Abstract
The chloroplast signal recognition particle (cpSRP) and its receptor (cpFtsY) function in thylakoid biogenesis to target integral membrane proteins to thylakoids. Unlike cytosolic SRP receptors in eukaryotes, cpFtsY partitions between thylakoid membranes and the soluble stroma. Based on sequence alignments, a membrane-binding motif identified in Escherichia coli FtsY appears to be conserved in cpFtsY, yet whether the proposed motif is responsible for the membrane-binding function of cpFtsY has yet to be shown experimentally. Our studies show that a small N-terminal region in cpFtsY stabilizes a membrane interaction critical to cpFtsY function in cpSRP-dependent protein targeting. This membrane-binding motif is both necessary and sufficient to direct cpFtsY and fused passenger proteins to thylakoids. Our results demonstrate that the cpFtsY membrane-binding motif may be functionally replaced by the corresponding region from E. coli, confirming that the membrane-binding motif is conserved among organellar and prokaryotic homologs. Furthermore, the capacity of cpFtsY for lipid binding correlates with liposome-induced GTP hydrolysis stimulation. Mutations that debilitate the membrane-binding motif in cpFtsY result in higher rates of GTP hydrolysis, suggesting that negative regulation is provided by the intact membrane-binding region in the absence of a bilayer. Furthermore, NMR and CD structural studies of the N-terminal region and the analogous region in the E. coli SRP receptor revealed a conformational change in secondary structure that takes place upon lipid binding. These studies suggest that the cpFtsY membrane-binding motif plays a critical role in the intramolecular communication that regulates cpSRP receptor functions at the membrane.
Proper compartmentalization of proteins relies on the ability of protein localization pathways to transport proteins efficiently from their sites of synthesis to their sites of function. The signal recognition particle (SRP)2 and its receptor function in every kingdom of life to target proteins to the endoplasmic reticulum (eukaryotes), cytoplasmic membrane (prokaryotes), and thylakoid membrane (chloroplasts) (1). The targeting function of SRP relies on a conserved 54-kDa SRP subunit (SRP54; Ffh in Escherichia coli and cpSRP54 in chloroplasts) as well as a conserved SRP receptor (SRα; FtsY in E. coli and cpFtsY in chloroplasts). For cytosolic SRPs (SRP54 and Ffh), interactions with a substrate signal sequence and an SRP RNA moiety are prerequisite for interaction with the SRP receptor (SRα and FtsY) (2). GTP binding and hydrolysis by both SRP54 and SRα coordinate substrate release from SRP to the translocon and release of SRP from SRα. In chloroplasts, cpFtsY functions along with a unique SRP (cpSRP) to post-translationally target nuclear encoded proteins to thylakoid membranes (3). Light-harvesting chlorophyll a/b-binding proteins (LHCPs) imported into the chloroplast stroma are bound by cpSRP to form a soluble targeting complex, which directs the LHCP substrate to the thylakoid membrane translocon Alb3 (Albino3) in a GTP- and cpFtsY-dependent manner (14, 36). Although many general steps of SRP protein targeting seem largely conserved across evolutionary boundaries, the nature and dynamics of the receptor appear to have diverged.
In eukaryotic systems, SRα is peripherally bound to the membrane through association with the integral membrane subunit SRβ. In contrast, no chloroplast or bacterial homolog of SRβ has been identified. cpFtsY and E. coli FtsY (EcFtsY) are found partitioned between the membrane and the stroma or cytosol, respectively. The membrane-binding capacity of EcFtsY serves to stimulate GTPase activity and appears critical in that only membrane-associated EcFtsY supports the release of nascent chains from SRP to the translocon (4, 5). However, the partitioning activity is not strictly required because EcFtsY tethered to the membrane is functional in vivo (37). Given the conserved nature of partitioning among bacterial and chloroplast SRP receptors, partitioning may play an, as of yet, unidentified role in protein targeting by SRP. Nevertheless, differences in lipid composition between bacterial and thylakoid membranes make it interesting to speculate that there are mechanistic differences in membrane partitioning.
Like many prokaryotic FtsY homologs (e.g. Thermus aquaticus), cpFtsY lacks the N-terminal acidic domain (A domain) implicated in EcFtsY membrane binding (6). Although the highly conserved FtsY GTPase domain (NG domain) of EcFtsY (EcFtsYNG) fails to support protein targeting, the addition of the last A domain residue, Phe-196 of a conserved double-Phe motif (EcFtsYNG+1), restores protein targeting in vivo (7). In vitro studies also show that EcFtsYNG+1 retains the capacity to bind membranes and support integration of SRP-dependent substrates, although at significantly reduced levels compared with full-length EcFtsY (8). A resolved structure of EcFtsYNG+1 suggests that the amphipathic nature of the region containing Phe-196 plays a critical role in membrane association (9). Furthermore, it has been demonstrated that liposomes stimulate GTP hydrolysis rates of SRP with EcFtsYNG+1, but not with EcFtsYNG, supporting the idea that the A domain in its entirety is not strictly required.
For cpFtsY, the necessity and functional role(s) of partitioning between a thylakoid-bound and a soluble phase, as well as the role of N-terminal residues in these functions, remain unknown. In addition, both the conformational state of membrane-bound cpFtsY and EcFtsY and the mechanism responsible for controlling membrane partitioning and altered GTPase activity remain unclear. Because of the gain of function exhibited by EcFtsYNG+1 and the conserved nature of the surrounding motif (9), it seems likely that this conserved region is necessary to support membrane binding and corresponding functions not only in EcFtsY but also in FtsY homologs.
To examine the functional role of the N-terminal region of cpFtsY, we have utilized deletion and point mutants in assays that reconstitute cpFtsY activities, including the cpSRP-dependent integration of LHCP. Together, our data indicate that the conserved lipid-binding motif identified in bacterial FtsY homologs is present in cpFtsY and is both necessary and sufficient for thylakoid binding and critical for LHCP targeting.
EXPERIMENTAL PROCEDURES
All reagents and enzymes used were purchased commercially. All primers were from Integrated DNA Technologies. The plasmid used for in vitro transcription/translation of pLHCP (psAB80XD/4) has been described (10). cpSRP43, cpFtsY, and cpSRP54 were prepared as described (38–40).
Construction of cpFtsY Clones for Transcription and Translation—cpFtsY clones for transcription/translation were designed to match the mature coding sequence of Arabidopsis thaliana cpFtsY starting with the predicted mature amino acid sequence CSAGPSGF. PCR amplification was used to create deletion and substitution mutants with incorporation of a Kozak sequence (Met-Ala) and restriction sites for insertion into pGEM-4Z. Expression clones were created by subcloning into pET-32b. The cpSRP43 transcription/translation clone was designed to match the mature coding sequence of A. thaliana cpSRP43 starting with Ala-Ala-Val-Gln-Arg-Asn and incorporating a Kozak sequence and restriction sites for insertion into pGEM-7Z. Chimeric sequence transcription/translation clones for Tha4TM-cpFtsY, Tha4TM-F48A, and Tha4TM-F48A/F49A are exact fusions constructed by overlap extension (11) of Pisum sativum Tha4 beginning with Ala-Phe-Phe-Gly-Leu-Gly and ending with Val-Phe-Gly-Pro-Lys-Lys and A. thaliana cpFtsY beginning with Met-Ala-Cys-Ser-Ala-Gly-Pro-Ser. Likewise, cpFtsY39–56-cp43, EcFtsY186–204-cp43, and cpFtsY39–56-RubSS constructs are exact fusions of mature A. thaliana cpFtsY construct residues 39–56 (Met-Ala-Cys-Ser-Ala-Gly-Pro-Ser-Gly-Phe-Phe-Thr-Arg-Leu-Gly-Arg-Leu-Ile) or E. coli FtsY residues 186–204 (Glu-Gln-Glu-Lys-Pro-Thr-Lys-Glu-Gly-Phe-Phe-Ala-Arg-Leu-Lys-Arg-Ser-Leu-Leu), a linker (Val-Phe-Gly-Pro-Lys-Lys), and either the predicted mature sequence of A. thaliana cpSRP43 or the P. sativum RubSS beginning Gln-Val-Trp-Pro-Pro-Ile-Gly-Lys. The RubSS transcription/translation clone includes the linker described above and the predicted mature sequence of the P. sativum RubSS. Kozak sequences and restriction sites for insertion into pGEM-4Z were included in the primer for all above-mentioned transcription/translation clones. All cloned sequences were verified by DNA sequencing (Molecular Resource Laboratory, University of Arkansas for Medical Sciences).
