Abstract
RecA and its ubiquitous homologs are crucial components in homologous recombination. Besides their eukaryotic nuclear counterparts, plants characteristically possess several bacterial-type RecA proteins localized to chloroplasts and/or mitochondria, but their roles are poorly understood. Here, we analyzed the role of the only mitochondrial RecA in the moss Physcomitrella patens. Disruption of the P. patens mitochondrial recA gene RECA1 caused serious defects in plant growth and development and abnormal mitochondrial morphology. Analyses of mitochondrial DNA in disruptants revealed that frequent DNA rearrangements occurred at multiple loci. Structural analysis suggests that the rearrangements, which in some cases were associated with partial deletions and amplifications of mitochondrial DNA, were due to aberrant recombination between short (<100 bp) direct and inverted repeats in which the sequences were not always identical. Such repeats are abundant in the mitochondrial genome, and interestingly many are located in group II introns. These results suggest that RECA1 does not promote but rather suppresses recombination among short repeats scattered throughout the mitochondrial genome, thereby maintaining mitochondrial genome stability. We propose that RecA-mediated homologous recombination plays a crucial role in suppression of short repeat-mediated genome rearrangements in plant mitochondria.
INTRODUCTION
Plant mitochondria play a vital role in the cell as the major producers of ATP via oxidative phosphorylation, and they provide metabolic intermediates that serve as substrates for the synthesis of nucleic acids, amino acids, and fatty acids. Plant mitochondrial genomes encode only a small number of RNAs and proteins, including rRNAs, tRNAs, some subunits of respiratory chain complexes, and a few ribosomal proteins (Oda et al., 1992; Unseld et al., 1997). Most of the reported mutations in mitochondrial genes affect plant growth and development (Newton et al., 2004). Characteristically, the rate of nucleotide substitutions in plant mitochondrial genomes is generally lower than that in plant nuclear and chloroplast genomes (Wolfe et al., 1987; Palmer and Herbon, 1988). Most of the reported mitochondrial mutations are rearrangements and deletions caused by aberrant recombination between short (generally <200 bp) repeats (Newton et al., 2004). These recombination events are the main source of mitochondrial mutations that confer cytoplasmic male sterility and nonchromosomal stripe, a maternally inherited mutation that confers variable leaf striping and poor growth (Newton et al., 2004), and they are also thought to contribute to the relatively rapid structural evolution of mitochondrial genomes (Small et al., 1989). In addition to the short repeats, which commonly exist in lower and higher plant mitochondrial genomes (Andre et al., 1992), higher plant mitochondrial genomes uniquely contain at least one pair of long repeats (>1 kb), which when oriented as direct repeats may be involved in frequent homologous recombination to generate subgenomic molecules (Lonsdale et al., 1984). Thus, recombination is deeply involved in the dynamics of plant mitochondrial genomes, although the mechanisms underlying these dynamics are still largely unknown.
RecA is a crucial component in homologous recombination and recombinational DNA repair in bacteria. RecA binds to DNA to form a nucleoprotein filament, which then aligns with a homologous DNA duplex to promote single-strand exchange (Lusetti and Cox, 2002). Mutation of recA confers a dramatic reduction not only in the efficiency of homologous recombination but also in the extent of cellular tolerance to DNA damage. This is because RecA has multiple functions in SOS induction (Little and Mount, 1982) and mutagenic lesion bypass synthesis during the SOS response (Pham et al., 2001), besides its direct role in recombinational repair. Recent studies suggest a significant role for RecA and other recombination proteins in the repair of stalled or collapsed replication forks (Cox et al., 2000; Lusetti and Cox, 2002).
Homologs of RecA have been identified in many prokaryotes and eukaryotes. The higher plant Arabidopsis thaliana features Rad51 and Dmc1, eukaryotic counterparts for nuclear DNA recombination/repair (Bishop et al., 1992; Shinohara et al., 1992), and several bacterial-type RecA homologs encoded in the nucleus, which have been shown to be targeted to chloroplasts (Cerutti et al., 1992; Cao et al., 1997) and/or mitochondria (Khazi et al., 2003; Shedge et al., 2007). RecA-like strand transfer activity has been detected in a stromal extract from pea (Pisum sativum) chloroplasts (Cerutti and Jagendorf, 1993) and in soybean (Glycine max) mitochondria (Manchekar et al., 2006). In the moss Physcomitrella patens, two RecA homologs were identified, a mitochondrial-targeted RecA (RECA1) and a chloroplast-targeted RecA (RECA2) (Odahara et al., 2007; Inouye et al., 2008). The RECA1 gene disruptant exhibits a lower rate of recovery of the mitochondrial DNA (mtDNA) from methyl methanesulfonate (MMS)-induced damage (Odahara et al., 2007), suggesting the involvement of RECA1 in the repair of mtDNA.
The nuclear genome of P. patens, in which gene targeting occurs with an efficiency similar to that observed in yeast (Schaefer, 2001), was recently sequenced (Rensing et al., 2008), and the chloroplast and the mitochondrial genomes were previously sequenced (Sugiura et al., 2003; Terasawa et al., 2007), enabling advanced genetic and molecular biological approaches to be used in this organism. Here, we analyzed the in vivo roles of the P. patens mitochondrial RecA protein RECA1 by targeted nuclear gene inactivation. Disruption of the RECA1 gene resulted in a remarkable defect in plant growth and gross rearrangements of the mitochondrial genome. Our structural analysis of the rearranged DNA showed that recombination among short dispersed repeats was induced in the disruptants. Based on our results, we discuss an important role of bacterial-type RecA in maintaining mitochondrial genome stability and the possible implications of our findings for the evolution of the plant mitochondrial genome.
RESULTS
Disruption of RECA1 Causes Serious Defects in Growth and Development of P. patens
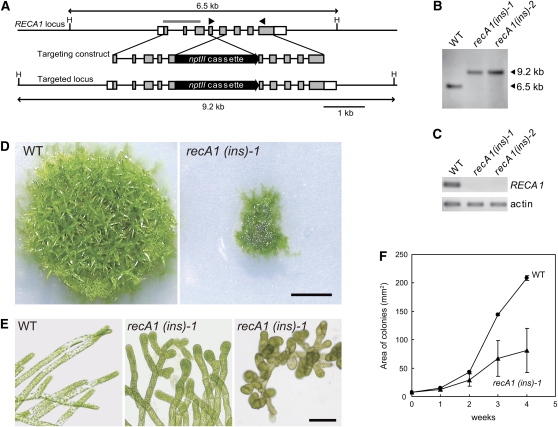
To investigate the role of RECA1 in planta, we generated RECA1 gene disruptants by homologous recombination using a targeting construct in which a neomycin phosphotransferase gene (nptII) cassette was inserted into the middle of the genomic RECA1 sequence (Figure 1A). Two of three independently obtained RECA1 disruptants [recA1(ins)-1 and -2] were selected for further analysis, as they showed very similar phenotypes. In these disruptants, proper integration of one copy of the nptII cassette into the genomic RECA1 locus and the absence of a RECA1 transcript originating downstream of the inserted nptII cassette were confirmed by DNA gel blot analysis (Figure 1B) and RT-PCR analysis (Figure 1C), respectively.
Figure 1.
Generation of RECA1 Insertional Disruptants and Their Growth and Development.
(A) Scheme for the disruption of the RECA1 locus. The boxes represent exons, and the lines between boxes represent introns and noncoding flanking sequences of RECA1. The coding regions are shaded in gray. The HindIII recognition sites are indicated by H. The location of the probe and the primers used in (B) and (C) are shown by a bold gray line and arrowheads, respectively. The nptII cassette consists of the cauliflower mosaic virus 35S promoter-driven neomycin phosphotransferase II gene.
(B) DNA gel blot analysis of the RECA1 locus in two RECA1 insertional disruptants [recA1(ins)-1 and recA1(ins)-2]. Total genomic DNA was digested with HindIII and hybridized to the RECA1 probe. The lengths of the bands are indicated on the right.
