Fibrillins are large cysteine-rich glycoproteins that are evolutionarily conserved from scyphozoans to mammals. Fibrillin assemblies (microfibrils) serve two key physiological functions: the function of a structural support that imparts tissue integrity and the function of a regulator of signaling events that instruct cellular performance (1, 2). The importance of microfibrils in organ formation and tissue homeostasis was originally underscored by the finding that mutations of human fibrillin-1 and fibrillin-2 are responsible for the pleiotropic manifestations of MFS2 (OMIM 154700) and congenital contractural arachnodactyly (OMIM 121050), respectively (3).
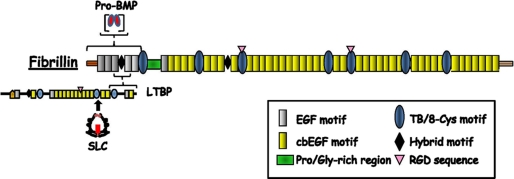
Fibrillins display a common modular structure that consists predominantly of cbEGF domains interspersed with TB/8-Cys modules (Fig. 1) (1, 2, 4). TB/8-Cys modules are unique to fibrillins and LTBPs. Fibrillins polymerize into microfibrils in which individual molecules are organized in a head-to-tail arrangement and interact laterally; furthermore, microfibrils associate or interact with additional proteins, such as elastin in elastic fibers (Fig. 2) (1, 2, 4, 5). Fibrillins also control the bioavailability of endogenous (local) TGFβ and BMP ligands by targeting the respective complexes to the ECM (Fig. 1) (1–3).
FIGURE 1.
Schematic representation of prototypical fibrillin and LTBPs (not in scale). Sites of interactions between fibrillin and TGFβ and BMP complexes are shown. For a detailed description of the structural features of fibrillins and LTBPs, see Hubmacher et al. (2). SLC, small latent complex.
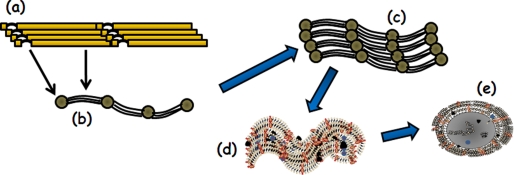
FIGURE 2.
Diagram highlighting the main steps in microfibril biogenesis. They include the polymerization of fibrillins in a head-to-tail organization (step a) that is visualized by electron microscopy as multiple strings with regularly spaced beads (step b). The beads correspond to the N-terminal regions of fibrillins containing the Gly/Pro-rich stretch (arch) and the sites interacting with TGFβ and BMP complexes, whereas the strings correspond to the central sequence of multiple cbEGF motifs interspersed with a few TB/8-Cys modules (see Fig. 1). Also shown are microfibrils (step c) growing into large macro-aggregates that are either devoid of elastin (step d) or associated with cross-linked elastin (gray core) in elastic fibers (step e). Orange, blue, and black circles depict microfibril- and elastin-interacting molecules; additionally, some microfibrils are shown buried within amorphous elastin. A detailed description of microfibril and elastic fiber biogenesis can be found in several recent reviews (2, 4, 5).
This article focuses on the instructive function of fibrillin-rich microfibrils in organ development and tissue homeostasis. A number of excellent reviews are available that describe in greater detail the structural and biosynthetic aspects of fibrillin assemblies (2, 4, 5).
Fibrillins Bind TGFβ and BMP Complexes
TGFβs 1–3 (hereafter collectively referred to as TGFβ) are secreted either as a small latent complex in which the bioactive homodimer is in noncovalent association with the processed N-terminal propeptide (LAP) or as a large latent complex in which LAP is covalently linked to LTBP1, LTBP3, or LTBP4 (6). Association with LAP blocks binding of the ligand to the receptors, whereas interaction with LTBP1 or LTBP4 promotes small latent complex targeting to fibrillin-rich microfibrils (Fig. 1). A variety of extracellular molecules (many of which interact with fibrillin-rich microfibrils) are involved in releasing TGFβ from the ECM, disrupting LAP-mediated latency, or inhibiting TGFβ activity (6, 7).
