Abstract
Inflamed cystic fibrosis (CF) human bronchial epithelia (HBE), or normal HBE exposed to supernatant from mucopurulent material (SMM) from CF airways, exhibit endoplasmic reticulum (ER)/Ca2+ store expansion and amplified Ca2+-mediated inflammation. HBE inflammation triggers an unfolded protein response (UPR) coupled to mRNA splicing of X-box binding protein-1 (XBP-1). Because spliced XBP-1 (XBP-1s) promotes ER expansion in other cellular models, we hypothesized that XBP-1s is responsible for the ER/Ca2+ store expansion in inflamed HBE. XBP-1s was increased in freshly isolated infected/inflamed CF in comparison with normal HBE. The link between airway epithelial inflammation, XBP-1s, and ER/Ca2+ store expansion was then addressed in murine airways challenged with phosphate-buffered saline or Pseudomonas aeruginosa. P. aeruginosa-challenged mice exhibited airway epithelial ER/Ca2+ store expansion, which correlated with airway inflammation. P. aeruginosa-induced airway inflammation triggered XBP-1s in ER stress-activated indicator (ERAI) mice. To evaluate the functional role of XBP-1s in airway inflammation linked to ER/Ca2+ store expansion, control, XBP-1s, or dominant negative XBP-1 (DN-XBP-1) stably expressing 16HBE14o- cell lines were used. Studies with cells transfected with an unfolded protein response element (UPRE) luciferase reporter plasmid confirmed that the UPRE was activated or inhibited by expression of XBP-1s or DN-XBP-1, respectively. Expression of XBP-1s induced ER/Ca2+ store expansion and potentiated bradykinin-increased interleukin (IL)-8 secretion, whereas expression of DN-XBP-1 inhibited bradykinin-dependent IL-8 secretion. In addition, expression of DN-XBP-1 blunted SMM-induced ER/Ca2+ store expansion and SMM-induced IL-8 secretion. These findings suggest that, in inflamed HBE, XBP-1s is responsible for the ER/Ca2+ store expansion that confers amplification of Ca2+-dependent inflammatory responses.
Chronic airway infection and inflammation are hallmarks of several airway diseases, including cystic fibrosis (CF)2 and chronic obstructive pulmonary disease (COPD). The altered airway environment resulting from infection and inflammation can, in turn, impact on the innate defense responses of the epithelia lining the airways. In accord with this notion, we have reported that the endoplasmic reticulum (ER) and its Ca2+ stores are expanded in native CF human airway epithelia. The ER expansion can be recapitulated in normal human bronchial epithelia (HBE) by exposing the cells to supernatant from mucopurulent material (SMM) from human CF airways (1, 2). The up-regulation of the ER Ca2+ stores is functionally relevant, because it provides a mechanism for amplification of intracellular Ca2+ (Ca2+i)-dependent secretion of inflammatory mediators in inflamed HBE (2). These previous studies illustrated that the ER Ca2+ stores expand when the demand to up-regulate the innate response is imposed upon the secretory pathway. However, the mechanism responsible for the ER Ca2+ storage expansion in inflamed airway epithelial cells remains elusive.
Because total protein synthesis is increased in inflamed HBE (2), and the increased synthesis of inflammatory mediators and defensive factors should result in an increased flux of newly synthesized, unfolded proteins into the ER, we have hypothesized that airway epithelial inflammation alters ER homeostasis and triggers an ER stress response, the unfolded protein response (UPR) (2–9). Eukaryotic cells exhibit three major UPR pathways, namely 1) inositol requiring enzyme 1 (IRE1), 2) activating transcription factor 6, and 3) PKR-like ER kinase/pancreatic eIF2α kinase. The present studies focused on the IRE1 branch of the UPR (for a recent review of UPR pathways, see Ref. 10). IRE1 is a transmembrane ER stress sensor, which exists in two isoforms, α and β. Activation of IRE1 by ER stress due to increases in unfolded proteins results in dissociation of the lumenal ER chaperone immunoglobulin-binding protein (BIP/GRP78) from a BIP/IRE1 complex (10) to facilitate the folding of the newly synthesized proteins. Dissociation of BIP from the lumenal domain of IRE1 results in IRE1 dimerization, transautophosphorylation, and activation of its C-terminal endoribonuclease activity (5, 7). The IRE1 endoribonuclease activity splices the leucine zipper transcription factor X-box binding protein-1 (XBP-1) mRNA via removal of a 26-nucleotide intron (11, 12). The spliced XBP-1 (XBP-1s) mRNA is translated into a potent transcription factor of 54 kDa (11, 12) that up-regulates genes encoding ER chaperones involved in protein folding (6, 13).
Data from several laboratories provide compelling evidence that activation of the UPR pathway mediated by XBP-1s couples to ER expansion. Studies with B lymphocytes and plasma cell differentiation have established a link between increased protein synthesis, UPR activation, and ER expansion. Differentiated plasma cells are programmed to secrete large quantities of immunoglobulins, a function accomplished by the acquisition of a highly developed ER, the development of which is dependent upon XBP-1 mRNA splicing (14–16). Studies demonstrating a direct link between XBP-1s, phospholipid biosynthesis, and ER expansion further supported a functional role for this UPR pathway regarding ER proliferation (17–19). In these studies, overexpression of XBP-1s was sufficient to increase cellular phospholipids and expand the ER compartment. Finally, deletion of XBP-1 in mouse models revealed its functional role in secretory organs such as the embryonic liver, pancreas, and salivary glands (20).
The role of XBP-1s in coordinating structural and functional features characteristic of secretory cells is likely relevant for inflammatory responses of airway epithelia. We have previously shown, utilizing the SMM model, that inflammation-triggered ER Ca2+ store expansion correlated with a 2.5-fold induction of the mRNA levels of XBP-1s in HBE (2). Based on these previous observations and the studies aforementioned, we hypothesized that inflammation-induced XBP-1s is the mechanism for the ER Ca2+ store expansion responsible for the amplification of Ca2+i-dependent inflammatory responses in inflamed HBE. The present studies utilized overexpression of XBP-1s or a dominant negative XBP-1 to evaluate the functional role of XBP-1s in airway epithelial inflammation coupled to ER Ca2+ store expansion. The relevance of XBP-1s in airway inflammation was further investigated in native airway epithelia from ER stress-activated indicator (ERAI) mice exposed to the bacterial pathogen Pseudomonas aeruginosa and freshly isolated normal versus CF human bronchial epithelia. Our findings are consistent with a functional role for XBP-1s in airway inflammatory responses.
