the site in Arabidopsis NEDD1 corresponding to human GCP-WD contains an SPII motif that does not constitute a canonical Cdk phosphorylation site. Therefore, future experiments will elucidate whether the function of Arabidopsis NEDD1 in MT organization is regulated by phosphorylation of the Ser-415 residue of this SPII motif in a cell cycle–dependent manner.
While the quest for molecular mechanisms that regulate the targeting of γTuRC to the centrosome continues in animal cells, future endeavors to elucidate acentrosomal MT nucleation and organization in plant cells must consider the contribution of NEDD1.
METHODS
Materials and Growth Conditions
The cDNA clone pdz37649 containing the full-length coding sequence of Arabidopsis thaliana NEDD1 was isolated and annotated at the RIKEN Genomic Sciences Center in Tsukuba, Japan (Seki et al., 1998,2002). The FLAG_413D07 (EYLTV188T3) line bearing the nedd1 mutation was generated and made available to us by the Genetic Resources Team of the Station de Génétique et Amélioration des Plantes at Institut National de la Recherche Agronomique Centre de Versailles in Versailles, France (Samson et al., 2002).
Plants were grown under similar conditions as described previously (Lee et al., 2007). Standard genetic crosses were performed between the mutant line and control plants of the ecotype Wassilewskija.
Phylogenetic Analysis of NEDD1/GCP-WD Proteins
Protein function domains were predicted using the Simple Modular Architecture Research Tool at EMBL (http://smart.embl-heidelberg.de/). Besides At NEDD1, and the rice (Oryza sativa) homolog Os NEDD1, four NEDD1 homologs have been annotated in the moss Physcomitrella patens, including Pp NEDD1a, Pp NEDD1b, Pp NEDD1c, and Pp NEDD1d. BLAST searches also identified two NEDD1 homologs, Sm NEDD1a and Sm NEDD1b, in the lycophyte Selaginella moellendorffii (http://genome.jgi-psf.org/Selmo1). Other NEDD1/GCP-WD proteins used for comparison included Hs GCP-WD of Homo sapiens, Xl NEDD1 of Xenopus laevis, Mm NEDD1 of Mus musculus, and Dgp71WD of Drosophila. melanogaster. Alignment of amino acid sequences was performed using the ClustalX program. The phylogenetic tree was deduced with the neighbor-joining method in PAUP*4 (http://paup.csit.fsu.edu/) and was rooted using the WD40 protein COP1 as an outgroup because COP1 has no homology with NEDD1 outside the WD40 repeats. Bootstrap support values were obtained from 1000 replicates.
Screening of T-DNA Insertion Mutant
The T-DNA insertion at the NEDD1 locus was confirmed by PCR using the gene-specific primer 188T3RP (5′-ACTGATTCTAATTTCCATGTCTTGC-3′) and the T-DNA border-specific primer Tag5 (5′-CTACAAATTGCCTTTTCTTATCGAC-3′). The gene-specific primers, 188T3RP and 188T3LP (5′-CTACCAGAATATAGCCATTATCCCC-3′), were used to detect the wild-type allele devoid of T-DNA insertion. Genomic DNA extraction and PCR conditions were performed according to published protocols (Krysan et al., 1996).
The T-DNA was also linked to the BAR and NPT II genes conferring resistance to the herbicide BASTA and the antibiotic kanamycin (Samson et al., 2002), respectively. Resistant seedlings were selected by spraying Finale (AgrEvo) at a 1:1000 dilution after plants had germinated on soil for 5 d or by germinating surface-sterilized seeds on half-strength Murashige and Skoog agar medium (MP Biomedicals) containing 25 μg/mL kanamycin.