BMPs are also secreted as complexes in which C-terminal cross-linked dimers are noncovalently associated with the prodomains (8). In contrast to TGFβ, however, BMPs can be targeted directly to microfibrils through the interaction between their prodomains and the N-terminal regions of fibrillins (Fig. 1) (9). Furthermore, BMP signaling can be activated through competitive displacement of the prodomain by type II receptors (10). Studies discussed below strongly suggest that the relative composition of fibrillin-rich microfibrils imparts contextual specificity to TGFβ and BMP signaling either by concentrating the ligands at sites of intended function (positive regulation) or by inhibiting their bioavailability (negative regulation).
Fibrillins in Vertebrate Development
Expression of fibrillin genes in lower and higher vertebrates is largely confined to mesenchyme derivatives (11–14). Studies of frog and zebrafish embryos, in particular, have correlated onset of fibrillin gene expression with the beginning of gastrulation (13, 14). Consistent with this observation, a recent study has demonstrated that Xenopus fibrillin (an ortholog of fibrillin-2) is required to complete the process of convergent extension in the presumptive notochord of the gastrulating embryo (15). Another study has associated notochord abnormalities and vascular malformations with morpholino-induced silencing of fibrillin-2 production in zebrafish embryos (14). By contrast, caudal vein dilation and impaired plexus formation were the sole manifestations of fibrillin-1 morphants (16). Interestingly, the phenotype of fibrillin-2 morphants faithfully mirrors that of mutant zebrafish embryos that were selected in a forward genetic screen for notochord sensitivity to lysyl oxidase inhibition (14). It has been argued that fibrillin-2 microfibrils may recruit the lysyl oxidase enzyme to properly assemble and stabilize the ECM of the notochordal sheath. It was also reasoned that impaired caudal vein morphogenesis in mutant zebrafish embryos may reflect a nonstructural (instructive) function of fibrillin-rich microfibrils. The recent report that lysyl oxidase inhibits TGFβ activity supports this last postulate (17).
Genetic investigations in mice have corroborated the notion that fibrillin-1 and fibrillin-2 play discrete roles in vertebrate morphogenesis. A case in point is the limb-patterning defect (syndactyly) of mice lacking fibrillin-2 gene (Fbn2) expression, a phenotype not seen in Fbn1-null mice even though both proteins are abundantly deposited in ECM of forming autopods (18, 19). Syndactyly is also observed in Bmp7-null mice but not in mice haploinsufficient for either Fbn2 or Bmp7; however, combined Fbn2 and Bmp7 haploinsufficiency yields syndactyly (18). Two lessons derive from these observations. First, in the developing autopod, the predominant effect of fibrillin-2 on BMP7 signaling is positive regulation. Second, despite robust expression, fibrillin-1 cannot compensate for loss of fibrillin-2.
The developing mouse aorta is another example of organ-specific roles of fibrillins. Whereas impaired maturation of the aortic matrix accounts for dissecting aneurysm and neonatal death of Fbn1-null mice, loss of fibrillin-2 has no impact on vessel maturation and, consequently, on postnatal survival and fitness (18, 19). However, mice lacking both fibrillins die at mid-gestation, significantly earlier than either of the parental strains, and exhibit a poorly developed aortic media, implying functional cooperation between fibrillins in promoting ECM assembly (19). Furthermore, half of Fbn1+/–;Fbn2–/– embryos die in utero, suggesting either that the total amount of microfibrils drives aortic matrix formation or that the fibrillins play functionally distinct roles in vessel morphogenesis (19). Ongoing studies of bone remodeling support the latter hypothesis. Specifically, these analyses have shown that Fbn2-null osteoblast cultures fail to mineralize due to heightened TGFβ signaling, whereas Fbn1-null osteoblasts differentiate properly despite enhanced TGFβ signaling because a greater amount of BMPs is no longer sequestered in the ECM.3
Temporal variation in gene expression can also influence the relative contribution of fibrillins to tissue morphogenesis and the phenotypic consequence of microfibril deficiency. Fibrillin-1 deficiency in mice leads to TGFβ-mediated failure of distal alveolar septation, which is evident in the first week of post-natal development and is maintained throughout adult life (20). By contrast, fibrillin-2 deficiency associates with a more proximal defect in lung branching morphogenesis that is most evident during late embryogenesis but resolves completely shortly after birth (18).4 These observations are reconciled by the predominantly fetal expression of fibrillin-2 in the developing lung and by the emergence of significant fibrillin-1 expression in the perinatal period (11). In this light, it appears that the regulatory role of fibrillins in the developing lung is uniquely shouldered by fibrillin-2 during fetal life and that fibrillin-1 can compensate for fibrillin-2 deficiency by virtue of its later expression.