Preparation of Chloroplasts and Radiolabeled Precursors—Intact chloroplasts were isolated from 10–12-day-old pea seedlings (P. sativum cv. Laxton's Progress) and used to prepare thylakoids and stroma as described (12). Chlorophyll content was determined as described previously (13). Thylakoids were isolated from lysed chloroplasts by centrifugation and salt-washed two times with 1 m potassium acetate in import buffer (50 mm HEPES-KOH (pH 8.0) and 0.33 m sorbitol) and two times with import buffer with 10 mm MgCl2 (IBM buffer) prior to use. For protease treatment, salt-washed thylakoids were diluted to 0.5 mg/ml chlorophyll in import buffer with 0.2 mg/ml thermolysin and 1 mm CaCl2, incubated for 40–60 min, combined with EDTA in import buffer to 20 mm EDTA, and applied to a 7.5% (v/v) Percoll gradient in import buffer containing 10 mm EDTA. Pellets were washed once with import buffer containing 10 mm EDTA and twice with IBM buffer. Protease-treated thylakoids were resuspended at 1 mg/ml chlorophyll in IBM buffer.
In vitro-transcribed capped RNA was translated in the presence of [35S]methionine using a wheat germ system to produce radiolabeled proteins (12). Constructs were labeled with ratios of labeled and unlabeled Met such that equal 35S signal represented equimolar protein. For example, to prepare equimolar amounts of two proteins with six and seven Met residues, respectively, the first construct would be prepared with 100% [35S]Met, whereas the second construct would be prepared with 86% [35S]Met and 14% unlabeled Met. Constructs were quantified by comparing the 35S signal from a given protein band as analyzed by SDS-PAGE and phosphorimaging. Equimolar amounts of proteins were added to each experiment (supplemental Fig. S2). Precursor LHCP translation products were diluted 2-fold with 30 mm unlabeled Met in import buffer prior to use.
Protein Integration Assays Using Isolated Salt-washed Thylakoids—Integration assays included salt-washed thylakoids (equal to 50 μg of chlorophyll) in IBM buffer, 5 mm ATP, 1 mm GTP, 12.5 μl of radiolabeled pLHCP translation products, and stromal extract (equivalent to 50 μg of chlorophyll) or 25 μl of radiolabeled cpFtsY translation products and recombinant cpSRP43 and cpSRP54. Stromal extract, containing cpSRP and cpFtsY, was used as a positive control. Import buffer was used to bring the final volume to 150 μl. The mixtures were incubated at 25 °C for 30 min with light. Membranes were collected by centrifugation at 3200 × g for 6 min at 4 °C and protease-treated with thermolysin. Protease-treated membranes were solubilized in SDS buffer and heated. Amounts equivalent to 10 μg of chlorophyll/assay were analyzed by SDS-PAGE and phosphorimaging.
Assays for Determining Membrane Binding and Partitioning—Partitioning assays included thylakoids (equal to 75 μg of chlorophyll) in IBM buffer and radiolabeled translation products. Reactions were incubated for 30 min in light at 25 °C. Thylakoids were centrifuged at 3200 × g for 6 min at 4 °C, washed in 1 ml of IBM buffer, and transferred to clean tubes. Thylakoids were then pelleted, solubilized in SDS buffer, and heated. Amounts equivalent to 7.5 μg of chlorophyll/sample were analyzed by SDS-PAGE and phosphorimaging.
cpFtsY Membrane Binding Saturation Curves—Salt-washed or protease-treated thylakoids (equal to 50 μg of chlorophyll) were incubated with 0, 1, 2, 4, 8, 16, 32, or 64 μg of cpFtsY in a final volume of 100 μl of IBM buffer. Thylakoids were re-isolated, washed, and resuspended to a final volume of 50 μl, and 5 μl of each sample was analyzed by SDS-PAGE. Separated samples were transferred to BioTrace™ polyvinylidene difluoride membrane (Pall Life Sciences) and incubated with rabbit anti-A. thaliana cpFtsY polyclonal antibodies (14). Horseradish peroxidase-labeled mouse IgG (SouthernBiotech) was used as a secondary antibody. Proteins reacting with antibodies were revealed by incubation with SuperSignal® West Pico chemiluminescent substrate (Pierce).
Imaging Acquisition—SDS-polyacrylamide gels were imaged using a Typhoon 8600 (GE Healthcare) and analyzed with IQ Solutions software (Molecular Dynamics). Western blots were imaged using a FluorChem™ 8900 (Alpha Innotech) and analyzed with the corresponding AlphaEase® FC StandAlone software.
Isothermal Titration Calorimetry (ITC)—The binding of GMP-PNP/GDP to cpFtsY or F48A was analyzed by measuring heat change during titration of nucleotide into a protein solution using a VP-ITC titration microcalorimeter (MicroCal Inc.). All solutions were degassed under vacuum and equilibrated at 25 °C prior to titration. The sample cell (1.4 ml) contained 0.1 mm protein in 10 mm Tris buffer (pH 7.0) and 50 mm KCl. The reference cell contained MilliQ water. Upon equilibration, 5 mm GMP-PNP/GDP was injected in 20 × 6-μl aliquots using the default injection rate. Titration curves were corrected for protein-free buffer and analyzed using Origin ITC software (MicroCal Inc.).
Liposome Preparation and Fluorescence Quenching Experiments—Soybean total extract (Avanti Polar Lipids) lipids were dissolved at 100 mg/ml in chloroform, dried under nitrogen, and vacuum-desiccated overnight. Lipid pellets were resuspended to 10 mg/ml (13 mm) in 10 mm HEPES-KOH (pH 7), 100 mm KCl, and 1 mm EDTA. The lipid solution was subjected to 15-s sonication/15-s rest cycles for 2 min. Liposomes were clarified by centrifugation at 11,700 × g for 10 min at 4 °C and stored at 4 °C for up to 1 month. Liposomes were sized (Avanti mini-extruder) by passing through polycarbonate filters seven times. Brominated lipids were obtained by bromine addition to the unsaturated carbons of the soybean phosphatidylcholine fatty acyl chain as described (15). The brominated lipid mixture was extruded through 80-nm polycarbonate membranes and homogenized via freeze/thaw cycles.
Fluorescence quenching was measured using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices) set for maximum sensitivity and 282-nm excitation/330-nm emission wavelengths. 10 μg of protein in 50 μl of 10 mm HEPES-KOH (pH 8) and 10 mm MgCl2 and 0–50 μl of liposomes were mixed and equilibrated for 20 min at 25 °C, and the fluorescence was measured. For each concentration, six measurements of five separate samples were acquired. Fluorescence quenching was estimated as the normalized value of (F0 – F)/F0, where F0 is the average fluorescence of the samples without liposomes, and F is the average fluorescence for each concentration.
