Abstract
Vasopressin controls renal water excretion largely through actions to regulate the water channel aquaporin-2 in collecting duct principal cells. Our knowledge of the mechanisms involved has increased markedly in recent years with the advent of methods for large-scale systems-level profiling such as protein mass spectrometry, yeast two-hybrid analysis, and oligonucleotide microarrays. Here we review this progress.
Regulation of water excretion by the kidney is one of the most visible aspects of everyday physiology. An outdoor tennis game on a hot summer day can result in substantial water losses by sweating, and the kidneys respond by reducing water excretion. In contrast, excessive intake of water, a frequent occurrence in everyday life, results in excretion of copious amounts of clear urine. These responses serve to exact tight control on the tonicity of body fluids, maintaining serum osmolality in the range of 290–294 mosmol/kg of H2O through the regulated return of water from the pro-urine in the renal collecting ducts to the bloodstream.
The importance of this process is highlighted when the regulation fails. For example, polyuria (rapid uncontrolled excretion of water) is a sometimes devastating consequence of lithium therapy for bipolar disorder. On the other side of the coin are water balance disorders that result from excessive renal water retention causing systemic hypo-osmolality or hyponatremia. Hyponatremia due to excessive water retention can be seen with severe congestive heart failure, hepatic cirrhosis, and the syndrome of inappropriate antidiuresis.
The chief regulator of water excretion is the peptide hormone AVP,2 whereas the chief molecular target for regulation is the water channel AQP2. In this minireview, we describe new progress in the understanding of the molecular mechanisms involved in regulation of AQP2 by AVP in collecting duct cells, with emphasis on new information derived from “systems-level” approaches involving large-scale profiling and screening techniques such as oligonucleotide arrays, protein mass spectrometry, and yeast two-hybrid analysis. Most of the progress with these techniques is in the identification of individual molecules involved in AVP signaling and binding interactions with AQP2. Additional related issues are addressed in several recent reviews (1–4).
Background: AVP and AQP2
An increase in blood osmolality triggers the neurohypophyseal release of AVP. Classic studies in isolated perfused renal collecting ducts demonstrated that AVP triggers a rapid increase in the osmotic water permeability of the collecting duct epithelium, explaining the dramatic fall in water excretion seen when AVP is administered in vivo (5). In isolated perfused rat renal IMCDs, a half-maximal increase is seen ∼8 min following AVP addition (6).
The increase in water permeability of the collecting duct epithelium is a consequence of recruitment of AQP2 to the apical plasma membrane of the collecting duct principal cells (7). In the absence of AVP, most of the cellular AQP2 resides in intracellular vesicles thought to be a subpopulation of recycling endosomes (8, 9). Dynamic water permeability measurements coupled with mathematical modeling (10, 11) have established that the AVP-induced redistribution of AQP2 into the apical plasma membrane occurs through two general processes: 1) acceleration of the rate of exocytic insertion of AQP2 into the plasma membrane and 2) deceleration of the endocytic removal of AQP2 from the apical plasma membrane. Brown et al. (2, 12) have demonstrated, in both the presence and absence of AVP, that the amount of AQP2 in the plasma membrane is a result of a balance between continuing endocytosis and exocytosis of AQP2. The effect of AVP to redistribute AQP2 to the plasma membrane can be mimicked by perturbations that decrease the intrinsic rate of endocytosis, such as expression of a dominant-negative form of dynamin (13).
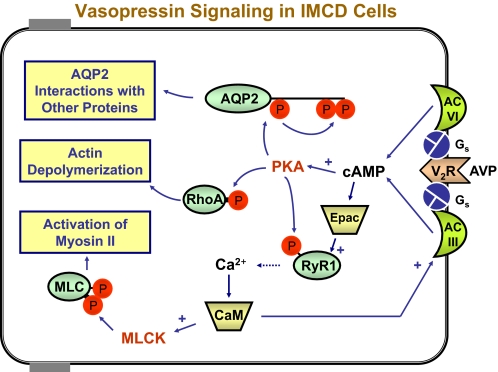
The general pathways involved in AVP signaling in collecting duct cells are diagrammed in Fig. 1. The V2R is a Gs-coupled receptor that binds AVP and activates two adenylyl cyclases, types III and VI, to increase intracellular cAMP. Inasmuch as exogenously added cAMP analogs reproduce the acute water permeability increase seen with AVP, it appears that the action of AVP in collecting ducts is mediated by cAMP (6). Downstream effects are believed to be mediated largely by activation of PKA, although other kinases likely play important roles. One substrate for PKA is AQP2 itself, which undergoes sequential phosphorylation of three C-terminal serines as a result of PKA-mediated phosphorylation of Ser256. This ultimately leads to interactions with proteins that modulate either AQP2 exocytosis or endocytosis.