Fibrillins in Disease Processes
Heterozygous mutations that affect the structure or decrease the synthesis of fibrillin-1 are responsible for MFS manifestations, which principally involve the ocular, skeletal, and cardiovascular systems (3). Progressive aortic root enlargement and abnormally thick and elongated valve leaflets are the major determinants of morbidity and mortality in MFS patients. Treatment of vascular disease in MFS includes regular imaging to monitor aneurysm progression, β-adrenergic blockade to slow aortic growth, and prophylactic surgery to prevent aortic complications. Extensive phenotypic variability, age-dependent onset of informative manifestations, a high degree of spontaneous mutations, and clinical overlap with several other conditions are all potential problems in MFS diagnosis and the timely management of cardiovascular complications, particularly in young children (30).
About 14% of MFS patients show chronic obstructive lung disease and a predisposition for pneumothorax, a process that was originally equated with destructive emphysema due to impaired tissue integrity (3). Fbn1 hypomorphic mice replicate this lung phenotype, as they display widening of the distal pre-alveolar saccules at birth without signs of inflammation or tissue destruction (21). As already mentioned, Neptune at al. (20) were the first to causally relate impaired lung development with constitutive Smad2/3 signaling in Fbn1 mutant mice and thus the first to provide direct proof for the involvement of fibrillin-rich microfibrils in the extracellular control of endogenous TGFβ bioavailability. Subsequent studies have associated promiscuous TGFβ signaling with the progression of mitral valve prolapse, muscle hypoplasia, and aortic aneurysm in Fbn1 mutant mice (22–24). Importantly, systemic administration of TGFβ-neutralizing antibodies to Fbn1 mutant mice improved all of these MFS manifestations (20, 22–24). This last finding led to the proposal that fibrillin-1 mutations in MFS preclude or decrease matrix sequestration of latent TGFβ, thus rendering it more prone to or accessible for activation (23).
Emerging evidence indicates that additional pathological events exacerbate TGFβ-driven disease progression in MFS, perhaps in an organ-specific manner. Recent investigations have reported that a C-terminal third fragment of fibrillin-1 can apparently displace LTBPs from microfibrils, thus contributing to large latent complex release from the ECM (25). This mechanism cannot, however, provide the sole or even predominant basis for increased TGFβ activity in MFS because improper Smad2/3 signaling is also seen in tissues and cultured cells from Fbn1-null mice (19, 26).
Other investigators have shown that addition to cell cultures of synthetic fibrillin-1 peptides or protein extracts from Fbn1 mutant aortas stimulates metalloproteinase production and macrophage chemotaxis (27, 28). In accordance with these findings, doxycycline administration to Fbn1 mutant mice improves aortic wall architecture and delays aneurysm rupture (29, 30).
More recent in vivo and in vitro analyses have implicated p38 MAPK activation as an early contributor to promiscuous Smad2/3 signaling in Fbn1-null aortas (26). Work in progress is addressing whether or not p38 MAPK is improperly activated through the non-canonical TGFβ signaling cascade and whether p38 MAPK stimulation also contributes to aortic disease in progressively severe mouse models of MFS (1). Finally, the aforementioned bone remodeling data have raised the possibility that impaired BMP sequestration in the ECM is another determinant of MFS pathogenesis.
Pathogenic Network of MFS-related Disorders
The above studies and additional evidence from human patients and genetically engineered mice (see below) support the new concept that microfibrils are part of a broader biological network consisting of molecules that interact with fibrillins and modulate or transduce signaling by TGFβ and BMP ligands (3). Patients with LDS (OMIM 609192) are a particularly informative example because of the extensive clinical overlap between this condition and MFS (31).
LDS is caused by heterozygous loss-of-function mutations in TGFβ receptors (TGFBR1 or TGFBR2) that culminate, however, in increased (as opposed to decreased) TGFβ signaling in the aortic wall by mechanisms that remain poorly defined (31, 32). This apparent paradox has been reconciled by arguing that heterozygous loss-of-function mutations in TGFβ receptor subunits either trigger unproductive compensatory events or have themselves gain-of-function properties (3, 33). For example, lower than threshold levels of TGFβ signaling during a temporally constrained developmental event might activate a compensatory loop that remains constitutively active in the absence of a normal complement of signal transducers or regulators (3). Alternatively, TGFβ receptor mutations may change the normal balance of opposing endocytic processes that regulate trafficking of the TGFβ receptors by favoring interactions with accessory proteins that promote recycling rather than degradation (33). Generation of LDS mutations in mice will test these possibilities, in addition to providing the experimental means to compare and contrast pathogenic mechanisms initiated by mutations in the extracellular and cell-surface components of the TGFβ signaling network.
