Abstract
These studies explore the connections between simvastatin, Rac1, and AMP-activated protein kinase (AMPK) pathways in cultured vascular endothelial cells and in arterial preparations isolated from statin-treated mice. In addition to their prominent effects on lipoprotein metabolism, statins can regulate the small GTPase Rac1, and may also affect the phosphorylation of the ubiquitous AMPK. We explored pathways of statin-modulated Rac1 and AMPK activation both in arterial preparations from statin-treated mice as well as in cultured endothelial cells. We treated adult mice with simvastatin daily for 2 weeks and then harvested and analyzed arterial preparations. Simvastatin treatment of mice led to a significant increase in AMPK and LKB1 phosphorylation and to a decrease in protein kinase A activity relative to control animals, associated with a marked increase in Rac1 activation. Exposure of bovine aortic endothelial cells to simvastatin for 24 h strikingly increased GTP-bound Rac1 and led to increased phosphorylation of AMPK as well as the AMPK kinase LKB1. These responses to simvastatin were blocked by mevalonate or geranylgeranyl pyrophosphate but not by farnesyl pyrophosphate. Small interfering RNA (siRNA)-mediated knockdown of AMPK abrogated simvastatin-induced Rac1 activation and LKB1 phosphorylation. Importantly, siRNA-mediated knockdown of the key AMPK kinase, calcium/calmodulin-dependent protein kinase kinase β, completely blocked simvastatin-induced endothelial cell migration and also abrogated statin-promoted phosphorylation of AMPK and LKB1, as did pharmacological inhibition with the specific calcium/calmodulin-dependent protein kinase β inhibitor STO-609. Moreover, siRNA-mediated knockdown of Rac1 completely blocked simvastatin-induced LKB1 phosphorylation, but without affecting simvastatin-induced AMPK phosphorylation. These findings establish a key role for simvastatin in activation of a novel Rac1-dependent signaling pathway in the vascular wall.
HMG-CoA2 reductase inhibitors, commonly known as statins, are widely prescribed for the prevention and treatment of hypercholesterolemia and cardiovascular diseases (1, 2). The salutary clinical effects of these drugs derive in part from their effects on the levels of serum lipoproteins, yet other statin responses appear to be mediated by alterations in vascular function involving the endothelial isoform of nitric-oxide synthase (3) and related signaling pathways. Inhibition of HMG-CoA reductase suppresses the cellular levels of its enzymatic product mevalonate, thereby attenuating formation both of cholesterol as well as the synthesis of distinct isoprenoid compounds such as farnesyl pyrophosphate (Fpp) and geranylgeranyl pyrophosphate (GGpp). Many key signaling proteins are covalently modified by these isoprenoids, which are the products of a metabolic pathway that diverges from the pathway that leads to cholesterol synthesis downstream of HMG-CoA reductase. These isoprenoid compounds can provide lipophilic anchors that facilitate membrane targeting and modulate protein-protein interactions of many key signaling proteins. One such iso-prenylated signaling protein is the GTP-binding cytoskeletonassociated protein Rac1, a member of the Rho GTPase small G protein family that undergoes geranylgeranylation at its C terminus. Statins also affect post-translational modification of another small GTPase, RhoA, that, like Rac1, is a geranylgeranylated protein that is an important determinant of vascular signaling (4–8). Rac1 has particularly important roles in vascular endothelial cells, where this cytoskeleton regulatory protein modulates activity of the endothelial isoform of nitric-oxide synthase (eNOS), a key determinant of vascular homeostasis (9). Rac1 activation in endothelial cells is influenced by the AMP-activated protein kinase (AMPK) (6), which itself is phosphorylated by the protein kinase LKB1 and by the calcium-calmodulin-dependent protein kinase β (CaMKKβ) (see review (10)). In recent years, numerous reports have described effects of statins on variety of these signaling proteins in different experimental systems (11–14).
Statins have been shown to promote the phosphorylation of AMPK (13), a heterotrimeric enzyme involved in the modulation of cellular energy pathways that has also been implicated in eNOS regulation (3, 15–17). AMPK was originally discovered and characterized as a cellular “energy sensor” that can be activated by increases in the intracellular AMP:ATP ratio (18). However, in recent years, it has become clear that AMPK is also regulated through AMP-independent pathways involving enzyme phosphorylation on threonine 172 of the enzyme's α subunit, leading to marked enzyme activation (19). Protein kinases that phosphorylate AMPK include the tumor suppressor LKB1 and the calcium/calmodulin-dependent kinase CaMKKβ. LKB1 itself is a phosphoprotein. The pathways that regulate LKB1 are incompletely understood, and a variety of upstream protein kinases have been implicated in LKB1 regulation (see review (20)). CaMKKβ is principally regulated by calcium binding, but this kinase may also be phosphorylated by the cAMP-dependent protein kinase PKA (21, 22). Another substrate for PKA in vascular cells is the actin-binding phosphoprotein VASP (23, 24); the phosphorylation state of VASP at its PKA site can serve as a surrogate marker for the activity of cAMP-dependent signaling pathways in the vascular wall (25). CaMKKβ has been shown to be involved in AMPK regulation in endothelial cells in response to receptor tyrosine kinase activation and via G protein-coupled receptor pathways (6). Activated AMPK directly phosphorylates eNOS, and this kinase thereby appears be an important determinant of NO-dependent signaling in endothelial cells. However, much remains to be learned about the molecular mechanisms whereby statins enhance AMPK activation.
In cultured cells, statins have been shown to inhibit the geranylgeranylation of Rac1, associated with an increase in Rac1 GTP binding and activation (26). The activation of Rac1 is a key step in eNOS activation: siRNA-mediated Rac1 “knockdown” in endothelial cells markedly suppresses receptor signaling to eNOS (5, 7). siRNA-mediated AMPK knockdown suppresses Rac1 activation, again leading to the attenuation of receptor-dependent activation of eNOS (6). The relationships among these various statin-modulated signaling pathways are incompletely characterized. The present studies identify CaMKKβ and LKB1 as critical determinants of simvastatin-dependent activation of AMPK- and Rac1-modulated signaling and reveal that Rac1 in turn regulates LKB1 phosphorylation.
EXPERIMENTAL PROCEDURES
Materials—Simvastatin, GGTI-298, and FTI-277 were from EMD Bioscience (San Diego, CA). Fetal bovine serum (FBS) was from HyClone (Logan, UT). Lipofectamine 2000 and all other cell culture reagents and media were from Invitrogen. Polyclonal antibodies directed against phospho-AMPK (Thr172), phospho-LKB1 (Ser428), phospho-ACC (Ser79), phospho-VASP (Ser157), total AMPK, LKB1, VASP, and ACC, and CaMKI protein were from Cell Signaling Technologies (Beverly, MA). CaMKKβ antibody was from Abnova (Walnut Creek, CA); Super Signal substrate for chemiluminescence detection and secondary antibodies conjugated with horseradish peroxidase was from Pierce. Protein determinations were made with the Bio-Rad protein assay kit. The Rac activity assay kit was from Millipore (Billerica, MA). The LKBtide peptide (SNLYHQGKFLQTFCGSPLYRRR (27)) was synthesized by the Harvard Medical School Biopolymers Laboratory. All other reagents and chemicals were from Sigma.