(C) RT-PCR analysis of the RECA1 transcript in RECA1 disruptants.The PCR was repeated twice with similar results.
(D) and (E) Morphology of colonies (D) and protonemata (E) in the wild-type background and of the RECA1 disruptant cultivated on agar medium for 4 weeks. Bars = 5 mm in (D) and 100 μm in (E).
(F) Growth rates of wild-type and RECA1 disruptant colonies. Data are expressed as mean ± sd (n = 3).
In the early stage of the formation of a P. patens colony starting from a small cluster of cells on an agar medium, filamentous cells called protonemata grow to make a network of filaments. Subsequently, gametophores resembling a small plant with leaves and root-like rhizoids develop from a fraction of the protonemata. To investigate the influence of RECA1 gene disruption on P. patens cell growth and development, RECA1 disruptant colonies formed on agar medium were compared with wild-type colonies. RECA1 disruptants exhibited obvious defects in growth rate; their surface area was significantly reduced compared with that of wild-type colonies (Figures 1D and 1F). Besides the reduction in growth rate, gametophores barely developed in RECA1 disruptant colonies cultivated for 4 weeks (Figure 1D). The protonemata of RECA1 disruptants were shorter than those of wild-type colonies, and in particular, the lengths of the apical cells were significantly reduced (Figure 1E). The protonemata of the RECA1 disruptant appeared darker than did those of wild-type plants because the chloroplast density was higher in RECA1 disruptant cells, which had a reduced volume compared with wild-type cells (Figure 1E). Judging from these cell morphologies, the elongation of disruptant cells appeared to be inhibited.
Abnormal Mitochondria in RECA1 Disruptant Cells
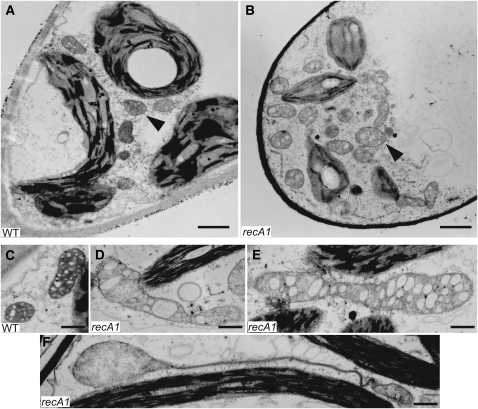
The RECA1 protein has been shown to be targeted to mitochondria (Odahara et al., 2007), strongly suggesting that this organelle is primarily affected by disruption of the RECA1 gene. Ultrastructural analysis of protonema by transmission electron microscopy revealed obvious abnormalities of mitochondrial structure in the RECA1 disruptant as follows. Whereas wild-type mitochondria contained developed cristae that resembled small rings (Figures 2A and 2C), by contrast, disruptant mitochondria were enlarged and contained vacuolated cristae (Figures 2B, 2D, and 2E). The disruptant mitochondrial matrix stained less intensely than did the wild-type matrix (Figures 2C to 2E), suggesting a lower electron density of the mitochondrial matrix. In addition, abnormally extended mitochondria were often observed in RECA1 disruptants (Figure 2F). The apparent abnormalities in the structure of the disruptant mitochondria revealed by transmission electron microscopy suggest that RECA1 disruptant mitochondria are dysfunctional. It should be added that the RECA1 disruption also affected the ultrastructure of chloroplasts, which were abnormally shaped and had reduced amounts of thylakoid membrane (Figure 2B). However, the defects of chloroplasts would not be a special case, as it is well established that photosynthetic function can be reduced in the mitochondrial electron transport mutant (Gu et al., 1993; Jiao et al., 2005).
Figure 2.
Ultrastructure of Mitochondria in the Protonemal Cell.
Transmission electron micrographs of protonemal cells of the wild-type background ([A] and [C]) and in RECA1 insertional disruptants [recA1(ins)-1] ([B] and [D] to [F]). The arrowheads indicate mitochondria. Bars = 1 μm in (A) and (B) and 500 nm in (C) to (F).
Specific Rearrangements of RECA1 Disruptant Mitochondrial DNA
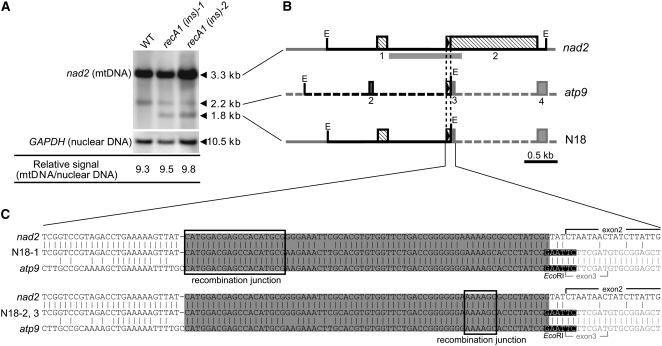
Escherichia coli RecA contributes to the maintenance of genomic DNA via recombinational DNA repair. Because RECA1 was suggested to participate in the repair of mtDNA, like E. coli RecA (Odahara et al., 2007), we examined the effect of the RECA1 disruption on mtDNA copy number. To estimate the copy number of mtDNA relative to that of nuclear DNA, we performed DNA gel blot analysis. The intensity of a 3.3-kb EcoRI fragment derived from the mitochondrial nad2 locus was divided by that of a 10.5-kb EcoRI fragment derived from the nuclear GAPDH locus (Figure 3A). There was no significant difference in the relative mtDNA copy number between wild-type and the two RECA1 disruptant strains (Figure 3A), indicating that the RECA1 disruptants bear a normal copy number of mtDNA at the nad2 locus. However, we observed two additional subsignals, a 2.2-kb EcoRI fragment seen in both the wild-type and the RECA1 disruptants and a 1.8-kb EcoRI fragment seen only in the disruptants (Figure 3A). Since the two RECA1 disruptants were independently obtained, the presence of this latter fragment suggests that a specific DNA rearrangement occurred in the disruptant mtDNA.
Figure 3.
Recombination of mtDNA in RECA1 Disruptants.
(A) DNA gel blots of total DNA from wild-type cells and RECA1 disruptants hybridized with the nad2 and GAPDH probes. Total DNA was digested with EcoRI. The intensity of nad2 bands (3.3 kb) relative to that of GAPDH on the blot is calculated and shown below the blots.
(B) The structures of nad2-related EcoRI DNA fragments that were revealed by cloning and DNA sequencing. The EcoRI fragments corresponding to the bands on the blot in (A) are represented by black lines, and flanking fragments are represented by gray lines. The position of the nad2 probe used in (A) is indicated by a thick gray line. The sequences corresponding to the nad2 and atp9 loci are shown by solid and broken lines, respectively. The EcoRI recognition sites are indicated by E. The boxes represent exons, and the lines between boxes represent introns and noncoding flanking sequences. Annotated numbers of the exons are shown under the boxes. The repeats (69 bp long, 91% identity) are indicated by the triangles in boxes.
(C) Nucleotide sequences of a region around the recombination junction in three independently obtained clones (N18-1 to -3), compared with those of the corresponding nad2 and atp9 loci. The 69-bp repeat sequences are shaded, and recombination is considered to occur within the boxed sequences.
To characterize this rearrangement, we analyzed the structures of the all three EcoRI fragments (3.3, 2.2. and 1.8 kb) that hybridized to the nad2 probe. Cloning and sequencing of the nad2-related DNA fragments indicated the following: the 3.3-kb fragment was a segment of the mitochondrial nad2 locus to which the nad2 probe was originally designed to hybridize, and the 2.2-kb fragment was a segment of the mitochondrial atp9 locus with a 69-bp sequence that is highly similar (91% identity) to a sequence in the nad2 locus, which explains why this fragment was detected using the nad2 probe. A clone of the RECA1 disruptant-specific 1.8-kb fragment (named N18-1) was a segment of a sequence essentially identical to the nad2 sequence except for a 69-bp sequence, in which the sequence identity was switched from nad2 to atp9 (Figures 3B and 3C). This shows that the 1.8-kb fragment was derived from recombination involving the nad2 and atp9 loci. Similar analysis of two other clones of the 1.8-kb fragments (N18-2 and -3) revealed that they were segments of a nad2-atp9 recombinant in which the recombination junction also resides within the 69-bp sequence but which was different from that of the clone N18-1 (Figure 3C). These results indicate an accumulation of DNA molecules derived from aberrant recombination between the 69-bp sequences present in both the nad2 and atp9 loci in RECA1 disruptant mitochondria.