FIGURE 1.
AVP signaling pathways in the renal IMCD. Occupation of the V2R by AVP (right) triggers signaling mechanisms that result in at least three downstream effects vital to AQP2 trafficking (yellow boxes on left). Adenylyl cyclases III (AC III) and VI (AC VI) produce cAMP, most of whose effects are mediated by PKA, including phosphorylation of AQP2 at Ser256, followed by downstream phosphorylation at Ser264 and Ser269 (top). These phosphorylation events alter binding interactions with regulatory proteins (see text). In addition, RhoA is inactivated, possibly due to PKA-mediated phosphorylation of RhoA (center) and leading to F-actin depolymerization. AVP also triggers spike-like increases in intracellular calcium due to either phosphorylation of RyR1 or Epac (RapGEF3) effects. Calcium mobilization causes calmodulin (CaM)-ependent phosphorylation of the MRLC (MLC) by MLCK, resulting in non-muscle myosin II activation and long-distance translocation of AQP2 vesicles to the apical region.
AVP stimulates depolymerization of F-actin in the subapical region of collecting duct cells (14). The dense cortical network of actin filaments is viewed as a barrier to movement of AQP2-containing vesicles to the apical plasma membrane, and actin depolymerization is therefore expected to facilitate exocytic insertion of AQP2-laden vesicles. The state of actin polymerization in the collecting duct is regulated by Rho family kinases (4). Rho activation appears to be associated with redistribution of AQP2 from the plasma membrane to intracellular compartments. Whether this is achieved by inhibition of AQP2 exocytosis or stimulation of AQP2 endocytosis has not been directly addressed. Rho has been shown to be phosphorylated by PKA at Ser188 in natural killer lymphocytes, an effect that triggers dissociation of Rho from membrane-associated effectors (15), chiefly Rho/Rac/Cdc42-activated kinases. However, phosphorylation of RhoA has not (as of this writing) been demonstrated in the renal collecting duct.
Binding of the V2R by AVP triggers an increase in intracellular calcium in native IMCD cells (16, 17). The V2R-elicited calcium mobilization appears to result from sensitization of ryanodine-inhibitable calcium channels, presumably RyR1, in the endoplasmic reticulum of collecting duct cells (18). The increase in intracellular calcium is in the form of a train of spike-like increases in calcium that occur in each cell independently of the calcium spike pattern in neighboring cells (19, 20). Enhancement of Ca2+ release by ryanodine receptors by cAMP-dependent phosphorylation has been demonstrated in pancreatic beta cells (21), and a similar mechanism may sensitize RyR1 to release Ca2+ in the IMCD (18), possibly by phosphorylation at Ser2730 (rat). However, Yip (22) has also demonstrated an Epac (RapGEF3)-dependent, PKA-independent mechanism for regulation of ryanodine-sensitive Ca2+ release in response to AVP or cAMP.
Several pieces of evidence support the view that V2R-mediated calcium mobilization is a key component of the mechanism by which AVP acutely increases osmotic water permeability. Either prevention of Ca2+ release with ryanodine or chelation of intracellular calcium with BAPTA inhibited the vasopressin action to increase water permeability in isolated perfused collecting ducts (18). Calmodulin inhibitors strongly attenuated the action of AVP to increase water permeability in isolated perfused IMCD segments (18). Downstream targets of Ca2+-calmodulin include both adenylyl cyclase type III (23) and MLCK (24), both of which are stimulated.