Duplex siRNA Targeting Constructs—Custom-designed duplex siRNA targeting constructs specific for Rac1, AMPK, and CaMKKβ have been extensively characterized in our earlier reports (6, 7) and purchased from Dharmacon, Inc. (Lafayette, CO) or Ambion (Austin, TX). LKB1 siRNA (Sc-35816) was from Santa Cruz Biotechnology, Inc., and the final siRNA concentration for human umbilical vein endothelial cells (HUVECs) are 100 nm. The duplex siRNA used as a negative control was reported previously (7).
Cell Culture and Transfection—Bovine aortic endothelial cells (BAECs) were obtained from Cell Applications, Inc. (San Diego, CA) and maintained in culture in Dulbecco's modified Eagle's medium supplemented with FBS (10% v/v) as described previously (28). BAECs were plated onto gelatin-coated culture dishes and studied prior to cell confluence between passage 5 and 9. siRNA transfections were performed as described previously in detail (7). HUVECs were from Genlantis, Inc. (San Diego) and maintained in EBM-2 medium supplemented with 2% FBS plus growth factors as recommended by the company (Lonza, Walkersville, MD). siRNA transfections for BAECs or HUVECs used 30 nm or 100 nm RNA, respectively; the cells were transfected using Lipofectamine 2000 (0.15%, v/v) following the protocol provided by the manufacturer 24 h after being split at a 1:5 ratio; Lipofectamine 2000 was removed by changing into fresh medium containing 10% FBS 5 h post-transfection, and cells were analyzed 48 h following transfection.
In Vivo Treatment and Tissue Harvesting—Male wild-type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME), and studied at 8–9 weeks of age. All procedures were performed according to protocols approved by the Harvard Medical School Standing Committee for Animal Experimentation. For these studies, mice received simvastatin (10 mg/kg/day) or phosphate-buffered saline by intraperitoneal injection daily for 2 weeks, during which time the animals were provided standard chow and water ad libitum. For immunoblot analyses, aortae were expeditiously isolated following animal sacrifice and homogenized in 300 μl of Nonidet P-40 buffer using a PowerGen Model 125 homogenizer (Fisher Scientific, Morris Plain, NJ). Cell lysates were collected and analyzed in immunoblots as described below. For the Rac1 activity assay, aortas from three mice were collected together and homogenized in a total of 600 μl of MLB buffer (25 mm HEPES, pH 7.5, 150 mm NaCl, 1% Igepal CA-630, 10 mm MgCl2, 1 mm EDTA, 10 glycerol, 2 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml soybean trypsin inhibitor, and 2 μg/ml lima trypsin inhibitor); the Rac activity assay was performed in the aortic homogenates according to the manufacturer's instructions.
Cell Treatments and Immunoblot Analysis—Simvastatin treatment of cultured endothelial cells used 10 μm drug for 24 h unless otherwise indicated. For immunoblots, treated BAECs were washed with phosphate-buffered saline, and cell lysates were collected in Nonidet P-40 buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.025% sodium deoxycholate, 1 mm EDTA, 2 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml soybean trypsin inhibitor, and 2 μg/ml lima trypsin inhibitor), resolved by SDS-PAGE, and transferred onto nitrocellulose membranes. Immunoblots were probed with specific antibodies as indicated. Immunoblot analyses of protein expression and phosphorylation were assessed as previously described in detail (29). Quantitative analyses of immunoblots were determined using a ChemiImager HD4000 (Alpha-Innotech, San Leandro, CA).
Rac1 Activity Assay—For statin treatments of BAECs, confluent cells in 100-mm dishes were incubated with simvastatin or vehicle for 24 h with or without other drugs as noted. For some experiments, duplex siRNA targeting constructs were transfected 24 h prior to simvastatin treatments, and simvastatin treatment was continued for another 24 h. 48 h following siRNA transfection, the cells were washed with ice-cold phosphate-buffered saline and lysed in MLB buffer provided by the manufacturer. The GTP-bound active form of Rac1 was isolated from cell lysates using a GST fusion protein containing the p21-binding domain of PAK-1 bound to glutathione-agarose, following protocols provided by the manufacturer. The beads were washed three times with MLB buffer, and the bound proteins were eluted with Laemmli sample buffer and analyzed for GTP-bound Rac1 in immunoblots probed with a Rac monoclonal antibody.
Activity Assays for LKB1 and CaMKKβ—Kinase activity assays were performed in cell lysates following immunoprecipitation from control or simvastatin-treated endothelial cells that had been transfected with different siRNA constructs. 24 h following siRNA transfection, the cells were treated with simvastatin (10 μm) or vehicle for 24 h and then harvested. Cell lysates were prepared by solubilizing cells with 900 μl of OG buffer (50 mm Tris-HCl, pH 7.4, 125 mm NaCl, 60 mm N-octyl-β-d-glucopyranoside, 0.5 mm EDTA, 2 mm dithiothreitol, 2 mm Na3VO4, 1 mm NaF, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml soybean trypsin inhibitor, and 2 μg/ml lima trypsin inhibitor) for 10 min at 4 °C; cell lysates were then incubated with either LKB1 or CaMKKβ antibodies for 1 h at 4 °C. Protein A/G-Sepharose beads were added to the supernatant, incubated for 1 h, and washed extensively with OG buffer. For the LKB1 immunocomplexes, LKB1 activity was assessed using LKBtide and γ-[32P]ATP, according to the manufacturer's protocol (Cell Signaling Inc.). CaMKKβ activity was assayed in immunocomplexes as previously described (30), using CaMK-1 as substrate. CaMKKβ activity was calculated based on the difference in γ-[32P]ATP incorporation into CaMK-1 in the presence and absence of Ca2+/calmodulin (30).
Endothelial Cell Migration Assay—Cell migration was assayed using a Transwell cell culture chamber, as we have previously described in detail (5). In brief, BAECs were transfected with control, AMPK-specific or CaMKKβ-specific siRNA, and migration experiments were performed 48 h after transfection. Simvastatin (10 μm) was added into the lower chamber, and the chambers were incubated at 37 °C overnight to allow cell migration; recovery and quantitation of migrated cells has been described previously (5, 6). Each treatment was performed and analyzed in duplicate.
Other Methods—Mean values for individual experiments were expressed as mean ± S.E. For quantitative analysis of experiments exploring the effects of both siRNA-mediated knockdown and drug effects, responses are normalized relative to the control siRNA and in the absence of drug treatment. Statistical differences were assessed by ANOVA. p < 0.05 was considered statistically significant.
RESULTS
Treatment of Mice with Simvastatin Increases Rac1 Activation, Promotes LKB1 and AMPK Phosphorylation, and Attenuates VASP Phosphorylation in Arterial Preparations—To explore the activation of Rac1 and related signaling proteins by simvastatin in vivo, mice were administered simvastatin by intraperitoneal injection daily for 2 weeks; phosphate-buffered saline was used as vehicle control. After animal sacrifice, aortae were harvested and the Rac1 activity assay was performed in tissue homogenates, as described in detail under “Experimental Procedures.” As shown in Fig. 1 (A and B), simvastatin treatment led to a significant increase in the activation of Rac1 (1.7 ± 0.1-fold increase compared with vehicle-treated mice, n = 3, p < 0.05), without any change in the overall abundance of Rac1. We extended these experiments to explore the effects of simvastatin on the phosphorylation of AMPK, VASP, and LKB1 by analyzing immunoblots in arterial preparations isolated from mice treated with simvastatin or vehicle daily for 2 weeks. Following drug or vehicle treatments, animals were sacrificed, aortae were harvested, and tissue homogenates were analyzed in immunoblots probed with antibodies directed against phosphothreonine172-AMPK, phosphoserine428-LKB1, phosphoserine157-VASP, total AMPK, LKB1, VASP, or actin, as shown in Fig. 1 (C and D). Simvastatin treatment of mice induced a significant increase in AMPK phosphorylation (2.2 ± 0.2-fold increase compared with vehicle, n = 7, p < 0.01) and LKB1 phosphorylation (1.9 ± 0.4-fold increase compared with vehicle, n = 3, p < 0.05), accompanied by a decrease in VASP phosphorylation (40 ± 8% decrease compared with vehicle, n = 3, p < 0.05), with no substantive change in the overall protein abundance of AMPK, VASP, LKB1, and actin.