GTPase Assays—GTPase activity assays were conducted at 22 °C and contained 100 nm cpFtsY or F48A, 0.5 μm [α-32P]GTP (400 Ci/mmol), and liposomes in a final volume of 5 μl of buffer (50 mm HEPES (pH 8.0), 150 mm potassium acetate, 10 mm potassium chloride, 2 mm magnesium acetate, 0.01% (v/v) C12E8 (octaethylene glycol mono-N-dodecyl ether), and 2 mm dithiothreitol). Aliquots were removed at frequent time points and spotted onto polyethyleneimine-cellulose thin layer plates as described (16).
NMR Structural Studies—All NMR spectra were acquired at 25 °C on a Bruker AVANCE DMX-500 MHz spectrometer equipped with a 5-mm triple resonance cryoprobe. NMR samples (∼1 mm concentration) were prepared both in 90% (v/v) H2O + 10% (v/v) D2O (pH 7.0) containing 100 mm NaCl and in Me2SO-d6. Two-dimensional 1H TOCSY and NOESY (17) data were acquired with 2048 data points in the f2 dimension and 512 increments in the f1 dimension over a spectral width corresponding to 12 ppm. Two-dimensional 1H TOCSY data were acquired with mixing times of 60 and 75 ms. NOE-based distance restraints were derived from two-dimensional 1H NOESY data obtained with various mixing times (200, 250, 300, and 350 ms). All NMR spectra were processed using XWIN-NMR and Sparky software (18). The backbone dihedral angle restraints derived from 3JNHαH coupling constants and the χ1 dihedral angles derived from the TOCSY data were used as additional constraints for the structure calculation (19).
Distance restraints were derived from the NOESY spectrum of the peptides. NOE cross-peak intensities were measured and converted into distance. Structure calculation was performed using ARIA-CNS (Version 1.2) (20). Several cycles of ARIA were performed using standard protocols by varying the chemical shift tolerance between 0.04 and 0.01 ppm. Assignments and violations were analyzed after each cycle. An ensemble of 12 structures was chosen (from a pool of 50 structures) on the basis of lowest energy terms associated with violation of experimentally derived constraints. The ensemble of the best overlapping structures (with least root mean square deviation) of peptides was viewed using MOLMOL (21).
Circular Dichroism—CD spectra were recorded on a Jasco J-715 spectropolarimeter using a sandwich quartz cell of 0.1-mm path length. Spectra were acquired at 0.1-nm intervals and at scan speeds of 10 nm/min. All measurements were made after incubation of peptides (50 μm final concentration) in appropriate concentrations of liposomes for 5 min at room temperature. The results are expressed as mean residue ellipticity ([θ]), defined as [θ] = θobs/nlc, where θobs is the observed ellipticity in degrees, n is the number of amino acids in the peptide, c is peptide concentration, and l is the light path (centimeters). Spectra were averaged over 10 scans and corrected for background absorption.
Sequence Alignments—Sequence alignments of A. thaliana chloroplast, E. coli, Thermotoga maritima, and T. aquaticus FtsY homologs were performed using ClustalW (22). Sequences were input in FASTA format, and ClustalW was run using default settings. Alignment files were viewed using Jalview Version 2.0 (23).
Organeller cpFtsY sequences were obtained by searching for short, nearly exact matches using protein-protein BLAST (24). Residues 41–366 of A. thaliana cpFtsY were blasted against Eukaryota with a word size of two and otherwise default settings. A non-redundant set of six cpFtsY sequences was obtained and aligned for a consensus sequence using ClustalW as described. A prokaryotic FtsY consensus sequence was obtained by blasting the same cpFtsY sequence against bacteria with 500 descriptions. Sequences were shortened to contain only the NG domain plus 25 N-terminal residues. Resulting sequences were reduced to a non-redundant set of 375 and aligned using ClustalW. The percentage of each residue represented in an alignment column represents the total number of appearances of an amino acid divided by the total number of residues in that column.
RESULTS
The N-terminal Region of Mature cpFtsY Is Necessary for LHCP Integration and Thylakoid Membrane Binding—To understand whether the cpFtsY N terminus is functionally important in targeting of LHCP by cpSRP, cpFtsY was replaced with N-terminal deletion mutants (Fig. 1) in assays that reconstitute LHCP integration into isolated thylakoids. Proper integration of LHCP results in a protease-resistant degradation product, as shown in Fig. 2A. Deletion of cpFtsY residues 41–46 had little effect on LHCP integration, whereas further deletions (Δ41–49, Δ41–52, and Δ41–56) decreased integration by ∼90% relative to cpFtsY.
FIGURE 1.
N-terminal deletions of mature cpFtsY (residues 41–366). The conserved NG domain is shaded. N-terminal residues Met and Ala (MA) are added for translation initiation in the recombinant cpFtsY constructs that lack a chloroplast transit peptide (residues 1–40).
FIGURE 2.
cpFtsY residues 47–49 (GFF) are required for LHCP integration and efficient thylakoid partitioning. A, integration of LHCP was reconstituted with salt-washed thylakoids using stromal extract or recombinant proteins and equimolar amounts of in vitro-translated cpFtsY construct as indicated. See “Experimental Procedures” and supplemental Fig. S1 for more information regarding the production of equimolar amounts of in vitro-translated constructs. Correctly integrated LHCP migrated as a protease-resistant (thermolysin) degradation product (DP). A lane of pLHCP (translation product (TP)) is shown for comparison. LHCP integration was calculated from a minimum of three separate experiments and is shown relative to the level of integration observed for the stroma. B, the membrane binding of radiolabeled cpFtsY constructs as indicated was examined by incubation with salt-washed (SW) thylakoids. Thylakoids were re-isolated, washed, and analyzed by SDS-PAGE and phosphorimaging. The level of each membrane-bound cpFtsY construct was calculated from three separate experiments and is shown relative to bound cpFtsY.
To address membrane-partitioning competency, salt-washed thylakoids were incubated with radiolabeled cpFtsY N-terminal deletion constructs and repurified to remove unbound protein. Deletion of the first six residues (Δ41–43 and Δ41–46) reduced membrane binding to 40–50% of that observed for cpFtsY (Fig. 2B). Further N-terminal deletions (Δ41–49, Δ41–52, and Δ41–56) reduced membrane binding to only 13% of that seen for cpFtsY, correlating with the precipitous drop in LHCP integration observed for the same cpFtsY deletions (Fig. 2, A and B).
Phe-48 and Phe-49 Are Required for Efficient Thylakoid Membrane Binding and LHCP Integration—cpFtsYNG+1 and cpFtsYNG+2, consisting of the cpFtsY NG domain (residues 50–366) and Phe-49 (+1) or Phe-48 and Phe-49 (+2), respectively (Fig. 1), were examined for their ability to support LHCP integration and bind thylakoids. Although cpFtsYNG+2 bound membranes with ∼50% lower efficiency than cpFtsY, this construct supported efficient (∼90% relative to cpFtsY) LHCP integration in vitro (Fig. 3, A and B). cpFtsYNG+1 associated with thylakoids with 25% the efficiency of cpFtsY and exhibited integration efficiency comparable with that found in assays conducted without added cpFtsY. These data imply that the cpFtsY N terminus plays an active role in thylakoid binding and that membrane binding retained by cpFtsYNG+1 is not productive in terms of supporting the role of cpFtsY in LHCP localization.
FIGURE 3.