EXPERIMENTAL PROCEDURES
Freshly Isolated Human Bronchial Epithelia
Human bronchial airway epithelial cells were obtained under the auspices of protocols approved by the Institutional Committee on the Protection of the Rights of Human Subjects. Bronchial epithelial cells were isolated from main stem or lobar bronchi from 3 normal and 3 CF human lungs as previously described (1, 2) and immediate total RNA extraction was performed according to standard methods for determination of XBP-1 mRNA splicing.
Pseudomonas aeruginosa Challenge of Mouse Airways
All mouse studies were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of North Carolina. P. aeruginosa strain PAK was grown as previously described (21) and used in the acute challenge studies. 5–6-Week-old wild type (C57BL/6 strain) or ERAI mice (RIKEN, Japan) were anesthetized and intratracheally instilled with 40 μl of P. aeruginosa suspension (2.5 × 107 colony forming units/ml for 1 × 106 colony forming units/40 μl; A600 = 0.060) or sterile phosphate-buffered saline (PBS) as control as previously reported (22). At 24 h post-exposure, the animals were euthanized and histological specimens obtained by gently perfusing the lungs with 10% neutral-buffered formalin via tracheal cannulation, and embedding the fixed tissue in paraffin. Sections were obtained for hematoxylin and eosin stain, immunofluorescence assessment of calreticulin, and Venus fluorescence.
Calreticulin Staining in Murine Airway Epithelia
Immunofluorescence of calreticulin was performed in lung sections from 5–6-week-old wild type mice (C57BL/6 strain) utilizing a primary rabbit anti-calreticulin antibody (Affinity Bioreagents) and a secondary goat anti-rabbit fluorescein isothiocyanate-conjugated antibody (Jackson ImmunoResearch Laboratories). Calreticulin fluorescence was assessed with a Leica 4D inverted laser scanning confocal microscope according to our method (1, 2).
Assessment of Venus Fluorescence in ERAI Mice Airway Epithelia
5–6-Week-old ERAI mice were sacrificed following a 24-h airway exposure to PBS or P. aeruginosa, their lungs were perfused with Tissue-Tek OCT Compound (Sakura Finetek, Inc.) and frozen at -80 °C. Frozen sections (10 μm thick) were obtained and differential interference contrast images and Venus fluorescence assessed with a Nikon TE2000 inverted fluorescent/differential interference contrast/phase microscope with dual cameras and rapidly switchable excitation and emission filters. The airway epithelial Venus fluorescence intensity was quantified with the Metamorph software and expressed per surface epithelial area.
Cell Culture
Wild type 16HBE14o- cells and 16HBE14o- cells stably expressing retroviral vectors were grown according to standard procedures and plated on 12-mm Transwell permeable supports (500,000 cells/well) for interleukin (IL)-8 secretion and ER Ca2+ store expansion studies. At this density, cultures become confluent, achieve air-liquid interface, and polarize within 24 h. For the retroviral expressing cell lines, cultures were maintained in antibiotic-selecting media (Geneticin® G418; Invitrogen).
Stable Expression of Control, XBP-1s, and Dominant Negative XBP-1 (DN-XBP-1) Vectors
50 μg of the control, pQCXIN retroviral vector (Clontech), or the pQCXIN retroviral vector containing XBP-1s (gi: 18148381) or a previously described DN-XBP-1 (23) (a gift from Dr. Laurie Glimcher, Harvard University) were packaged into retroviruses as previously described (24). 16HBE14o- cells were infected with retroviruses in the presence of Polybrene and positive cells were selected with neomycin G418 (Invitrogen).
Supernatant from Mucopurulent Material from CF Airways
Mucopurulent material was harvested from the lumens of excised human CF lungs infected with P. aeruginosa at the North Carolina Cystic Fibrosis Center as previously described (1, 2). SMM from several patients was pooled to assure homogeneous stimulus throughout the experiments. 30 μl of SMM or PBS (a control for SMM) were applied to the mucosal surface of the wild type and each stably expressing 16HBE14o- cell line to induce ER Ca2+ store expansion and an inflammatory response.
IL-8 Secretion
Serosal media were collected following exposure of polarized 16HBE14o- cultures to either mucosal PBS or SMM or mucosal exposure to PBS or 5 μm bradykinin (BK). IL-8 was measured by ELISA (R&D Systems) in duplicate as previously reported (2).
XBP-1 mRNA Splicing
16HBE14o- cells were exposed to mucosal PBS or SMM for 6 and 24 h and washed with PBS before using cells for RNA isolation. The assessment of XBP-1 mRNA splicing was performed as previously described (2). Total RNA was isolated using RNeasy (Qiagen kit) and 200 ng of RNA was reverse transcribed using SuperScript II RNase H+ (Invitrogen) using 250 ng of random primers. PCR primers (forward: GGA GTT AGG ACA GCG CTT GG; reverse: GGA AGG GCA TTT GAA GAA CA) were designed to amplify a 270-base pair (bp) region of XBP-1 (NM_005080) and PCR products were column purified using a QIAquick PCR Purification Kit (Qiagen) and digested with PstI. PstI-digested products were run in a 2% agarose gel, transferred, and cross-linked to a membrane and probed (Megaprime DNA labeling kit from Amersham Biosciences with the 270-bp PCR product from PBS-treated samples). Data were quantified with a Storm phosphorimaging system (GE Healthcare).
Luciferase Assay
16HBE14o- cells stably expressing XBP-1s, DN-XBP-1, or control vector were transfected with a previously described reporter plasmid containing a luciferase reporter UPR element (UPRE-luciferase; a gift from Dr. Laurie Glimcher, Harvard University) (23) and CMV-LacZ using FuGENE HD Transfection Reagent (Roche) 2 h after plating in 96-well plates. Cells were challenged with vehicle or 5 μg/ml tunicamycin (a pharmacological ER stress inducer) at 48 h and luciferase activity was assayed 24 h later.
Assessment of ER Ca2+ Store Expansion in 16HBE14o- Cultures
Morphological—ER density, studied by confocal immunofluorescence analyses of calreticulin expression in 4% paraformaldehyde-fixed polarized 16HBE14o- cultures and quantification of the calreticulin fluorescence intensity were performed as previously reported (1). To visualize the nuclei, cultures were also stained with 4′,6-diamidino-2-phenylindole.