FIGURE 1.
Rac1 activation and phosphorylation of key signaling proteins in arterial preparations isolated from statin-treated mice. A, the results from a Rac1 activation assay and immunoblot analyses performed in arterial preparations isolated from mice treated with intraperitoneal injections of simvastatin (10 mg/kg) or vehicle daily for 2 weeks. Rac1 activity was assayed using the GST-PAK pulldown technique described in the text. Rac1 and actin expression were determined in aliquots of the arterial homogenate by probing immunoblots with Rac1 or actin antibodies, as shown. The experiment shown is representative of three similar experiments that yielded equivalent results. B, pooled data from three experiments, quantitating the relative abundance of active Rac1 in arterial preparations of mice treated as described in A. Rac1 activity in vehicle-treated mice was defined as 1.0. *, p < 0.05 for simvastatin versus vehicle treatment. C, the results of immunoblots analyzed in arterial preparations isolated from mice treated with simvastatin or vehicle, as described in A. Immunoblots were probed with antibodies against phospho-AMPK, phospho-LKB1, phospho-VASP, total AMPK, LKB1, VASP, or actin, as shown. The blots shown are representative of at least three similar experiments that gave equivalent results; D, pooled data from at least three experiments, using digital chemiluminescence to quantitate levels of phospho-AMPK, phospho-LKB1, and phospho-VASP in immunoblots analyzed in arterial preparation from mice treated with simvastatin daily for 2 weeks. The signal for phospho-protein in vehicle-treated mice was defined as 1.0. *, p < 0.05 for simvastatin versus vehicle treatment.
Isoprenoid Metabolites Affect Simvastatin-induced Activation of Rac1—To explore in greater detail the intracellular pathways whereby in vivo treatments with simvastatin lead to the activation of Rac1, we performed experiments in cultured vascular endothelial cells. Fig. 2 shows results from an experiment in which BAECs were treated with simvastatin (10 μm) or vehicle for 24 h, and then Rac1 activation was assayed in cell lysates, as described above. As shown in Fig. 2, simvastatin addition dramatically stimulates Rac1 activation: there is a 34 ± 18-fold increase in Rac1 activation in BAECs treated with simvastatin (n = 4, p < 0.05). Following statin treatment, there was no change in the small fraction of Rac1 protein found in membrane fractions in these cells (data not shown), with the bulk of Rac1 remaining in the cytosol, as we have previously reported (7). A key control to establish specificity for the effects of statins on Rac1 activation is to document reversal of the statin response by the addition of mevalonate, which is the immediate product of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase enzyme that is inhibited by statin treatment. As can be seen in Fig. 2 (A and B), mevalonate completely abolishes the effects of simvastatin on Rac1 activation. We next explored the effect of geranylgeranyl pyrophosphate (GGpp), an isoprenoid metabolite that is downstream of mevalonate and directly modifies small G proteins, including Rac1. Treatment of BAECs with GGpp partially reverses the effects of simvastatin on Rac1 activation (Fig. 2, A and B). We then tested the effects of the geranylgeranyl transferase (GGT) inhibitor GGTI on simvastatin-induced Rac1 activation. We found that treatment of cells with GGTI significantly enhances GTP-bound Rac1 abundance (2.1 ± 0.5-fold, n = 4, p < 0.05). As seen for simvastatin, the stimulatory effect of GGTI on Rac1 activation was reversed by treating cells with the GGT product geranylgeranyl pyrophosphate. Treatment of cells with the farnesyl transferase inhibitor FTI had no effect on Rac1 activity (Fig. 2, C and D).
FIGURE 2.
Simvastatin and activation of Rac1 in cultured endothelial cells. This figure shows the results of Rac1 activity assays in BAECs, measuring Rac1 activation using the GST-PAK pulldown method described in the text. In A, BAECs were incubated for 24 h with 10 μm simvastatin, with or without mevalonate (400 μm), geranylgeranyl pyrophosphate (GGpp, 10 μm), or squalene (50 μm). Active Rac1 and total Rac were detected in immunoblots probed with an anti-Rac antibody. This experiment was repeated four times, with equivalent results. B, pooled data from four experiments identical in design to the experiment shown in A, normalizing the signals of active Rac1 to total Rac1; the normalized Rac1 activity in vehicle-treated cells was defined as 1.0. *, p < 0.05. C, a representative Rac1 activity assay in cells treated for 24 h with the geranylgeranyl transferase inhibitor GGTI-298 (GGTI, 10 μm), either alone or in the presence of GGpp (10 μm); the final lane shows Rac1 activity in cells treated with the farnesyl transferase inhibitor FTI-277 (FTI, 10 μm). This experiment was repeated four times with equivalent results, and D shows the results from pooled data from these three experiments. Rac1 activity in vehicle treated cells was defined as 1.0; *, p < 0.05.
Simvastatin Promotes Phosphorylation of AMPK and LKB1 and Dephosphorylation of VASP in Cultured Endothelial Cells—We used phosphorylation state-specific antibodies to probe immunoblots prepared from BAECs treated with varying concentrations of simvastatin for 24 h (Fig. 3, A and B). We found that simvastatin promotes the dose-dependent phosphorylation of AMPK and LKB1 at Thr172 and Ser428, respectively. In parallel, simvastatin treatment promotes the dose-dependent phosphorylation of acetyl-CoA carboxylase, a key substrate for AMPK-dependent phosphorylation (Fig. 3). We also found that simvastatin promotes the dose-dependent dephosphorylation of VASP at Ser157, a VASP residue that undergoes preferential phosphorylation by PKA.
FIGURE 3.
Dose response for simvastatin-modulated phosphorylation responses of AMPK, ACC, LKB1, and VASP in cultured endothelial cells. BAECs were treated for 24 h with varying doses of simvastatin as shown; cells were harvested and cell lysates analyzed in immunoblots probed with antibodies directed against phospho-AMPK, phospho-ACC, phospho-LKB1, phospho-VASP, total AMPK, VASP, ACC, and actin as shown. A, a representative experiment, which was repeated five times with equivalent results; B, pooled data, quantitating the immunoblot signals using digital chemiluminescence imaging, and defining for each antibody. The basal phosphorylation in vehicle-treated cells was 1.0. *, statistical significance at the p < 0.05 level (ANOVA).