Mice with targeted inactivation of genes coding for microfibril-associated proteins that are involved in the extracellular control of TGFβ signaling include those lacking biglycan, fibulin-4, or MAGP1 (microfibril-associated glycoprotein-1) (34–36). In contrast to the first two mutant strains, Magp1-null mice exhibit a complex phenotype, which in many aspects is opposite to that of Fbn1 mutant mice and which is apparently associated with reduced TGFβ signaling (36). Thus, preferential interactions of the forming microfibrils with positive and negative modulators of TGFβ activity may contribute to establishing the signaling threshold for various physiological or pathological processes.
Clinical Applications
The notion of TGFβ antagonism has been extended to the systemic treatment of Fbn1 mutant mice with losartan, an angiotensin II type 1 receptor antagonist that also blunts TGFβ signaling (23). The treatment not only counteracted the emergence of histological signs of aortic aneurysm in Fbn1 mutant mice but also improved alveolar septation and muscle hypoplasia in these animals (23, 24). Although the precise mechanism whereby losartan exerts systemic TGFβ blockade remains to be elucidated, these proof-of-principle experiments have indicated that TGFβ antagonism is a general strategy against disease progression in MFS and related disorders of the TGFβ signaling network. Indeed, losartan treatment rescued impaired muscle regeneration in both fibrillin-1 and dystrophin mutant mice (24). Importantly, therapy with losartan significantly reduced the rate of aortic growth in a small cohort of children affected by a particularly severe and rapidly progressive MFS (37). On average, these patients showed a marked reduction in aortic root growth rate and in the rate of change in deviation of aortic root dimension from normal when indexed to age and body size after losartan therapy and compared with their performance on prior medical regimens (37). Although these findings are extremely encouraging, both theoretical and practical limitations are apparent that justify pursuing the identification of additional biological targets that may prove amenable to combinatorial therapies in MFS patients who may be refractive or resistant to losartan treatment.
Conclusions and Perspectives
The studies outlined in this minireview underscore the extraordinary progress made during the past decade in our understanding of the multiple roles that extracellular microfibrils play in organ development and disease processes. Fibrillins provide contextual specificity to TGFβ and BMP signals that promote matrix formation and remodeling, in addition to imparting structural properties to connective tissues. A new paradigm has thus emerged whereby the fibrillins are integral components of a broader biological network of extracellular, cell-surface, and signaling molecules that orchestrate morphogenetic and homeostatic programs in multiple organ systems.
The above paradigm has already translated into novel therapeutic opportunities in MFS. Indeed, losartan treatment in MFS represents the first instance in which the life-threatening consequences of mutations in a structural component of the ECM have been mitigated using pharmacological means of intervention. As such, the MFS experience is bound to influence therapeutic efforts for other human diseases in which gene- or cell-based strategies are difficult or impossible to implement.
The findings we have described have also raised new questions that are likely to be the focus of future investigations. Relevant to MFS pathogenesis, it would be important to identify the mechanisms responsible for constitutive TGFβ activation and losartan action in various organ systems, the nature of the cellular events downstream of improper TGFβ signaling in different tissues, and the potential contribution of other signaling pathways to disease progression. Another unresolved issue is the mechanism that targets TGFβ and BMP complexes to individual fibrillin molecules in a stage- and tissue-specific manner and with discrete consequences for tissue morphogenesis and homeostasis.
The ultimate challenge is to unravel the manner in which disease processes integrate the complex (and even opposing) roles of fibrillin-rich microfibrils, as well as the balance between cooperating and antagonistic signals by matrix-bound TGFβ and BMP ligands. For example, it remains possible and even likely that selected manifestations in MFS may reflect decreased (rather than increased) TGFβ signaling alone and/or in combination with dysregulated BMP signaling. A refined understanding of such disease-causing events will delineate therapeutic windows, opportunities, and limitations, particularly as they apply to the clinical management of organ-specific manifestations in MFS and related disorders of the connective tissue.