Transcriptomic and Proteomic Profiling of the Renal Collecting Duct
To piece together a comprehensive model of how osmotic water transport is regulated at a molecular level in the renal collecting duct, a gene expression “parts list” is needed. A comprehensive list of genes expressed in native IMCD cells from rats has been generated by transcriptomic profiling using oligonucleotide arrays (IMCD Transcriptome Database, dir.nhlbi.nih.gov/papers/lkem/imcdtr/) (25). About 8000 transcripts (of ∼20,000 open reading frames in the rat genome) were expressed above background levels. Over 2000 of these have been directly confirmed at the protein level by large-scale LC-MS/MS studies (IMCD Proteome Database, dir.nhlbi.nih.gov/papers/lkem/imcd/) (26). Similar profiling studies have been completed in AVP-responsive mouse mpkCCD cells (mpkCCD Transcriptome Database, dir.nhlbi.nih.gov/papers/lkem/mpkccdtr/default.aspx) (27).
Phosphoproteomic Profiling Reveals Novel AQP2 Phosphorylation Sites
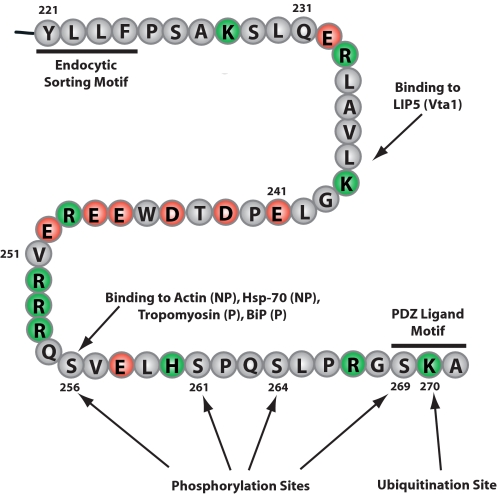
Protein phosphorylation is critical to biochemical regulation in eukaryotic cells. Within the past few years, rapid developments in affinity chromatographic enrichment of phosphopeptides, as well as more accurate and sensitive tandem mass spectrometers, have ushered in a new era of “global” analysis of protein phosphorylation. Consequently, it has been possible to address the following questions. 1) Which proteins in renal collecting duct cells are phosphorylated? 2) What are the phosphorylation sites? 3) Which sites undergo a change in phosphorylation in response to vasopressin. Hoffert et al. (28) identified 714 phosphorylation sites on 223 unique phosphoproteins in isolated rat IMCD tubule segments. These identifications included four serines in AQP2 (Ser256, Ser261, Ser264, and Ser269) within the terminal 16 amino acids of the C-terminal tail (Fig. 2). Ser261, Ser264, and Ser269 had not previously been identified. The Ser256 site (29) had already been shown to be regulated by AVP (30) and to play an important role in AQP2 trafficking to and from the apical plasma membrane (31, 32). Ser256 can be directly phosphorylated by PKA in vitro (33, 34). Other basophilic kinases may also be capable of phosphorylating Ser256, as reviewed recently by Brown et al. (2). The kinases responsible for phosphorylation at Ser261, Ser264, and Ser269 are as yet unidentified.
FIGURE 2.
C-terminal tail of AQP2. Shown are the C-terminal 51 amino acids of rat AQP2, demonstrating relevant post-translational modifications and binding interactions. Binding interactions for actin and hsp70 are favored by lack of phosphorylation at Ser256 (NP), whereas binding interactions for tropomyosin 5b and BiP are favored by the presence of phosphorylation at Ser256 (P).
In AQP2, Ser261 phosphorylation was decreased by vasopressin treatment by ∼60% (28, 35). Lu et al. (36) have demonstrated that mutating Ser261 did not affect regulated or constitutive AQP2 trafficking in transfected LLC-PK1 cells. Thus, the role of phosphorylation at Ser261 in AVP signaling and AQP2 trafficking, if any, remains to be determined.