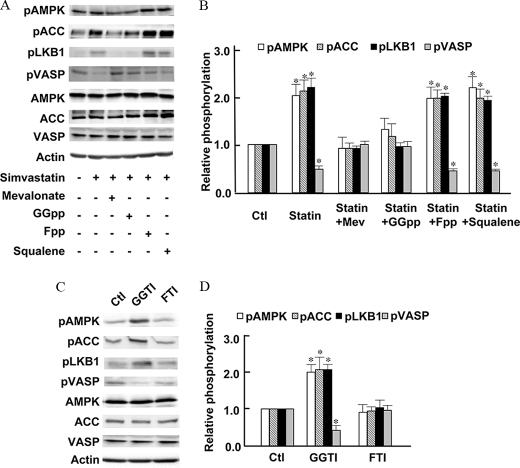
Effects of Isoprenoid Metabolites on Simvastatin-modulated Phosphorylation of AMPK, LKB1, and VASP—We next explored the effects of isoprenoid metabolites and the cholesterol precursor squalene on simvastatin-induced AMPK phosphorylation. BAECs were treated for 24 h with simvastatin plus the HMG-CoA product mevalonate; also studied were the isoprenoids geranylgeranyl pyrophosphate and farnesyl pyrophosphate, as well as the cholesterol precursor squalene. As shown in Fig. 4 (A and B), simvastatin-promoted phosphorylation of AMPK, ACC, and LKB1, as well as the dephosphorylation of VASP, were blocked by addition of mevalonate, but there was no effect either of the cholesterol precursor squalene or of farnesyl pyrophosphate. As was shown for simvastatin-induced Rac1 activation (Fig. 2), addition of GGpp but not Fpp reversed simvastatin-induced LKB1, AMPK, and ACC phosphorylation. In similar fashion, GGpp but not Fpp blocked the statin-induced dephosphorylation of VASP on the proteins PKA phosphorylation site (Fig. 4, A and B).
FIGURE 4.
Effects of isoprenoid metabolites on simvastatin-modulated phosphorylations of signaling proteins in endothelial cells. A, a representative immunoblot analyzed from BAECs treated for 24 h with simvastatin (10μm), either alone or in the presence of mevalonate (400 μm), GGpp (10 μm), Fpp (10 μm), or squalene (50 μm), as indicated. The cell lysates were resolved by SDS-PAGE and analyzed in immunoblots probed separately with antibodies directed against phospho-AMPK, phospho-ACC, phospho-LKB1, phospho-VASP, total AMPK, VASP, ACC, or actin, as shown. The experiment shown in A was repeated six times with equivalent results; pooled data are shown in B, and the asterisk indicates p < 0.01. In C, BAECs were incubated with the geranylgeranyl transferase inhibitor GGTI-298 (GGTI, 10 μm) alone or plus GGpp (10 μm), or with the farnesyl transferase inhibitor FTI-277 (10 μm), using experimental conditions identical to those used in Fig. 2C. The cell lysates were resolved by SDS-PAGE and analyzed in immunoblots probed separately with antibodies directed against phospho-AMPK, phospho-ACC, phospho-LKB1, phospho-VASP, total AMPK, ACC, VASP, or actin, as indicated. This experiment was repeated four times with equivalent results, and quantitative analyses of pooled data are shown in D.*, p < 0.05 by ANOVA.
GGTI but Not FTI Mimics the Effects of Simvastatin—Because geranylgeranyl pyrophosphate can reverse the simvastatin-modulated phosphorylations of AMPK, LKB1, ACC, and VASP (Fig. 4, A and B), we explored the effects of the geranylgeranyl transferase inhibitor GGTI and farnesyl transferase inhibitor FTI on phosphorylation of these proteins. As shown in Fig. 4 (C and D), treatment of BAECs with the geranylgeranyl transferase inhibitor GGTI significantly increases phosphorylation of AMPK, ACC, and LKB1 (by 2.1 ± 0.2-fold, 1.9 ± 0.1-fold, and 2.0 ± 0.3-fold, respectively, p < 0.05, n = 4 for each) and also promotes the dephosphorylation of VASP (51 ± 6% decrease in VASP phosphorylation, n = 4, p < 0.05). By contrast, the farnesyl transferase inhibitor FTI has no effect either on the phosphorylation of AMPK, ACC, or LKB1, or on the dephosphorylation of VASP.
siRNA-mediated AMPK Knockdown Attenuates Rac1 Activation by Simvastatin—We next used siRNA approaches to explore the role of AMPK in simvastatin-mediated Rac1 activation. Transfection of AMPK siRNA was able to knock down AMPK protein abundance by 90% without affecting the level of Rac protein expression (Fig. 5), as we have shown previously (6). siRNA-mediated AMPK knockdown did not change basal Rac1 activity, but significantly attenuated simvastatin-induced Rac1 activation (Fig. 5, A and B), suggesting that AMPK is required for the stimulatory effect of simvastatin on Rac1. siRNA-mediated AMPK knockdown significantly attenuated LKB1 and AMPK phosphorylation but did not affect VASP dephosphorylation by statin (Fig. 5, C and D), indicating an effect of AMPK on LKB1 phosphorylation.
FIGURE 5.
Effects of siRNA-mediated AMPK knockdown on simvastatin-modulated Rac1 activation and signaling protein phosphorylation. Cultured endothelial cells were transfected with a previously characterized siRNA targeting construct directed against AMPK or with control siRNA; 24 h after transfection, cells were incubated with simvastatin (10 μm) or vehicle for another 24 h, then harvested. In A and B, Rac1 activity was assayed in cell lysates using the GST-PAK pulldown method described in the text. A, a representative experiment that was repeated four times with equivalent results; quantitative analyses of pooled data from four experiments is shown in B; *, p < 0.05. C and D, results of immunoblot analyses in cells transfected with AMPK siRNA or control siRNA, treated with simvastatin for 24 h as above; immunoblots were probed with antibodies directed against pLKB1, pACC, pACC, pVASP, total AMPK, ACC, VASP, CaMKKβ, Rac, or actin, as indicated. C, results from a representative experiment; D, quantitative analyses of pooled data; basal phosphorylation in vehicle-treated/control siRNA-transfected cells was defined as 1.0. *, p < 0.05 (ANOVA, n = 4).
siRNA-mediated Rac1 Knockdown Blocks Simvastatin-induced LKB1 Phosphorylation, but Not AMPK Phosphorylation—We next explored the effect of siRNA-mediated knockdown of Rac1 on simvastatin-modulated phosphorylations of LKB1, AMPK, and VASP. As shown in Fig. 6, transfection of Rac1 siRNA knocked down Rac protein abundance by 90% without affecting the level of AMPK, VASP, CaMKKβ, ACC, and actin. siRNA-mediated Rac1 knockdown effectively abrogated simvastatin-induced LKB1 phosphorylation, indicating that Rac1 is required for LKB1 phosphorylation. By contrast, siRNA-mediated Rac1 down-regulation changed neither basal nor simvastatin induced-AMPK phosphorylation or VASP dephosphorylation.
FIGURE 6.
Differential effects of siRNA-mediated Rac1 knockdown on simvastatin-promoted phosphorylation of LKB1 and AMPK. BAECs were transfected with a siRNA construct targeting Rac1 or with control siRNA and treated 24 h later with simvastatin (10 μm), then incubated another 24 h and harvested. Cell lysates were resolved by SDS-PAGE and analyzed in immunoblots probed separately with specific antibodies directed against phospho-LKB1, phospho-AMPK, phospho-ACC, phospho-VASP, total AMPK, VASP, CaMKKβ, ACC, Rac, or actin, as indicated. A, a representative experiment; B, quantitative analyses of pooled data; *, p < 0.05 (ANOVA; n = 5).