Functional—Polarized 16HBE14o- cultures were luminally exposed for 48 h to 30 μl of mucosal PBS or SMM. Cultures were subsequently loaded with fura-2/AM and 100 μm mucosal UTP-dependent ER-derived Ca2+i mobilization was measured by microfluorimetry as we have previously described (1, 2). ER-derived Ca2+i signals were quantified by subtracting the peak value from the UTP-dependent Ca2+i response (in the presence of nominally Ca2+ free conditions) from the baseline Ca2+i value prior to UTP. The resulting value represents an index of agonist-dependent ER Ca2+ release as previously reported (1). Data are expressed as Δ340/380 (peak-baseline values) fura-2 fluorescence.
Protein-disulfide Isomerase (PDI) Western Blotting—Polarized 16HBE14o- cultures expressing control, XBP-1s, or DN-XBP-1 vectors were luminally exposed for 48 h to 30 μl of mucosal PBS or SMM. Cultures were lysed, subjected to SDS-gel electrophoresis on 10% polyacrylamide gels, and proteins were blotted to nitrocellulose membranes as previously reported (1). PDI was probed with a mouse anti-PDI monoclonal antibody (1:1,000 dilution; Stressgen) and visualized by enhanced chemiluminescence detection. The PDI signal was quantified with the Metamorph software.
Statistics
Southern blots are representative of 3 individual normal and 3 CF human donors (for native HBE studies) or 3 experiments with 16HBE14o- cultures. Morphological studies with mouse airways exposed to PBS or P. aeruginosa are representative of several sections from two experiments. Data from bar graphs represent the mean ± S.E. from at least three to four experiments and were analyzed by unpaired t test or two-way analysis of variance. Statistical significance was defined with p < 0.05.
RESULTS
Airway Inflammation Activates the UPR in Vivo—We have reported that ER Ca2+ stores are expanded in native HBE harvested directly from infected/inflamed CF airways as compared with HBE harvested from uninfected/non-inflamed donors (1). To address whether the up-regulation of ER Ca2+ stores in CF airways was associated with an increased XBP-1 mRNA splicing, we measured the levels of XBP-1s in freshly isolated normal and infected/inflamed CF HBE. Fig. 1A depicts a representative Southern blot illustrating that XBP-1s is increased in CF as compared with normal airway epithelia. The data compiled from XBP-1 mRNA splicing from normal and CF epithelia are shown in Fig. 1B. These findings suggest that the IRE1-dependent XBP-1 UPR pathway is activated in native infected/inflamed CF airway epithelia.
FIGURE 1.
Native CF HBE exhibit increased XBP-1 mRNA splicing. A, Southern blot illustrating that XBP-1 mRNA splicing is increased in native infected/inflamed CF HBE as compared with non-infected/inflamed normal native HBE. Data are representative of three tissue codes from normal and CF HBE. B, compilation of the XBP-1 mRNA splicing data expressed as a percentage of XBP-1 mRNA splicing from normal epithelia.
Stronger data linking inflammation with airway epithelial ER expansion in vivo were provided by findings from wild type mouse airways exposed to 40 μl of PBS or PBS containing 1 × 106 colony forming units of P. aeruginosa strain PAK (21). Although this is a model of acute pneumonia (21), it also induces airway inflammation 24 h after infection, regardless of whether the bacteria have been cleared from the airways. Histological analyses of P. aeruginosa-challenged lungs at 24 h revealed areas that were clearly inflamed (Fig. 2B) and areas that showed no evidence of inflammation (Fig. 2C). Inflamed areas were characterized by an increased thickness of the airway wall and an increased number of nuclei (Fig. 2B) corresponding to infiltration of inflammatory cells seen with hematoxylin and eosin staining (Fig. 2E). Illustrative hematoxylin and eosin staining from non-inflamed airways challenged with PBS or P. aeruginosa is shown in Fig. 2, D and F, respectively. As visualized by calreticulin staining, ER expansion was only observed in epithelia from airways that were inflamed 24 h post-inoculation with P. aeruginosa (Fig. 2B). ER expansion was not observed in airway sites that were not inflamed post-inoculation with P. aeruginosa (Fig. 2C). The epithelial calreticulin staining in these airways was similar to that of PBS-exposed airways (Fig. 2A). Together with the findings from Fig. 1, these data suggest that epithelial ER expansion and UPR activation are a feature of inflamed airways. These findings are particularly significant to CF airways disease, because P. aeruginosa is the dominant pathogen in CF.
FIGURE 2.
Airway inflammation induced by P. aeruginosa infection promotes ER Ca2+ store expansion in murine airway epithelia in vivo. A and D, epithelia without airway inflammation from PBS-exposed wild type mice; B and E, epithelia from P. aeruginosa-infected mice in the presence of airway inflammation. C and F, epithelia from P. aeruginosa-infected mice in the absence of airway inflammation. A–C, green stain, calreticulin (ER Ca2+ store marker); blue stain,4′,6-diamidino-2-phenylindole-labeled nuclei. D–F, hematoxylin and eosin stain. Bar, 10 μm. P.a., P. aeruginosa.
To test this association further, studies were conducted with ERAI mice, which exhibit ER stress-dependent XBP-1 mRNA splicing coupled to expression of the fluorescent protein Venus, a variant of green fluorescent protein (25). The same protocol used for the experiments illustrated in Fig. 2 was utilized in these studies. XBP-1 splicing, as reflected by increased Venus fluorescence, was increased 24 h post P. aeruginosa infection in airway epithelia from inflamed airways that showed increased thickness of the airway wall, as evidenced by an increased number of cells (Fig. 3, C and D) and parenchyma destruction (data not shown). In contrast, XBP-1 splicing/Venus expression was low in non-inflamed airway sites exhibiting thin airway walls and a healthy parenchyma in both PBS- and P. aeruginosa-challenged airways (Fig. 3, A, B, E, and F). Quantification of Venus fluorescence intensity, expressed per surface airway epithelial area, strengthened the notion that XBP-1 splicing was increased in airway epithelia from inflamed airways (Fig. 3G). Together with the findings from Fig. 2, these data suggest that airway inflammatory responses up-regulate the levels of XBP-1s, which are associated with ER expansion in airway epithelia.
FIGURE 3.
Airway inflammation induced by P. aeruginosa infection promotes XBP-1 mRNA splicing in murine airway epithelia in vivo. Differential interference contrast (A, C, and E) and Venus expression (B, D, and F) in airway epithelia from non-inflamed, PBS-challenged (A and B), inflamed, P. aeruginosa (P.a.)-challenged (C and D) and non-inflamed, P.a.-challenged (E and F) ERAI mice airways. Arrows point to airway epithelia. Bar, 20 μm. G, compiled data for Venus fluorescence intensity, expressed per surface epithelial area, from all groups. *, p < 0.05, P.a.-challenged and inflamed versus PBS-challenged, non-inflamed.