cpFtsY double-Phe motif (Phe-48 and Phe-49) plays a critical role in LHCP integration and thylakoid partitioning. A, integration of radiolabeled LHCP was reconstituted as described in the legend to Fig. 2A. Integration efficiency was calculated from three separate experiments and is presented relative to integration observed in the presence of the stroma. TP, translation product; DP, degradation product. B, the membrane binding of each radiolabeled cpFtsY construct indicated was examined by incubation with salt-washed (SW) thylakoids as described in the legend to Fig. 2B. The level of each cpFtsY construct bound to membranes was calculated from three separate experiments and is shown relative to bound cpFtsY.
cpFtsY constructs with Phe-48, Phe-49, or both replaced with alanine (F48A, F49A, or F48A/F49A) were examined for LHCP integration and membrane-binding defects. Strikingly, the F48A mutation reduced LHCP integration efficiency by nearly 80%, whereas F49A exhibited a 40% decrease in integration efficiency (Fig. 4A). The results using the F48A/F49A double mutant closely resembled those obtained with F48A. Thylakoid binding with F48A, F49A, and F48A/F49A mutations was reduced by ∼75, 60, and 75%, respectively (Fig. 4B). These data suggest that the conserved double-Phe motif is critical for the membrane-binding function of cpFtsY and supporting cpFtsY function in LHCP targeting. In particular, mutation of Phe-48 to Ala abolishes the ability of cpFtsY to support LHCP integration.
FIGURE 4.
cpFtsY Phe-48 plays a critical role in LHCP integration and thylakoid partitioning. A, integration of radiolabeled LHCP was reconstituted as described in the legend to Fig. 2A. Integration efficiency was calculated from three separate experiments and is presented relative to integration observed in the presence of the stroma. TP, translation product; DP, degradation product. B, the membrane binding of each radiolabeled cpFtsY construct indicated was examined by incubation with salt-washed (SW) thylakoids as described in the legend to Fig. 2B. The level of each cpFtsY construct bound to membranes was calculated from three separate experiments and is shown relative to bound cpFtsY.
Alanine Substitution of Phe-48 Does Not Affect Nucleotide Binding—Mature cpFtsY is composed primarily of the GTPase active NG domain. To ensure that the F48A mutation did not induce large global structural changes, we used ITC to compare the binding affinities of cpFtsY and F48A for both GMP-PNP and GDP (Fig. 5). Interaction of GMP-PNP with cpFtsY and F48A was exothermic and proceeded with changes in enthalpy of –3.4 and –3.3 kcal·mol–1, respectively. The number of binding sites (n) for GMP-PNP on cpFtsY and F48A was estimated to be 0.98 ± 0.01 and 0.96 ± 0.01, respectively. GMP-PNP bound to cpFtsY and F48A with similar affinity (Kd ∼ 1.4 μm), which is in agreement with previous studies (40). The binding affinity of GDP (Kd ∼ 1.2 μm) for cpFtsY and F48A was similar to that of GMP-PNP. Taken together, these results suggest that the global structure of F48A is intact, with minimal structural differences between cpFtsY and F48A.
FIGURE 5.
Alanine substitution of Phe-48 does not affect nucleotide binding. ITC curves show binding of GMP-PNP or GDP with cpFtsY or F48A at 25 °C. The upper and lower panels show the raw and integrated data, respectively, of the titration of the protein with nucleotide as indicated. The solid lines in the lower panels represent the best fit curve of the data (MicroCal Origin). Background corrections were made in all spectra.
Liposomes Stimulate Basal Hydrolysis of cpFtsY, but Not F48A—In the presence of SRP, GTP hydrolysis by EcFtsY, but not by EcFtsYNG, is stimulated by the addition of liposomes (4). Fig. 6A shows that liposomes stimulated basal GTP hydrolysis by cpFtsY, but not by F48A. Importantly, F48A exhibited a GTP hydrolysis rate that was four times greater than cpFtsY in the absence of liposomes and did not respond to a rise in liposome concentration (Fig. 6A). This corresponds to the earlier observations that the NG domain of EcFtsY has a higher basal hydrolysis rate than that of EcFtsYNG+1 (4, 25). Taken together, these data confirm that Phe-48 is part of a structurally distinct, lipid-responsive domain. Interestingly, this lipid-responsive domain also appears to repress GTP hydrolysis when in solution, thereby limiting futile GTP hydrolysis by cpFtsY when not engaged in protein-targeting activities at the membrane.
FIGURE 6.
F48A has an elevated GTP hydrolysis rate in solution, and GTP hydrolysis by cpFtsY, but not by F48A, is stimulated by liposomes. A, GTPase assays were performed with 100 nm cpFtsY (dark gray bars) or F48A (light gray bars) and 0.5 μm GTP in the presence of soybean liposomes as indicated. Activity levels shown are the average of a minimum of two separate experiments. Variation between independent assays of equivalent conditions was <15% in all cases. B, liposome binding estimated from fluorescence quenching suggests that cpFtsY (▪) has a higher binding affinity for the lipid membrane than F48A (▾). Fluorescence quenching, which requires the close proximity of Trp and the brominated acyl chain, suggests partial insertion of the protein into the bilayer. Fluorescence quenching was calculated from three separate experiments and is represented in relative fluorescence units (RFU).
The N Terminus of cpFtsY Partially Inserts into the Lipid Bilayer during Membrane Association—In contrast to SecY/FtsY interaction in the bacterial system (8), no proteinaceous thylakoid component has been identified to provide a binding site for cpFtsY to the thylakoid membrane. Neither protease treatment of salt-washed thylakoids3 nor pretreatment of thylakoid membranes with antiserum against SecY or Alb3 prevents cpFtsY from partitioning to the thylakoid membrane (14). Taken together, these results suggest that cpFtsY is able to bind thylakoids through interaction with the lipid bilayer.
To determine whether membrane binding by cpFtsY was affected by the F48A mutation, soybean liposomes containing brominated acyl chains were used to examine the interaction of cpFtsY or the F48A mutant with lipid bilayers. Bromine quenching of cpFtsY Trp fluorescence served as an indicator of protein/bilayer interactions (15). As shown in Fig. 6B, quenching of cpFtsY Trp fluorescence increased with the amount of brominated lipid in the assay. One of two Trp residues in cpFtsY, Trp-88, is positioned spatially closer to the putative lipid-binding site (26). Because quenching requires that Trp be in close proximity to the brominated acyl chains, these data suggest that cpFtsY partially inserts into the bilayer. In contrast, brominated lipids exhibited a greatly reduced ability to quench Trp fluorescence of the F48A mutant, indicating impairment in lipid binding of F48A, which mirrors the loss of thylakoid binding. Together, these results suggest that the conserved membrane-binding domain is capable of partial insertion into the lipid bilayer.