Simvastatin-promoted LKB1 and AMPK Phosphorylation Is Dependent on CaMKKβ—We have previously used siRNA methods and pharmacological approaches to show that receptor-modulated AMPK phosphorylation is dependent on CaMKKβ (6). We extended these approaches to explore the role of CaMKKβ in statin-modulated LKB1 and AMPK phosphorylation. Transfection of CaMKKβ siRNA specifically knocked down CaMKKβ protein abundance by 90%, as shown in Fig. 7 and our previous report (6), and did not change other protein expression level such as AMPK. VASP, ACC, and Rac. siRNA-mediated CaMKKβ down-regulation did not affect the basal phosphorylation of LKB1, AMPK, or VASP. However, siRNA-mediated knockdown of CaMKKβ totally blocked statin-induced LKB1 and AMPK phosphorylation (Fig. 7, A and B). Similarly, the CaMKKβ inhibitor STO-609 attenuated stain-induced LKB1 and AMPK phosphorylation (Fig. 7, C and D). CaMKKβ knockdown did not affect statin-promoted VASP dephosphorylation (Fig. 7).
FIGURE 7.
CaMKKβ is a key determinant of statin-dependent AMPK and LKB1 phosphorylation. In the experiments shown in A and B, cultured endothelial cells were transfected with control or CaMKKβ siRNA; 24 h later simvastatin (10 μm) was added, and cells were harvested 24 h later. Cell lysates were resolved by SDS-PAGE and analyzes in immunoblots probed with antibodies directed against phospho-LKB1, phospho-AMPK phospho-VASP, AMPK, VASP, ACC, Rac, actin, and CaMKKβ, as shown. A, a representative experiment; B, pooled data from four experiments showing quantitative analyses of phosphoprotein abundance in the presence and absence of simvastatin, for control and CaMKKβ–transfected cells. Basal AMPK phosphorylation in vehicle-treated cells was defined as 1.0; *, p < 0.05 (ANOVA, n = 4). In C and D, BAECs were treated with or without simvastatin with or without the CaMKKβ inhibitor STO-609 (10 μm). Immunoblots prepared from treated cells were probed with antibodies as shown. C, a representative experiment that was repeated three times with similar results; D, results of pooled data, analyzed and presented as in B.
Effects of Simvastatin on Kinase Activities of LKB1 and CaMKKβ—We performed kinase activity assays to further validate the effects of simvastatin on the CaMKKβ and LKB1 pathways, because there is not necessarily a clear correlation between phosphorylation and enzyme activity for these proteins (11, 31). In endothelial cells transfected with control siRNA, simvastatin treatment (10 μm, 24 h) led to a striking increase in LKB1 activity, assayed using the LKBtide as a substrate for the LKB1 immunoprecipitated from endothelial cell lysates using a LKB1-specific antibody. LKB1 phosphorylation activity assays analyzed in immunoprecipitated lysates from vehicle-treated cells showed 0.16 mol of phosphate incorporated per mol of LKBtide versus 0.47 mol of phosphate/mol LKBtide in lysates immunoprecipitated from statin-treated cells (p < 0.05, n = 5; Fig. 8 shows normalized data). Similarly, there was an increase in CaMKKβ activity following simvastatin treatment of endothelial cells, as assayed by the incorporation of γ-[32P]ATP into the CaMKKβ substrate CaMKI following immunoprecipitation of the kinase from endothelial cell lysates using the CaMKKβ antibody. The CaMKKβ activity assay analyzed in lysates from vehicle-treated cells yielded 0.07 mol of phosphate incorporated per mol of CaMK1 versus 0.13 mol of phosphate/mol of CaMK1 in immunoprecipitates from statin-treated cells (p < 0.05, n = 5). Fig. 8 shows normalized data from multiple experiments. siRNA-mediated knockdown of either AMPK or CaMKKβ completely blocked the statin-induced increase in LKB1 activity (Fig. 8A). In contrast, siRNA-mediated knockdown of AMPK did not block the simvastatin-induced increase in CaMKKβ activity (Fig. 8B).
FIGURE 8.
Effects of simvastatin on kinase activities of LKB1 and CaMKKβ. This figure shows the results of kinase activity assays analyzed in LKB1 and CaMKKβ immunoprecipitates prepared from lysates of simvastatin-treated endothelial cells. To compare the different kinase assays from multiple experiments, this graph shows normalized data, with activity of untreated control siRNA-transfected cells defined as 1.0. Absolute values for the LKB1 phosphorylation activity assay showed 0.16 mol of phosphate incorporated per mol of LKBtide versus 0.47 mol of phosphate/mol LKBtide for statin-treated cells (p < 0.05, n = 5). The CaMKKβ phosphorylation activity assay in preparations from vehicle-treated cells showed 0.07 mol of phosphate incorporated per mol of CaMK1 versus 0.13 mol of phosphate/mol of CaMK1 for statin-treated cells (p < 0.05, n = 5). Please see the text for a detailed description of these analyses. A, data pooled from five independent experiments, presenting results of LKB1 activity assays analyzed in immunoprecipitates prepared from lysates of control or simvastatin-treated endothelial cells that had been transfected with siRNA targeting constructs as shown. B, results of CaMKKβ activity assays analyzed in immunoprecipitates prepared from lysates of control or simvastatin-treated endothelial cells transfected with siRNA targeting constructs as shown. For both panels, the data shown represent results of five independent experiments, each analyzed in duplicate. *, p < 0.05 compared with untreated control siRNA-transfected cells.
siRNA-mediated Knockdown of CaMKKβ Blocks Simvastatin-promoted Endothelial Cell Migration—We have previously used siRNA methods to explore the roles of Rac1, AMPK, and other signaling proteins in endothelial cell migration (5, 7). As shown in Fig. 9, simvastatin induced a 1.9-fold increase in endothelial cell migration (n = 6, p < 0.05). However, simvastatin-induced cell migration was completely attenuated by siRNA-mediated knockdown of CaMKKβ, AMPK, or Rac1; there was no effect of control siRNA on simvastatin-induced cell migration.
FIGURE 9.
siRNA-mediated knockdown of CaMKKβ, AMPK, or Rac1 blocks simvastatin-promoted endothelial cell migration. Endothelial cell migration was measured using a Transwell system in BAECs transfected with duplex siRNA constructs targeting CaMKKβ, AMPK, or Rac1 siRNA, or with control siRNA, as described under “Experimental Procedures.” The Migration Index represents the number of migratory cells/number of migratory cells in vehicle-treated control siRNA-transfected BAECs (Ctl). Each data point represents the mean ± S.E. from six independent experiments. *, p < 0.01 versus vehicle-treated untransfected control cells.
LKB1 Is Required for Simvastatin-induced AMPK Phosphorylation—We next explored a possible role for the kinase LKB1 in statin-induced AMPK phosphorylation in cultured endothelial cells. The commercially available antibodies against total LKB1 fail to detect a reliable specific signal in bovine endothelial cells (data not shown), despite the strong and reproducible signal detected by the LKB1 phosphorylation state-specific antibody shown above. Therefore, for our analyses of LKB1 knockdown, we studied cultured HUVECs. As shown in Fig. 10, siRNA-mediated knockdown of LKB1 blocked statin-induced AMPK phosphorylation without change total AMPK level, suggesting that LKB1, in addition to CaMKKβ (Fig. 7), is involved in statin-promoted AMPK phosphorylation.