Studies using quantitative LC-MS/MS and immunoblotting with phospho-specific antibodies have demonstrated that vasopressin strongly increases phosphorylation of AQP2 at Ser264 (37) and Ser269 (34) in rat IMCD cell suspensions (Fig. 2). Vasopressin-mediated increases in AQP2 phosphorylation at Ser264 (t½ = 4.2 min) and Ser269 (t½ = 3.2 min) occurred significantly more slowly than the increase in Ser256 phosphorylation (t½ = 41 s) (34). These response times correlate well with the dynamics of the water permeability response to AVP in isolated perfused IMCD segments (initial increase at 35 s, half-maximal response at 8 min) (6), indicating plausible roles in regulation of AQP2 trafficking. Interestingly, immunogold electron microscopy in rat renal IMCDs revealed that AQP2 phosphorylated at Ser269 was present only in the apical plasma membrane, whereas AQP2 phosphorylated at any of the other three C-terminal sites was found throughout the cell in intracellular vesicles as well as in the plasma membrane (34). Mutation of the Ser269 site to aspartate, mimicking the charge state of a phosphorylated serine, resulted in constitutive localization of AQP2 in the plasma membrane, suggesting that AVP-mediated phosphorylation of AQP2 at Ser269 is involved in regulated plasma membrane retention of AQP2.
Vasopressin-mediated increases in phosphorylation at all three sites (Ser256, Ser264, and Ser269) are reproduced when the collecting ducts are stimulated instead by a cAMP analog, indicating a probable causative role for cAMP. Although only the Ser256 site was phosphorylated in vitro by purified PKA catalytic subunit, the PKA antagonist H-89 blocked vasopressin-stimulated phosphorylation at Ser256, Ser264, and Ser269 (34). The explanation for this finding is that PKA-mediated phosphorylation at Ser256 is a prerequisite for phosphorylation at Ser264 and Ser269 by unknown kinases.
Phosphoproteomic profiling also identified novel phosphorylation sites in the vasopressin-regulated urea transporter UT-A1/3 (Ser35, Ser62, Ser63, and Ser486) that are hypothetically involved in urea transport regulation in the IMCD (28) and identified a number of regulatory proteins that underwent significant changes in phosphorylation state in response to short-term vasopressin treatment. One example is scaffold attachment factor B, a protein involved in RNA processing. The site on scaffold attachment factor B that was down-regulated in response to vasopressin (Ser309; NCBI accession number NP_071789.1) is a putative ERK1 phosphorylation site, an observation consistent with the finding that vasopressin decreases ERK1/2 activation in rat renal IMCD cells (20). Data from the LC-MS/MS-based phosphoproteomic studies in collecting duct can be browsed at the Collecting Duct Phosphoprotein Database (dir.nhlbi.nih.gov/papers/lkem/cdpd/).
Protein Binding Interactions Involving AQP2
The C terminus of AQP2 (Fig. 2) is critical for regulation of its trafficking (1, 3). AQP2 trafficking is presumably mediated by interactions with proteins that are part of the exocytic or endocytic apparatus. Both yeast two-hybrid analysis and affinity-based isolation of proteins followed by LC-MS/MS analysis have been used to identify AQP2-interacting proteins.
Noda et al. (38) used an immunoaffinity approach in which extracts of rat kidney papillae were passed over a column covalently coupled with an anti-AQP2 antibody. Bound proteins were identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. They identified several AQP2-interacting proteins that formed a so-called “multiprotein motor complex.” Member proteins in this complex (aside from AQP2) included actin, MRLC isoforms 2A and 2B, α-tropomyosin 5b, annexins A2 and A6, gelsolin, α-actinin 4, αII-spectrin, and myosin IIA.
Lu et al. (39) utilized yeast two-hybrid screening of a human kidney cDNA library and identified the abundant chaperone proteins hsc70 and hsp70 as AQP2-interacting proteins. The interaction of AQP2 with hsc70 was confirmed by mass spectrometry of proteins pulled down from a rat kidney papilla extract using a glutathione S-transferase-AQP2 C-terminal fusion protein as bait. Both co-immunoprecipitation from AQP2-transfected LLC-PK1 cells and direct binding of purified hsp70 and hsc70 to the glutathione S-transferase-tagged AQP2 C terminus confirmed these interactions. Functional knockdown of hsc70 activity in AQP2-expressing cells resulted in membrane accumulation of AQP2, suggesting that hsc70/hsp70 may play a role in endocytic retrieval of AQP2 from the apical plasma membrane.