Genetic Suppression/Complementation of the nedd1-1 Mutation
The genomic fragment used for the complementation experiment was based on the annotated At5g05970 locus. It included a 5.7-kb DNA fragment encompassing the entire coding sequence and a 2-kb promoter sequence upstream of the start codon. Two primers, 5′-CACCTTATTCTTCGTCGACTTATGATATAG-3′ and 5′-AAGCCTTTGTCTGCGCTCTTGATTC-3′, were used in the PCR reaction to amplify the combined fragment from Arabidopsis genomic DNA, using Phusion High-Fidelity DNA polymerase (New England Biolabs). The PCR product was first cloned into the vector pENTR/D-TOPO (Invitrogen) and then via the Gateway LR Clonase reaction (Invitrogen) into the binary vector pGWB16 (Nakagawa et al., 2007). Having pGWB16 as the vector would allow NEDD1 to be tagged with 4 X c-Myc when expressed. The resulting construct pGWAt5g05970 was transformed into Agrobacterium tumefaciens GV3101 cells and then to the heterozygous NEDD1/nedd1 mutant plants by the standard floral dip method (Clough and Bent, 1998). As a control for the complementation test, the original pGWB16 vector was also transformed into the NEDD1/nedd1 plants. Transformants were selected on half-strength Murashige and Skoog agar medium containing 20 μg/mL hygromycin (Invitrogen).
Mutant Characterization
To determine the phenotype of mutant ovules, anthers were removed from flowers of stage 12, in which petals were level with long stamens, but sepals were not open (Smyth et al., 1990). Pistils were collected 48 h after emasculation. Ovules were dissected from pistils and cleared overnight in Hoyer's solution (Liu and Meinke, 1998). The dissected pistils were observed on a Zeiss Axioplan microscope under differential interference contrast optics, and images were acquired on a Zeiss Axiocam HRC CCD camera using the Axiovision program.
To determine whether a mutation segregating at meiosis was responsible for the pollen grain phenotype, we crossed a NEDD1/nedd1 plant to a qrt1/qrt1 plant (Preuss et al., 1993). The NEDD1/nedd1 qrt1/qrt1 plants were selected in subsequent generations by PCR-based genotyping at the NEDD1 locus and by phenotyping the mutation at the QRT locus. The nuclei of pollen grains were visualized by staining with the dye DAPI according to a published protocol (Park et al., 1998). The dye aniline blue was used to stain callose in dividing microspores (Park and Twell, 2001). Fluorescent signals of DAPI (for DNA) and aniline blue (for callose) staining were observed using standard Chroma filter sets. To monitor pollen grain viability, pollen grains were collected by dissecting anthers from flowers of stage 12 in Alexander staining solution on microscope slides (Alexander, 1969).
Antibody Preparation
The NEDD1 cDNA fragment encoding amino acids 253 to 577 was excised from the pdz37649 cDNA clone by BamHI and EcoRI digestion. This fragment was ligated into the GST vector pGEX4T1 (GE Healthcare Bio-Sciences) at the same restriction sites. GST-AtNEDD1 fusion protein was expressed in the Escherichia coli strain BL21 (DE3) pLysS (EMD Chemicals) and purified using the glutathione-sepharose affinity resin (Pierce) according to the manufacturer's instructions. Purified GST-AtNEDD1 fusion protein was then used as an antigen to raise polyclonal antibodies in two rabbits at the Comparative Pathology Laboratory of the University of California, Davis. Antibodies specific to NEDD1 were purified by a blot purification method (Olmsted, 1981) after the anti-GST antibodies were depleted by incubation with GST protein. The depletion of anti-GST antibodies was confirmed by immunoblotting using GST alone.
Fluorescence Microscopy
Immunolocalization experiments in root tip cells were performed as described previously (Lee and Liu, 2000). Immunolocalization in pollen grains was performed according to published studies (Terasaka and Niitsu, 1990; Lee et al., 2007). To test the specificity of antibodies, the antigen GST-AtNEDD1 was added to the purified antibodies and used as the control in immunolocalization experiments. In addition to the rabbit anti-GST-AtNEDD1 antibodies reported here, antibodies used in this study included mouse DM1A anti-α-tubulin (Sigma-Aldrich), rabbit anti-γ-tubulin (Liu et al., 1993), mouse 9E10 anti-c-myc (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), fluorescein isothiocyanate–conjugated goat anti-rabbit (Sigma-Aldrich), and Texas Red X–conjugated goat anti-mouse IgG (Invitrogen).