FIGURE 10.
siRNA-mediated LKB1 knockdown attenuates simvastatin-induced AMPK phosphorylation. This figure shows results of an immunoblot experiment performed in HUVECs. Cells were transfected with control or LKB1 siRNA, and 24 h later simvastatin (10 μm) was added; after another 24 h, cells were harvested, and cell lysates were analyzed in immunoblots probed with antibodies directed against phospho-AMPK, LKB, or actin, as indicated. An experiment representative of three similar experiments is shown in A, and B shows quantitative results from pooled data. Basal AMPK phosphorylation in vehicle-treated cells was defined as 1.0; *, p < 0.05.
DISCUSSION
These studies have explored the effects of simvastatin on key endothelial signaling pathways, and have identified novel points of connection in statin-mediated responses involving AMPK, Rac1, and the AMPK kinases CaMKKβ and LKB1 (Fig. 11). Treatment of mice with simvastatin leads to a significant increase in Rac1 activity (Fig. 1), providing evidence for the first time in vivo of a statin effect on Rac1 activation. This finding is consistent with observations on statin-dependent Rac1 activation studied in in vitro models (24) (Fig. 2). These in vivo studies also document an increase in LKB1 and AMPK phosphorylation in arterial preparations isolated from mice following chronic administration of simvastatin (Fig. 1). This increase in LKB1 and AMPK phosphorylation in statin-treated animals is accompanied by a decrease in phosphorylation of the PKA substrate VASP, suggesting that statin treatment may modulate cAMP responses in the vascular wall.
FIGURE 11.
Simvastatin-induced Rac1 activation pathways in endothelial cells. This figure integrates the findings of these studies exploring the signaling pathways leading to Rac1 activation that are initiated by simvastatin. Simvastatin treatment of mice or of cultured endothelial cells leads to a marked enhancement in Rac1 activation, accompanied by increased phosphorylation of AMPK and LKB1. CaMKKβ appears to hold the key to all statin effects, both on the phosphorylation of LKB1 and of AMPK, as well as the downstream activation of Rac1: siRNA-mediated CaMKKβ knockdown or pharmacological inhibition of CaMKKβ completely blocks all these responses. siRNA-mediated knockdown of Rac1 has no effect on AMPK phosphorylation, but Rac1 knockdown completely blocks LKB1 phosphorylation. The relationship between LKB1 and AMPK is complex: LKB1 is a known AMPK kinase, yet AMPK knockdown blocks statin-induced LKB phosphorylation. Moreover, knockdown of the AMPK kinase CaMKKβ also blocks statin-induced phosphorylation of LKB1 and completely suppresses AMPK phosphorylation. siRNA-mediated LKB1 knockdown blocks statin-induced AMPK phosphorylation. It thus appears that neither LKB1 nor CaMKKβ alone are sufficient for statin-induced Rac1 activation, yet these AMPK kinases are themselves differentially regulated. While Rac1 is clearly downstream of LKB1 and CaMKKβ, siRNA-mediated knockdown of Rac1 blocks phosphorylation of LKB1 but not AMPK. The inter-relationships between these AMPK kinases and their differential modulation by Rac1 identify new levels of control in the pathways leading to statin-dependent modulation of vascular signaling.
Although these in vivo analyses of signaling protein activation help to establish the biological relevance of statin effects on Rac1 and AMPK pathways, we turned to studies of cultured endothelial cells to gain further mechanistic insight into the roles of statins in modulating these and related vascular signaling pathways. Treatment of cultured endothelial cells for 24 h with simvastatin leads to a striking 34-fold increase in Rac1 activity (Fig. 2). Importantly, statin-promoted Rac1 activation is completely inhibited by addition of mevalonate (Fig. 2), the metabolite synthesized by HMG-CoA reductase, providing strong evidence that the statin effect on Rac1 activation is a direct consequence of HMG-CoA reductase inhibition. There are many biologically active metabolites downstream of mevalonate, and these studies provide several lines of evidence that the effect of simvastatin on Rac1 activation involves enzymatic geranylgeranylation. Inhibition of geranylgeranyl transferase, but not of farnesyl transferase, leads to Rac1 activation (Fig. 2). Addition of the geranylgeranyl transferase product GGpp (geranylgeranyl pyrophosphate) reverses statin-promoted Rac1 activation (Fig. 2). However, neither farnesyl pyrophosphate nor the cholesterol precursor squalene affects statin-modulated Rac1 activation or phosphorylation responses (Figs. 2 and 4). Taken together, these observations strongly implicate protein geranylgeranylation as the key molecular event that is inhibited by statin treatment and leads to Rac1 activation.
Because Rac1 is itself geranylgeranylated, it is tempting to speculate that statin-dependent Rac1 activation directly involves statin-mediated inhibition of Rac1 geranylgeranylation. Although our data are consistent with this hypothesis, it is also possible that other geranylgeranylated proteins may be involved. The list of candidate geranylgeranylated signaling proteins is long: other members of the Rho GTPase family, G protein γ subunits, and many other signaling pathways involve geranylgeranylation and may also be affected by statins (32). Previous reports in cultured endothelial cells have provided conflicting evidence on the effects of statins on the small GTPase RhoA. Some studies found that statins can inhibit RhoA geranylgeranylation and attenuate RhoA translocation and activation (33, 34), whereas other studies have provided contrary evidence indicating that statins can promote RhoA activation (35). Moreover, Rac1 and RhoA often have opposing effects in vascular physiological responses in the vascular wall (36), and the dramatic statin-induced 34-fold increase in Rac1 activation seen in these studies (Fig. 2) is not inconsistent with there being a concomitant decrease in RhoA activity elicited by the same treatment. The mechanisms whereby statins inhibit RhoA or activate Rac1 in endothelial cells remain incompletely defined, and it seems plausible that statin effects on endothelial signaling pathways involve complexly interacting molecular loci that are influenced by post-translational modifications or protein-protein interactions among key signaling proteins. For example, statins have been shown to decrease inhibitory association of Rac1 with RhoGDI in monocytes (32); this protein-protein association depends on Rac1 geranylgeranylation (37, 38), and dissociation of RhoGDI from Rac1 is required for full Rac1 activation. Adding another level of complexity to statin-mediated effects on cellular signaling, some of the Rac1-associated regulatory proteins are themselves complexly modulated by phosphorylation pathways (39, 40). siRNA-mediated knockdown of Rac1 does not attenuate statin-promoted AMPK phosphorylation or VASP dephosphorylation, suggesting that Rac1 is not required for these phosphorylation responses.
These studies have established that simvastatin influences the phosphorylation and dephosphorylation of multiple vascular signaling proteins, both in mice chronically treated with simvastatin (Fig. 1), or in cultured endothelial cells treated with simvastatin for 24 h (Fig. 3). Simvastatin treatment of endothelial cells promotes a significant increase in the phosphorylation of AMPK as well as the AMPK kinase LKB1 (Fig. 3). In contrast, statin treatment leads to the dephosphorylation of the phosphoprotein VASP, a well known substrate for cyclic nucleotide-dependent protein kinases. The VASP phosphorylation state-specific antibody used in these studies detects phosphorylation of VASP at Ser157, the site that preferentially undergoes phosphorylation by the cAMP-dependent protein kinase (PKA) (23, 24). This statin-promoted decrease in VASP phosphorylation suggests that statins attenuate cAMP-dependent signaling pathways in these cells. This effect of simvastatin on cAMP pathways appears to be due to HMG-CoA reductase inhibition and effects on protein geranylgeranylation, as the statin-promoted suppression of VASP phosphorylation is completely reversed by mevalonate or by GGpp, but not by Fpp (Fig. 4). Likewise, the effect of simvastatin on LKB1 phosphorylation is likely to involve geranylgeranylation rather than farnesylation pathways, because GGpp but not Fpp reverse the statin-induced increase in LKB1 phosphorylation (Fig. 4).