Zwang et al. (40) incorporated a “bait peptide” pulldown approach with synthetic AQP2 peptides to identify phosphorylation-dependent binding partners of AQP2 by LC-MS/MS. This study confirmed previously identified interactions between AQP2 and hsc70 (39), hsp70-1 and hsp70-2 (39), and annexin II (38, 41), all of which bound more avidly to the unphosphorylated AQP2 peptide. In contrast, hsp70-5 (also known as BiP, found in both the endoplasmic reticulum and cytosol) bound preferentially to the Ser256-phosphorylated AQP2 peptide, suggesting that “phosphorylation at Ser256 may regulate AQP2 trafficking in part by mediating differential binding of hsp70 family proteins to the COOH-terminal tail” (40). Other novel AQP2 interactors identified included RhoGDI2 and PP1c, both of which preferentially bound to the unphosphorylated AQP2 peptide.
Additional yeast two-hybrid screens have identified two other proteins that interact with the C-terminal tail of AQP2, viz. LIP5 (lysosomal trafficking regulator-interacting protein-5), a late endosomal protein (Fig. 2) (42), and AKAP220 (also called AKAP11; NCBI accession number NM_012773), a PKA-anchoring protein (43). AKAP11 was identified as the most abundant AKAP transcript in the rat renal IMCD (25). An additional AQP2-interacting protein is MAL (44).
Role of the Actin Cytoskeleton in AQP2 Trafficking
Noda et al. (45) recently investigated the relevance of the “force-generating motor complex” discovered by mass spectrometry of AQP2-interacting proteins (see above), focusing on the role of tropomyosin 5b (also known as tropomyosin 1). The authors showed that when AQP2 in intracellular vesicles becomes phosphorylated at Ser256, it binds tropomyosin 5b. This depletes free tropomyosin from the vicinity of the AQP2 vesicle, thereby fostering local F-actin depolymerization, in effect cutting a hole in the cortical actin network around the vesicle. As a consequence, the AQP2 vesicles can cut their way through the cortical actin barrier that would otherwise prevent them from reaching the plasma membrane.
AVP also can affect the actin cytoskeleton in another way, namely stimulation of phosphorylation of the MRLC as demonstrated initially by two-dimensional electrophoresis coupled with protein mass spectrometry (24). Studies with phospho-specific antibodies demonstrated that the phosphorylation sites include the Thr18 and Ser19 residues already known to be a target of MLCK and showed that phosphorylation at these sites increases in native IMCD cells in response to AVP. Phosphorylated MRLC activates conventional myosins, including non-muscle myosins IIA and IIB, both of which are expressed in the renal collecting duct (25). Studies in isolated perfused IMCD segments showed that the ability of AVP to increase osmotic water permeability was markedly decreased either by MLCK inhibitors (24) or by the myosin II inhibitor blebbistatin (46). Activation of myosin II isoforms in collecting duct cells mediates the well known effect of AVP to alter cell shape (cells become taller) and hypothetically play roles (along with microtubules) in long-distance translocation of AQP2-laden vesicles from throughout the cell to the apical region, where they can fuse with the plasma membrane. We propose that this could occur via myosin-dependent “flow” of the cortical actin network along the basolateral-apical axis of the cell in a manner similar to the process described for polarization in fertilized eggs (47).
In addition to the role of myosin II as discussed above, other myosins may be important for AQP2 vesicle trafficking. Myosin VB has been implicated in recycling of AQP2 after endocytosis (9). In addition to myosins IIA and IIB and MRLC, immunoisolated AQP2 vesicles were found to entrain myosins 1C, VI, and IXB (8). Specific roles for these myosins in AQP2 trafficking have not been ascertained.
Role of PDZ Domain Interactions in Regulation of AQP2 Localization
Noda et al. (48) have established that AQP2 undergoes binding interactions with a PDZ domain-containing protein called SPA-1 (signal-induced proliferation-associated protein-1) via its C-terminal PDZ ligand domain (-Ser269-Lys270-Ala271). AQP2 trafficking to the apical plasma membrane was found to be impaired in collecting ducts from SPA-1-deficient mice. SPA-1 is a Rap GTPase-activating protein, which could hypothetically mediate localized Rap inactivation in the vicinity of AQP2, thereby reducing local ERK activity (20). Localized ERK inactivation could hypothetically play a role in phosphorylation of AQP2 at Ser261, a potential target site for proline-directed kinases, including ERK (28).