Wide-field fluorescence images were acquired with an Eclipse E600 microscope equipped with epifluorescence optics (Nikon), a CCD camera (Hamamatsu Photonics), and the MetaMorph software package (Molecular Devices). Images were assembled in Adobe Photoshop.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database under the following accession numbers: NP_196216, NP_001062722, XP_001771119, XP_001751568, XP_001768421, XP_001752892, NP_690869, NP_001087857, NP_032708, NP_609902, and NP_180854.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Viability Tests of the Pollen Grains of Tetrads Produced in Plants with the qrt/qrt Mutation Background.
Supplemental Figure 2. Defects in the Development of the Female Gametophyte (Embryo Sac) Caused by the nedd1 Mutation.
Supplemental Data Set 1. Protein Sequence Alignments Used to Generate the Phylogenetic Tree Presented in Figure 1B.
Supplementary Material
Acknowledgments
We thank Masatomo Kobayashi and his team at the Experimental Plant Division of the RIKEN Genomic Biosciences Center in Tsukuba, Japan for providing the cDNA clone used in this study; the Genetic Resources Team of the Station de Génétique et Amélioration des Plantes at Institut National de la Recherche Agronomique Centre de Versailles in Versailles, France for the FLAG line; and Tsuyoshi Nakagawa at the Research Institute of Molecular Genetics of Shimane University in Matsue, Japan for the pGWB vectors. We thank Gabriela Pagnussat and Quy Ngo in Venkatesan Sundaresan's laboratory for their expert help on analyses of female gametophytes and Keith Markus at the City University of New York for assistance on statistical analyses. We also thank Lindsay Kiyama for correcting grammatical errors in the manuscript. This work was supported by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Science, U.S. Department of Energy (Contract DE-FG02-04ER15554 to B.L.) and the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (Grant 2005-35304-16031).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Bo Liu (bliu@ucdavis.edu).
Online version contains Web-only data.
References
- Alexander, M.P. (1969). Differential staining of the aborted and non aborted pollen. Stain Technol. 44 117–122. [DOI] [PubMed] [Google Scholar]
- Anders, A., Lourenco, P.C.C., and Sawin, K.E. (2006). Noncore components of the fission yeast γ-tubulin complex. Mol. Biol. Cell 17 5075–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binarova, P., Cenklova, V., Prochazkova, J., Doskocilova, A., Volc, J., Vrlik, M., and Bogre, L. (2006). γ-Tubulin is essential for acentrosomal microtubule nucleation and coordination of late mitotic events in Arabidopsis. Plant Cell 18 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R.C., and Lemmon, B.E. (1991. a). Pollen development in orchids 3. A novel generative pole microtubular system predicts unequal pollen mitosis. J. Cell Sci. 99 273–281. [Google Scholar]
- Brown, R.C., and Lemmon, B.E. (1991. b). Pollen development in orchids 5. A generative cell domain involved in spatial control of the hemispherical cell plate. J. Cell Sci. 100 559–566. [Google Scholar]
- Brown, R.C. and Lemmon, B.E. (1992). Cytoplasmic domain: A model for spatial control of cytokinesis in reproductive cells of plants. EMSA Bull. 22: 48–53. [Google Scholar]
- Brown, R.C., and Lemmon, B.E. (2007). The pleiotrophic plant MTOC: An evolutionary perspective. J. Integr. Plant Biol. 49 1142–1153. [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Erhardt, M., Stoppin-Mellet, V., Campagne, S., Canaday, J., Mutterer, J., Fabian, T., Sauter, M., Muller, T., Peter, C., Lambert, A.M., and Schmit, A.C. (2002). The plant Spc98p homologue colocalizes with γ-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J. Cell Sci. 115 2423–2431. [DOI] [PubMed] [Google Scholar]