Suppression of RECA1 Disruptant Phenotypes by Overexpression of RECA1 cDNA
In the RECA1 disruptant, which was constructed by insertion of the nptII cassette into the middle of the coding region, a truncated form of the RECA1 protein could be produced from the remaining 5′ half of the coding region (Figure 1A). The recombination between short repeats in the disruptant mitochondria described above might be caused by aberrant activity of the putative truncated RECA1 protein. To eliminate this possibility, we analyzed a RECA1 null disruptant in which the complete coding sequence of RECA1 was removed and replaced with a hygromycin phosphotransferase gene cassette (Odahara et al., 2007). The null disruptant showed phenotypes very similar to those of the insertional disruptant described above (Figures 1D and 1E), in terms of defects in growth and development as well as morphological abnormality of the protonema (Figure 4A). The nad2-atp9 recombinants accumulated in the null disruptant (Figure 4B) as well as in the insertional disruptant (Figure 3A). For further confirmation, the RECA1 cDNA was introduced into the nuclear genome of the null disruptant and expressed constitutively. The resulting RECA1 overexpressor grew normally (Figure 4A), and the signal specific to the nad2-atp9 recombinant almost disappeared in this strain (Figure 4B). We therefore conclude that the phenotypes of the RECA1 disruptant, including mtDNA rearrangement, were due to the absence of a functional RECA1 gene.
Figure 4.
Phenotypes of the RECA1 Null Disruptant and the RECA1 cDNA Overexpressor in the RECA1 Null Background.
(A) Growth and morphology of colonies and protonemata of the wild-type background, the RECA1 null disruptant (recA1-1), and the RECA1 cDNA overexpressor in the RECA1 null background (RECA1 cDNA+/ recA1-1). Colonies were cultivated on agar plates for 4 weeks. Bars = 5 mm in top panels and 50 μm in bottom panels.
(B) DNA gel blot analysis of the mtDNA configuration at the nad2 locus using the nad2 probe. Total genomic DNA was digested with EcoRI. The asterisk indicates the DNA fragment corresponding to nad2-atp9 recombinant DNA molecules.
[See online article for color version of this figure.]
Frequent and Multiple Rearrangements of RECA1 Disruptant mtDNA
The nad2-atp9 recombinant DNA that accumulated in RECA1 disruptant mitochondria was a result of aberrant recombination between 69-bp repeats sharing <100% sequence identity. To determine whether other short repeats participate in rearrangements of RECA1 disruptant mtDNA, we searched for repeats in the P. patens mtDNA sequence (Terasawa et al., 2007) using REPuter, a program that identifies repeated sequences (Kurtz and Schleiermacher, 1999). We identified 96 pairs of repeats longer than 40 bp. Among these, the longest is 90 bp, and Table 1 lists 20 sequences with E-values smaller than 5.00 × 10−11, which includes the nad2-atp9 repeats (see R10 in Table 1). Intriguingly, many of these repeats (∼60%) are located in introns and are oriented as direct repeats (Table 1).
Table 1.
Repeated Sequences in the P. patens Mitochondrial Genome (E-Values <5.00 × 10−11, >40 bp)
| Repeat-1
|
Repeat-2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Length (bp) | Positiona | Locusb | Length (bp) | Position | Locus | Orientationc | Distance (bp)d | E-valuee |
| R1 | 79 | 79449 | nad9 intron | 79 | 90131 | nad7 2nd intron | DR | 0 | 8.54E-39 |
| R2 | 78 | 43155 | nad5 1st intron | 78 | 79453 | nad9 intron | DR | 1 | 3.17E-35 |
| R3 | 83 | 43147 | nad5 1st intron | 84 | 90126 | nad7 2nd intron | DR | 3 | 7.00E-34 |
| R4 | 90 | 49768 | nad4 intron | 88 | 73530 | nad1 2nd intron | DR | 6 | 3.48E-31 |
| R5 | 57 | 43453 | nad5 1st intron | 57 | 70207 | coxIII intron | DR | 1 | 1.02E-22 |
| R6 | 56 | 50033 | nad4 intron | 56 | 73795 | nad1 2nd intron | DR | 1 | 4.01E-22 |
| R7 | 73 | 40892 | tRNA-Leu (CAA) | 73 | 40973 | tRNA-Leu (TAA) | DR | 6 | 1.64E-21 |
| R8 | 50 | 50374 | nad4 intron | 50 | 74200 | nad1 2nd intron | DR | 0 | 2.46E-21 |
| R9 | 47 | 20077 | ccmF intron | 47 | 102359 | atp9 1st intron | DR | 0 | 1.58E-19 |
| R10 | 69 | 52599 | nad2 intron | 69 | 103603 | atp9 2nd intron | DR | 6 | 2.95E-19 |
| R11 | 51 | 70213 | coxIII intron | 51 | 80506 | nad9 intron | DR | 1 | 3.74E-19 |
| R12 | 48 | 944 | – | 48 | 9303 | coxI 1st intron | DR | 1 | 2.25E-17 |
| R13 | 51 | 43459 | nad5 1st intron | 51 | 80506 | nad9 intron | DR | 2 | 9.04E-17 |
| R14 | 58 | 80499 | nad9 intron | 58 | 90545 | nad7 2nd intron | DR | 4 | 9.81E-17 |
| R15 | 62 | 1640 | – | 62 | 41363 | – | IR | 6 | 2.48E-15 |
| R16 | 62 | 43448 | nad5 1st intron | 62 | 90541 | nad7 2nd intron | DR | 6 | 2.48E-15 |
| R17 | 49 | 58275 | atp6 intron | 50 | 104767 | atp9 3rd intron | IR | 3 | 4.25E-14 |
| R18 | 53 | 70213 | coxIII intron | 53 | 90552 | nad7 2nd intron | DR | 5 | 4.59E-12 |
| R19 | 46 | 27033 | nad4L intron | 46 | 29552 | sdh3 intron | DR | 3 | 8.42E-12 |
| R20 | 48 | 12919 | – | 47 | 101024 | – | IR | 4 | 4.72E-11 |
Smallest number of the position of the repeated sequences corresponding to the P. patens mtDNA sequence accession number AB251495 (Terasawa et al., 2007)
Locus of the repeated sequences. –, intergenic region. All the introns listed in this table are group II introns.
DR, direct repeat; IR, inverted repeat.
Differences between Repeat-1 and Repeat-2.
Calculated E-value.