Studies using LC-MS/MS to identify apically biotinylated proteins in the renal IMCD have also pointed to a role for PDZ domain interactions in apical targeting (49). Most of the integral membrane proteins identified by apical surface biotinylation possessed C-terminal PDZ ligand motifs, including AQP2, low density lipoprotein receptor-related protein-4 (gene name Lrp4), γ-glutamyl carboxylase (Gacx), ATP-binding cassette subfamily A2 (Abca2), tyrosine kinase receptor 3 (Tyro3), Na+/H+ exchanger 2 (Slc9a2), orphan G-protein-coupled receptor 64 (Gpr64), and the Na+/Cl–-dependent taurine transporter (Slc6a6). Furthermore, several peripheral membrane proteins were identified in IMCDs via their attachment to apically biotinylated proteins, including three PDZ domain-containing proteins: Semacap3 (a RING finger protein with putative ubiquitin ligase activity; gene name Pdzrn3), SPA-1-like protein (a probable Rap GTPase-activating protein (Sipa1l1), and nitric-oxide synthase-1 (Nos1). The role of PDZ domain interactions in epithelial cell polarity determination has been reviewed recently (50).
The AQP2 phosphorylation site at Ser269 is part of the AQP2 PDZ ligand motif (Fig. 2), and phosphorylation at this site could theoretically alter PDZ domain interactions that are involved in targeting of AQP2 to the apical plasma membrane. Similarly, ubiquitination of Lys270 could alter AQP2 distribution in collecting duct cells by altering interactions with PDZ domain-containing proteins.
Conclusion
In this brief review, we have described the role of discovery approaches involving large-scale transcriptomic or proteomic data acquisition in fostering progress in a specific area of molecular physiology, viz. the regulation of the AQP2 water channel by AVP. On the horizon are additional approaches based on better techniques in protein mass spectrometry as well as “next-generation” nucleotide sequencing that will add to the rapidly expanding treasure trove of information. In this minireview, we have emphasized easily interpreted observations (“the low-hanging fruit”). However, taking maximal advantage of large-scale data sets in the future will require computational methods capable of finding patterns in the data that speak to mechanism and causality, i.e. formal computational systems biology.
Supplementary Material
This work was authored by National Institutes of Health staff. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: AVP, arginine vasopressin; AQP2, aquaporin-2; IMCD, inner medullary collecting duct; V2R, vasopressin type 2 receptor; PKA, protein kinase A; RyR1, ryanodine receptor type 1; BAPTA, 1,2-bis(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; MLCK, myosin light chain kinase; LC-MS/MS, liquid chromatography-tandem mass spectrometry; ERK, extracellular signal-regulated kinase; MRLC, myosin regulatory light chain.
References
- 1.Boone, M., and Deen, P. M. (2008) Pfluegers Arch. 456 1005–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, D., Hasler, U., Nunes, P., Bouley, R., and Lu, H. A. J. (2008) Curr. Opin. Nephrol. Hypertens. 17 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noda, Y., and Sasaki, S. (2006) Biochim. Biophys. Acta 1758 1117–1125 [DOI] [PubMed] [Google Scholar]
- 4.Valenti, G., Procino, G., Tamma, G., Carmosino, M., and Svelto, M. (2005) Endocrinology 146 5063–5070 [DOI] [PubMed] [Google Scholar]
- 5.Grantham, J. J., and Burg, M. B. (1966) Am. J. Physiol. 211 255–259 [DOI] [PubMed] [Google Scholar]