- Fujita, A., Vardy, L., Garcia, M.A., and Toda, T. (2002). A fourth component of the fission yeast γ-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when γ-tubulin function is compromised. Mol. Biol. Cell 13 2360–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R.N., Martin, O.C., and Zheng, Y.X. (2003). Characterization of a new γTuRC subunit with WD repeats. Mol. Biol. Cell 14 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren, L., Remy, M.H., Bazin, I., Callebaut, I., Wright, M., and Merdes, A. (2006). NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job, D., Valiron, O., and Oakley, B. (2003). Microtubule nucleation. Curr. Opin. Cell Biol. 15 111–117. [DOI] [PubMed] [Google Scholar]
- Krysan, P.H., Young, J.C., Tax, F., and Sussman, M.R. (1996). Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc. Natl. Acad. Sci. USA 93 8145–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S., Matsuzaki, T., Yoshida, Y., and Noda, M. (1994). Molecular cloning and biological activity of a novel developmentally regulated gene encoding a protein with β-transducin-like structure. J. Biol. Chem. 269 11318–11326. [PubMed] [Google Scholar]
- Lee, Y.R.J., Li, Y., and Liu, B. (2007). Two homologous phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis in Arabidopsis. Plant Cell 19 2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.R.J., and Liu, B. (2000). Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr. Biol. 10 797–800. [DOI] [PubMed] [Google Scholar]
- Liu, B., Joshi, H.C., and Palevitz, B.A. (1995). Experimental manipulation of γ-tubulin distribution in Arabidopsis using anti-microtubule drugs. Cell Motil. Cytoskeleton 31 113–129. [DOI] [PubMed] [Google Scholar]
- Liu, B., Joshi, H.C., Wilson, T.J., Silflow, C.D., Palevitz, B.A., and Snustad, D.P. (1994). γ-Tubulin in Arabidopsis-gene sequence, immunoblot, and immunofluorescence studies. Plant Cell 6 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B., Marc, J., Joshi, H.C., and Palevitz, B.A. (1993). A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J. Cell Sci. 104 1217–1228. [DOI] [PubMed] [Google Scholar]
- Liu, C.-M., and Meinke, D.W. (1998). The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 16 21–31. [DOI] [PubMed] [Google Scholar]
- Liu, L., and Wiese, C. (2008). Xenopus NEDD1 is required for microtubule organization in Xenopus egg extracts. J. Cell Sci. 121 578–589. [DOI] [PubMed] [Google Scholar]
- Lüders, J., Patel, U.K., and Stearns, T. (2006). GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8 137–147. [DOI] [PubMed] [Google Scholar]
- Manning, J., and Kumar, S. (2007). NEDD1: Function in microtubule nucleation, spindle assembly and beyond. Int. J. Biochem. Cell Biol. 39 7–11. [DOI] [PubMed] [Google Scholar]
- Motose, H., Tominaga, R., Wada, T., Sugiyama, M., and Watanabe, Y. (2008). A NIMA-related protein kinase suppresses ectopic outgrowth of epidermal cells through its kinase activity and the association with microtubules. Plant J. 54 829–844. [DOI] [PubMed] [Google Scholar]
- Murata, T., Sonobe, S., Baskin, T.I., Hyodo, S., Hasezawa, S., Nagata, T., Horio, T., and Hasebe, M. (2005). Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat. Cell Biol. 7 961–968. [DOI] [PubMed] [Google Scholar]
- Murata, T., Tanahashi, T., Nishiyama, T., Yamaguchi, K., and Hasebe, M. (2007). How do plants organize microtubules without a centrosome? J. Integr. Plant Biol. 49 1154–1163. [Google Scholar]