Inflammation of Polarized 16HBE14o- Cultures Triggers a UPR Coupled to XBP-1 mRNA Splicing—We have previously shown that XBP-1s induced by luminal exposure of primary cultures of HBE to an inflammatory stimulus (SMM) is associated with a hyperinflammatory phenotype dependent on ER Ca2+ store expansion (2), a notion further reinforced by the data presented in Figs. 1, 2, 3. However, the UPR acts through a variety of mechanisms and the functional link between XBP-1s and ER Ca2+ store expansion has not been established. Hence, the present study was designed to address the hypothesis that XBP-1s induced by airway inflammation is the mechanism for the hyperinflammatory airway epithelial phenotype coupled to ER Ca2+ store expansion (2). To test this hypothesis, we utilized 16HBE14o- epithelia, a cell line immortalized from human bronchial epithelium (26), which, unlike primary cultures of well differentiated HBE, can be easily used for manipulations of XBP-1 levels. We first addressed whether mucosal SMM exposure induced inflammation, as indexed by an increased IL-8 secretion, in polarized 16HBE14o- cultures grown to confluence under the air-liquid interface conditions. SMM triggered a time-dependent increase in the IL-8 secretory response as compared with PBS-treated 16HBE14o- cultures (Fig. 4A), mimicking its effect on IL-8 secretion as we have reported for primary cultures of HBE (2). The cumulative IL-8 secretion from PBS-treated 16HBE14o- cultures has been previously reported in primary cultures of HBE (2). It likely reflects a combination of the epithelial response to agonists present in the culturing media and the volume of PBS applied to the mucosal surface, which constitutes a volume control for the SMM exposure.
FIGURE 4.
SMM exposure induces IL-8 secretion coupled to XBP-1 mRNA splicing in polarized 16HBE14o- cultures. A, time course for mucosal PBS or SMM on serosal IL-8 secretion; *, p < 0.05, SMM versus PBS-treated cultures. B, Southern blot illustrating the time course for mucosal PBS or SMM on XBP-1 mRNA splicing.
The increased SMM-dependent IL-8 secretion was associated with increases in XBP-1 mRNA splicing in 16HBE14o- cultures (Fig. 4B). Mucosal SMM exposure increased XBP-1 mRNA splicing at 6 and 24 h as compared with PBS-exposed cultures. Thus, this is a valid model for further studies.
16HBE14o- Cells Expressing Empty, XBP-1s, and DN-XBP-1 Vectors—We used the retroviral vectors pQCXIN (control) or pQCXIN vectors expressing XBP-1s or a DN-XBP-1 shown to completely abolish transactivation of the UPR responsive element by XBP-1s and to inhibit the induction of XBP-1 target genes (23). Stable overexpressing cell lines were generated to directly test the role of the XBP-1 during airway epithelial inflammation coupled with ER Ca2+ store expansion. To confirm the presence of constitutive activation and inhibition of the unfolded protein response element (UPRE) in our stable 16HBE14o- cell lines expressing XBP-1s or DN-XBP-1, we used a UPRE luciferase reporter construct. The UPRE reporter construct and a CMV-LacZ plasmid (to normalize for transfection efficiency) were transfected into the cells and baseline and tunicamycin-stimulated luciferase activity was assessed 72 h later. Because tunicamycin inhibits N-glycosylation, which leads to accumulation of unfolded proteins in the ER compartment, it activates the UPR. Fig. 5 illustrates that in cells expressing the empty vector, UPRE activity was low and tunicamycin-induced UPR activation promoted a 7.5-fold increase in UPRE activity. Expression of XBP-1s induced constitutive activation of the UPRE, and tunicamycin had only a minimal additional effect on the UPRE activity induced by XBP-1s expression alone. In contrast, expression of DN-XBP-1 completely inhibited tunicamycin-induced UPRE activity. Although previous studies with mouse embryonic fibroblasts have shown that both the XBP-1 and the activating transcription factor 6 UPR pathways can couple to UPRE activation (23), our findings suggest that, in 16HBE14o- cultures, UPR target genes can be significantly up-regulated or inhibited by overexpression of XBP-1s or DN-XBP-1, respectively. These data confirmed that these stably expressing cell lines could be used to address the functional role of XBP-1s on airway inflammatory responses dependent on ER Ca2+ store expansion.
FIGURE 5.
Constitutive activation or inhibition of the UPRE in airway epithelia expressing XBP-1s or DN-XBP-1. A UPRE luciferase reporter was transfected into stable 16HBE14o- cell lines expressing control vector, XBP-1s, or DN-XBP-1. Cultures were exposed 48 h later to vehicle or 5 μg/ml tunicamycin for an additional 24 h. Luciferase activity, assessed by conventional methods, was normalized to CMV-LacZ. Data are expressed as fold-change of relative luciferase activity versus untreated empty vector-expressing cultures.
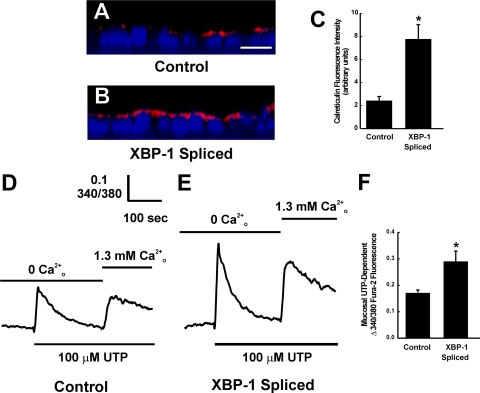
XBP-1s Induces Morphological and Functional ER Ca2+ Store Expansion in Polarized 16HBE14o- Cultures—Studies were then conducted with polarized 16HBE14o- cultures stably expressing the same empty and XBP-1s vectors utilized in the UPRE luciferase studies described in Fig. 5. Cells were plated onto membrane-permeable supports and ER Ca2+ store expression was assessed 48 h later by fluorescence intensity of calreticulin (a marker of ER Ca2+ stores) as we have previously reported (1). In comparison with control cultures expressing empty vector, cultures expressing XBP-1s exhibited an increased fluorescence intensity of calreticulin (Fig. 6, A and B). The compiled data from calreticulin fluorescence intensity is shown in Fig. 6C. These data suggested that expression of XBP-1s alone was sufficient to increase the density of ER Ca2+ stores in 16HBE14o- cells.
FIGURE 6.
