Abstract
The cAMP signaling pathway plays an essential role in modulating the apoptotic response to various stress stimuli. Until now, it was attributed exclusively to the activity of the G-protein-responsive transmembrane adenylyl cyclase. In addition to transmembrane AC, mammalian cells possess a second source of cAMP, the ubiquitously expressed soluble adenylyl cyclase (sAC). However, the role of this cyclase in apoptosis was unknown. A mitochondrial localization of this cyclase has recently been demonstrated, which led us to the hypothesis that sAC may play a role in apoptosis through modulation of mitochondria-dependent apoptosis. To prove this hypothesis, apoptosis was induced by simulated in vitro ischemia or by acidosis, which is an important component of ischemia. Suppression of sAC activity with the selective inhibitor KH7 or sAC knockdown by small interfering RNA transfection abolished endothelial apoptosis. Furthermore, pharmacological inhibition or knockdown of protein kinase A, an important cAMP target, demonstrated a significant anti-apoptotic effect. Analysis of the underlying mechanisms revealed (i) the translocation of sAC to mitochondria under acidic stress and (ii) activation of the mitochondrial pathway of apoptosis, i.e. cytochrome c release and caspase-9 cleavage. sAC inhibition or knockdown abolished the activation of the mitochondrial pathway of apoptosis. Analysis of mitochondrial co-localization of Bcl-2 family proteins demonstrated sAC- and protein kinase A-dependent translocation of Bax to mitochondria. Taken together, these results suggest the important role of sAC in modulating the mitochondria-dependent pathway of apoptosis in endothelial cells.
Increasing evidence suggests that apoptosis of endothelial cells (EC)3 may be responsible for acute and chronic vascular diseases, e.g. through atherogenesis (1), endothelial dysfunction (2), or thrombosis (3). Within several signaling mechanisms, a cAMP-dependent signaling pathway plays a substantial role in mediating apoptotic cell death induced by various stress factors. Elevation of the cellular cAMP either by forskolin-induced stimulation of the G-protein-responsive transmembrane adenylyl cyclase (tmAC) or by treatment with cAMP analogs has been shown to lead to both induction and suppression of apoptosis in different cell types (4–7). This discrepancy may be due to differences in cell types and experimental models. Alternatively, a lack of specificity of tmAC-induced signals, especially directed to distant intracellular targets like mitochondria, may be a cause of the discrepancy. Indeed, the classical model of cAMP signaling requires the diffusion of cAMP from plasma membrane-localized tmAC to targets localized throughout the cell. Diffusion of cAMP throughout the cytosol makes it difficult to selectively activate distally localized targets without also activating more proximal targets. Therefore, such diffusion of cAMP would likely diminish specificity, selectivity, and signal strength. This model is further complicated by the presence of phosphodiesterases, which degrade cAMP, thus preventing its diffusion.
In addition to tmAC, a second source of cAMP, soluble adenylyl cyclase (sAC), was demonstrated for mammalian cells (8, 9). Cytosolic localization of sAC provides both specificity and selectivity by permitting generation of cAMP proximal to intracellular targets. Furthermore, this model for cAMP action incorporates phosphodiesterases, which would act to limit diffusion and prevent nonspecific effector activation.
Whether sAC participates in apoptosis was unknown. A previous report demonstrated that sAC is co-localized with mitochondria (10). Because mitochondria play a fundamental role in apoptosis (11), we hypothesized that sAC may influence the development of apoptosis by modulating the mitochondrial pathway of apoptosis. Therefore, we aimed to examine the role of sAC in apoptotic cell death, especially its role in the modulation of the mitochondria-dependent pathway of apoptosis. For this purpose, apoptosis was induced in rat coronary EC by simulated in vitro ischemia or by acidosis. By applying pharmacological inhibition of sAC or small interfering RNA (siRNA)-mediated sAC knockdown, we found that sAC activity is required for the induction of apoptosis by ischemia or acidosis. Additionally, translocation of sAC to mitochondria and the sAC-dependent release of cytochrome c suggest that this cyclase specifically regulates the mitochondrial pathway of apoptosis.
EXPERIMENTAL PROCEDURES
Cell Culture—Coronary EC were isolated from 250–300-g male Wistar rats and maintained in Earle's minimal essential medium 199 supplemented with 10% fetal calf serum and 10% newborn calf serum (12). The purity of the cell culture (>98% EC) was confirmed by immunochemical staining with antibodies against von Willebrand factor and by uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled acetylated low density lipoprotein as described previously (13). Experiments were performed with monolayers reaching 90% confluence.
Experimental Protocols—To induce apoptosis, cells were exposed to acidosis (pH 6.4) in M199 medium supplemented with 2% serum for 3 h at 37 °C. Analysis of the buffer pH after acidic treatment did not reveal any significant alteration. Alternatively, apoptosis was induced by treatment with simulated ischemia (glucose-free anoxia at pH 6.4) for 2 h at 37 °C as described previously (14). KH7 (10 μmol/liter; ChemDiv Inc.) (15), 2′,5′-dideoxyadenosine (50 μmol/liter; Calbiochem), H-89 (3 μmol/liter; Calbiochem), or adenosine cyclic 3′:5′-monophosphorothioate (30 μmol/liter; Calbiochem) was applied simultaneously with acidosis or simulated ischemia. At the end of the experiments, floating cells were collected and used together with attached cells for further analyses. We found that ≤5% of the cells were floating at the end of the experiments.
Caspase-3 Activity Assay—Caspase-3 activity in cell extracts was detected using a colorimetric caspase-3 cellular activity assay kit (Calbiochem) based on the cleavage of the synthetic caspase substrate-1 linked to the chromophore p-nitroanilide (acetyl-DEVD-p-nitroanilide). Preparation of cell extracts and analysis of caspase-3 activity were performed according to the manufacturer's protocol. The amount of hydrolyzed substrate was measured as absorbance at 405 nm. The activity of caspase-3 was calculated as the maximal increase in absorbance per 0.5 × 106 cells within 30 min.
Hoechst 33342 and Propidium Iodide Staining—To distinguish between apoptotic and necrotic cells, nuclei were stained with Hoechst 33342 and propidium iodide as described previously (16). For quantitative assay, a blind analysis of 200–300 nuclei from four to five randomized fields was applied. Cells were scored as apoptotic when nuclei stained with Hoechst 33342 produced unequivocal bright blue fluorescence due to chromatin condensation.