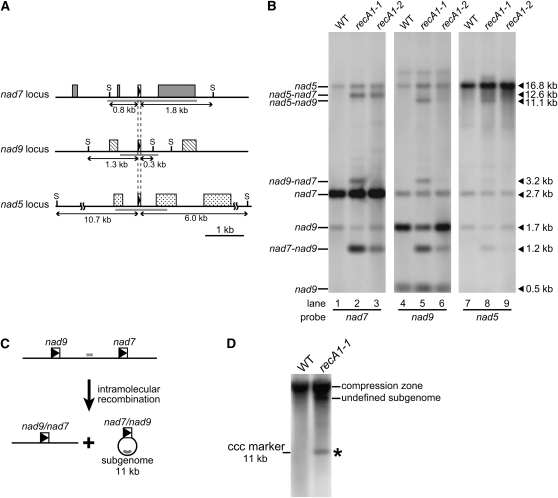
We then focused on three copies of repeats located in the nad5, nad7, and nad9 genes (78 to 84 bp long, 96 to 100% identity; R1, R2, and R3 in Table 1; Figure 5A), all of which are oriented as direct repeats, and analyzed the accumulation of DNA molecules resulting from recombination among them. As shown in Figure 5B, through gel blot analysis using SacII-digested genomic DNA and probes hybridizing to the nad5, nad7, and nad9 loci, we identified four novel DNA fragments (12.6, 11.1, 3.2, and 1.2 kb) that specifically accumulated in RECA1 null disruptants. We conclude that these novel RECA1 disruptant-specific DNA fragments are bona fide DNAs that arose from recombination between the repeats because (1) the sizes of these fragments were consistent with the predicted sizes of DNA fragments created by recombination between the repeats (Figures 5A and 5B), even when the genomic DNA was digested with other restriction enzymes (e.g., DraI and EcoRI; see Supplemental Figure 1 online), and (2) these novel DNA fragments hybridized to probes used to detect both repeat loci (e.g., a 1.2-kb SacII fragment, which was assumed to be a nad7-nad9 recombinant, hybridized to both the nad7 and nad9 probes; Figure 5B). Accumulation of the predicted recombinants in RECA1 disruptants was also confirmed by PCR using primers that specifically amplify recombinants and by DNA sequencing of the PCR products. All possible recombinants involving the three copies of the repeats, except for the nad7-nad5 recombinant and the nad9-nad5 recombinant, were detectable in this blot (Figure 5B); an explanation for the absence of signals for the two recombinants is provided below (see Discussion). A notable finding in this blot is that the amount of the nad7-nad9 recombinant in the recA1-1 strain (1.2 kb, lane 5 in Figure 5B) was equivalent to as much as 40% of the amount of the nad9 segment in the wild-type background (1.7 kb, lane 4 in Figure 5B), and the amount of the nad9 segment was correspondingly decreased in the recA1-1 strain (1.7 kb, lane 5 in Figure 5B), suggesting that ∼40% of the nad9 locus was converted to the nad7-nad9 recombinant as a consequence of recombination between the 79-bp repeats in the nad7 and nad9 genes. By contrast, the amount of the nad7 segment in the recA1-1 strain (2.7 kb, lane 2 in Figure 5B) was almost the same as that in the wild-type background (2.7 kb, lane 1 in Figure 5B) in spite of the conversion of a significant amount of the nad7 segment in the nad7-nad9 recombinant (1.2 kb, lane 2 in Figure 5B). This means that the nad7 locus was amplified in the recA1-1 strain. These results show that frequent and multiple rearrangements, which were likely to be caused by aberrant recombination among the dispersed repeats, occurred in the RECA1 disruptant mtDNA, resulting in gene truncation and amplification.
Figure 5.
Frequent and Multiple Rearrangements and Repeat-Mediated Deletion of mtDNA.
(A) Schematic representation of the structures of the three copies of repeats located at the nad7, nad9, and nad5 loci and the flanking region. The repeats (78 to 84 bp, 96 to 100% identity) are indicated by the triangles in the boxes. The probes used in (B) and the SacII recognition sites are indicated by bold gray lines and S, respectively.
(B) Analysis of the mtDNA configuration at the nad7, nad9, and nad5 loci. Hybridizations using the probes indicated below the blots were performed with SacII-digested total genomic DNA of the wild-type background and of two RECA1 null disruptants (recA1-1 and recA1-2). The presumed structures and the lengths of the major bands are indicated on the left and right, respectively.
(C) Scheme for the production of deleted mtDNA by intramolecular recombination between direct repeats. R1 direct repeats shown by the triangles in the boxes are located in both the nad7 and nad9 loci, and intramolecular recombination between them generates an 11-kb circular DNA molecule. The probe used in (D) is shown by a thick gray line.
(D) Detection of subgenomic molecules by DNA gel blot analysis. Hybridization using the probe designed to detect the genomic region between nad9 and nad7 was performed with total genomic DNA of the wild-type background and the RECA1 null disruptant (recA1-1) extracted by the CTAB method. The asterisk indicates subgenomic molecules that comigrated with an 11-kb covalently closed circular (ccc) plasmid.
Repeated Sequence-Mediated Deletion in RECA1 Disruptant mtDNA
Recombination between direct repeats in mtDNA can occur intermolecularly or intramolecularly. However, it is difficult to determine how the recombined DNA regions abnormally accumulating in the RECA1 disruptants were generated, since the DNA was fragmented by digestion with restriction enzymes. The distance between the repeats in the nad9 and nad7 loci, in which efficient recombination occurred in the disruptant as shown in Figures 5A and 5B, is 11 kb. To obtain evidence for intramolecular recombination between the nad9 and nad7 loci, we examined the generation of an 11-kb circular DNA molecule, which is an expected product of intramolecular recombination (Figure 5C).
DNA gel blot analysis was performed with total DNA extracted from protonema of wild-type and recA1-1 strains by the cetyltrimethylammonium bromide (CTAB) method, a routine method for extraction of total genomic DNA, and a probe that hybridizes to a region between nad9 and nad7. We detected a specific signal indicative of the predicted 11-kb covalently closed circular DNA in the RECA1 disruptant (Figure 5D). A more intense signal was detected for DNA prepared from the disruptant by the alkaline lysis method (see Supplemental Figure 2 online), an efficient extraction method for circular plasmids in E. coli (Sambrook et al., 1989), suggesting that the DNA has a closed circular structure. Furthermore, another signal was detected for RECA1 disruptant DNA prepared by the CTAB method (Figure 5D), as well as for that prepared by the alkaline lysis method (see Supplemental Figure 2 online); this might correspond to an undefined subgenome derived from intramolecular recombination between direct repeats other than the nad9-nad7 repeats and which contains a sequence recognized by the probe. These results demonstrate that intramolecular recombination between direct repeats occurred, and thereby subgenomic molecules with a deletion of part of the mitochondrial genome were indeed generated in RECA1 disruptant cells.
Recombination between Inverted Repeats
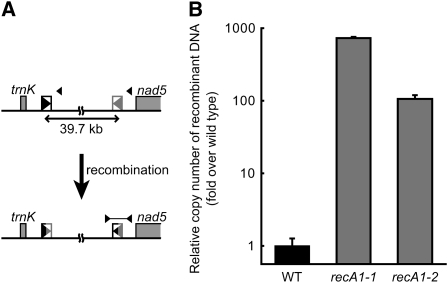
As all the repeats examined above were direct repeats, we tested whether inverted repeats are also involved in rearrangements. By DNA gel blot analysis, we first tried to detect a DNA fragment resulting from recombination between the inverted R15 repeats (62 bp long, 90% identity; Table 1), which are located in intergenic regions. However, there was no apparent accumulation of the DNA fragment in the RECA1 disruptants as well as in wild-type plants. We then developed a real-time PCR method with primers that specifically amplify the DNA resulting from recombination between the inverted repeats (Figure 6A) and thereby quantified the relative copy number of the recombined DNA in the wild-type background and in RECA1 disruptants. In RECA1 disruptants, the calculated relative copy number of the recombined DNA per that of nuclear DNA was found to be 100- to 700-fold higher than in the wild-type background (Figure 6B). This result indicates that aberrant recombination was significantly induced between inverted repeats as well as between direct repeats in RECA1 disruptant mitochondria and hence demonstrates that repeat orientation is not a prerequisite for the aberrant recombination. With the elevated recombination frequency in the RECA1 disruptants, the recombined DNA was still not detectable by DNA gel blot analysis as described above, probably because of a relatively low intrinsic tendency of recombination between R15 repeats whose E-value is larger than those of R1-R3 and R10 (see Table 1).
Figure 6.
Effect of the RECA1 Disruption on the Rate of Recombination between Inverted Repeats in mtDNA.
(A) Schematic representation of recombination between R15 inverted repeats. The repeats (62 bp, 90% identity) and flanking genes are indicated by triangles in boxes and gray boxes, respectively. A DNA segment containing the recombination junction can be amplified by PCR using specific primers (shown by arrowheads connected by horizontal line).
(B) Relative copy number of the recombined DNA per nuclear DNA, measured by quantitative real-time PCR. Recombinant DNA in the wild-type background was given a value of 1. Data from three independent PCR reactions are expressed as mean ± se (n = 3).