Expression of XBP-1s promotes morphological and functional expansion of ER Ca2+ stores in airway epithelia. A and B, XZ confocal images illustrating the immunofluorescent stain of calreticulin in polarized 16HBE14o- cultures expressing control vector or XBP-1s vector, respectively. Calreticulin is stained in red. Nuclei are stained in blue with 4′,6-diamidino-2-phenylindole. Bar, 10 μm. C, compiled data for calreticulin fluorescence from the two groups. D and E, representative tracings from 100 μm mucosal UTP-induced intracellular Ca2+ mobilization in polarized 16HBE14o- cultures loaded with fura-2 and expressing control vector or XBP-1s vector, respectively. First and second phases of Ca2+ mobilization in the absence or presence of extracellular Ca2+ illustrate ER Ca2+ release and capacitative calcium entry, respectively. F, compiled data for mucosal UTP-dependent ER Ca2+ release from the two groups.
We next addressed whether XBP-1s-increased ER Ca2+ store density was linked to a greater functional ER Ca2+ storage by investigating mucosal UTP-dependent ER Ca2+ mobilization as we have previously reported (1). UTP-induced ER Ca2+ release, as indexed by the larger Δ340/380 fura-2 signal in the absence of extracellular Ca2+, was increased in XBP-1s expressing cultures as compared with control cultures (Fig. 6, D and E). The compiled data from these measurements are depicted in Fig. 6F. Although the present studies focused on ER Ca2+ release, mucosal UTP-activated capacitative calcium entry (illustrated as the second phase of Ca2+ mobilization upon addition of 1.3 mm extracellular Ca2+), also appeared larger in XBP-1s expressing cells (Fig. 6E). These findings suggest that expression of XBP-1s promotes morphological and functional expansion of the ER Ca2+ stores in polarized cultures of 16HBE14o-.
Additional morphological and functional evidence for the role of XBP-1s in ER Ca2+ store expansion was obtained by studies with the control and DN-XBP-1 vectors. Polarized 16HBE14o- cultures expressing the empty vector or the DN-XBP-1 vector were plated on permeable support and exposed for 48 h to mucosal PBS or SMM (to induce ER/Ca2+ store expansion). In control cultures expressing empty vector, calreticulin fluorescence intensity was up-regulated by SMM exposure in comparison with PBS exposure (Fig. 7A, top panels). In contrast, SMM-increased calreticulin fluorescence was blunted in cultures expressing the DN-XBP-1 (Fig. 7A, bottom panels). The compiled data from calreticulin fluorescence intensity from these studies are shown in Fig. 7B. These data suggested that expression of DN-XBP-1 inhibits the ER expansion induced by SMM in 16HBE14o- cultures.
FIGURE 7.
Expression of DN-XBP-1 inhibits SMM-induced morphological and functional expansion of ER Ca2+ stores in airway epithelia. A, XZ confocal images illustrating the immunofluorescent stain of calreticulin in polarized 16HBE14o- cultures expressing control vector or DN-XBP-1 vector and exposed for 48 h to mucosal PBS or SMM. Calreticulin is stained in red. Nuclei are stained in blue with 4′,6-diamidino-2-phenylindole. Bar, 10 μm. B, compiled data for calreticulin fluorescence from the four groups. C–F, representative tracings from 100 μm mucosal UTP-induced intracellular Ca2+ mobilization in polarized 16HBE14o- cultures loaded with fura-2 and expressing control vector (C and D) or DN-XBP-1 vector (E and F). Cultures were exposed for 48 h to mucosal PBS (C and E) or SMM (D and F). First and second phases of Ca2+ mobilization in the absence or presence of extracellular Ca2+ illustrate ER Ca2+ release and capacitative calcium entry, respectively. G, compiled data for mucosal UTP-dependent ER Ca2+ release from the four groups.
We next evaluated mucosal UTP-induced ER Ca2+ mobilization in cultures expressing control vector or DN-XBP-1 vector utilizing the same experimental protocol used for the studies depicted in Fig. 6, D and E. In cultures expressing the empty vector, SMM exposure resulted in a larger mucosal UTP-triggered ER Ca2+ release (Fig. 7D) as compared with PBS-exposed cultures (Fig. 7C), reproducing our previous observations in primary cultures of HBE exposed to SMM (1). We next addressed the effect of DN-XBP-1 expression in this response. SMM-dependent up-regulation of UTP-sensitive ER Ca2+ stores was inhibited in DN-XBP-1-expressing cultures as compared with cultures expressing the control vector (Fig. 7, E and F). The compiled data for mucosal UTP-mobilized ER Ca2+ are illustrated in Fig. 7G. Together with the data from Fig. 6, these results indicate that XBP-1s induced by airway inflammation is functionally necessary for the up-regulation of ER Ca2+ stores in airway epithelia.
To further investigate the role of XBP-1s in SMM-triggered ER expansion, Western blot analyses of PDI (an additional ER marker) were conducted in 16HBE14o- cultures expressing control, XBP-1s, or DN-XBP-1 vectors. We have elected to use PDI as an additional ER marker because we have previously shown that native chronically infected/inflamed CF human airway epithelia exhibit an up-regulation of PDI in addition to up-regulation of calreticulin (2). Fig. 8A illustrates that, in control cultures, mucosal SMM exposure increased the expression of PDI as compared with PBS exposure. In addition, cultures expressing XBP-1s exhibited up-regulation of PDI in the absence of SMM exposure and mimicked the stimulatory effect of SMM on PDI levels (Fig. 8A, compare control versus XBP-1s expressing cultures exposed to PBS or SMM). Finally, expression of DN-XBP-1 blocked the PDI up-regulation induced by SMM (Fig. 8A). Compilation of the PDI signals from the six experimental groups is shown in Fig. 8B. The evaluation of PDI expression provides additional evidence, independently of calreticulin, that SMM induces ER expansion and that this effect is mediated by XBP-1s in cultures of 16HBE14o-.
FIGURE 8.
Additional evidence that SMM-induced airway epithelial ER expansion is mediated by XBP-1s. A, representative Western blot of the ER marker PDI from polarized 16HBE14o- cultures expressing control, XBP-1s, or DN-XBP-1 vectors and exposed for 48 h to mucosal PBS or SMM. B, compiled data from the Western blot PDI signals from all groups.