- 6.Wall, S. M., Han, J. S., Chou, C.-L., and Knepper, M. A. (1992) Am. J. Physiol. 262 F989–F998 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen, S., Chou, C.-L., Marples, D., Christensen, E. I., Kishore, B. K., and Knepper, M. A. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barile, M., Pisitkun, T., Yu, M. J., Chou, C.-L., Verbalis, M. J., Shen, R. F., and Knepper, M. A. (2005) Mol. Cell. Proteomics 4 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedvetsky, P. I., Stefan, E., Frische, S., Santamaria, K., Wiesner, B., Valenti, G., Hammer, J. A., III, Nielsen, S., Goldenring, J. R., Rosenthal, W., and Klussmann, E. (2007) Traffic 8 110–123 [DOI] [PubMed] [Google Scholar]
- 10.Nielsen, S., and Knepper, M. A. (1993) Am. J. Physiol. 265 F204–F213 [DOI] [PubMed] [Google Scholar]
- 11.Knepper, M. A., and Nielsen, S. (1993) Am. J. Physiol. 265 F214–F224 [DOI] [PubMed] [Google Scholar]
- 12.Brown, D. (2003) Am. J. Physiol. 284 F893–F901 [DOI] [PubMed] [Google Scholar]
- 13.Lu, H., Sun, T.-X., Bouley, R., Blackburn, K., McLaughlin, M., and Brown, D. (2004) Am. J. Physiol. 286 F233–F243 [DOI] [PubMed] [Google Scholar]
- 14.Simon, H., Gao, Y., Franki, N., and Hays, R. M. (1993) Am. J. Physiol. 265 C757–C762 [DOI] [PubMed] [Google Scholar]
- 15.Lang, P., Gesbert, F., Espine-Carmagnat, M., Stancou, R., Pouchelet, M., and Bertoglio, J. (1996) EMBO J. 15 510–519 [PMC free article] [PubMed] [Google Scholar]
- 16.Star, R. A., Nonoguchi, H., Balaban, R., and Knepper, M. A. (1988) J. Clin. Investig. 81 1879–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champigneulle, A., Siga, E., Vassent, G., and Imbert-Teboul, M. (1993) Am. J. Physiol. 265 F35–F45 [DOI] [PubMed] [Google Scholar]
- 18.Chou, C.-L., Yip, K.-P., Michea, L., Kador, K., Ferraris, J., Wade, J. B., and Knepper, M. A. (2000) J. Biol. Chem. 275 36839–36846 [DOI] [PubMed] [Google Scholar]
- 19.Yip, K.-P. (2002) J. Physiol. (Lond.) 538 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisitkun, T., Jacob, V., Schleicher, S. M., Chou, C.-L., Yu, M. J., and Knepper, M. A. (2008) Am. J. Physiol. 295 F1030–F1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam, M. S., Leibiger, I., Leibiger, B., Rossi, D., Sorrentino, V., Ekstrom, T. J., Westerblad, H., Andrade, F. H., and Berggren, P. O. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 6145–6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip, K.-P. (2006) Am. J. Physiol. 291 F882–F890 [DOI] [PubMed] [Google Scholar]
- 23.Hoffert, J. D., Chou, C.-L., Fenton, R. A., and Knepper, M. A. (2005) J. Biol. Chem. 280 13624–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou, C.-L., Christensen, B. M., Frische, S., Vorum, H., Desai, R. A., Hoffert, J. D., de Lanerolle, P., Nielsen, S., and Knepper, M. A. (2004) J. Biol. Chem. 279 49026–49035 [DOI] [PubMed] [Google Scholar]
- 25.Uawithya, P., Pisitkun, T., Ruttenberg, B. E., and Knepper, M. A. (2008) Physiol. Genomics 32 229–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisitkun, T., Bieniek, J., Tchapyjnikov, D., Wang, G., Wu, W. W., Shen, R. F., and Knepper, M. A. (2006) Physiol. Genomics 5 760–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu, M.-J., Miller, R. T., Uawithya, P., Rinschen, M. M., Khositseth, S., Braucht, D. W. W., Chou, C.-L., Pisitkun, T., Nelson, R. D., and Knepper, M. A. (2009) Proc. Natl. Acad. Sci. U. S. A. 106 2441–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffert, J. D., Pisitkun, T., Wang, G., Shen, R. F., and Knepper, M. A. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 7159–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwahara, M., Fushimi, K., Terada, Y., Bai, L., Marumo, F., and Sasaki, S. (1995) J. Biol. Chem. 270 10384–10387 [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto, G., Zelenina, M., Li, D., Yasui, M., Aperia, A., Nielsen, S., and Nairn, A. C. (1999) Am. J. Physiol. 276 F254–F259 [DOI] [PubMed] [Google Scholar]