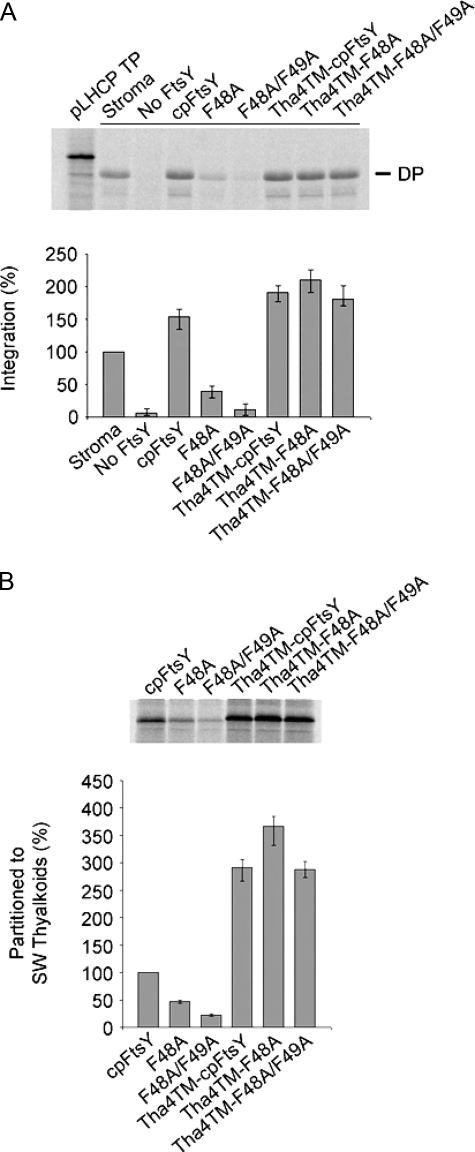
N-terminal Fusion of a Spontaneously Inserting Transmembrane Domain Complements F48A—If cpFtsY mutants lose activity due to loss of membrane binding, then a transmembrane anchor may restore activity. To better understand the influence of Phe → Ala loss-of-function mutants and to differentiate between a reduction in membrane binding and other potential causes for their inability to support LHCP integrations, we fused the transmembrane portion of P. sativum Tha4 to the N termini of mature cpFtsY, F48A, and F48A/F49A. P. sativum Tha4 is a spontaneously inserting thylakoid membrane component of the twin-arginine translocase protein-targeting pathway (41). Tha4TM-cpFtsY exhibited a 25% increase in LHCP integration compared with cpFtsY (Fig. 7A). Furthermore, fusion of Tha4TM to F48A and F48A/F49A completely restored their ability to support LHCP integration. It should be noted that fusion of P. sativum Tha4TM (residues 87–111) restored membrane binding to cpFtsY constructs F48A (Tha4TM-F48A) and F48A/F49A (Tha4TM-F48A/F49A) (Fig. 7B). These data strongly suggest that F48A is incapable of supporting LHCP integration due to a loss of thylakoid-binding capacity, which can be restored by fusing a transmembrane domain to the N terminus.
FIGURE 7.
Tethering cpFtsY(F48A) to the thylakoid restores cpSRP targeting. A, integration of radiolabeled LHCP was reconstituted as described in the legend to Fig. 2A. Integration efficiency was calculated from three separate experiments and is presented relative to integration observed in the presence of the stroma. TP, translation product; DP, degradation product. B, the membrane binding of radiolabeled cpFtsY constructs was examined by incubation with salt-washed (SW) thylakoids as described in the legend to Fig. 2B. The level of each cpFtsY construct bound to membranes was calculated from three separate experiments and is shown relative to bound cpFtsY.
The N Terminus of cpFtsY Is Necessary and Sufficient to Promote Thylakoid Binding—Although we have clearly established that cpFtsY residues 39–56 play a critical role in cpFtsY binding to the thylakoid, the possibility exists that this region is not the thylakoid-binding site per se, but instead facilitates membrane binding by a separate region of cpFtsY. However, if the membrane-binding activity is housed in cpFtsY residues 39–56, then we should expect this region to be able to bind thylakoids independently of the remainder of the protein and even, perhaps, localize a fused moiety to thylakoids. To examine this possibility, cpFtsY (residues 39–56) and the analogous region in EcFtsY (residues 186–204) were fused to the N terminus of the soluble protein cpSRP43 (cpFtsY39–56-cp43). cpSRP43 (cp43) exhibited low background binding to protease-treated thylakoid membranes (Fig. 8), whereas cpFtsY39–56-cp43 stably associated with membranes at a level of >10-fold that of cp43 alone. Similarly, cpFtsY39–56-RubSS led to a nearly 3-fold increase in thylakoid binding.3 Membrane localization of cp43 fused to the cpFtsY membrane-binding region was severely reduced by alanine replacement of Phe-48, Phe-49, or Phe-48/Phe-49, which reflects similar reductions in membrane localization of full-length cpFtsY point mutants (Figs. 4B and 8). Importantly, fusion of the analogous region from EcFtsY (residues 186–204) to cp43 resulted in a 6-fold increase in membrane binding of cp43. Alanine replacement of either Phe-195 or Phe-196 in the EcFtsY region resulted in complete loss of cp43 localization to thylakoid membranes. These data demonstrate that the N-terminal residues 39–56 of cpFtsY or the analogous region of EcFtsY are sufficient for tethering unrelated proteins to thylakoid membranes and do not require the NG domain to promote protein binding to thylakoids.
FIGURE 8.
The membrane active N terminus of cpFtsY is necessary and sufficient for targeting proteins to the thylakoid membrane. The membrane binding of equimolar radiolabeled cpFtsY, cp43, and chimeric constructs of either cpFtsY39–56 (cpFtsYpep) or EcFtsY186–204 (EcFtsYpep) with cp43 was examined by incubation with protease-treated thylakoids. Thylakoids were re-isolated, washed, and examined by SDS-PAGE and phosphorimaging. The level of each construct was calculated from three separate experiments and is shown relative to bound cpFtsY.
cpFtsY N-terminal Peptide Structures Reveal Potential Membrane Interaction Domains—Although multiple crystal structures of FtsY homologs have been published, the local conformation of the region immediately N-terminal to the double-Phe motif remains unresolved (9, 26–30). In this context, we determined the three-dimensional solution structures of cpFtsY39–56 and cpFtsY39–56(F48A) peptides using multidimensional NMR techniques (Fig. 9, Panel I, A). The cpFtsY39–56 peptide is mostly unstructured. However, a segment composed of Phe-48 to Leu-52 assumes an α-helical conformation (Fig. 9, Panel I, B). Helical conformation in this segment of cpFtsY39–56 is supported by several i to i+4 NOEs in the two-dimensional 1H NOESY spectrum. The root mean square deviation of the backbone heavy atom structured helical segment (residues 48–52) is 0.22 ± 0.03 Å.
FIGURE 9.
NMR structural studies of cpFtsY peptides. Panel I, cpFtsY39–56 peptide; Panel II, cpFtsY39–56(F48A) peptide. A, ensemble of the 12 lowest energy structures; B, ribbon diagram depicting the backbone fold; C, depiction of the distribution of hydrophobic residues.
Several NOEs between the γ-hydrogen of Arg-51 and the ring protons of Phe-48 strongly suggest a side chain interaction between the aromatic ring of Phe-48 and the positively charged guanido group of Arg-51 (Fig. 9, Panel I, B). This interaction decreases the freedom of the aromatic ring of Phe-48 and provides a microenvironment conducive to the formation of a hydrophobic core consisting of Phe-48, Phe-49, Leu-52, and Leu-55. The positively charged guanidino groups of Arg-51 and Arg-54 together with the hydrophobic core generate a local amphipathic structure (Fig. 9, Panel I, C).
The three-dimensional solution structure of cpFtsY39–56(F48A) shows i to i+3 interactions (characterizing a 310 helix) between the backbone atoms of Ala-48 to Arg-51 (Fig. 9, Panel II, A and B). The side chain interaction observed between residues 48 and 51, which is crucial for the packing of the hydrophobic core in cpFtsY39–56, is missing in cpFtsY39–56(F48A) (Fig. 9, Panel II, C). Comparison of the three-dimensional solution structures of the wild-type cpFtsY and cpFtsY(F48A) peptides suggests that the prominent projection of the hydrophobic side chain at position 48 and the unique asymmetric distribution of residues at the N terminus may be crucial for interaction with the membrane.
CD Reveals Liposome-induced Structural Changes in cpFtsY and EcFtsY Peptides—The backbone conformations of cpFtsY39–56 and cpFtsY39–56(F48A) were examined in the presence of soybean liposomes using far-UV CD. Surprisingly, the presence of the α-helical segment is not reflected in the far-UV CD spectrum of the cpFtsY39–56 peptide. The CD spectrum of cpFtsY39–56 shows a positive ellipticity at ∼218 nm and two negative ellipticity bands centered at 205 and 232 nm, but the 208 and 222 nm bands characteristic of the α-helical conformation are not present (Fig. 10, Panel I, line A). Such anomalies in CD spectra have been attributed to the contribution(s) of aromatic side chains to absorption in the far-UV region (42, 43). The combined absorption effects of the Phe doublet appear to dominate and mask the far-UV CD signal(s) typical of α-helices. This is obvious from the CD spectrum of cpFtsY39–56(F48A), which shows the signature α-helix bands at 208 and 222 nm (Fig. 10, Panel I, line B). The CD spectrum for EcFtsY186–204 is nearly identical to that for the cpFtsY39–56 peptide, as it also contains two Phe residues (Fig. 10, Panel II, line C).