Supplementary Material
Acknowledgments
We thank current and past members of our laboratories for many helpful discussions and Karen Johnson for organizing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant AR049698. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: MFS, Marfan syndrome; cbEGF, calcium-binding epidermal growth factor-like; TGFβ, transforming growth factor-β; TB/8-Cys, TGF-binding protein or 8-cysteine-containing domain; LTBP, latent TGFβ-binding protein; BMP, bone morphogenetic protein; ECM, extracellular matrix; LAP, latency-associated protein; MAPK, mitogen-activated protein kinase; LDS, Loeys-Dietz syndrome.
F. Ramirez, unpublished data.
F. Ramirez and H. C. Dietz, unpublished data.
References
- 1.Ramirez, F., Sakai, L. Y., Dietz, H. C., and Rifkin, D. B. (2004) Physiol. Genomics 19 151–154 [DOI] [PubMed] [Google Scholar]
- 2.Hubmacher, D., Tiedemann, K., and Reinhardt, D. P. (2006) Curr. Top. Dev. Biol. 75 93–122 [DOI] [PubMed] [Google Scholar]
- 3.Ramirez, F., and Dietz, H. C. (2007) Curr. Opin. Genet. Dev. 17 252–258 [DOI] [PubMed] [Google Scholar]
- 4.Kielty, C. M., Sherratt, M. J., Marson, A., and Baldock, C. (2005) Adv. Protein Chem. 70 406–436 [DOI] [PubMed] [Google Scholar]
- 5.Wagenseil, J. E., and Mecham, R. P. (2007) Birth Defects Res. Part C Embryo Today Rev. 81 229–240 [DOI] [PubMed] [Google Scholar]
- 6.Rifkin, D. B. (2005) J. Biol. Chem. 280 7409–7412 [DOI] [PubMed] [Google Scholar]
- 7.ten Dijke, P., and Arthur, H. M. (2007) Nat. Rev. Mol. Cell Biol. 8 857–869 [DOI] [PubMed] [Google Scholar]
- 8.Katagiri, T., Suda, T., and Miyazono, K. (2008) in The TGF-β Family (Derynck, R., and Miyazono, K., eds) pp. 121–149, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 9.Sengle, G., Charbonneau, N. L., Ono, R. N., Sasaki, T., Alvarez, J., Keene, D. R., Bächinger, H. P., and Sakai, L. Y. (2008) J. Biol. Chem. 283 13874–13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengle, G., Ono, R. N., Lyons, K. M., Bächinger, H. P., and Sakai, L. Y. (2008) J. Mol. Biol. 381 1025–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, H., Hu, W., and Ramirez, F. (1995) J. Cell Biol. 129 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quondamatteo, F., Reinhardt, D. P., Charbonneau, N. L., Pophal, G., Sakai, L. Y., and Herken, R. (2002) Matrix Biol. 21 637–646 [DOI] [PubMed] [Google Scholar]
- 13.Skoglund, P., Dzamba, B., Coffman, C. R., Harris, W. A., and Keller, R. (2006) Dev. Dyn. 235 1974–1983 [DOI] [PubMed] [Google Scholar]
- 14.Gansner, J. M., Madsen, E. C., Mecham, R. P., and Gitlin, J. D. (2008) Dev. Dyn. 237 2844–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoglund, P., and Keller, R. (2007) Dev. Biol. 301 404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, E., Larson, J. D., and Ekker, S. C. (2006) Blood 107 4364–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atsawasuwan, P., Mochida, Y., Katafuchi, M., Kaku, M., Fong, K. S., Csiszar, K., and Yamauchi, M. (2008) J. Biol. Chem. 283 34229–34240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arteaga-Solis, E., Gayraud, B., Lee, S. Y., Shum, L., Sakai, L., and Ramirez, F. (2001) J. Cell Biol. 154 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carta, L., Pereira, L., Arteaga-Solis, E., Lee-Arteaga, S. Y., Lenart, B., Starcher, B., Merkel, C. A., Sukoyan, M., Kerkis, A., Hazeki, N., Keene, D. R., Sakai, L. Y., and Ramirez, F. (2006) J. Biol. Chem. 281 8016–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neptune, E. R., Frischmeyer, P. A., Arking, D. E., Myers, L., Bunton, T. E., Gayraud, B., Ramirez, F., Sakai, L. Y., and Dietz, H. C. (2003) Nat. Genet. 33 407–411 [DOI] [PubMed] [Google Scholar]