TUNEL Staining—TUNEL staining using the in situ cell death detection kit (TMR red) from Roche Diagnostics GmbH (Mannheim, Germany) was performed according to the manufacturer's instructions. Samples were analyzed with a Leica TCS SP2 confocal microscope. Four culture dishes (each with 200–300 cells) per group were scored for TUNEL analysis.
Western Blotting—Cells were lysed in buffer containing 50 mmol/liter Tris-HCl (pH 8.0), 150 mmol/liter NaCl, 0.1% SDS, 1.0% Igepal, 0.5% sodium deoxycholate, 0.1 mmol/liter phenylmethylsulfonyl fluoride, and 1:100 protease inhibitor mixture (Sigma). Protein concentrations were quantified using a Pierce protein assay kit. Equal amounts of total proteins were separated on SDS-polyacrylamide gels. Separated proteins were transferred to nitrocellulose membrane. The primary antibodies used were sAC (clone R21 kindly provided by Dr. J. Buck, Cornell University) (10); cytochrome c (Sigma); cytochrome oxidase IV (Molecular Probes); actin (Chemicon International); caspase-3, caspase-9, Bax, Bad, BclxL, Bcl-2, and protein kinase Aα (PKAα) catalytic subunit (Cell Signaling); tmAC-6 (Abcam); and Bim (Stressgen). Specific bands were visualized after incubation with peroxidase-linked/horseradish peroxidase-labeled secondary antibodies by chemiluminescence using an ECL+ kit (Amersham Biosciences). Equivalent sample loading was confirmed by stripping membranes with Restore Western blot stripping buffer (Pierce), followed by treatment with antibodies against actin.
Subcellular Fractionation—Subcellular fractionation was performed essentially as described by Vander Heiden et al. (17). Briefly, cells were permeabilized by resuspension in buffer containing 210 mmol/liter sucrose, 20 mmol/liter HEPES-KOH (pH 7.5), 10 mmol/liter KCl, 1.5 mmol/liter MgCl2, 1.0 mmol/liter EDTA, 1.0 mmol/liter EGTA, 1.0 mmol/liter dithiothreitol, 0.1 mmol/liter phenylmethylsulfonyl fluoride, 1:100 protease inhibitor mixture, and 0.04% digitonin. To sediment nuclei and cellular debris, cell suspensions were centrifuged at 600 × g for 10 min at 4 °C. The supernatant was centrifuged at 15,000 × g for 30 min at 4 °C. After this centrifugation, the pellet was defined as the mitochondrial fraction and the supernatant as the cytosolic mitochondria-free fraction. The purity of the cytosolic fraction was confirmed by the absence of cytochrome oxidase IV.
siRNA Transfection—Knockdown of sAC was achieved by treatment of EC with siRNA duplexes corresponding to the sAC RNA sequence (Dharmacon catalog no. M-095523-00) or to the catalytic subunit of PKAα (Cell Signaling catalog no. 6406). In the control group, EC were treated with non-targeting siRNA duplexes (Dharmacon catalog no. D-001810-01). Cells were transfected according to the manufacturers' instructions. Briefly, cells were seeded 1 day before transfection in M199 medium containing 10% fetal bovine serum without antibiotics. Targeting siRNA or non-targeting siRNA was mixed with Oligofectamine (Invitrogen) in Opti-MEM I (Invitrogen) for 15 min at room temperature and then added to the culture medium at a final concentration of 60–100 nmol of either oligonucleotide/liter. Cells were incubated at 37 °C for 72 h. Protein expression was determined by Western blotting using a specific antibody for sAC, which revealed 89 ± 4% (n = 5) reduction of sAC and 86 ± 6% (n = 4) reduction of PKAα.
Immunocytochemistry and Confocal Imaging—Cells attached to culture dishes were washed twice with phosphate-buffered saline and fixed with 4% paraformaldehyde. For mitochondrial detection, cells were treated for 15 min with 0.5 mg/ml MitoTracker Red (Molecular Probes). After washing with phosphate-buffered saline, the cells were additionally fixed for 5 min with 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, and incubated with mouse monoclonal anti-sAC antibody, followed by treatment with Alexa 488-conjugated donkey anti-mouse IgG (Dianova). The cells were examined with a Leica TCS SP2 laser scanning confocal microscope. Series of confocal optical sections were taken at 0.5-μm intervals using a Leica Planapo 63/1.4 objective lens. Each recorded image was taken using dual-channel scanning and consisted of 1024 × 1024 pixels.
For quantitative analysis of co-localization of sAC with mitochondria, four cultures per group were used. The confocal settings had been standardized for all experimental groups to ensure that the images collected demonstrated a full range of fluorescence intensity from 0 to 255 intensity levels and were kept constant for recording of data in all measurements. Ten randomly selected fields with 80–120 cells per field were investigated per each culture. Confocal images were transferred as binary data to a Silicon Graphics workstation to display the histograms of fluorescence intensity distribution and to calculate the percentage of co-localization in the Voxel Shop and Colocalization programs (Bitplane AG, Zürich, Switzerland).
Cellular cAMP Analysis—Analysis of the total cellular cAMP content was performed immediately after 2 h of acidic treatment using the cAMP (direct) enzyme immunoassay kit from Assay Designs. Preparation of cell extracts and cAMP measurement were performed according to the manufacturer's protocol. The measured absorbance at 405 nm was used to calculate the concentration of cAMP, applying a calibration curve.
Statistical Analysis—Data are given as means ± S.E. Comparison of means between the groups was performed by one-way analysis of variance, followed by the Bonferroni post hoc test. Statistical significance was accepted when p was <0.05.