- Murphy, S.M., Preble, A.M., Patel, U.K., O'Connell, K.L., Dias, D.P., Moritz, M., Agard, D., Stults, J.T., and Stearns, T. (2001). GCP5 and GCP6: Two new members of the human γ-tubulin complex. Mol. Biol. Cell 12 3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, T., Kurose, T., Hino, T., Tanaka, K., Kawamukai, M., Niwa, Y., Toyooka, K., Matsuoka, K., Jinbo, T., and Kimura, T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104 34–41. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., and Hashimoto, T. (2009). A spiral3 mutation in the Arabidopsis γ-tubulin-containing complex subunit causes abnormal microtubule branching-angle distribution and right-handed helical growth. J. Cell Sci ., in press. [DOI] [PubMed]
- Olmsted, J.B. (1981). Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J. Biol. Chem. 256 11955–11957. [PubMed] [Google Scholar]
- Park, S.K., Howden, R., and Twell, D. (1998). The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125 3789–3799. [DOI] [PubMed] [Google Scholar]
- Park, S.K., and Twell, D. (2001). Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol. 126 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuglia, M., Azimzadeh, J., Goussot, M., Camilleri, C., Belcram, K., Evrard, J.L., Schmit, A.C., Guerche, P., and Bouchez, D. (2006). γ-Tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, D., Lemieux, B., Yen, G., and Davis, R.W. (1993). A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 7 974–985. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina, B., and Merdes, A. (2007). γ-Tubulin complexes and microtubule organization. Curr. Opin. Cell Biol. 19 24–30. [DOI] [PubMed] [Google Scholar]
- Samson, F., Brunaud, V., Balzergue, S., Dubreucq, B., Lepiniec, L., Pelletier, G., Caboche, M., and Lecharny, A. (2002). FLAGdb/FST: A database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res. 30 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit, A.C. (2002). Acentrosomal microtubule nucleation in higher plants. Int. Rev. Cytol. 220 257–289. [DOI] [PubMed] [Google Scholar]
- Seki, M., Carninci, P., Nishiyama, Y., Hayashizaki, Y., and Shinozaki, K. (1998). High-efficiency cloning of Arabidopsis full-length cDNA by biotinylated CAP trapper. Plant J. 15 707–720. [DOI] [PubMed] [Google Scholar]
- Seki, M., et al. (2002). Functional annotation of a full-length Arabidopsis cDNA collection. Science 296 141–145. [DOI] [PubMed] [Google Scholar]
- Seltzer, V., Janski, N., Canaday, J., Herzog, E., Erhardt, M., Evrard, J.L., and Schmit, A.C. (2007). Arabidopsis GCP2 and GCP3 are part of a soluble gamma-tubulin complex and have nuclear envelope targeting domains. Plant J. 52 322–331. [DOI] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M. (1990). Early flower development in Arabidopsis. Plant Cell 2 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka, O., and Niitsu, T. (1990). Unequal cell division and chromatin differentiation in pollen grain cells II: Microtubule dynamics associated with the unequal cell division. Bot. Mag . (Tokyo) 103 133–142. [Google Scholar]
- Venkatram, S., Tasto, J.J., Feoktistova, A., Jennings, J.L., Link, A.J., and Gould, K.L. (2004). Identification and characterization of two novel proteins affecting fission yeast γ-tubulin complex function. Mol. Biol. Cell 15 2287–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vérollet, C., Colombié, N., Daubon, T., Bourbon, H.M., Wright, M., and Raynaud-Messina, B. (2006). Drosophila melanogaster γ-TuRC is dispensable for targeting γ-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 172 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh, D.B., Kern, J.W., Hancock, W.O., Howard, J., and Davis, T.N. (2002). Reconstitution and characterization of budding yeast γ-tubulin complex. Mol. Biol. Cell 13 1144–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C., and Zheng, Y. (2006). Microtubule nucleation: γ-Tubulin and beyond. J. Cell Sci. 119 4143–4153. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Wong, M.L., Alberts, B., and Mitchison, T. (1995). Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 378 578–583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.