We have previously shown that CaMKKβ is required for receptor-modulated AMPK phosphorylation stimulated by agonists such as vascular endothelial growth factor or sphingosine 1-phosphate (6); these agonists promote a level of phosphorylation similar to that seen in response to simvastatin treatment in these studies. The present studies establish (Fig. 8) that simvastatin treatment increases CaMKKβ activity. Importantly, the statin-promoted increase in CaMKKβ activity is not blocked by siRNA-mediated AMPK knockdown (Fig. 8B). These observations provide addition support for the placement of CaMKKβ “upstream” of AMPK, as had been previously postulated based on analyses of phosphorylation responses (6). Of course, the observation that upstream kinases (such as CaMKKβ) show a statin-modulated increase in kinase activity does not necessarily mean that the “downstream” kinases (such as AMPK) are necessarily phosphorylated directly by these proteins. It is possible, even likely, that intervening signaling proteins are involved in these pathways. Interestingly, CaMKKβ knockdown did not affect statin-promoted VASP dephosphorylation (Fig. 7), suggesting that CaMKKβ regulation is not upstream of this cyclic nucleotide-modulated response. Clearly, CaMKKβ is a critical determinant of the many other effects of simvastatin that were measured in these studies: either the CaMKKβ inhibitor STO-609 or siRNA-mediated CaMKKβ knockdown effectively abrogated all statin-mediated responses in these cells.
siRNA methodologies permitted us to further elucidate the pathways involved in statin-promoted Rac1 activation in cultured endothelial cells by permitting us to selectively knock down key signaling proteins. These studies implicate AMPK as a key determinant of statin-promoted Rac1 activation and endothelial migration. siRNA-mediated knockdown of AMPK significantly attenuated statin-promoted Rac1 activation (Fig. 5). In contrast, statin-induced phosphorylation of AMPK is completely unaffected by siRNA-mediated knockdown of Rac1 (Fig. 6). These results are consistent with our previous observations (6), which showed that receptor-dependent activation of Rac1 is AMPK-dependent, but AMPK activation is unaffected by knockdown of Rac1.
Our finding that simvastatin treatment leads to phosphorylation of AMPK and LKB1 in cultured endothelial cells and in murine aorta is consistent with previous reports (11, 13). Other previous studies have reported effects of statins on AMPK phosphorylation in short term treatments of cultured endothelial cells (12), but we found the most reproducible simvastatin responses only after 24 h of simvastatin treatment. We found in our studies that simvastatin-induced phosphorylation of AMPK totally depends on its ability to inhibit HMG-CoA reductase and geranylgeranylation. Mevalonate, the immediate product of HMG-CoA reductase, completely inhibits the stimulatory effect of simvastatin on AMPK and LKB1 phosphorylation, as does the precursor geranylgeranyl pyrophosphate. In contrast to a recent report (11), we found that CaMKKβ is a critical determinant of the statin-induced increase in AMPK and LKB1 phosphorylation: both the specific CaMKKβ inhibitor STO-609 as well as siRNA-mediated knockdown of CaMKKβ significantly attenuate these statin responses, consistent with our previous report showing that CaMKKβ modulates agonist-modulated AMPK activation in endothelial cells (6). Taken together, these data suggest that CaMKKβ is located upstream of LKB1 and AMPK in the pathway leading from statins to phosphorylation of these proteins.
There are several possible explanations for the discrepancy between our observations and the findings from a recent publication reporting that statin-induced AMPK phosphorylation is independent of CaMKKβ (11). Unfortunately, this previous report did not report any experiments using siRNA methods to knock down CaMKKβ, whereas our studies (Fig. 7) clearly establish that siRNA-mediated knock down of CaMKKβ abrogates the effects of statins on AMPK activation. Furthermore, the previous report (11) failed to provide a positive control to support their negative data using the CaMKKβ inhibitor STO-609. Other reasons for the discrepancy may relate to more subtle differences in culture conditions and/or experimental design; in any event, negative results should be interpreted with caution. As was found in this previous report, we too find that LKB1 is involved in regulation of AMPK by statins: siRNA-mediated knockdown of LKB1 blocks statin-induced AMPK phosphorylation. Importantly, we found further that siRNA-mediated knockdown of AMPK attenuates statin-promoted LKB1 phosphorylation (Fig. 5) as well as the statin-induced increase in LKB1 activity (Fig. 8A). Moreover, we found that siRNA-mediated Rac1 knockdown completely blocks statin-promoted LKB1 phosphorylation, but Rac1 knockdown has no effect whatsoever on AMPK phosphorylation. The regulation of LKB1 is incompletely understood: LKB1 can undergo autophosphorylation and may be phosphorylated by other kinases, including AMPK (41). Clearly, the signaling pathways intersect in complex ways, but our findings suggest that Rac1 is necessary for statin-promoted phosphorylation of LKB1 but not of AMPK (Fig. 6).
These studies demonstrate that simvastatin treatment increases the CaMKKβ-dependent phosphorylation of AMPK and LKB1 and enhances Rac1 activity both in cultured endothelial cells and in mouse aorta. These findings identify new points for pharmacological regulation of vascular signaling pathways and uncover new complexity in understanding the pleiotropic effects of statins in the vascular wall.
This work was supported, in whole or in part, by National Institutes of Health Grants HL46457, HL48743, and GM36259.
Footnotes
The abbreviations used are: HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; AMPK, 5′-AMP-activated protein kinase; siRNA, small interfering RNA; FBS, fetal bovine serum; BAECs, bovine aortic endothelial cells; HUVECs, human umbilical vein endothelial cells; PKA, cAMP-dependent protein kinase; VASP, vasodilator-stimulated phosphoprotein; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; FTI, farnesyl transferase inhibitor; FPP, farnesyl pyrophosphate; GGT, geranylgeranyl-transferase; GGTI, geranylgeranyl-transferase inhibitor; GGPP, geranylgeranyl pyrophosphate; PAK, p21-activated kinase; ACC, acetyl-CoA carboxylase; ANOVA, analysis of variance; GST, glutathione S-transferase; eNOS, endothelial isoform of nitric-oxide synthase.