FIGURE 10.
CD structural studies of cpFtsY and EcFtsY peptides. Panels I and II show the far-UV CD spectra. Panel I, cpFtsY39–56 (lines A and A′; blue) and cpFtsY39–56(F48A) (lines B and B′; green); Panel II, EcFtsY186–204 (lines C and C′; cyan); EcFtsY186–204(F195A) (lines D and D′; purple), and EcFtsY186–204(F196A) (lines E and E′; orange). In both Panels I and II, lines A′ through E′ indicate the corresponding spectra in the presence of liposomes. Panel III shows the shift in secondary structure as a function of liposome concentration for cpFtsY39–56 (blue closed circle), cpFtsY39–56(F48A) (green open square), EcFtsY186–204 (cyan closed square), EcFtsY186–204(F195A) (purple open triangle), and EcFtsY186–204(F196A) (orange cross).
The CD spectra of the wild-type peptides in the presence of ∼50 μm soybean liposomes are significantly different from those obtained in the absence of liposomes (Fig. 10, Panels I and II, compare lines A and A′ and lines C and C′). The spectra for both cpFtsY39–56 and EcFtsY186–204 show positive ellipticity bands at ∼199 nm and prominent negative bands centered at ∼225 nm (Fig. 10, Panels I and II, lines A′ and C′), suggesting that portions of these peptides assume a β-turn type of structure in the presence of liposomes (31). Hence, liposome interaction within both cpFtsY39–56 and EcFtsY186–204 induces a conformational switch from helix to a putative β-turn type of structure. Induction of the structural change requires a higher concentration of liposomes for cpFtsY39–56(F48A), EcFtsY186–204(F195A), and EcFtsY186–204(F196A) compared with cpFtsY39–56 and EcFtsY186–204, suggesting that liposomes have a weaker influence on these alanine replacements (Fig. 10, Panel III). The apparent Kd values (calculated from molar ellipticity changes at 208 nm as a function of liposome concentration) are 130 nm for cpFtsY and 200 nm for F48A (supplemental Fig. S2). Taken together, these data suggest that the regions in cpFtsY and EcFtsY containing the double-Phe motif respond to lipid bilayers by changing local backbone conformation.
Point Substitution of Phe-48 Reveals Structural Requirements—To examine the characteristics of Phe-48 important for function, we produced substitution mutants using amino acids differing in side chain length, charge, polarity, aromaticity, and secondary structure propensity (Ala, Gly, Val, Leu, Glu, Gln, Lys, Tyr, and Trp). LHCP integration assays performed with each mutant (Fig. 11A) revealed that small nonpolar side chain replacements (F48A and F48G) and polar side chain replacements (F48E and F48Q) resulted in severe integration defects. Larger nonpolar side chain replacements (F48L and F48V) exhibited integration efficiencies closer to cpFtsY: 98 and 72%, respectively. Valine appears to be the smallest residue that can serve as a functional replacement for Phe-48. Residue replacements containing aromatic rings (F48W and F48Y) also maintained high levels of integration (104 and 85% of cpFtsY, respectively). The thylakoid-binding capabilities of each mutant mirrored LHCP integration efficiency (Fig. 11B). Only F48L, F48W, F48V, and F48Y maintained sufficient membrane binding to support LHCP integration. Sequence alignments revealed a high degree of conservation of hydrophobic residues at the same positions in bacterial and chloroplast FtsY homologs (Fig. 12). Residues frequently found in alignment with the conserved double-Phe motif include Trp, Leu, and Val, all of which are functional replacements for cpFtsY Phe-48 (Fig. 11, A and B). Alignment of 375 bacterial FtsY sequences revealed a strong conservation of two Leu residues (Fig. 12) positioned to contribute to an amphipathic helix. Similarly, there are also conserved positively charged residues in positions compatible with the formation of an amphipathic helix in this region (Fig. 12). In comparison, this pattern of residues is not conserved in eukaryotic SRα homologs, perhaps because of the presence of the integral membrane receptor SRβ, which binds SRα to anchor it to the membrane.
FIGURE 11.
cpFtsY Phe-48 substitution studies. A, integration of radiolabeled LHCP was reconstituted as described in the legend to Fig. 2A. Integration efficiency was calculated from three separate experiments and is presented relative to integration observed in the presence of the stroma. TP, translation product; DP, degradation product. B, the membrane binding of each radiolabeled cpFtsY construct was examined by incubation with salt-washed (SW) thylakoids as described in the legend to Fig. 2B. The level of each cpFtsY construct bound to membranes was calculated from three separate experiments and is shown relative to bound cpFtsY.
FIGURE 12.
Membrane-binding motif residues are highly conserved among organellar and prokaryotic FtsY receptors. Upper panel, the A. thaliana cpFtsY double-Phe region was aligned with the corresponding regions of E. coli, T. maritima, and T. aquaticus FtsY receptors using ClustalW. Hydrophobic (boxed) and positively charged residues (+) thought to be important for lipid binding are indicated. ClustalW was used to generate consensus sequences for prokaryotic and organellar FtsY receptors. Lower panel, the relative abundance of each hydrophobic or positively charged residue at the position indicated is shown.
To examine whether the conserved positively charged residues (Arg-51 and Arg-54) and hydrophobic residues (Leu-52 and Ile-56) are also critical for cpFtsY function, we used the following point mutants in integration and membrane partition assays: R51A/R54A, L52A, L52Q, and I56A. As shown in Fig. 13A, alteration of any of these conserved residues decreased integration efficiency by 70% or more. Likewise, mutation of any of the hydrophobic residues (Leu-52, Ile-56, Phe-48, and Phe-49) to alanine or a charged amino acid decreased membrane binding by 40–75% (Fig. 13B). The double mutant R51A/R54A exhibited an appreciable loss of both membrane binding (∼60% loss) and LHCP integration. These data suggest that conserved positively charged and hydrophobic residues following Phe-48 and Phe-49 in the cpFtsY membrane-binding motif (Arg-51, Arg-54, Leu-52, and Ile-56) play a role in membrane partitioning, although the extent to which each residue is involved remains to be explored.
FIGURE 13.
Conserved residues in the cpFtsY membrane-binding motif are involved in thylakoid binding and LHCP integration. A, integration of radiolabeled LHCP was reconstituted as described in the legend to Fig. 2A. Integration efficiency was calculated from three separate experiments and is presented relative to integration observed in the presence of the stroma. TP, translation product; DP, degradation product. B, the membrane binding of each radiolabeled cpFtsY construct was examined by incubation with salt-washed (SW) thylakoids as described in the legend to Fig. 2B. The level of each cpFtsY construct bound to membranes was calculated from three separate experiments and is shown relative to bound cpFtsY.
DISCUSSION
SRP receptor homologs in E. coli (EcFtsY) and chloroplasts (cpFtsY) partition between membrane-bound and membrane-soluble phases. Previous studies have shown that membrane association is critical for EcFtsY function, and a membrane-binding motif has been identified for EcFtsY (9). However, the necessity and mechanism of membrane binding for cpFtsY during protein targeting remains uncertain. Our results demonstrate that cpFtsY in chloroplasts must interact with the thylakoid membrane to support cpSRP-dependent targeting. Furthermore, membrane binding takes place through a conserved amphipathic helix located at the N terminus of cpFtsY that is both necessary and sufficient for interaction with the thylakoid membrane. Characterization of the cpFtsY membrane-binding domain clearly reveals residues that are well tolerated in conserved positions. Importantly, we correlate loss of membrane binding with loss of GTP hydrolysis regulation where GTP hydrolysis activity is normally switched off in solution and then switched on by interaction with membranes.