- 21.Gayraud, B., Keene, D. R., Sakai, L. Y., and Ramirez, F. (2000) J. Cell Biol. 150 667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng, C. M., Cheng, A., Myers, L. A., Martinez-Murillo, F., Jie, C., Bedja, D., Gabrielson, K. L., Hausladen, J. M., Mecham, R. P., Judge, D. P., and Dietz, H. C. (2004) J. Clin. Investig. 114 1586–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habashi, J. P., Judge, D. P., Holm, T. M., Cohn, R. D., Loeys, B. L., Cooper, T. K., Myers, L., Klein, E. C., Liu, G., Calvi, C., Podowski, M., Neptune, E. R., Halushka, M. K., Bedja, D., Garielson, K., Rifkin, D. B., Carta, L., Ramirez, F., Huso, D. L., and Dietz, H. C. (2006) Science 312 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn, R. D., van Erp, C., Habashi, J. P., Soleimani A. A., Klein, E. C., Lisi M. T., Gamradt, M., ap Rhys, C. M., Holm, T. M., Loeys, B. L., Ramirez, F., Judge, D. P., Ward, C. W., and Dietz, H. C. (2007) Nat. Med. 13 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry, S. S., Cain, S. A., Morgan, A., Dallas, S. L., Shuttleworth, C. A., and Kielty, C. M. (2007) J. Cell Biol. 176 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carta, L., Smaldone, S., Zilberberg, L., Loch, D., Dietz, H. C., Rifkin, D. B., and Ramirez, F. (2009) J. Biol. Chem. 284 5630–5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booms, P., Pregla, R., Ney, A., Barthel, F., Reinhardt, D. P., Pletschacher, A., Mundlow, S., and Robinson, P. N. (2005) Hum. Genet. 116 51–61 [DOI] [PubMed] [Google Scholar]
- 28.Guo, G., Booms, P., Halushka, M., Dietz, H. C., Ney, A., Stricker, S., Hecht, J., Mundlos, S., and Robinson, P. N. (2006) Circulation 114 1855–1862 [DOI] [PubMed] [Google Scholar]
- 29.Chung, A. W. Y., Yang, H. H. C., Radomski, M. W., and van Breemen, C. (2008) Circ. Res. 102 e73–e85 [DOI] [PubMed] [Google Scholar]
- 30.Xiong, W., Knispel, R. A., Dietz, H. C., Ramirez, F., and Baxter, B. T. (2008) J. Vasc. Surg. 47 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeys, B. L., Chen, J., Neptune, E. R., Judge, D. P., Podowski, M., Holm, T., Meyers, J., Leitch, C. C., Katsanis, N., Sharifi, N., Xu, F. L, Myers, L. A., Spevak, P. J., Cameron, D. E, De Backer, J., Hellemans, J., Chen, Y., Davis, E. C., Webb, C. L., Kress, W., Coucke, P., Rifkin, D. B., De Paepe, A. M., and Dietz, H. C. (2005) Nat. Genet. 37 275–281 [DOI] [PubMed] [Google Scholar]
- 32.Maleszewski, J. J., Miller, D. V., Lu, J., Dietz, H. C., and Halushka, M. K. (2009) Am. J. Surg. Pathol. 33 194–201 [DOI] [PubMed] [Google Scholar]
- 33.Jones, J. A., Spinale, F. G., and Ikonomidis, J. S. (2009) J. Vasc. Res. 46 119–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi, Y., Stuelten, C. H., Kilts, T., Wadhwa, S., Iozzo, R. V., Robey, P. G., Chen, X.-D., and Young, M. F. (2005) J. Biol. Chem. 280 30481–30489 [DOI] [PubMed] [Google Scholar]
- 35.Hanada, K., Vermeij, M., Garinis, G. A., de Waard, M. C., Kunen, M. G. S., Myers, L., Maas, A., Duncker, D. J., Meijers, C., Dietz, H. C., Kanaar, R., and Essers, J. (2007) Circ. Res. 100 738–746 [DOI] [PubMed] [Google Scholar]
- 36.Weinbaum, J. S., Broekelmann, T. J., Pierce, R. A., Werneck, C. C., Segade, F., Craft, C. S., Knutsen, R. H., and Mecham, R. P. (2008) J. Biol. Chem. 283 25533–25543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooke, B. S., Habashi, J. P., Judge, D. P., Patel, N., Loeys, B., and Dietz, H. C. (2008) N. Engl. J. Med. 358 2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.