DISCUSSION
Here, we showed that disruption of a P. patens mitochondrial recA gene (RECA1) caused a serious defect in plant growth and development as well as a morphological abnormality in mitochondria. Further analyses of the disruptants revealed frequent and dynamic rearrangements involving multiple loci of the disruptant mtDNA (Figures 3 and 5). The rearrangements were caused by recombination among short (<100 bp) dispersed repeated sequences. Our results suggest a role for RECA1 in suppressing recombination among the short repeats that are abundantly scattered throughout the P. patens mitochondrial genome.
Our analyses showed that most of the repeats in the P. patens mtDNA are located in introns. Recombination between the repeats thus causes truncation (and in some cases, chimerization) of genes, as shown in this article (Figures 3 and 5). The recombination simultaneously generates subgenomes containing the truncated gene. The subgenome exists in some proportion relative to the normal genome, a condition known as heteroplasmy, and when the amount of the normal genome falls below a threshold level required for normal function of mitochondria, a mutant phenotype is seen. The effect of mtDNA heteroplasmy is discussed in a number of studies in maize (Zea mays; Gu et al., 1993, 1994; Marienfeld and Newton, 1994). The phenotypes of the RECA1 disruptant, including the defect in plant growth and development as well as mitochondrial abnormalities, may be caused by the effect of mtDNA heteroplasmy involving the subgenomes with the truncated gene, which were derived from the induced aberrant recombination. In addition, we demonstrated that intramolecular recombination between direct repeats occurred, resulting in the generation of two subgenomic mtDNA molecules, one of which is shown in Figure 5D. If one subgenome becomes predominant over the parental normal genome, the dose of genes existing in another subgenome will decrease. When the gene dosage falls below the threshold level required for normal function of mitochondria, the phenotypic defect of the RECA1 disruptant could be seen. The apparent absence of a signal corresponding to one of the two reciprocal molecules resulting from recombination between the nad7 and nad5 loci as well as between the nad9 and nad5 loci (Figure 5B) might be due to the difference in stability between the molecules. This could result in a decrease in the dose of genes existing in the unstable molecule. Such a decrease in the gene dosage might affect the phenotypes of the RECA1 disruptant; however, it was reported that plant mitochondria are likely to tolerate some extent of variation in gene dosages (Allen et al., 2007).
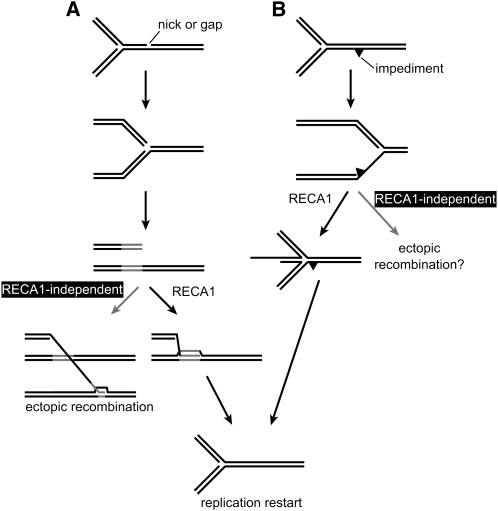
What is the molecular basis for the suppression of mtDNA rearrangements by RECA1? Functional similarity between RECA1 and E. coli RecA is expected from their protein sequence similarity (Odahara et al., 2007). Indeed, plant mitochondrial recA genes, including RECA1, can partially complement the E. coli recA mutation (Khazi et al., 2003; Odahara et al., 2007), and strand transfer activity that is probably related to RecA has been detected in partially purified plant mitochondrial fractions (Manchekar et al., 2006). In an early model of recombinational DNA repair in E. coli, RecA binds to single-stranded DNA resulting from the processing of double-strand DNA ends and then promotes strand exchange between homologous sequences to make D-loops (Kowalczykowski et al., 1994). Later studies suggested that RecA and other recombination proteins function in the repair of collapsed or stalled replication forks, which are caused by lesions on the template DNA (Cox et al., 2000; Lusetti and Cox, 2002). A collapsed replication fork may be repaired via a D-loop formed by RecA according to the conventional early model of recombinational DNA repair, whereas in the context of repair of a stalled replication fork, RecA is, in some situations, proposed to participate in regression of the fork (Seigneur et al., 2000; Robu et al., 2001) and/or maintenance of the regressed fork (Courcelle and Hanawalt, 2003). Based on models proposed for E. coli, a possible explanation for the suppression of DNA rearrangements by RECA1 is illustrated in Figure 7. A collapsed replication fork may be repaired via a D-loop formed by RECA1 to restart replication (Figure 7A). In the absence of RECA1, the DNA end could recombine with a short similar sequence in an ectopic locus by the action of an uncharacterized recombination system that is independent of RECA1 (Figure 7A). On the other hand, a stalled replication fork may be processed by the action of RECA1 via regression of the fork (Figure 7B). Without RECA1, an unrepaired stalled replication fork may lead to a rearrangement that involves short repeats (Figure 7B) because stalled forks have been suggested to be a significant cause of ectopic DNA rearrangements mediated by template-switching during replication in bacteria (Slack et al., 2006) and human cells (Lee et al., 2007). The lower rate of mtDNA repair in the RECA1 disruptant following MMS treatment (Odahara et al., 2007) supports a role for RECA1 in the repair of collapsed or stalled replication forks in mitochondria because replication at MMS-damaged DNA and at single-strand nicks introduced by AP-endonuclease in base excision repair of MMS-damaged bases are suggested to cause stalling and collapse of replication forks, respectively, and RecA-dependent homologous recombination is thought to be essential for fork repair (Nowosielska et al., 2006).
Figure 7.
A Model for the Repression of DNA Rearrangements by RECA1 in the Repair of a Collapsed or Stalled Replication Fork.
(A) Repair of a collapsed replication fork by RECA1. A collapsed replication fork can result from the DNA polymerase encountering a nick or a gap on the template DNA. RECA1 may be loaded onto the DNA, and a D-loop is formed between the DNA end and a sister chromosome to restart replication. In the absence of RECA1, the DNA end could initiate recombination independent of RECA1 with a similar short sequence at an ectopic locus.
(B) Repair of a stalled replication fork by RECA1. A replication fork can be stalled by an impediment on the template DNA. RECA1 may promote regression of the stalled fork and/or maintain the regressed fork. After removing the impediment, replication can be restarted. In the absence of RECA1, the presence of the stalled fork leads to ectopic recombination.
Several Arabidopsis nuclear mutations influence the amount of specific DNA molecules generated by recombination between relatively short repeats in mitochondria. Mutation of MutS homolog 1 (MSH1), which encodes a homolog of E. coli MutS targeted to mitochondria, confers an accumulation of two kinds of mitochondrial DNA: one product is generated by aberrant recombination between two 249-bp repeats; another is generated by recombination between two 335-bp repeats (Abdelnoor et al., 2003; Shedge et al., 2007). A similar accumulation of recombined mitochondrial DNA molecules has been observed in an Organellar Single-stranded DNA Binding protein 1 (OSB1) mutant of Arabidopsis (Zaegel et al., 2006). In this mutant, four pairs of repeats were reported to be involved in the recombination event: a pair of 435-bp repeats and a pair of 556-bp repeats as well as the two repeat pairs that were studied in the MSH1 mutant described above. Recently, mutation of RECA3, one of three nuclear recA homologs in Arabidopsis, was reported to induce recombination between the 249-bp repeats in mitochondria (Shedge et al., 2007). Any pair of repeats involved in rearrangements in these Arabidopsis mutants is >200 bp and exactly identical, whereas the repeats involved in rearrangements in RECA1 disruptants are <100 bp and contain some mismatches. Furthermore, the few hot spots for the recombination observed in the MSH1 and RECA3 mutants suggests a role for MSH1 and RECA3 in suppression of specific recombination events, whereas the multiple hot spots for recombination in RECA1 disruptant mtDNA suggest a role for RECA1 in suppression of a broad variety of recombination. These differences in repeats between Arabidopsis and P. patens may imply that the features of recombination induced in the RECA1 disruptant are distinct from those in the Arabidopsis mutants.