In cpFtsY, Phe in the NG+2 position (Phe-48) of the conserved double-Phe motif is an essential component for functional binding of cpFtsY to thylakoids. Although EcFtsYNG+1 appears to be sufficient in vivo to maintain cell viability (7), in vitro results indicate a significant reduction in the ability of this construct to support integration of SRP-dependent substrates (8). Our results showing that cpFtsYNG+2 is considerably more active than cpFtsYNG+1 suggest that EcFtsYNG+2 would exhibit greater activity than EcFtsYNG+1. Regardless, both Phe residues in EcFtsY likely contribute to membrane binding because removal of Phe-195 or Phe-196 in proteins directed to the membrane by residues 186–204 of EcFtsY results in loss of this function (Fig. 8). Furthermore, our results, including the EcFtsY peptide structural data, suggest that Phe-195 and Phe-196 play roles comparable with cpFtsY Phe-48 and Phe-49 in promoting functional association with target membranes (Figs. 8 and 10).
It is noteworthy that the N terminus of cpFtsY appears to provide little specificity for thylakoid lipids, but rather exhibits a more generic lipid-binding activity. Thylakoid membranes and soybean total extract liposomes have vastly different lipid compositions, yet cpFtsY is capable of interacting with both by a mechanism that is sensitive to Phe-48 mutation (Figs. 4B, 6B, and 8). Given that the lipid composition of the thylakoid and inner envelope is quite similar (32), it would be expected that cpFtsY is able to bind both the thylakoid and inner envelope. Membrane specificity for the targeting mechanism is therefore likely to stem from interaction of cpSRP, cpFtsY, or targeting substrates with proteins that reside at the target membrane. We hypothesize that membrane specificity in chloroplasts is provided by interactions between the membrane translocon Alb3 and cpSRP components because a complex composed of cpSRP and cpFtsY specifically copurifies Alb3 in the presence of GMP-PNP (14). In addition, cpSRP43 alone exhibits the ability to bind Alb3 (33). In this context, it will be important to determine required interactions between cpSRP and Alb3 and to understand whether lipid binding by cpFtsY influences Alb3 association with cpSRP targeting components.
Although multiple crystal structures of FtsY homologs have been published, the local conformation of the cpFtsY double-Phe motif in its native state remains uncertain (9, 27–30, 34). Currently available structures of cpFtsY lack the N-terminal 23 residues of the mature protein, including the membrane-binding motif (29, 30). A resolved structure of EcFtsYNG+1 suggests that the region containing Phe-196 is α-helical in nature (9). In agreement with these data, the NMR structure of cpFtsY39–56 confirms that residues 48–52 form a helical structure. The three-dimensional structure of cpFtsY39–56 also shows that Phe-48 is located in a hydrophobic core lined by positively charged residues. The aromatic ring of Phe-48 projects out of the core, and its rotational freedom is restricted by interaction with the positively charged guanido group of Arg-51. The phenyl ring of Phe-48, together with the asymmetric distribution of the hydrophobic core and the positively charged residues Arg-51 and Arg-54, appears to provide a microenvironment crucial for association between cpFtsY and the thylakoid membrane. Amino acids with shorter hydrophobic side chains (e.g. Ala and Gly) at position 48 may be buried in the hydrophobic core and therefore unable to access the membrane. Similarly, substitution of Phe-48 with a charged group (Asp, Glu, Lys, or Arg) does not energetically favor interaction with nonpolar membrane regions. The other nonpolar residues in the hydrophobic core (Phe-49, Leu-52, and Ile-56), along with the nearby charged residues, may support or stabilize partial insertion of this region into the membrane.
We have also provided evidence for partial insertion of the helix into the thylakoid membrane (Fig. 6B). We predict a model for cpFtsY/membrane association whereby initial binding takes place between the N terminus and the lipid bilayer via the amphipathic helix of the membrane-binding motif and a secondary step requires partial insertion of the membrane-binding motif to stabilize membrane association. Far-UV CD data clearly show that membrane interaction of the cpFtsY N-terminal residues is accompanied by a conformational switch (helix to a β-turn-like structure) in the N-terminal segment of cpFtsY (Fig. 10). The functional relevance of this backbone change is supported by data identifying functional residue replacements for Phe-48 (Fig. 11). Correlation of integration and thylakoid-binding defects with a reduction in lipid-induced conformational changes, combined with the conserved nature of this structural motif, suggests that the lipid-induced conformational change in the cpFtsY N terminus serves as a functional switch to communicate a membrane-bound state and to induce or enhance associated activities.
Lipid binding by the N terminus of cpFtsY appears to play a key role in the SRP targeting cycle by influencing the GTP-hydrolyzing activity of the adjacent NG domain. It has been demonstrated that the presence of liposomes increases the hydrolysis rates of SRP with EcFtsYNG+1, but not with EcFtsYNG (4). Importantly, mutations in the lipid-binding region of cpFtsY (e.g. F48A) uncouple the requirement for membrane interaction from increased GTP hydrolysis; GTP hydrolysis is elevated in the F48A mutant without the need for lipids (Fig. 6A). It is interesting that Bahari et al. (35) did not find a higher basal hydrolysis rate for EcFtsYNG compared with either EcFtsY or EcFtsYNG+1. One possibility is that the membrane-binding motif in EcFtsY is not used to regulate GTP hydrolysis. However, conservation in sequence and structure of chloroplast and prokaryotic NG domains argues against this (9, 28–30, 34). Moreover, the results of Bahari et al. are not supported in the literature because three other reports demonstrate that FtsY from E. coli and T. aquaticus exhibits higher basal hydrolysis rates upon removal of the double-Phe motif-containing A domain (4, 25, 26). Regardless, we have clearly demonstrated that mutations in the cpFtsY membrane-binding motif that result in loss of membrane-binding activity also result in loss of membrane-induced GTPase stimulation and higher rates of GTP hydrolysis in solution. In this context, fusion of a membrane anchor to F48A, which restores its ability to support LHCP integration, further supports a model in which cpFtsY membrane binding must be coupled with elevated cpFtsY GTP hydrolysis activity (Fig. 7A). By structurally linking lipid binding to the ability of the SRP receptor to both bind SRP and hydrolyze GTP, futile hydrolysis in the absence of target membranes would be minimized. We speculate that during membrane association, the N terminus of cpFtsY shifts and may serve as a membrane sensor for the GTPase domain. It is attractive to envision that this conserved membrane-binding motif plays a universal role in regulating GTP hydrolysis in plastid and prokaryotic SRP-based protein targeting.
Supplementary Material
Acknowledgments
We thank R. E. Koeppe II for valuable suggestions on the manuscript, R. Gilmore and E. Mandon for assistance with GTP hydrolysis protocols, and K. Cline for Tha4TM clone templates.
This work was supported, in whole or in part, by National Institutes of Health Grant P20RR15569 (to R. L. G. and T. K. S. K.) from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources. This work was also supported by Department of Energy Grant DE-FG02-01ER15161 (to R. L. H., T. K. S. K., and R. L. G.), the Arkansas Bioscience Institute, and the National Science Foundation GK-12 Outreach funded by National Science Foundation Grant 0139570.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
This article was selected as a Paper of the Week.