Inhibition of XBP-1 Signaling in Polarized 16HBE14o- Cultures Blunts Inflammatory Responses—We next investigated whether expression of DN-XBP-1 blunted SMM-triggered IL-8 secretion in polarized cultures of 16HBE14o-. As predicted, in cultures expressing empty vector, SMM induced a greater IL-8 secretory response in comparison with PBS (Fig. 9A). In contrast, SMM-promoted IL-8 secretion was inhibited in cultures expressing the DN-XBP-1 (Fig. 9A).
FIGURE 9.
XBP-1s constitutively promotes IL-8 secretion and plays a functional role in SMM- and BK-stimulated IL-8 secretion in airway epithelia. A, serosal IL-8 secretion from polarized 16HBE14o- cultures expressing control vector or DN-XBP-1 vector exposed for 24 h to mucosal PBS or SMM. *, p < 0.05 control vector + SMM versus control vector + PBS. B, serosal IL-8 levels from polarized 16HBE14o- cultures expressing control, XBP-1s, or DN-XBP-1 vectors resulting from 8 h exposure to vehicle or 5 μm mucosal BK. *, p < 0.05 XBP-1s versus control vector.
The data from Figs. 6, 7, 8, 9 strongly suggest that XBP-1s may mediate a hyperinflammatory phenotype resultant from ER Ca2+ store expansion induced by SMM in airway epithelia. Because we have previously shown that a hyperinflammatory response was, in part, typified by an increased mucosal BK-dependent IL-8 secretion in HBE (2), we investigated IL-8 secretory responses induced by mucosal BK in cultures of 16HBE14o- expressing control, XBP-1s, or DN-XBP-1 vectors. In control vector-expressing cultures, mucosal BK triggered a minor increase in IL-8 secretion in comparison with vehicle-treated cultures (Fig. 9B). On the other hand, XBP-1s expressing cultures exhibited a higher IL-8 secretory response as compared with control vector expressing cultures in the absence of BK, and BK-induced IL-8 secretion was potentiated by expression of XBP-1s (Fig. 9B). Finally, expression of DN-XBP-1 inhibited mucosal BK-induced IL-8 secretion (Fig. 9B). These data suggest that constitutive activation of XBP-1s induces an increased IL-8 secretory response. Moreover, these findings are in agreement with our previous observations (2) that, whereas mucosal BK has a minor effect on IL-8 secretion in non-inflamed HBE (i.e. PBS-exposed HBE exhibiting normal levels of XBP-1s and ER Ca2+ stores), its effect on IL-8 secretion is potentiated by inflammation (i.e. SMM-exposed HBE exhibiting increased levels of XBP-1s and expanded ER Ca2+ stores).
DISCUSSION
Our previous studies have shown that inflammation of human airway epithelia, a condition associated with an increase in total protein synthesis (2), expands the ER compartment and its Ca2+ stores (1, 2). Based on the role of XBP-1s in coordinating structural and functional features characteristic of secretory cells, we hypothesized that XBP-1 function would be likely relevant for inflammatory responses of airway epithelia. The data presented here suggest that increases in XBP-1s are, in fact, relevant for airway disease characterized by infection and inflammation, because freshly isolated human bronchial airway epithelia from chronically infected and inflamed CF lungs exhibited higher levels of XBP-1s (Fig. 1). This notion was further strengthened by the demonstration that P. aeruginosa-induced airway inflammation triggered ER Ca2+ store expansion (Fig. 2) and XBP-1 mRNA splicing in vivo, based on the increased Venus fluorescence of ERAI mice (Fig. 3).
Additional evidence for a functional role of XBP-1s in airway inflammatory responses was provided by the following findings. First, airway epithelial expression of XBP-1s constitutively activated UPRE and potentiated UPRE activity pharmacologically induced by tunicamycin (Fig. 5) and promoted morphological and functional ER Ca2+ store expansion (Figs. 6 and 8). Second, airway epithelial expression of DN-XBP-1 completely inhibited the UPRE activity induced by tunicamycin (Fig. 5) and inhibited SMM-induced morphological and functional ER Ca2+ store expansion (Figs. 7 and 8). Third, SMM- and BK-induced IL-8 secretion was blunted in airway epithelial cultures expressing DN-XBP-1 (Fig. 9, A and B). Finally, airway epithelial cultures expressing XBP-1s exhibited increased basal and BK-induced IL-8 secretory responses (Fig. 9B).
Although we found that freshly isolated CF airway epithelia exhibited increased levels of XBP-1s (Fig. 1), previous work from our laboratory and other investigators suggests that mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) are not responsible for the higher levels of XBP-1s found in infected/inflamed CF epithelia in the present study. For instance, whereas CF human bronchial epithelia homozygous for the most common CFTR mutation (ΔF508 CFTR) had an increased IL-8 secretion in short-term primary cultures, their higher IL-8 secretion was lost in long-term cultures (2). Moreover, exposure of long-term CF cultures to SMM reproduced the increased IL-8 secretion found in short-term CF cultures, and this response was associated with the ability of SMM to induce XBP-1s (2). Dissociation of CFTR mutations from XBP-1 mRNA splicing has been suggested by follow-up studies. For example, no differences in XBP-1 mRNA splicing were found in passage 1 normal as compared with CF airway epithelial cultures (27). In addition, dissociation of the ΔF508 CFTR mutation from IRE1/XBP-1 signaling and airway epithelial inflammatory responses has been further illustrated in a recent study showing no significant differences in basal and P. aeruginosa (or flagellin)-induced IL-8 secretion, intracellular Ca2+ mobilization, and IRE1 activity in CF15 cells overexpressing wild type or ΔF508 CFTR (28). In contrast, an exaggerated expression of recombinant ΔF508 CFTR in Calu-3 cells has been shown to induce increased levels of XBP-1s (29). The lack or presence of activation of the IRE1/XBP-1 pathway in the last two studies may result from cell-specific and/or differences in ΔF508 CFTR expression levels induced in cell lines. The relevance of in vitro-promoted exaggerated expression of ΔF508 CFTR-triggered XBP-1s in airway epithelial inflammatory responses remains to be established. Nevertheless, the findings from primary cultures of normal and CF epithelia expressing endogenous CFTR mutations suggest that airway epithelial inflammation, rather than mutated CFTR, is responsible for induction of XBP-1s.
Our findings are the first demonstration that XBP-1s plays a functional role in airway epithelial inflammatory responses involving up-regulation of ER Ca2+ stores. In addition, our observations are in agreement with recent data implicating the XBP-1-mediated UPR pathway in innate immunity. For example, recent findings have linked ER stress responses mediated by XBP-1 to intestinal inflammation, suggesting this pathway is also relevant for human inflammatory bowel disease (30). These studies were based on a XBP-1 conditional knock-out mouse strain, which was mated to Villin-Cre transgenic mice. The intestinal epithelia of XBP-1-/- mice exhibited loss of paneth cells, and as a consequence, a decrease in defensive secretory proteins, but also an ∼25% decrease in AB-PAS staining of intestinal epithelial mucous substances (30). These and our present observations demonstrate the functional importance of XBP-1 in secretory responses activated during epithelial inflammation.