Arabidopsis has two mitochondrial RecA homologs, RECA2 (which also localizes to chloroplasts) and RECA3. It was reported that disruption of RECA3 results in viable and phenotypically normal plants but induces aberrant recombination in the mitochondrial genome, whereas disruption of RECA2 results in lethality (Shedge et al., 2007). RECA3 uniquely has a truncation in the C-terminal end important for strand exchange and substitutions in conserved amino acids important for ATP binding/hydrolyzing and monomer–monomer interaction (Shedge et al., 2007). The existence of two mitochondrial RecA proteins is reminiscent of functional diversification. Shedge et al. (2007) suggested a role for RECA3 in maintaining mitochondrial genome stability; however, the functional relationship between the two RecA proteins and the contribution of RECA2 to recombination in the mitochondrial genome remain unclear. Our subcellular localization analyses of P. patens RecA proteins (Odahara et al., 2007; Inouye et al., 2008) and survey of the P. patens nuclear genome sequence (Rensing et al., 2008) show that P. patens has a single mitochondrial RecA. Thus, our analyses of RECA1 clearly show a role for RecA in plant mitochondria, and here we propose that one of the primary roles of plant mitochondrial RecA is to suppress recombination among the short repeats (<100 bp) scattered throughout the genome.
Our analysis of P. patens mtDNA showed that most of the 96 pairs of repeats exceeding 40 bp are located in group II introns (Table 1), which are known to be transposable elements (Lambowitz and Zimmerly, 2004). This interesting feature may have arisen by an increase in the number of copies of group II introns by retrotransposition and subsequent base substitutions, except in regions where there are functional constraints on intron splicing, resulting in the conserved short repeats scattered throughout the mtDNA. Therefore, our results suggest that RecA suppresses mitochondrial genome instability caused by this increase in the copy number of repeats, which were amplified via retrotransposition of group II introns. It is possible that genome stabilization by RecA could ensure an increase in the copy number of group II introns.
In this report, we showed abnormally induced rearrangements of the mitochondrial genome in the RECA1 disruptant. Plant mitochondrial genomes were reported to exhibit a slow rate of nucleotide substitution (Wolfe et al., 1987; Palmer and Herbon, 1988); however, they are occasionally rearranged by recombination between short (<1 kb, in most case <200 bp) repeats (Newton et al., 2004). This suggests that plant mitochondrial genomes can potentially undergo rearrangements. Our results suggest that mtDNA rearrangements might be normally suppressed to a low level by the functioning of a mitochondrial RecA, and when its activity is attenuated, frequent rearrangements might be induced. In the mitochondrial genomes of plants, especially higher plants, short repeats are abundant. RecA-mediated homologous recombination can play a crucial role in suppression of recombination among such dispersed repeats in plant mitochondria, and the role is likely to be indispensable for maintaining mitochondrial genome stability.
METHODS
Plant Material and Growth Conditions
Protonemata of Physcomitrella patens Bruch and Schimp subsp patens were cultured on cellophane-covered BCDATG agar medium (Nishiyama et al., 2000). Protonemata were cultivated at 25°C under constant light conditions (70 μmol m−2 s−1). To compare the growth rate of RECA1 disruptants with that of wild-type cells, tissues were homogenized using Ultra-Turrax (IKA), and protonemata were subsequently cultivated as described above at least twice, and small pieces (∼1.5 mm in diameter) of fresh protonemata were then transferred onto BCDATG agar medium without cellophane and cultivated.
DNA and RNA Extraction
Genomic DNA was extracted by the CTAB method (Murray and Thompson, 1980) from protonemata cultivated for 4 d after inoculation on agar medium.
The alkaline lysis method (Sambrook et al., 1989) was applied for efficient extraction of a species of small circular DNA from protonemata. Protonemata (200 mg) ground in liquid nitrogen were mixed with 400 μL alkaline buffer (0.2 M NaOH and 1% SDS) and incubated on ice for 5 min and then neutralized by adding 300 μL neutralization buffer (3 M potassium acetate and 2 M acetate) and incubated on ice for 5 min. DNA was purified by phenol:chloroform extraction and isopropanol precipitation. For DNA gel blot analysis, 10 ng of the extracted DNA was used.
Total RNA was extracted from protonemata using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer's protocol and treated with DNaseI to remove contaminating genomic DNA.
Moss Transformation
Isolation of protoplasts and subsequent polyethylene glycol–mediated transformation of P. patens were performed according to Nishiyama et al. (2000). To generate stable transformants, 10 μg of linearized targeting construct was introduced, and the resulting transformants were subsequently selected in medium containing 20 μg/mL G418 or 30 μg/mL hygromycin B.
Construction of Vectors for RECA1 Disruption and RECA1 cDNA Overexpression
To generate a RECA1 insertional disruptant, a 1.6-kb fragment spanning the 5′ region and a 1.5-kb fragment spanning the 3′ region of the genomic RECA1 were amplified by PCR using the following primers (restriction sites are underlined): P1 (5′-CCCTGATCAGGTACCCCCGGGGACATGGCGGGAGCTCTGCGAGTACTTAG-3′) and P2 (5′-CGGGAATTCATCGACACCAGTAGAGCG-3′) for amplification of the 1.6-kb fragment, and P3 (5′-GCGTCTAGATTCACGTGATTGCACAGG-3′) and P4 (5′-CCCAAGCTTCACCATGGGATCCCCTGTCACCTCTAGATTATCAGCATC-3′) for amplification of the 1.5-kb fragment. The resulting 1.6-kb fragment was cleaved with KpnI and EcoRI, and the 1.5-kb fragment was cleaved with XbaI and ligated to KpnI/EcoRI- and XbaI-cleaved pTN3 (Nishiyama et al., 2000), respectively. The resulting plasmid containing the targeting construct was named pMAK124 and linearized before introduction into P. patens protoplasts.
For ectopic expression of RECA1 cDNA in the RECA1 null background, plasmid pPpMADS2-7133 carrying a genomic fragment of Pp MADS2 (Henschel et al., 2002), which is interrupted by a DNA fragment containing the E7133 promoter (Mitsuhara et al., 1996), the nos terminator, and the nptII gene expression cassette, was used. The coding region of the RECA1 cDNA was amplified by PCR with cDNA prepared from P. patens total RNA and the primers P1 and P5 (5′-GGCGCCCGGGCTACCCTGTCACCTCTAG-3′), digested with XmaI, and inserted into the XmaI site between the E7133 promoter and the nos terminator of pPpMADS2-7133. The resulting plasmid pMAK136 was linearized and then introduced into protoplasts of the RECA1 null disruptant (Odahara et al., 2007). Two stably transformed strains were obtained, and integration of the introduced DNA into the genomic DNA and overexpression of RECA1 cDNA were confirmed by PCR and RT-PCR analysis, respectively, according to Odahara et al. (2007). Since these two strains exhibited very similar phenotypes, only one strain was used for further analyses.