Footnotes
The abbreviations used are: SRP, signal recognition particle; cpSRP, chloroplast SRP; SR, SRP receptor; cpFtsY, chloroplast FtsY; LHCP, light-harvesting chlorophyll-binding protein; EcFtsY, E. coli FtsY; Tha4TM, Tha4 transmembrane domain; cp43, cpSRP43; RubSS, ribulose-bisphosphate carboxylase/oxygenase small subunit; ITC, isothermal titration calorimetry; GMP-PNP, guanosine 5′-(β,γ-iminotriphosphate); TOCSY, total correlation spectroscopy; NOE, nuclear Overhauser effect; NOESY, NOE spectroscopy.
N. J. Marty, A. D. Kight, N. E. Lewis, R. L. Henry, and R. L. Goforth, unpublished data.
References
- 1.Pool, M. (2005) Mol. Membr. Biol. 22 3–15 [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw, N., Neher, S. B., Booth, D. S., and Walter, P. (2009) Science 323 127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry, R., Goforth, R. L., and Schunemann, D. (2007) in The Enzymes: Molecular Machines Involved in Protein Transport across Cellular Membranes (Tamanoi, F., Dalbey, R., and Koehler, C., eds) Vol. 25, pp. 493–521, Elsevier, St. Louis, MO [Google Scholar]
- 4.de Leeuw, E., te Kaat, K., Moser, C., Menestrina, G., Demel, R., de Kruijff, B., Oudega, B., Luirink, J., and Sinning, I. (2000) EMBO J. 19 531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valent, Q. A., Scotti, P. A., High, S., De Gier, J.-W. L., Von Heijne, G., Lentzen, G., Wintermeyer, W., Oudega, B., and Luirink, J. (1998) EMBO J. 17 2504–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsson, T., and Zwieb, C. (1999) Nucleic Acids Res. 27 169–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eitan, A., and Bibi, E. (2004) J. Bacteriol. 186 2492–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelini, S., Boy, D., Schiltz, E., and Koch, H.-G. (2006) J. Cell Biol. 174 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parlitz, R., Eitan, A., Stjepanovic, G., Bahari, L., Bange, G., Bibi, E., and Sinning, I. (2007) J. Biol. Chem. 282 32176–32184 [DOI] [PubMed] [Google Scholar]
- 10.Cline, K., Fulsom, D. R., and Viitanen, P. V. (1989) J. Biol. Chem. 264 14225–14232 [PubMed] [Google Scholar]
- 11.Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K., and Pease, L. R. (1989) Gene (Amst.) 77 61–68 [DOI] [PubMed] [Google Scholar]
- 12.Cline, K., Henry, R., Li, C., and Yuan, J. (1993) EMBO J. 12 4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnon, D. I. (1949) Plant Physiol. 24 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore, M., Goforth, R. L., Mori, H., and Henry, R. (2003) J. Cell Biol. 162 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carney, J., East, J. M., Mall, S., Marius, P., Powl, A. M., Wright, J. N., and Lee, A. G. (2006) Curr. Protocols Protein Sci., Unit 19.2 [DOI] [PubMed]
- 16.Connolly, T., and Gilmore, R. (1993) J. Cell Biol. 123 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuthrich, K. (1986) NMR of Proteins and Nucleic Acids, John Wiley & Sons, Inc., Hoboken, NJ
- 18.Goddard, T. D., and Kneller, D. G. (1997) Sparky3, University of California, San Francisco
- 19.Wang, Y., Nip, A. M., and Wishart, D. S. (1997) J. Biomol. NMR 10 373–382 [DOI] [PubMed] [Google Scholar]
- 20.Linge, J. P., O'Donoghue, S. I., and Nilges, M. (2001) Methods Enzymol. 339 71–90 [DOI] [PubMed] [Google Scholar]
- 21.Koradi, R., Billeter, M., and Wuthrich, K. (1996) J. Mol. Graphics 14 51–55, 29-32 [DOI] [PubMed] [Google Scholar]
- 22.Chenna, R., Sugawara, H., Koike, T., Lopez, R., Gibson, T. J., Higgins, D. G., and Thompson, J. D. (2003) Nucleic Acids Res. 31 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clamp, M., Cuff, J., Searle, S. M., and Barton, G. J. (2004) Bioinformatics 20 426–427 [DOI] [PubMed] [Google Scholar]
- 24.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. (1997) Nucleic Acids Res. 25 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neher, S. B., Bradshaw, N., Floor, S. N., Gross, J. D., and Walter, P. (2008) Nat. Struct. Mol. Biol. 15 916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawronski-Salerno, J., Coon, J. S., Focia, V. P. J., and Freymann, D. M. (2007) Proteins Struct. Funct. Bioinformat. 66 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egea, P. F., Shan, S.-o., Napetschnig, J., Savage, D. F., Walter, P., and Stroud, R. M. (2004) Nature 427 215–221 [DOI] [PubMed] [Google Scholar]
- 28.Focia, P. J., Shepotinovskaya, I. V., Seidler, J. A., and Freymann, D. M. (2004) Science 303 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stengel, K. F., Holdermann, I., Wild, K., and Sinning, I. (2007) FEBS Lett. 581 5671–5676 [DOI] [PubMed] [Google Scholar]
- 30.Chandrasekar, S., Chartron, J., Jaru-Ampornpan, P., and Shan, S.-o. (2008) J. Mol. Biol. 375 425–436 [DOI] [PubMed] [Google Scholar]
- 31.Bush, C. A., Sarkar, S. K., and Kopple, K. D. (1978) Biochem. J. 17 4951–4954 [DOI] [PubMed] [Google Scholar]
- 32.Douce, R., and Joyard, J. (1996) in Advances in Photosynthesis: Oxygenic Photosynthesis: The Light Reactions (Ort, D., and Yocum, C., eds) Vol. 4, pp. 69–101, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 33.Tzvetkova-Chevolleau, T., Hutin, C., Noel, L. D., Goforth, R., Carde, J.-P., Caffarri, S., Sinning, I., Groves, M., Teulon, J.-M., Hoffman, N. E., Henry, R., Havaux, M., and Nussaume, L. (2007) Plant Cell 19 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egea, P. F., Tsuruta, H., de Leon, G. P., Napetschnig, J., Walter, P., and Stroud, R. M. (2008) PLoS One 3 e3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahari, L., Parlitz, R., Eitan, A., Stjepanovic, G., Bochkareva, E. S., Sinning, I., and Bibi, E. (2007) J. Biol. Chem. 282 32168–32175 [DOI] [PubMed] [Google Scholar]
- 36.Asakura, Y., Hirohashi, T., Kikuchi, S., Belcher, S., Osborne, E., Yano, S., Terashima, I., Barkan, A., and Nakai, M. (2004) Plant Cell 16 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelazny, A., Seluanov, A., Cooper, A., and Bibi, E. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 6025–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan, J., Kight, A., Goforth, R. L., Moore, M., Peterson, E. C., Sakon, J., and Henry, R. (2002) J. Biol. Chem. 277 32400–32404 [DOI] [PubMed] [Google Scholar]
- 39.Goforth, R. L., Peterson, E. C., Yuan, J., Moore, M. J., Kight, A. D., Lohse, M. B., Sakon, J., and Henry, R. L. (2004) J. Biol. Chem. 279 43077–43084 [DOI] [PubMed] [Google Scholar]
- 40.Jaru-Ampornpan, P., Chandrasekar, S., and Shan, S.-o. (2007) Mol. Biol. Cell. 18 2636–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dabney-Smith, C., Mori, H., and Cline, K. (2006) J. Biol. Chem. 281 5476–5483 [DOI] [PubMed] [Google Scholar]
- 42.Viguera, A. R., and Serrano, L. (1995) Biochem J. 34 8771–8779 [DOI] [PubMed] [Google Scholar]
- 43.Sreerama, N., Manning, M. C., Powers, M. E., Zhang, J.-X., Goldenberg, D. P., and Woody, R. W. (1999) Biochem J. 38 10814–10822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.