RESULTS
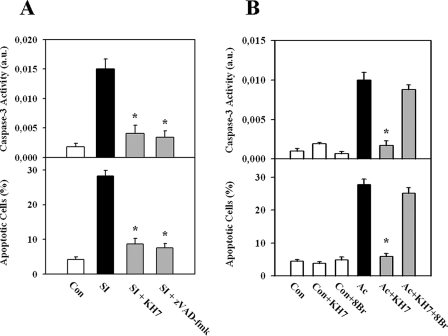
sAC Is Involved in Simulated Ischemia- or Acidosis-induced Apoptosis of EC—Under control conditions, i.e. incubation of EC in cell culture medium supplied with 2% serum for 16 h, only a few apoptotic cells (4.2 ± 0.7%, n = 10) and no significant caspase-3 activity were found. Exposure to simulated in vitro ischemia, i.e. glucose-free anoxia in combination with acidosis (pH 6.4), for 3 h led to a significant increase in the number of apoptotic cells (28.3 ± 1.6%, n = 11) and caspase-3 activity (Fig. 1A). To examine the role of sAC in EC apoptosis, treatment with the selective sAC inhibitor KH7 (10 μmol/liter) was performed (15). Previous studies demonstrated that KH7 at a similar range of concentrations inhibits sAC in various cell types (15, 18). In our model, KH7 applied at concentrations of 1–100 μmol/liter revealed a marked anti-apoptotic effect and no necrotic effect at 10 μmol/liter (data not shown). Inhibition of sAC with KH7 during ischemic treatment abolished the acidosis-induced increase in the caspase-3 activity and significantly reduced the number of apoptotic cells (Fig. 1A). Similarly, treatment with the pan-caspase inhibitor benzyloxycarbonyl-VAD-fluoromethyl ketone during simulated ischemia prevented EC apoptosis, demonstrating the caspase-dependent apoptosis in EC induced by ischemic stress.
FIGURE 1.
Apoptosis of EC is abolished by suppression of sAC. A, exposure to simulated ischemia (SI; glucose-free anoxia at pH 6.4) induced an increase in caspase-3 activity and the number of apoptotic cells (Hoechst 33342 staining of nuclei) compared with control cells (Con), which was suppressed by inhibition of sAC (10 μmol/liter KH7). A similar effect was found upon treatment with the pan-caspase inhibitor benzyloxycarbonyl-VAD-fluoromethyl ketone (zVAD-fmk; 50 μmol/liter). B, exposure to acidosis (Ac) induced an increase in caspase-3 activity and the number of apoptotic cells (Hoechst 33342 staining of nuclei), which was suppressed by inhibition of sAC (10 μmol/liter KH7). The effects of KH7 treatment were abolished by addition of the cAMP analog 8-bromo-cAMP (8Br; 30 μmol/liter). Values are means ± S.E. (n = 7–11). *, p < 0.05 versus simulated ischemia or acidosis. a.u., arbitrary units.
Because acidosis is an important component of ischemia and can trigger apoptosis in various cell types (19–21), we further examined whether sAC may play a role in acidosis-induced apoptosis. Similar to the ischemic treatment, inhibition of sAC with KH7 abolished the acidosis-induced rise in caspase-3 activity and reduced the number of apoptotic cells (Fig. 1B).
To determine whether the anti-apoptotic effect of KH7 may be reversed by an external supply of cAMP, treatment with the cAMP analog 8-bromo-cAMP (30 μmol/liter) was carried out during acidosis. The effect of KH7 was completely abolished (Fig. 1B).
To rule out the possibility of nonspecific inhibitory effects of KH7 on caspase-3, we performed a test in which KH7 was applied together with recombinant caspase-3 in the caspase-3 activity assay. The test demonstrated no significant effect of KH7 on caspase-3 activity (data not shown).
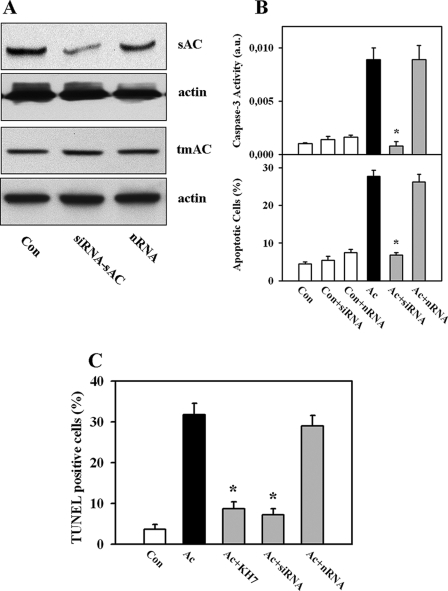
Knockdown of sAC Prevents Acidosis-induced Apoptosis of EC—To further support a role of sAC in apoptosis of coronary EC, expression of sAC was suppressed by pretreatment with sAC-specific siRNA. Transfection of EC with siRNA reduced the 50-kDa sAC protein by 89 ± 4% (Fig. 2A). In contrast, no significant effect was found on expression of tmAC-6, which is the main tmAC isoform expressed in non-myocyte cells in heart (22). Analysis of apoptosis revealed that the acidosis-induced activation of caspase-3 and the rise in apoptotic cell number were significantly suppressed by siRNA treatment, whereas treatment with non-targeting siRNA had no effect on these parameters (Fig. 2B). The extent of the apoptosis suppression induced by sAC knockdown was comparable with pharmacological inhibition of sAC by KH7 treatment (Fig. 1B). Therefore, sAC knockdown and the pharmacological inhibition strongly support the involvement of sAC in apoptosis of EC under acidic stress.
FIGURE 2.
Knockdown of sAC prevents acidosis-induced apoptosis of EC. A, Western blot of sAC and tmAC-6 prepared from extracts of EC after treatment with sAC-specific siRNA (siRNA-sAC) or non-targeting siRNA (nRNA) for 72 h. Data are representative of three to four independent experiments with similar results. Con, control cells. B, knockdown of sAC by treatment with siRNA prevents the acidosis (Ac)-induced increase in caspase-3 activity and the number apoptotic cells. No significant effects were found after equal treatment with non-targeting siRNA. Values are means ± S.E. (n = 6–11). *, p < 0.05 versus acidosis. a.u., arbitrary units. C, statistical analysis of the number of TUNEL-positive apoptotic EC. Conditions were similar to those described for Figs. 1B and 2B. Values are means ± S.E. (n = 4). *, p < 0.05 versus acidosis.
To substantiate the apoptosis analysis, TUNEL staining of the nuclei was performed (supplemental Fig. 1). This apoptosis detection method confirmed our data obtained by Hoechst 33342 staining and caspase-3 activity measurement and demonstrated similar alterations of EC apoptosis (Fig. 2C).