The finding that XBP-1s is functionally linked to ER Ca2+ store expansion in inflamed airway epithelia is in agreement with earlier data suggesting a role for the UPR in the coordination of the synthesis of phospholipids and new membranes and the up-regulation of a wide spectrum of genes of the secretory pathway (31, 32). Further demonstration of a UPR-dependent pathway responsible for ER proliferation was obtained by studies indicating a direct link between XBP-1s, phospholipid biosynthesis, and ER expansion (17–19). Following UPR activation, new membranes are produced and the resulting increased ER volume serves to dilute unfolded proteins and prepare the compartment to receive newly synthesized folding components, thereby restoring ER homeostasis (32). In addition to ER expansion, XBP-1s has been shown to increase protein synthesis, ribosomal number, lysosomal content, Golgi compartment, mitochondrial mass and function, and cell size (17). Additional evidence for the importance of XBP-1 in secretory processes has been provided by deletion of XBP-1 in mice, which has revealed the functional role of this transcription factor in secretory organs (20).
We speculate that the resulting XBP-1s-induced ER/Ca2+ store expansion can influence the adaptive response of airway epithelia during infection/inflammation in different ways. From a host defense mechanism, XBP-1-dependent ER Ca2+ store expansion can provide a beneficial protective function for infected/inflamed airways due to an up-regulated Ca2+-mediated mucociliary clearance. Previous studies suggested that the Ca2+i-activated apical Cl- conductance in human airway epithelia is not saturated at normal Ca2+i levels (33). In fact, a positive correlation between apical purinoceptor activation-dependent Ca2+i signals and apical Cl- conductance was found in both normal and CF human airway epithelia, e.g. the larger the apical Ca2+i signals, the larger the Cl- secretory response (33). Such a response may be particularly beneficial to CF patients, who depend on Ca2+-activated Cl- channels to compensate for the lack of CFTR-dependent Cl- secretion (34). However, an extra consequence of XBP-1-induced ER/Ca2+ store expansion is the amplification of airway inflammation, which may beneficially or adversely affect the homeostasis of normal and obstructed airways. For example, although exposure of airways to infectious/inflammatory stimuli can trigger Ca2+-dependent hyperinflammation and increasingly recruit inflammatory cells, this response should contribute to clear the airway of infection in airways with a normal mucociliary clearance. In contrast, such an amplified inflammatory response may be unfavorable for CF or COPD patients suffering from airway obstruction. In this scenario, the chronic but ineffective XBP-1-dependent ER Ca2+ store expansion-mediated hyperinflammatory response of CF and COPD airways may be harmful for patients.
Recent structural studies with the core region of the ER luminal domain of IRE1 suggest that dimerization of this domain, resulting from IRE1 activation during ER stress, creates a groove reminiscent of the peptide binding domains of histocompatibility complexes (35). Designing IRE1 modulators may be a therapeutic strategy for regulating XBP-1s-dependent signaling and may lead to therapies aimed at controlling the excessive inflammation of infected/inflamed airways via targeted reduction of Ca2+-mediated airway inflammation. Future studies with mouse models exhibiting specific deletion of XBP-1 in airway epithelia will address the role of this UPR pathway in airway inflammation in vivo, and whether it is beneficial or adverse for normal and obstructed airways.
In summary, our findings suggest a model whereby airway epithelial infection/inflammation triggers a UPR due to ER stress resulting from an increased demand for newly synthesized, unfolded inflammatory mediators and epithelial repair proteins. This UPR is characterized, in part, by IRE1-mediated XBP-1 mRNA splicing, which is directly responsible for ER Ca2+ store expansion and up-regulation of the protein secretory pathway. Collectively, these alterations provide a mechanism for the ER-derived Ca2+-mediated hyperinflammation we have previously reported in infected/inflamed CF and SMM-exposed human airway epithelia (2).
Acknowledgments
We thank Dr. Laurie Glimcher (Harvard University) for providing the UPRE-luciferase construct and the DN-XBP-1 plasmid; Dr. Masayuki Miura (University of Tokyo, Japan) and the RIKEN Institute (Japan) for providing the ERAI mice; Dr. Scott Randell (University of North Carolina CF Center) for providing normal and CF freshly isolated airway epithelia; Lisa Jones (University of North Carolina CF Center) for molecular biology assistance; Jason Tatreau (University of North Carolina CF Center) for assistance toward the UPRE-luciferase studies; Wendy Salmon (University of North Carolina Michael Hooker Microscopy Core Facility) for assistance with Venus fluorescence studies; Kimberly Burns, Tracey Eldred, and Donald Joyner (University of North Carolina CF Center) for histological sections of mouse airways; Lisa Brown for editorial assistance; and the University of North Carolina Immunotechnology Core Facility for determination of IL-8.
This work was supported by Cystic Fibrosis Foundation Grant RIBEIR07G0 (to C. M. P. R.).
Footnotes
The abbreviations used are: CF, cystic fibrosis; BIP/GRP78, immunoglobulin-binding protein; BK, bradykinin; Ca2+i, intracellular Ca2+; CFTR, cystic fibrosis transmembrane conductance regulator; COPD, chronic obstructive pulmonary disease; DN-XBP-1, dominant negative XBP-1; ER, endoplasmic reticulum; ERAI, ER stress-activated indicator; HBE, human bronchial epithelia; IL-8, interleukin-8; IRE1, inositol requiring enzyme 1; PDI, protein-disulfide isomerase; SMM, supernatant from mucopurulent material from CF airways; UPR, unfolded protein response; UPRE, unfolded protein response element; XBP-1, X-box binding protein-1; XBP-1s, spliced XBP-1; PBS, phosphate-buffered saline; CMV, cytomegalovirus.