DNA Gel Blot Analysis
Total genomic DNA was separated on a 0.7% agarose gel and blotted onto a nylon membrane. To detect nuclear-encoded RECA1 (shown in Figure 1) and mitochondrial encoded genes (shown in Figures 4 and 5), 4 and 0.5 μg of total genomic DNA were used, respectively. To prepare probes, PCR was performed using the PCR DIG Probe Synthesis Kit (Roche) and the following primers: P1 and P6 (5′-GTAATACGACTCACTATAGGCAATGTCCTTTTCC-3′) for the RECA1 probe; P7 (5′-CATGGTGGGATCGGCTAAG-3′) and P8 (5′-TAATACGACTCACTATAGGGCGAGATAGGAGCATCTGTACC-3′) for the GAPDH probe; P9 (5′-GCCTAGGAGGGCGCGTTTGGGAAGACG-3′) and P10 (5′-CCCAGACACATAACTATAGTGCTAGCCG-3′) for the nad2 probe; P11 (5′-TACTAGGTAGTTGTGTAGCAGGTG-3′) and P12 (5′-ACCCATAATCCCGAGAGCTAATCC-3′) for the nad5 probe; P13 (5′-CGGGTTAGGGGTACGACAGATAGCG-3′) and P14 (5′-TAATACGACTCACTATAGGGCGAGTAGTTCTATCTATCTACCTCTCC-3′) for the nad7 probe; P15 (5′-TAGAAGTCTGCAACGAAACGGGCACG-3′) and P16 (5′-CGAAGTGGATTACCTAGTCTCCCAG-3′) for the nad9 probe; and P17 (5′-TCGTCATACGAGCGGAGCTCGAA-3′) and P18 (5′-CGTAGCCAACAAAGGCTCTGAGAG-3′) for a probe to detect the genomic region between nad9 and nad7. Hybridization of the probes was performed at 39°C, and the membranes were washed in 2× SSC with 0.1% SDS at 25°C and 0.5× SSC with 0.1% SDS at 65°C. Detection of the DIG-labeled probes was performed with Anti-DIG-Alkaline Phosphatase (Roche) and AttoPhos (Promega).
For quantitative estimation of mtDNA and nuclear DNA shown in Figure 3, 4 μg of EcoRI-digested total genomic DNA was separated on a 0.7% agarose gel and blotted onto a nylon membrane. The blot was initially hybridized to the DIG-labeled GAPDH probe and then washed. The blot was subsequently rehybridized to the DIG-labeled nad2 probe. The intensities of the signals of GAPDH and nad2 (3.3 kb) on the blot were measured by Typhoon 9210 and ImageQuant (GE Healthcare). The relative copy number of mtDNA per nucleus was calculated by dividing the intensity of the signal of nad2 by that of GAPDH.
RT-PCR Analysis
Reverse transcription was performed using 1 μg of total RNA and an oligo(dT) primer, after which PCR was performed. To amplify a segment of RECA1, the primers P19 (5′-GGGAAGCTTCGGGTAGTGGAGATCTTTGG-3′) and P20 (5′-GGTGGTGATACTTTGTTC-3′) were used, and to obtain internal controls, the primers P21 (5′-CATGTTCGAGACGTTCAACGTGCCG-3′) and P22 (5′-GATGGACCAGATTCATCGTACTCGC-3′) were used to amplify a segment of the actin gene. In these experiments, PCR amplification was performed in the exponential amplification phase, and the quantities of amplification products were compared using SYBR Green I (TaKaRa) staining.
Transmission Electron Microscopy
Protonemata of each strain cultivated on agar medium were fixed in 2% glutaraldehyde in 20 mM sodium cacodylate, pH 7.2, and in 2% osmium tetroxide in 20 mM sodium cacodylate, pH 7.2, dehydrated with ethanol, and embedded in Spurr's resin. Thin sections were stained with uranyl acetate and lead citrate and observed with a JEM-1200EX electron microscope (JEOL).
Cloning and Sequencing of the nad2-related mtDNA Fragment
EcoRI-digested total genomic DNA (4 μg) of the RECA1 disruptant was separated on a 1.0% agarose gel, and the 3.3-, 2.2-, and 1.8-kb DNA fragments were recovered. Genomic DNA libraries were constructed by cloning these EcoRI fragments into the MfeI site of the cloning vector pMSC5 (MoBiTec), and these were introduced into E. coli cells by electroporation. Colonies were screened by hybridization (Sambrook et al., 1989) using the DIG-labeled nad2 probe, and positive clones were selected and sequenced.
Identification of Repeats in P. patens Mitochondrial DNA
Repeated sequences in P. patens mtDNA (accession number AB251495) were identified with the REPuter program (http://bibiserv.techfak.uni-bielefeld.de/reputer/) with a maximal 6 bp of mismatch permitted. Palindromic sequences were eliminated from the repeats.
PCR Analysis of the DNA Created by Recombination among Repeats in nad5, nad7, and nad9
To analyze the accumulation level of the DNA resulting from recombination among repeats in the nad5, nad7, and nad9 locus, PCR was performed using wild-type, recA1-1, and recA1-2 total genomic DNA extracted by the CTAB method and the following primers: P11 and P14 for the nad5-nad7 recombinant, P13 and P12 for the nad7-nad5 recombinant, P11 and P16 for the nad5-nad9 recombinant, P15 and P12 for the nad9-nad5 recombinant, P13 and P16 for the nad7-nad9 recombinant, P15 and P14 for the nad9-nad7 recombinant, and P9 and P10 for the nad2 locus as an internal control. PCR amplification was performed in the exponential amplification phase. The quantities of amplification products from two independent PCR reactions were compared using agarose gel electrophoresis and ethidium bromide staining. The products were then sequenced, and the recombination junctions were confirmed.
Real-Time PCR Analysis of the DNA Created by Recombination between the R15 Repeats
Real-time PCR was performed with the Applied Biosystems 7500 Fast Real-Time PCR System and POWER SYBR Green Master Mix (Applied Biosystems International) using total genomic DNA extracted by the CTAB method and the primers P23 (5′-CAACCGTCTTCTGTGTCTAGGTC-3′) and P24 (5′-GAAACCGGCCTGCATTACATG-3′) for the 91-bp segment of the nuclear-encoded actin gene, and P25 (5′-TACTAGTCCAATGTCGCCATAAGG-3′) and P26 (5′-ATTCTCGACCACAGGAATTTCC-3′) for the 134-bp segment of the DNA that was generated by recombination between the R15 repeats. The relative copy number of the recombined DNA per nuclear DNA was calculated with SDS software (Applied Biosystems International) using standards consisting of serial dilutions of genomic DNA from the wild-type background to quantify the actin gene and that from recA1-1 for the recombined DNA.
Accession Number
Sequence data from this article can be found in the GenBank/EMBL database under accession number AB284534 (RECA1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Multiple Rearrangements of RECA1 Disruptant mtDNA.
Supplemental Figure 2. Repeat-Mediated Deletion of RECA1 Disruptant mtDNA.
Supplementary Material
Acknowledgments
We thank Tomomichi Fujita and Mitsuyasu Hasebe for technical advice as well as Keiko Umezu and Hisaji Maki for helpful discussion. This work was supported by Japan Society for the Promotion of Science Fellowships (08575 to M.O.), by a Grant-in-Aid for Creative Scientific Research (17GS0314 to Y.S.) from the Japan Society for the Promotion of Science, by the program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), and by the Frontier Project “Adaptation and Evolution of Extremophiles.”