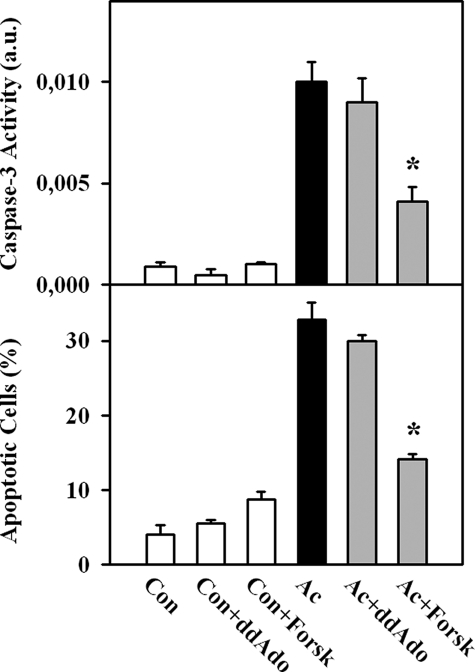
tmAC Is Not Involved in Acidosis-induced Apoptosis of EC—To test whether tmAC may participate in apoptosis, treatment with 50 μmol/liter 2′,5′-dideoxyadenosine (ddAdo), a potent inhibitor of tmACs (23), was performed. No significant alteration in apoptotic parameters under control conditions or under acidic stress was detected after this treatment (Fig. 3). Similarly, no changes in cAMP content were found upon treatment with ddAdo (Fig. 4A). In contrast, treatment with ddAdo prevented a forskolin-induced rise in cAMP content (Fig. 4B), demonstrating a sufficient potency of ddAdo to inhibit tmAC. Therefore, these data suggest a minor role of tmAC under acidic stress. Artificial stimulation of tmAC during acidic stress, i.e. forskolin treatment, significantly suppressed apoptosis of EC (Fig. 3).
FIGURE 3.
Inhibition of tmAC by treatment with ddAdo (50 μmol/liter) had no effect on acidosis-induced apoptosis (Ac), whereas stimulation of tmAC with forskolin (Forsk; 3 μmol/liter) significantly suppressed EC apoptosis. Values are means ± S.E. (n = 6–8). *, p < 0.05 versus acidosis. Con, control cells; a.u., arbitrary units.
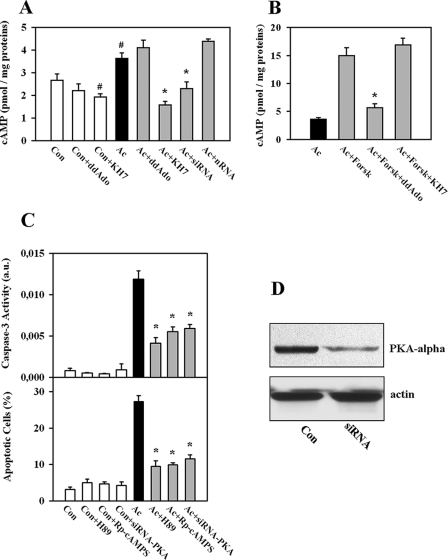
FIGURE 4.
Role of the cAMP-PKA pathway in acidosis-induced EC apoptosis. A, knockdown (siRNA transfection) or inhibition of sAC with KH7 (10 μmol/liter) during treatment with acidosis (Ac) significantly reduced the cellular content of cAMP. No effects were seen upon treatment with non-targeting siRNA (nRNA) or after inhibition of tmAC by ddAdo (50 μmol/liter). Con, control cells. B, treatment with forskolin (Forsk; 3 μmol/liter, 2 h) elevated cAMP content. This effect was abolished by tmAC inhibition with ddAdo (50 μmol/liter) but not by inhibition of sAC with KH7. C, knockdown of the catalytic subunit of PKAα (siRNA-PKA) or inhibition of PKA with H-89 (3 μmol/liter) or with (Rp)-adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS; 30 μmol/liter) during acidic exposure significantly attenuated acidosis-induced apoptosis of EC. Values are means ± S.E. (n = 4–6). #, p < 0.05 versus the control; *, p < 0.05 versus acidosis. a.u., arbitrary units. D, shown is a Western blot of the catalytic subunit of PKAα prepared from extracts of EC after treatment with PKA-specific siRNA for 72 h. Data are representative of three independent experiments with similar results.
The cAMP-PKA Pathway Is Involved in Acidosis-induced Apoptosis of EC—Conversion of ATP to cAMP is the main function of adenylyl cyclases. Therefore, we investigated whether inhibition of sAC corresponds to alteration of cellular cAMP content. Treatment with acidosis slightly but significantly increased the cellular cAMP concentration compared with the control (Fig. 4A). Knockdown of sAC or inhibition by KH7 treatment significantly reduced the cellular cAMP level during acidic stress (Fig. 4A). The effect of KH7 was not due to the nonspecific action on tmAC because KH7 had no effect on forskolin-induced cAMP elevation (Fig. 4B). This finding is in agreement with previous data demonstrating that KH7 has no effect on tmAC when applied at concentrations lower than 100 μmol/liter (18).
Because PKA is a major intracellular target for cAMP, we examined whether inhibition of PKA may affect the apoptosis rate in our model. Treatment either with the ATP-binding site inhibitor H-89 (3 μmol/liter) or with the cAMP-binding site inhibitor (Rp)-adenosine 3′,5′-cyclic monophosphorothioate (30 μmol/liter) during acidic exposure significantly reduced caspase-3 activity and the number of apoptotic cells (Fig. 4C). Furthermore, knockdown of the catalytic subunit of PKAα (Fig. 4D) similarly suppressed apoptosis of EC (Fig. 4C). Thus, PKA seems to play an essential role in the regulation of acidosis-induced apoptosis downstream of sAC.