References
- 1.Ribeiro, C. M. P., Paradiso, A. M., Carew, M. A., Shears, S. B., and Boucher, R. C. (2005) J. Biol. Chem. 280 10202-10209 [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro, C. M. P., Paradiso, A. M., Schwab, U., Perez-Vilar, J., Jones, L., O'Neal, W., and Boucher, R. C. (2005) J. Biol. Chem. 280 17798-17806 [DOI] [PubMed] [Google Scholar]
- 3.Kaufman, R. J. (1999) Genes Dev. 13 1211-1233 [DOI] [PubMed] [Google Scholar]
- 4.Mori, K. (2000) Cell 101 451-454 [DOI] [PubMed] [Google Scholar]
- 5.Patil, C., and Walter, P. (2001) Curr. Opin. Cell Biol. 13 349-355 [DOI] [PubMed] [Google Scholar]
- 6.Lee, A. S. (2001) Trends Biochem. Sci. 26 504-510 [DOI] [PubMed] [Google Scholar]
- 7.Kaufman, R. J., Scheuner, D., Schroeder, M., Shen, X., Lee, K., Liu, C. Y., and Arnold, S. M. (2002) Nat. Rev. Mol. Cell. Biol. 3 411-421 [DOI] [PubMed] [Google Scholar]
- 8.Rutkowski, D. T., and Kaufman, R. J. (2004) Trends Cell Biol. 14 20-28 [DOI] [PubMed] [Google Scholar]
- 9.Zhang, K., and Kaufman, R. J. (2004) J. Biol. Chem. 279 25935-25938 [DOI] [PubMed] [Google Scholar]
- 10.Ron, D., and Walter, P. (2007) Nat. Rev. Mol. Cell. Biol. 8 519-529 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida, H., Matsui, T., Yamamoto, A., Okada, T., and Mori, K. (2001) Cell 107 881-891 [DOI] [PubMed] [Google Scholar]
- 12.Calfon, M., Zeng, H., Urano, F., Till, J. H., Hubbard, S. R., Harding, H. P., Clark, S. G., and Ron, D. (2002) Nature 415 92-96 [DOI] [PubMed] [Google Scholar]
- 13.Urano, F., Bertolotti, A., and Ron, D. (2000) J. Cell Sci. 113 3697-3702 [DOI] [PubMed] [Google Scholar]
- 14.Wiest, D. L., Burkhardt, J. K., Hester, S., Hortsch, M., Meyer, D. I., and Argon, Y. (1990) J. Cell Biol. 110 1501-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reimold, A. M., Iwakoshi, N. N., Manis, J., Vallabhajosyula, P., Szomolanyi-Tsuda, E., Gravallese, E. M., Friend, D., Grusby, M. J., Alt, F., and Glimcher, L. H. (2001) Nature 412 300-307 [DOI] [PubMed] [Google Scholar]
- 16.Iwakoshi, N. N., Lee, A. H., Vallabhajosyula, P., Otipoby, K. L., Rajewsky, K., and Glimcher, L. H. (2003) Nat. Immunol. 4 321-329 [DOI] [PubMed] [Google Scholar]
- 17.Shaffer, A. L., Shapiro-Shelef, M., Iwakoshi, N. N., Lee, A. H., Qian, S. B., Zhao, H., Yu, X., Yang, L., Tan, B. K., Rosenwald, A., Hurt, E. M., Petroulakis, E., Sonenberg, N., Yewdell, J. W., Calame, K., Glimcher, L. H., and Staudt, L. M. (2004) Immunity 21 81-93 [DOI] [PubMed] [Google Scholar]
- 18.Sriburi, R., Jackowski, S., Mori, K., and Brewer, J. W. (2004) J. Cell Biol. 167 35-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ron, D., and Hampton, R. Y. (2004) J. Cell Biol. 167 23-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, A. H., Chu, G. C., Iwakoshi, N. N., and Glimcher, L. H. (2005) EMBO J. 24 4368-4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, R. S., Wolfgang, M. C., and Lory, S. (2004) Infect. Immun. 72 1677-1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mall, M., Grubb, B. R., Harkema, J. R., O'Neal, W. K., and Boucher, R. C. (2004) Nat. Med. 10 487-493 [DOI] [PubMed] [Google Scholar]
- 23.Lee, A. H., Iwakoshi, N. N., and Glimcher, L. H. (2003) Mol. Cell. Biol. 23 7448-7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, L. G., Mewshaw, J. P., Ni, H., Friedmann, T., Boucher, R. C., and Olsen, J. C. (1998) J. Virol. 72 8861-8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwawaki, T., Akai, R., Kohno, K., and Miura, M. (2004) Nat. Med. 10 98-102 [DOI] [PubMed] [Google Scholar]
- 26.Cozens, A. L., Yezzi, M. J., Kunzelmann, K., Ohrui, T., Chin, L., Eng, K., Finkbeiner, W. E., Widdicombe, J. H., and Gruenert, D. C. (1994) Am. J. Respir. Cell Mol. Biol. 10 38-47 [DOI] [PubMed] [Google Scholar]
- 27.Nanua, S., Sajjan, U., Keshavjee, S., and Hershenson, M. B. (2006) Biochem. Biophys. Res. Commun. 343 135-143 [DOI] [PubMed] [Google Scholar]
- 28.Hybiske, K., Fu, Z., Schwarzer, C., Tseng, J., Do, J., Huang, N., and Machen, T. E. (2007) Am. J. Physiol. 293 L1250-L1260 [DOI] [PubMed] [Google Scholar]
- 29.Bartoszewski, R., Rab, A., Jurkuvenaite, A., Mazur, M., Wakefield, J., Collawn, J. F., and Bebok, Z. (2008) Am. J. Respir. Cell Mol. Biol. 39 448-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaser, A., Lee, A. H., Franke, A., Glickman, J. N., Zeissig, S., Tilg, H., Nieuwenhuis, E. E., Higgins, D. E., Schreiber, S., Glimcher, L. H., and Blumberg, R. S. (2008) Cell 134 743-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox, J. S., Chapman, R. E., and Walter, P. (1997) Mol. Biol. Cell 8 1805-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travers, K. J., Patil, C. K., Wodicka, L., Lockhart, D. J., Weissman, J. S., and Walter, P. (2000) Cell 101 249-258 [DOI] [PubMed] [Google Scholar]
- 33.Paradiso, A. M., Ribeiro, C. M. P., and Boucher, R. C. (2001) J. Gen. Physiol. 117 53-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro, C. M. P., Paradiso, A. M., Lazarowski, E., and Boucher, R. C. (2001) in Cilia, Mucus and Mucociliary Interactions (Salathe, M., ed) pp. 303-314, Marcel Dekker, Inc., New York
- 35.Credle, J. J., Finer-Moore, J. S., Papa, F. R., Stroud, R. M., and Walter, P. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18773-18784 [DOI] [PMC free article] [PubMed] [Google Scholar]