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Yasuhiko Sekine (ysekine@rikkyo.ac.jp).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Abdelnoor, R.V., Yule, R., Elo, A., Christensen, A.C., Meyer-Gauen, G., and Mackenzie, S.A. (2003). Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proc. Natl. Acad. Sci. USA 100 5968–5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J.O., et al. (2007). Comparisons among two fertile and three male-sterile mitochondrial genomes of maize. Genetics 177 1173–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre, C., Levy, A., and Walbot, V. (1992). Small repeated sequences and the structure of plant mitochondrial genomes. Trends Genet. 8 128–132. [DOI] [PubMed] [Google Scholar]
- Bishop, D.K., Park, D., Xu, L., and Kleckner, N. (1992). DMC1: A meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69 439–456. [DOI] [PubMed] [Google Scholar]
- Cao, J., Combs, C., and Jagendorf, A.T. (1997). The chloroplast-located homolog of bacterial DNA recombinase. Plant Cell Physiol. 38 1319–1325. [DOI] [PubMed] [Google Scholar]
- Cerutti, H., and Jagendorf, A.T. (1993). DNA strand-transfer activity in pea (Pisum sativum L.) chloroplasts. Plant Physiol. 102 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti, H., Osman, M., Grandoni, P., and Jagendorf, A.T. (1992). A homolog of Escherichia coli RecA protein in plastids of higher plants. Proc. Natl. Acad. Sci. USA 89 8068–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcelle, J., and Hanawalt, P.C. (2003). RecA-dependent recovery of arrested DNA replication forks. Annu. Rev. Genet. 37 611–646. [DOI] [PubMed] [Google Scholar]
- Cox, M.M., Goodman, M.F., Kreuzer, K.N., Sherratt, D.J., Sandler, S.J., and Marians, K.J. (2000). The importance of repairing stalled replication forks. Nature 404 37–41. [DOI] [PubMed] [Google Scholar]
- Gu, J., Miles, D., and Newton, K.J. (1993). Analysis of leaf sectors in the NCS6 mitochondrial mutant of maize. Plant Cell 5 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J., Dempsey, S., and Newton, K.J. (1994). Rescue of a maize mitochondrial cytochrome oxidase mutant by tissue culture. Plant J. 6 787–794. [DOI] [PubMed] [Google Scholar]
- Henschel, K., Kofuji, R., Hasebe, M., Saedler, H., Munster, T., and Theissen, G. (2002). Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol. Biol. Evol. 19 801–814. [DOI] [PubMed] [Google Scholar]
- Inouye, T., Odahara, M., Fujita, T., Hasebe, M., and Sekine, Y. (2008). Expression and complementation analyses of a chloroplast-localized homolog of bacterial RecA in the moss Physcomitrella patens. Biosci. Biotechnol. Biochem. 72 1340–1347. [DOI] [PubMed] [Google Scholar]
- Jiao, S., Thornsberry, J.M., Elthon, T.E., and Newton, K.J. (2005). Biochemical and molecular characterization of photosystem I deficiency in the NCS6 mitochondrial mutant of maize. Plant Mol. Biol. 57 303–313. [DOI] [PubMed] [Google Scholar]
- Khazi, F.R., Edmondson, A.C., and Nielsen, B.L. (2003). An Arabidopsis homologue of bacterial RecA that complements an E. coli recA deletion is targeted to plant mitochondria. Mol. Genet. Genomics 269 454–463. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski, S.C., Dixon, D.A., Eggleston, A.K., Lauder, S.D., and Rehrauer, W.M. (1994). Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58 401–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz, S., and Schleiermacher, C. (1999). REPuter: Fast computation of maximal repeats in complete genomes. Bioinformatics 15 426–427. [DOI] [PubMed] [Google Scholar]
- Lambowitz, A.M., and Zimmerly, S. (2004). Mobile group II introns. Annu. Rev. Genet. 38 1–35. [DOI] [PubMed] [Google Scholar]
- Lee, J.A., Carvalho, C.M., and Lupski, J.R. (2007). A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131 1235–1247. [DOI] [PubMed] [Google Scholar]
- Little, J.W., and Mount, D.W. (1982). The SOS regulatory system of Escherichia coli. Cell 29 11–22. [DOI] [PubMed] [Google Scholar]
- Lonsdale, D.M., Hodge, T.P., and Fauron, C.M. (1984). The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 12 9249–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusetti, S.L., and Cox, M.M. (2002). The bacterial RecA protein and the recombinational DNA repair of stalled replication forks. Annu. Rev. Biochem. 71 71–100. [DOI] [PubMed] [Google Scholar]
- Manchekar, M., Scissum-Gunn, K., Song, D., Khazi, F., McLean, S.L., and Nielsen, B.L. (2006). DNA recombination activity in soybean mitochondria. J. Mol. Biol. 356 288–299. [DOI] [PubMed] [Google Scholar]
- Marienfeld, J.R., and Newton, K.J. (1994). The maize NCS2 abnormal growth mutant has a chimeric nad4-nad7 mitochondrial gene and is associated with reduced complex I function. Genetics 138 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhara, I., et al. (1996). Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol. 37 49–59. [DOI] [PubMed] [Google Scholar]
- Murray, M.G., and Thompson, W.F. (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, K.J., Gabay-Laughnan, S., and Paepe, R.D. (2004). Mitochondrial mutation in plants. In Plant Mitochondria, D.A. Day, A.H. Millar, and J. Whelan, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 121–141.
- Nishiyama, T., Hiwatashi, Y., Sakakibara, I., Kato, M., and Hasebe, M. (2000). Tagged mutagenesis and gene-trap in the moss, Physcomitrella patens by shuttle mutagenesis. DNA Res. 7 9–17. [DOI] [PubMed] [Google Scholar]
- Nowosielska, A., Smith, S.A., Engelward, B.P., and Marinus, M.G. (2006). Homologous recombination prevents methylation-induced toxicity in Escherichia coli. Nucleic Acids Res. 34 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda, K., Yamato, K., Ohta, E., Nakamura, Y., Takemura, M., Nozato, N., Akashi, K., Kanegae, T., Ogura, Y., Kohchi, T., and Ohyama, K. (1992). Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. A primitive form of plant mitochondrial genome. J. Mol. Biol. 223 1–7. [DOI] [PubMed] [Google Scholar]
- Odahara, M., Inouye, T., Fujita, T., Hasebe, M., and Sekine, Y. (2007). Involvement of mitochondrial-targeted RecA in the repair of mitochondrial DNA in the moss, Physcomitrella patens. Genes Genet. Syst. 82 43–51. [DOI] [PubMed] [Google Scholar]
- Palmer, J.D., and Herbon, L.A. (1988). Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J. Mol. Evol. 28 87–97. [DOI] [PubMed] [Google Scholar]
- Pham, P., Bertram, J.G., O'Donnell, M., Woodgate, R., and Goodman, M.F. (2001). A model for SOS-lesion-targeted mutations in Escherichia coli. Nature 409 366–370. [DOI] [PubMed] [Google Scholar]
- Rensing, S.A., et al. (2008). The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319 64–69. [DOI] [PubMed] [Google Scholar]
- Robu, M.E., Inman, R.B., and Cox, M.M. (2001). RecA protein promotes the regression of stalled replication forks in vitro. Proc. Natl. Acad. Sci. USA 98 8211–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schaefer, D.G. (2001). Gene targeting in Physcomitrella patens. Curr. Opin. Plant Biol. 4 143–150. [DOI] [PubMed] [Google Scholar]
- Seigneur, M., Ehrlich, S.D., and Michel, B. (2000). RuvABC-dependent double-strand breaks in dnaBts mutants require recA. Mol. Microbiol. 38 565–574. [DOI] [PubMed] [Google Scholar]
- Shedge, V., Arrieta-Montiel, M., Christensen, A.C., and Mackenzie, S.A. (2007). Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant Cell 19 1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara, A., Ogawa, H., and Ogawa, T. (1992). Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69 457–470. [DOI] [PubMed] [Google Scholar]
- Slack, A., Thornton, P.C., Magner, D.B., Rosenberg, S.M., and Hastings, P.J. (2006). On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2 e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, I., Suffolk, R., and Leaver, C.J. (1989). Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58 69–76. [DOI] [PubMed] [Google Scholar]
- Sugiura, C., Kobayashi, Y., Aoki, S., Sugita, C., and Sugita, M. (2003). Complete chloroplast DNA sequence of the moss Physcomitrella patens: Evidence for the loss and relocation of rpoA from the chloroplast to the nucleus. Nucleic Acids Res. 31 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa, K., Odahara, M., Kabeya, Y., Kikugawa, T., Sekine, Y., Fujiwara, M., and Sato, N. (2007). The mitochondrial genome of the moss Physcomitrella patens sheds new light on mitochondrial evolution in land plants. Mol. Biol. Evol. 24 699–709. [DOI] [PubMed] [Google Scholar]
- Unseld, M., Marienfeld, J.R., Brandt, P., and Brennicke, A. (1997). The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 15 57–61. [DOI] [PubMed] [Google Scholar]
- Wolfe, K.H., Li, W.H., and Sharp, P.M. (1987). Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. USA 84 9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaegel, V., Guermann, B., Le Ret, M., Andres, C., Meyer, D., Erhardt, M., Canaday, J., Gualberto, J.M., and Imbault, P. (2006). The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis. Plant Cell 18 3548–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.