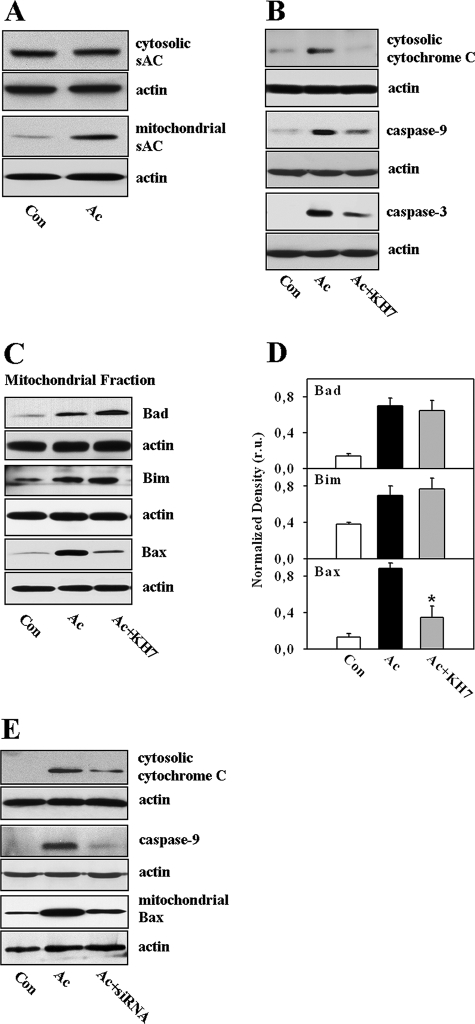
Mitochondria Play an Essential Role in sAC-dependent Apoptosis—The mitochondrial localization of sAC was demonstrated previously in several cell types (10), which supposes the involvement of mitochondria in the pro-apoptotic action of sAC. To test this hypothesis, we first investigated the mitochondrial co-localization of sAC in EC. For this purpose, immunocytochemistry (supplemental Figs. 2–4) and Western blot (Fig. 5A) analyses were carried out. Under control conditions, only a low level of mitochondrial localization of sAC was found in coronary EC. Treatment with acidosis dramatically enhanced the mitochondrial localization of sAC, indicating the acidosis-induced mitochondrial translocation of sAC. In addition, the release of cytochrome c from mitochondria was examined. Upon acidic treatment, cytosolic cytochrome c was markedly increased, accompanied by cleavage of downstream caspases, i.e. caspase-9 and caspase-3 (Fig. 5B). Treatment with KH7 abolished the acidosis-induced cytochrome c release and caspase cleavage.
FIGURE 5.
sAC controls the mitochondrial pathway of apoptosis. A, Western blot analysis of sAC in cytosolic and mitochondrial fractions before (control (Con)) and after treatment with acidosis (Ac). B, Western blot analysis of cytosolic cytochrome c and cleaved caspase-9 and caspase-3 prepared from extracts of control EC or EC exposed to acidosis with or without KH7 treatment (10 μmol/liter). C and D, Western blot analysis of Bad, Bim, and Bax in mitochondrial fractions of EC, followed by band optical density analysis. Values are means ± S.E. (n = 4). *, p < 0.05 versus acidosis. r.u., relative units. E, Western blot analysis of cytosolic cytochrome c, cleaved caspase-9, and mitochondrial Bax prepared from extracts of control EC or EC exposed to acidosis without (Ac) or after knockdown of sAC (siRNA). All Western blot data are representative of three to five independent experiments with similar results.
To understand more about the underlying cellular mechanisms of the sAC-dependent regulation of the mitochondrial pathway of apoptosis, the mitochondrial co-localization of the pro- and anti-apoptotic Bcl-2 family proteins, i.e. Bad, Bax, Bim, Bcl-2, and BclxL, was investigated. A marked mitochondrial translocation of Bax, Bad, and Bim was found upon acidic treatment. However, only Bax translocation was prevented by inhibition of sAC with KH7 treatment (Fig. 5, C–D). No significant alteration in mitochondrial targeting of BclxL and Bcl-2 was found in all protocols (data not shown). Similar to KH7 treatment, knockdown of sAC also abolished activation of mitochondrial apoptosis and Bax translocation (Fig. 5E).
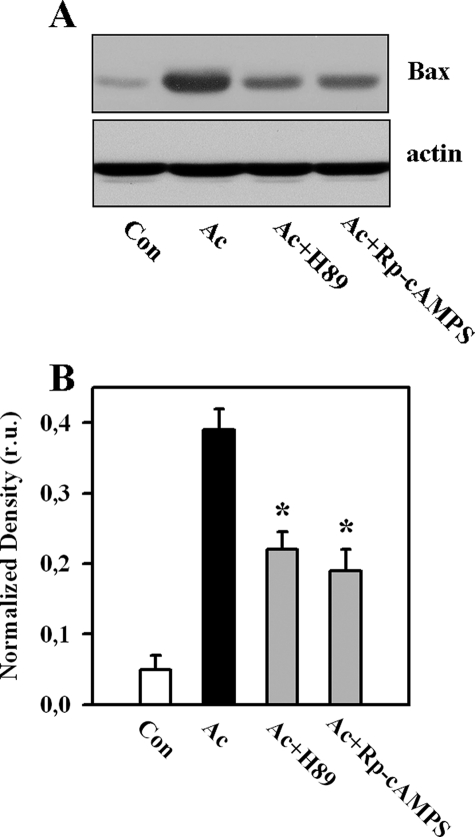
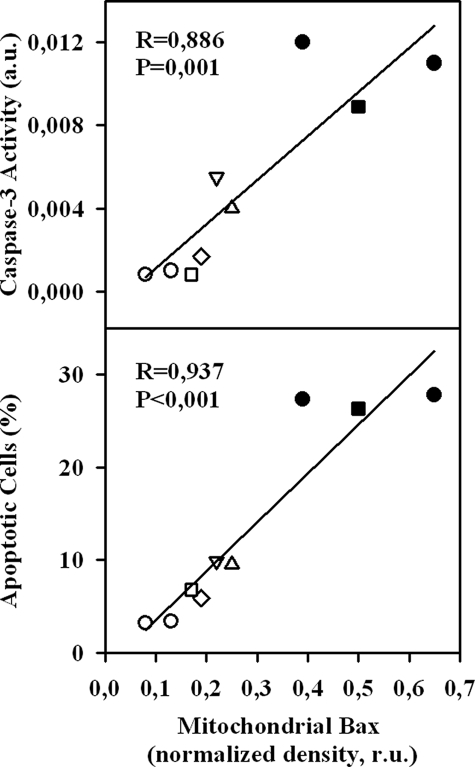
It has been shown previously that PKA may promote Bax translocation to mitochondria by phosphorylation at Ser60 (24). Therefore, we examined whether the sAC-dependent mitochondrial translocation of Bax also requires the active PKA. We found that inhibition of PKA by treatment with 3 μmol/liter H-89 or 30 μmol/liter adenosine cyclic 3′:5′-monophosphorothioate significantly reduced the mitochondrial translocation of Bax under acidic stress (Fig. 6). To demonstrate further the importance of mitochondrial Bax translocation for apoptosis in our model, a linear regression analysis was performed, which revealed a significant positive correlation between the amount of mitochondrial Bax and the degree of apoptosis (caspase-3 activity and the number of apoptotic cells) under different experimental conditions (Fig. 7).
FIGURE 6.