References
- 1.Halcox, J. P., and Deanfield, J. E. (2004) Circulation 109 II42–II48 [DOI] [PubMed] [Google Scholar]
- 2.Jain, M. K., and Ridker, P. M. (2005) Nat. Rev. Drug. Discov. 4 977–987 [DOI] [PubMed] [Google Scholar]
- 3.Chen, Z. P., Mitchelhill, K. I., Michell, B. J., Stapleton, D., Rodriguez-Crespo, I., Witters, L. A., Power, D., Ortiz de Montellano, P. R., and Kemp, B. E. (1999) FEBS Lett. 443 285–289 [DOI] [PubMed] [Google Scholar]
- 4.Sawada, N., Salomone, S., Kim, H. H., Kwiatkowski, D. J., and Liao, J. K. (2008) Circ. Res. 103 360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kou, R., and Michel, T. (2007) J. Biol. Chem. 282 32719–32729 [DOI] [PubMed] [Google Scholar]
- 6.Levine, Y. C., Li, G. K., and Michel, T. (2007) J. Biol. Chem. 282 20351–20364 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, E., Kou, R., and Michel, T. (2006) J. Biol. Chem. 281 3210–3216 [DOI] [PubMed] [Google Scholar]
- 8.Abid, M. R., Tsai, J. C., Spokes, K. C., Deshpande, S. S., Irani, K., and Aird, W. C. (2001) FASEB J. 15 2548–2550 [DOI] [PubMed] [Google Scholar]
- 9.Moncada, S., and Higgs, A. (1993) N. Engl. J. Med. 329 2002–2012 [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen, S. B., and Rose, A. J. (2008) Front. Biosci. 13 5589–5604 [DOI] [PubMed] [Google Scholar]
- 11.Choi, H. C., Song, P., Xie, Z., Wu, Y., Xu, J., Zhang, M., Dong, Y., Wang, S., Lau, K., and Zou, M. H. (2008) J. Biol. Chem. 283 20186–20197 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Fisslthaler, B., Fleming, I., Keseru, B., Walsh, K., and Busse, R. (2007) Circ. Res. 100 e12–e21 [DOI] [PubMed] [Google Scholar]
- 13.Sun, W., Lee, T. S., Zhu, M., Gu, C., Wang, Y., Zhu, Y., and Shyy, J. Y. (2006) Circulation 114 2655–2662 [DOI] [PubMed] [Google Scholar]
- 14.Xenos, E. S., Stevens, S. L., Freeman, M. B., Cassada, D. C., and Goldman, M. H. (2005) Ann. Vasc. Surg. 19 386–392 [DOI] [PubMed] [Google Scholar]
- 15.Thors, B., Halldorsson, H., and Thorgeirsson, G. (2004) FEBS Lett. 573 175–180 [DOI] [PubMed] [Google Scholar]
- 16.Fleming, I., Schulz, C., Fichtlscherer, B., Kemp, B. E., Fisslthaler, B., and Busse, R. (2003) Thromb. Haemost. 90 863–871 [DOI] [PubMed] [Google Scholar]
- 17.Zou, M. H., Hou, X. Y., Shi, C. M., Nagata, D., Walsh, K., and Cohen, R. A. (2002) J. Biol. Chem. 277 32552–32557 [DOI] [PubMed] [Google Scholar]
- 18.Hardie, D. G., and Carling, D. (1997) Eur. J. Biochem. 246 259–273 [DOI] [PubMed] [Google Scholar]
- 19.Hawley, S. A., Davison, M., Woods, A., Davies, S. P., Beri, R. K., Carling, D., and Hardie, D. G. (1996) J. Biol. Chem. 271 27879–27887 [DOI] [PubMed] [Google Scholar]
- 20.Alessi, D. R., Sakamoto, K., and Bayascas, J. R. (2006) Annu. Rev. Biochem. 75 137–163 [DOI] [PubMed] [Google Scholar]
- 21.Matsushita, M., and Nairn, A. C. (1999) J. Biol. Chem. 274 10086–10093 [DOI] [PubMed] [Google Scholar]
- 22.Davare, M. A., Saneyoshi, T., Guire, E. S., Nygaard, S. C., and Soderling, T. R. (2004) J. Biol. Chem. 279 52191–52199 [DOI] [PubMed] [Google Scholar]
- 23.Krause, M., Dent, E. W., Bear, J. E., Loureiro, J. J., and Gertler, F. B. (2003) Annu. Rev. Cell Dev. Biol. 19 541–564 [DOI] [PubMed] [Google Scholar]
- 24.Harbeck, B., Huttelmaier, S., Schluter, K., Jockusch, B. M., and Illenberger, S. (2000) J. Biol. Chem. 275 30817–30825 [DOI] [PubMed] [Google Scholar]
- 25.Comerford, K. M., Lawrence, D. W., Synnestvedt, K., Levi, B. P., and Colgan, S. P. (2002) FASEB J. 16 583–585 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson, J. R., Dudek, S. M., Birukov, K. G., Ye, S. Q., Grigoryev, D. N., Girgis, R. E., and Garcia, J. G. (2004) Am. J. Respir. Cell Mol. Biol. 30 662–670 [DOI] [PubMed] [Google Scholar]
- 27.Lizcano, J. M., Goransson, O., Toth, R., Deak, M., Morrice, N. A., Boudeau, J., Hawley, S. A., Udd, L., Makela, T. P., Hardie, D. G., and Alessi, D. R. (2004) EMBO J. 23 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel, T., Li, G. K., and Busconi, L. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 6252–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kou, R., Igarashi, J., and Michel, T. (2002) Biochemistry 41 4982–4988 [DOI] [PubMed] [Google Scholar]
- 30.Jensen, T. E., Rose, A. J., Jorgensen, S. B., Brandt, N., Schjerling, P., Wojtaszewski, J. F., and Richter, E. A. (2007) Am. J. Physiol. Endocrinol. Metab. 292 1308–1317 [DOI] [PubMed] [Google Scholar]
- 31.Fogarty, S., and Hardie, D. G. (2009) J. Biol. Chem. 284 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordle, A., Koenigsknecht-Talboo, J., Wilkinson, B., Limpert, A., and Landreth, G. (2005) J. Biol. Chem. 280 34202–34209 [DOI] [PubMed] [Google Scholar]
- 33.Gabriel, M., van Nieuw Amerongen, G. P., Van Hinsbergh, V. W., Amerongen, A. V., and Zentner, A. (2006) J. Biomater. Sci. Polym. Ed. 17 567–577 [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Perera, O., Perez-Sala, D., Soria, E., and Lamas, S. (2000) Circ. Res. 87 616–622 [DOI] [PubMed] [Google Scholar]
- 35.Ohkawara, H., Ishibashi, T., Sakamoto, T., Sugimoto, K., Nagata, K., Yokoyama, K., Sakamoto, N., Kamioka, M., Matsuoka, I., Fukuhara, S., Sugimoto, N., Takuwa, Y., and Maruyama, Y. (2005) J. Biol. Chem. 280 10182–10188 [DOI] [PubMed] [Google Scholar]
- 36.Brandes, R. P. (2005) Circ. Res. 96 927–929 [DOI] [PubMed] [Google Scholar]
- 37.Di-Poi, N., Faure, J., Grizot, S., Molnar, G., Pick, E., and Dagher, M. C. (2001) Biochemistry 40 10014–10022 [DOI] [PubMed] [Google Scholar]
- 38.Lian, L. Y., Barsukov, I., Golovanov, A. P., Hawkins, D. I., Badii, R., Sze, K. H., Keep, N. H., Bokoch, G. M., and Roberts, G. C. (2000) Structure 8 47–55 [DOI] [PubMed] [Google Scholar]
- 39.Price, L. S., Langeslag, M., ten Klooster, J. P., Hordijk, P. L., Jalink, K., and Collard, J. G. (2003) J. Biol. Chem. 278 39413–39421 [DOI] [PubMed] [Google Scholar]
- 40.DerMardirossian, C., Schnelzer, A., and Bokoch, G. M. (2004) Mol. Cell 15 117–127 [DOI] [PubMed] [Google Scholar]
- 41.Sapkota, G. P., Boudeau, J., Deak, M., Kieloch, A., Morrice, N., and Alessi, D. R. (2002) Biochem. J. 362 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]