- 31.Katsura, T., Gustafson, C. E., Ausiello, D. A., and Brown, D. (1997) Am. J. Physiol. 272 F817–F822 [PubMed] [Google Scholar]
- 32.Fushimi, K., Sasaki, S., and Marumo, F. (1997) J. Biol. Chem. 272 14800–14804 [DOI] [PubMed] [Google Scholar]
- 33.Procino, G., Carmosino, M., Marin, O., Brunati, A. M., Contri, A., Pinna, L. A., Mannucci, R., Nielsen, S., Kwon, T. H., Svelto, M., and Valenti, G. (2003) FASEB J. 17 1886–1888 [DOI] [PubMed] [Google Scholar]
- 34.Hoffert, J. D., Fenton, R. A., Moeller, H. B., Simons, B., Tchapyjnikov, D., McDill, B. W., Yu, M. J., Pisitkun, T., Chen, F., and Knepper, M. A. (2008) J. Biol. Chem. 283 24617–24627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffert, J. D., Nielsen, J., Yu, M. J., Pisitkun, T., Schleicher, S. M., Nielsen, S., and Knepper, M. A. (2007) Am. J. Physiol. 292 F691–F700 [DOI] [PubMed] [Google Scholar]
- 36.Lu, H. J., Matsuzaki, T., Bouley, R., Hasler, U., Qin, Q. H., and Brown, D. (2008) Am. J. Physiol. 295 F290–F294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffert, J. D., Wang, G., Pisitkun, T., Shen, R. F., and Knepper, M. A. (2007) J. Proteome Res. 6 3501–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noda, Y., Horikawa, S., Katayama, Y., and Sasaki, S. (2005) Biochem. Biophys. Res. Commun. 330 1041–1047 [DOI] [PubMed] [Google Scholar]
- 39.Lu, H. A. J., Sun, T.-X., Matsuzaki, T., Yi, X.-H., Eswara, J., Bouley, R., McKee, M., and Brown, D. (2007) J. Biol. Chem. 282 28721–28732 [DOI] [PubMed] [Google Scholar]
- 40.Zwang, N. A., Hoffert, J. D., Pisitkun, T., Moeller, H. B., Fenton, R. A., and Knepper, M. A. (2009) J. Proteome Res. 8 1540–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamma, G., Procino, G., Mola, M. G., Svelto, M., and Valenti, G. (2008) Pfluegers Arch. 456 729–736 [DOI] [PubMed] [Google Scholar]
- 42.van Balkom, B. W., Boone, M., Hendriks, G., Kamsteeg, E. J., Robben, J. H., Stronks, H. C., van der Voorde, A., van Herp, F., van der Sluijs, P., and Deen, P. M. T. (2009) J. Am. Soc. Nephrol. 20 in press [DOI] [PMC free article] [PubMed]
- 43.Okutsu, R., Rai, T., Kikuchi, A., Ohno, M., Uchida, K., Sasaki, S., and Uchida, S. (2008) Kidney Int. 74 1429–1433 [DOI] [PubMed] [Google Scholar]
- 44.Kamsteeg, E. J., Duffield, A. S., Konings, I. B., Spencer, J., Pagel, P., Deen, P. M., and Caplan, M. J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 16696–16701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noda, Y., Horikawa, S., Kanda, E., Yamashita, M., Meng, H., Eto, K., Li, Y., Kuwahara, M., Hirai, K., Pack, C., Kinjo, M., Okabe, S., and Sasaki, S. (2008) J. Cell Biol. 182 587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chou, C.-L., Yu, M. J., Kassai, E. M., Morris, R. G., Hoffert, J. D., Wall, S. M., and Knepper, M. A. (2008) Am. J. Physiol. 295 F192–F201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro, E., Nance, J., and Priess, J. R. (2004) Dev. Cell 7 413–424 [DOI] [PubMed] [Google Scholar]
- 48.Noda, Y., Horikawa, S., Furukawa, T., Hirai, K., Katayama, Y., Asai, T., Kuwahara, M., Katagiri, K., Kinashi, T., Hattori, M., Minato, N., and Sasaki, S. (2004) FEBS Lett. 568 139–145 [DOI] [PubMed] [Google Scholar]
- 49.Yu, M. J., Pisitkun, T., Wang, G., Shen, R. F., and Knepper, M. A. (2006) Mol. Cell. Proteomics 5 2131–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brone, B., and Eggermont, J. (2005) Am. J. Physiol. 288 C20–C29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.