Western blot analysis of Bax in mitochondrial fractions (A), followed by band optical density analysis (B), from control EC (Con) or EC exposed to acidosis without (Ac) or with treatment with inhibitors of PKA: 3 μmol/liter H-89 or 30 μmol/liter (Rp)-adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS). Western blot data are representative of four independent experiments with similar results. Values are means ± S.E. (n = 4). *, p < 0.05 versus acidosis. r.u., relative units.
FIGURE 7.
Linear regression analysis of band optical density of Bax in mitochondrial fractions detected by Western blotting and parameters of apoptosis. ○, control; •, acidosis; ▵, acidosis + H-89; ▿, acidosis + (Rp)-adenosine 3′,5′-cyclic monophosphorothioate; ⋄, acidosis + KH7; □, acidosis + sAC siRNA; ▪, acidosis + non-targeting siRNA. a.u., arbitrary units; r.u., relative units.
DISCUSSION
The aim of this study was to investigate whether sAC plays a role in apoptosis of coronary EC induced by ischemic or acidic treatment and the signaling pathways that might be involved. The main findings are as follows. (i) sAC plays a key role in simulated ischemia- or acidosis-induced EC apoptosis. (ii) The signaling pathway consists of the acidosis-induced translocation of sAC to mitochondria and the activation of the mitochondrial pathway of apoptosis, i.e. translocation of Bax to mitochondria, release of cytochrome c, and cleavage of caspase-9.
To examine the role of sAC, we chose two models simulating common clinical situations, i.e. ischemia and acidosis. Ischemia has been shown to be an important trigger of apoptosis (25, 26), and ischemic acidosis is its essential pro-apoptotic component (27). Besides ischemia, tissue acidification is relevant to several pathologies, e.g. tumors (28) and inflammatory disease (29). The pro-apoptotic effect of acidosis was demonstrated in various cell types (19–21) as well as in the whole heart during cardioplegia (30) and in cardiac samples obtained from patients undergoing cardiac surgery (27). Furthermore, cytosolic acidification seems to play a fundamental role in death receptor- and mitochondria-dependent pathways of apoptosis (31, 32). In our models of simulated ischemia or extracellular acidosis, a profound cytosolic acidification, i.e. pH ∼6.5, was described previously (14, 33), and its importance for apoptosis of coronary EC has been demonstrated (33).
In contrast to the pro-apoptotic effect of the severe extracellular acidification (pH 6.4) observed in our study, mild acidosis (pH 7.0) has been shown to produce an opposite, anti-apoptotic effect (34). Obviously, this mild form of extracellular acidification is not sufficient to induce apoptosis. In fact, there seems to be a correlation between the observed extracellular pH value and the apoptosis rate. As demonstrated in human atrial tissue and neonatal rat cardiac myocytes, extracellular pH values of ∼7.0 do not induce apoptosis, whereas extracellular pH values of <6.5 accompany a significant increase in apoptosis (19, 27). The precise cellular mechanism of acidosis-induced apoptosis was poorly understood until now.
In this study, we analyzed the role of the cAMP-dependent signaling pathway. Although the involvement of this pathway in mediating apoptosis was demonstrated in several reports, the data are still controversial. Apoptosis induced by serum deprivation (35) or by treatment with Fas ligand or staurosporine (7) could be suppressed by elevation of cAMP through activation of tmAC. Alternatively, several studies demonstrated that cellular elevation of cAMP itself may lead to apoptosis and suppress proliferation (5, 6). The basis for this discrepancy is still unclear and may be due to differences in cell types or experimental models. Apart from the nonspecific treatment with cAMP analogs, the majority of these studies focused on tmAC signaling. Whether the sAC-derived pool of cAMP may also play a role in apoptosis remained unknown. Therefore, in this study, we challenged the hypothesis that sAC may be involved in apoptosis induced by an ischemic milieu. Applying two different tools, i.e. pharmacological inhibition of sAC with KH7 and sAC knockdown by siRNA treatment, we demonstrated that sAC plays a key role in ischemia- or acidosis-induced apoptosis. In contrast, tmAC seems not to be involved in acidosis-induced apoptosis in coronary EC because inhibition of this cyclase with ddAdo had no effects on cAMP content and apoptotic parameters (Figs. 3 and 4A).
Interestingly, pharmacological stimulation of tmAC during acidic stress by treatment with forskolin elevated cellular cAMP content and suppressed acidosis-induced apoptosis, demonstrating an anti-apoptotic effect of tmAC in our model. This finding contradicts the pro-apoptotic role of sAC. The reason for the opposite effects of these two cyclases is still unclear. Increasing evidence suggests that specificity of signal transduction is dependent on compartmentalization of the second messenger and its effectors within multiple signaling microdomains. Regarding cAMP, such compartmentalization, either close to the plasma membrane (tmAC source) or in the vicinity of intracellular organelles (sAC source), i.e. mitochondria and nuclei (10), permits cAMP to mediate various and independent cellular effects. Indeed, a recent study has demonstrated that cAMP produced by tmAC and sAC leads to different effects on PC12 cell migration (36). Furthermore, using pulmonary EC, Sayner et al. (37) observed opposite effects of tmAC and sAC activation on the microvascular EC barrier. In agreement with these reports, the results of this study further emphasize the significance of the cellular compartmentalization of the cAMP pools for triggering or preventing apoptosis.
Besides cAMP compartmentalization, a spatial pattern of PKA distribution within the cell plays a significant role in the regulation of cAMP signal transduction specificity. PKA signaling is highly controlled by PKA-anchoring proteins, which tether PKA pools and localize them at discrete subcellular microdomains in close proximity to kinase-specific downstream substrates (38, 39). Because of the different subcellular localization of sAC and tmAC pools of cAMP, recruitment of PKA to different targets may lead to opposite, i.e. pro- or anti-apoptotic, effects.
The activity of mammalian sAC has been shown to be stimulated by bicarbonate and divalent cations, i.e. Ca2+, Mg2+, and Mn2+ (9, 40). The bicarbonate-dependent mechanism of sAC activation seems irrelevant to our model because the bicarbonate content in the incubation medium was not elevated. It has been shown previously that incubation of coronary EC at extracellular pH 6.4 leads to Ca2+ overload (33, 41), which may contribute to activation of sAC. Although we did not analyze the specific activity of sAC, investigation of cellular cAMP revealed a significant sAC-dependent elevation of this nucleotide under acidic stress.
Mitochondria seem to be an important target in sAC-dependent apoptosis. The role of mitochondria in ischemia- or acidosis-induced apoptosis was demonstrated previously (27, 42). In agreement with these data, in this study, we observed activation of the mitochondrial pathway of apoptosis. Furthermore, (i) mitochondrial translocation of sAC and (ii) prevention of cytochrome c release, caspase-9 cleavage, and mitochondrial Bax translocation by inhibition of sAC clearly demonstrate that activation of the mitochondrial pathway is under the control of sAC. Although in our model the majority of cells (∼93%) demonstrated a high level (≥80%) of mitochondrial co-localization with sAC under acidic stress, only ∼30% of the cells became apoptotic. Thus, although sAC translocation may participate in activation of the mitochondrial pathway of apoptosis, it does not undoubtedly lead to cell death.
How sAC may control the mitochondrial pathway of apoptosis is still obscure. Increasing evidence suggests that translocation of pro-apoptotic members of the Bcl-2 protein family, e.g. Bax, from the cytosol to mitochondria, followed by permeabilization of the outer mitochondrial membrane, is an important initial mechanism for the release of mitochondrial cytochrome c (43). Considering our finding that inhibition of sAC prevents translocation of Bax to mitochondria (Fig. 5), one may suggest that sAC participates in the activation and translocation of Bax to mitochondria. In agreement with this finding, previous reports demonstrated several mechanisms for cAMP-dependent Bax regulation. First, cAMP-dependent PKA activation may inhibit Akt (44), a prosurvival kinase that inactivates Bax through Ser184 phosphorylation (24). Second, elevation of cAMP may lead to activation of protein phosphatase 2A (45), which in turn may lead to Bax activation through its dephosphorylation (46). Furthermore, Arokium et al. (24) reported recently that PKA may promote mitochondrial Bax translocation through its phosphorylation at Ser60. In agreement with this finding, the data of our study demonstrate that inhibition of PKA during acidosis (i) significantly attenuated apoptosis and (ii) reduced Bax translocation to mitochondria. These findings therefore argue for PKA as a critical link between sAC and mitochondrial Bax translocation.
In conclusion, sAC plays a key role in apoptosis of coronary EC exposed to ischemic or acidic stress. Activation of the mitochondrial pathway of apoptosis seems to be an underlying mechanism of the pro-apoptotic action of sAC. Further investigation of the role of sAC in different apoptosis models and cell types may reveal new therapeutic approaches for the treatment of apoptosis-dependent pathologies, e.g. tumor, neurodegenerative, and ischemic diseases.
Supplementary Material
Acknowledgments
We thank Dr. J. Buck for kindly providing antibodies for sAC. We thank Dr. M. Schaefer (University of Leipzig) for reviewing the manuscript. The technical help of G. Scheibel and K. Rezny is gratefully acknowledged.
This work was supported by Grant LA 1159/6-1 from the Deutsche Forschungsgemeinschaft and by Forum Grant F620-08 from the Ruhr-Universität Bochum.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
Footnotes
The abbreviations used are: EC, endothelial cell(s); tmAC, transmembrane adenylyl cyclase; sAC, soluble adenylyl cyclase; siRNA, small interfering RNA; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; PKA, protein kinase A; ddAdo, 2′,5′-dideoxyadenosine.
References
- 1.Chen, J., Mehta, J. L., Haider, N., Zhang, X., Narula, J., and Li, D. (2004) Circ. Res. 94 370–376 [DOI] [PubMed] [Google Scholar]
- 2.Werner, N., Wassmann, S., Ahlers, P., Kosiol, S., and Nickenig, G. (2006) Arterioscler. Thromb. Vasc. Biol. 26 112–116 [DOI] [PubMed] [Google Scholar]
- 3.Bombeli, T., Karsan, A., Tait, J. F., and Harlan, J. M. (1997) Blood 89 2429–2442 [PubMed] [Google Scholar]
- 4.Kwak, H. J., Park, K. M., Choi, H. E., Chung, K. S., Lim, H. J., and Park, H. Y. (2008) Cell. Signal. 20 803–814 [DOI] [PubMed] [Google Scholar]
- 5.Smith, P. G., Wang, F., Wilkinson, K. N., Savage, K. J., Klein, U., Neuberg, D. S., Bollag, G., Shipp, M. A., and Aguiar, R. (2005) Blood 105 308–316 [DOI] [PubMed] [Google Scholar]
- 6.Zhang, L., and Insel, P. A. (2004) J. Biol. Chem. 279 20858–20865 [DOI] [PubMed] [Google Scholar]
- 7.Rudolph, J. A., Poccia, J. L., and Cohen, M. B. (2004) J. Biol. Chem. 279 14828–14834 [DOI] [PubMed] [Google Scholar]
- 8.Buck, J., Sinclair, M. L., Schapal, L., Cann, M. J., and Levin, L. R. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., Cann, M. J., Litvin, T. N., Iourgenko, V., Sinclair, M. L., Levin, L. R., and Buck, J. (2000) Science 289 625–628 [DOI] [PubMed] [Google Scholar]
- 10.Zippin, J. H., Chen, Y., Nahirney, P., Kamenetsky, M., Wuttke, M. S., Fischman, D. A., Levin, L. R., and Buck, J. (2003) FASEB J. 17 82–84 [DOI] [PubMed] [Google Scholar]
- 11.Vander Heiden, M. G., and Thompson, C. B. (1999) Nat. Cell Biol. 1 E209–E216 [DOI] [PubMed] [Google Scholar]
- 12.Piper, H. M., Spahr, R., Mertens, S., Krützfeldt, A., and Watanabe, H. (1990) in Cell Culture Techniques in Heart and Vessel Research (Piper, H. M., ed) pp. 158–177, Springer, Heidelberg
- 13.Ando, H., Kubin, T., Schaper, W., and Schaper, J. (1999) Am. J. Physiol. Heart Circ. Physiol. 276 1755–1768 [DOI] [PubMed] [Google Scholar]
- 14.Kumar, S., Kasseckert, S., Kostin, S., Abdallah, Y., Piper, H. M., Steinhoff, G., Reusch, H. P., and Ladilov, Y. (2007) J. Cell. Mol. Med. 11 798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess, K. C., Jones, B. H., Marquez, B., Chen, Y., Ord, T. S., Kamenetsky, M., Miyamoto, C., Zippin, J. H., Kopf, G. S., Suarez, S. S., Levin, L. R., Williams, C. J., Buck, J., and Moss, S. B. (2005) Dev. Cell 9 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terminella, C., Tollefson, K., Kroczynski, J., Pelli, J., and Cutaia, M. (2002) Am. J. Physiol. Lung Cell. Mol. Physiol. 283 1291–1302 [DOI] [PubMed] [Google Scholar]
- 17.Vander Heiden, M. G., Chandel, N. S., Williamson, E. K., Schumacker P. T., and Thompson, C. B. (1997) Cell 91 627–637 [DOI] [PubMed] [Google Scholar]
- 18.Han, H., Stessin, A., Roberts, J., Hess, K., Gautam, N., Kamenetsky, M., Lou, O., Hyde, E., Nathan, N., Muller, W. A., Buck, J., Levin, L. R., and Nathan, C. (2005) J. Exp. Med. 202 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster, K. A., Discher, D. J., Kaiser, S., Hernandez, O., Sato, B., and Bishopric, N. H. (1999) J. Clin. Investig. 104 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoyama, K., Burns, D. M., Suh, S. W., Garnier, P., Matsumori, Y., Shiina, H., and Swanson, R. A. (2005) J. Cereb. Blood Flow Metab. 25 358–370 [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb, R. A., Giesing, H. A., Zhu, J. Y., Engler, R. L., and Babior, B. M. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 5965–5968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu, H. J., Unnerstall, J. R., and Green, R. D. (1995) FEBS Lett. 374 89–94 [DOI] [PubMed] [Google Scholar]
- 23.Desaubry, L., Shoshani, I., and Johnson, R. A. (1996) J. Biol. Chem. 271 2380–2382 [DOI] [PubMed] [Google Scholar]
- 24.Arokium, H., Ouerfelli, H., Velours, G., Camougrand, N., Vallette, F. M., and Manon, S. (2007) J. Biol. Chem. 282 35104–35112 [DOI] [PubMed] [Google Scholar]
- 25.Scarabelli, T. M., Stephanou, A., Pasini, E., Comini, L., Raddino, R., Knight, R. A., and Latchman, D. S. (2002) Circ. Res. 90 745–748 [DOI] [PubMed] [Google Scholar]
- 26.Borutaite, V., Jekabsone, A., Morkuniene, R., and Brown, G. C. (2003) J. Mol. Cell. Cardiol. 35 357–366 [DOI] [PubMed] [Google Scholar]
- 27.Thatte, H. S., Rhee, J. H., Zagarins, S. E., Treanor, P. R., Birjiniuk, V., Crittenden, M. D., and Khuri, S. F. (2004) Ann. Thorac. Surg. 77 1376–1383 [DOI] [PubMed] [Google Scholar]
- 28.Tannock, I. F., and Rotin, D. (1989) Cancer Res. 49 4373–4384 [PubMed] [Google Scholar]
- 29.Simmen, H. P., and Blaser, J. (1993) Am. J. Surg. 166 24–27 [DOI] [PubMed] [Google Scholar]
- 30.Khabbaz, K. R., Feng, J., Boodhwani, M., Clements, R. T., Bianchi, C., and Sellke, F. W. (2008) J. Thorac. Cardiovasc. Surg. 135 139–146 [DOI] [PubMed] [Google Scholar]
- 31.Tafani, M., Cohn, J. A., Karpinich, N. O., Rothman, R. J., Russo, M. A., and Farber, J. L. (2002) J. Biol. Chem. 277 49569–49576 [DOI] [PubMed] [Google Scholar]
- 32.Lagadic-Gossmann, D., Huc, L., and Lecureur, V. (2004) Cell Death Differ. 11 953–961 [DOI] [PubMed] [Google Scholar]
- 33.Kumar, S., Kasseckert, S., Kostin, S., Abdallah, Y., Schafer, C., Kaminski, A., Reusch, H. P., Piper, H. M., Steinhoff, G., and Ladilov, Y. (2007) Cardiovasc. Res. 73 172–180 [DOI] [PubMed] [Google Scholar]
- 34.D'Arcangelo, D., Facchiano, F., Barlucchi, L. M., Melillo, G., Illi, B., Testolin, L., Gaetano, C., and Capogrossi, M. C. (2000) Circ. Res. 86 312–318 [DOI] [PubMed] [Google Scholar]
- 35.Leone, V., di Palma, A., Ricchi, P., Acquaviva, F., Giannouli, M., Di Prisco, A. M., Iuliano, F., and Acquaviva, A. M. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 293 673–681 [DOI] [PubMed] [Google Scholar]
- 36.Young, J. J., Mehdi, A., Stohl, L. L., Levin, L. R., Buck, J., Wagner, J. A., and Stessin, A. M. (2008) J. Neurosci. Res. 86 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayner, S. L., Alexeyev, M., Dessauer, C. W., and Stevens, T. (2006) Circ. Res. 98 675–681 [DOI] [PubMed] [Google Scholar]
- 38.Scott, J. D., Stofko, R. E., McDonald, J. R., Comer, J. D., Vitalis, E. A., and Mangili, J. A. (1990) J. Biol. Chem. 265 21561–21566 [PubMed] [Google Scholar]
- 39.Rubin, C. S. (1994) Biochim. Biophys. Acta 1224 467–479 [PubMed] [Google Scholar]
- 40.Geng, W., Wang, Z., Zhang, J., Reed, B. Y., Pak, C. Y., and Moe, O. W. (2005) Am. J. Physiol. Cell Physiol. 288 1305–1316 [DOI] [PubMed] [Google Scholar]
- 41.Ladilov, Y., Schäfer, C., Held, A., Schäfer, M., Noll, T., and Piper, H. M. (2000) Cardiovasc. Res. 47 394–403 [DOI] [PubMed] [Google Scholar]
- 42.Weiss, J. N., Korge, P., Honda, H. M., and Ping, P. (2003) Circ. Res. 93 292–301 [DOI] [PubMed] [Google Scholar]
- 43.Er, E., Oliver, L., Cartron, P. F., Juin, P., Manon, S., and Vallette, F. M. (2006) Biochim. Biophys. Acta 1757 1301–1311 [DOI] [PubMed] [Google Scholar]
- 44.Lou, L., Urbani, J., Ribeiro-Neto, F., and Altschuler, D. L. (2002) J. Biol. Chem. 277 32799–32806 [DOI] [PubMed] [Google Scholar]
- 45.Moon, E. Y., and Lerner, A. (2003) Blood 101 4122–4130 [DOI] [PubMed] [Google Scholar]
- 46.Xin, M., and Deng, X. (2006) J. Biol. Chem. 281 18859–18867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.